Abstract
Mitochondrial neurogastrointestinal encephalopathy (MNGIE) is characterized by significant gastrointestinal dysmotility. Early and long-term nutritional therapy is highly recommended. We report a case of MNGIE in a patient who was undergoing long-term nutrition therapy. The patient was diagnosed with a serious symptom of fatty liver and hyperlipidemia complications, along with homozygous mutation of the thymidine phosphorylase (TYMP) gene (c.217G > A). To our knowledge, this is the first report of such a case. Herein, we describe preventive measures for the aforementioned complications and mitochondrial disease-specific nutritional therapy.
Keywords: Mitochondrial neurogastrointestinal encephalopathy syndrome, TYMP gene, Nutrition therapy, Complications
Introduction
Mitochondrial neurogastrointestinal encephalopathy (MNGIE) is caused by mutations in the nuclear gene TYMP. The TYMP gene is located on chromosome 22q13.33, and it encodes thymidine phosphorylase, which catalyzes thymidine phosphorylation or the transition of deoxyuridine to thymine or uracil. Therefore, TYMP dysfunction is responsible for the instability and dysfunction of the respiratory chain in mitochondrial DNA [1]. MNGIE is a multisystemic autosomal recessive disorder clinically characterized by ptosis, progressive external ophthalmoplegia, gastrointestinal dysmotility, cachexia, peripheral neuropathy, and leukoencephalopathy [1]. To our knowledge, fewer than 200 cases of MNGIE have been reported in the literature [2]. Garone et al. [1] reviewed 102 cases of MNGIE that occurred between 1988 and 2011. They found that the mean onset age was 32.4 years, life expectancy was approximately 35 years, and approximately 33% of the patients were born to consanguineous parents. In addition, more than half of the patients were initially diagnosed with concomitant gastrointestinal diseases, and gastrointestinal symptoms appeared to eventually affect almost all the patients [3]. Therefore, rational nutrition support for the patients was extremely important. The case report was approved by the ethics committee of the Peking Union Medical School Hospital.
Case
The patient was a 32-year-old Chinese man who was admitted to the Peking Union Medical School hospital because he could not eat. After his admission, the patient was found to have had intermittent episodes of intestinal obstruction for 2 years and had been receiving enteral nutrition (EN) and intermittent parenteral nutrition (PN) therapy. His parents were consanguineous.
His physical examination result indicated a height of 162 cm, BMI of 13.3 kg/m2, blood pressure of 90/50 mmHg, and heart rate of 140 bpm. In addition, cachexia, drooping of the levatorpalpebrae muscle, mirror tongue, positive sound of water vibration in the stomach, loss of limbs with peripheral sensory hyperalgesia, and reduced tendon reflexes were detected.
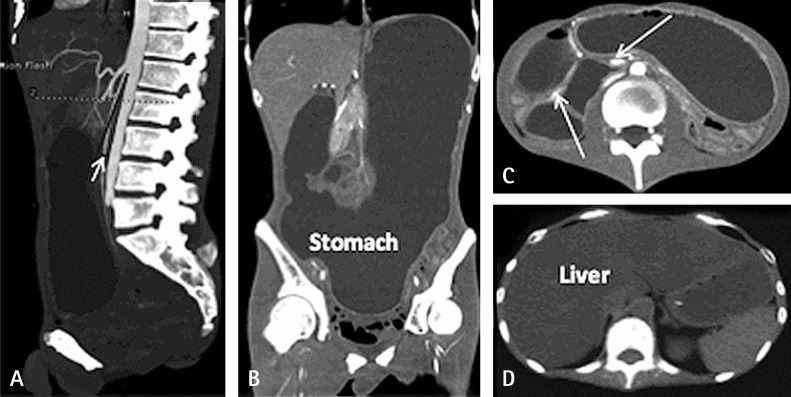
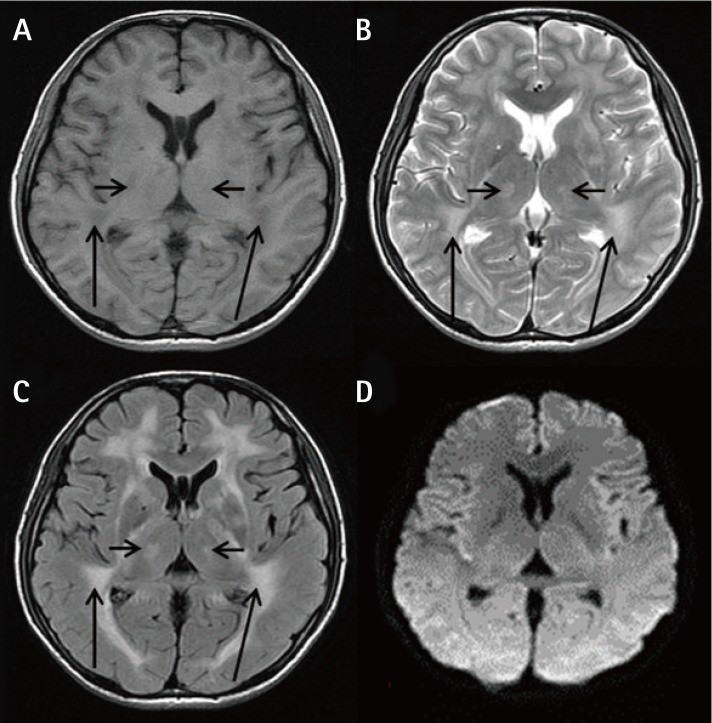
The laboratory test results demonstrated a triglyceride (TG) level of 18.8 mmol/L. His abdominal and pelvic computed tomography scan revealed a small angle between the superior mesenteric artery and abdominal aorta (Figure 1), low stomach tension (Figures 1A and 1B), a partial horizontal segment of the duodenum under pressure (Figures 1A and 1C), and significantly expansive intestines with thickened walls (Figure 1C), as well as a severe fatty liver (Figure 1D). An MRI scan of the brain presented white matter changes (Figure 2). A genetic test revealed a homozygous TYMP gene (c.217G > A) mutation in the nuclear DNA. Accordingly, MNGIE was diagnosed. Our patient was treated with capacity expansion, water-and-electrolyte balance maintenance, gastrointestinal decompression, total PN (TPN) therapy, containing energy at 33.69 kcal/kg/day, and fat emulsion suspension. Seven days later, TG was administered at 4.88 mmol/L, as well as a Calvin injection with supplements of energy at 40 kcal/kg/day, soybean fat emulsion at 1.94 g/kg, and l-carnitine at 1 g. Ten days later, the patient was administered TG at 15.17 mmol/L. Thereafter, an individualized formulation was administered, containing energy at 46 kcal/kg/day, structured triacylglycerol emulsion at 1.43 g/kg, and l-carnitine at 3 g. After 4 days, the TG dose was reduced to 11.09 mmol/L. Two weeks after admission, both EN and PN were provided, together with supplements of vitamins B1, B6, and B12; vitamin C; folic acid; Q10; and probiotics. The details of the examination and nutrition therapy are shown in Table 1. One month later, the patient was discharged on his own and was provided with Weaver (Nestlé) at 600 kcal/day only. He died 5 days after discharge.
Figure 1. Abdominal and pelvic computed tomography scans. (A): The angle between the abdominal aorta and superior mesenteric artery (short white arrow) was significantly smaller. (A) and (B): The position of the stomach was significantly lower. The stomach and intestines were significantly dilated with large quantities of liquid. (C): The gastrointestinal wall was uniformly thick with homogeneous enhancement (long white arrows). (D): The density of the whole liver was significantly reduced, and the volume was elevated.
Figure 2. Cranial MRI showing cerebral white matter changes. (A): A T1-weighted image showing bilateral cerebral white matter fibers (long black arrows) and the bilateral thalamus (short black arrows) with diffuse, symmetrical, low-signal intensity. (B) and (C): T2-weighted (B) and fluid-attenuated inversion recovery images (C) showing bilateral cerebral white matter fibers(long black arrows) and the bilateral thalamus(short black arrows) with diffuse, symmetrical, high-signal intensity. (D): No significant diffusion is observed on the diffusion-weighted.
Table 1. Biochemical test results and nutrition therapy of the patient.
| Hospital Stay, days | -7 | -1 | 1 | 8 | 12 | 16 | 22 |
|---|---|---|---|---|---|---|---|
| TG, mmol/L | 18.08 | 4.88 | 15.61 | 15.17 | 11.09 | ||
| ALT, U/L | 78 | 46 | 50 | 44 | 47 | 41 | 15 |
| Hemoglobin, g/L | 77 | 74 | 80 | 113 | 97 | 74 | |
| Total energy per day, kcal | 1612 | 1283 | 1179 | 1400 | 1800 | 1710 | 1610 |
| NPC, kcal | 1432 | 1147 | 1043 | 1220 | 1620 | 1550 | 1450 |
| Fat, g | 68 | 51 | 51 | 68 | 68 | 50 | 50 |
| Fat, g/kg | 1.94 | 1.46 | 1.46 | 1.94 | 1.94 | 1.43 | 1.43 |
| Glucose, g | 205 | 172 | 147 | 130 | 230 | 275 | 225 |
| Glucose, g/kg | 5.86 | 4.06 | 4.2 | 3.71 | 6.57 | 7.86 | 6.43 |
| Amino acids, g | 45 | 34 | 34 | 45 | 45 | 40 | 40 |
| Amino acids, g/kg | 1.29 | 0.97 | 0.97 | 1.29 | 1.29 | 1.14 | 1.14 |
| Proportion of energy supply by glucose, % | 57 | 60 | 56 | 43 | 57 | 71 | 62 |
| Proportion of energy supply by fat, % | 43 | 40 | 46 | 57 | 43 | 29 | 38 |
TG: triglyceride, ALT: alanine aminotransferase, NPC: daily intake of non-protein calories, Fat (g/kg): grams of fat intake per kilogram of body weight, Glucose (g/kg): grams of glucose intake per kilogram of body weight, Amino acids (g/kg): grams of amino acids intake per kilogram of body weight.
Discussion
In the present case, according to the clinical features and homozygous mutation of the TYMP gene (c.217G > A), the patient was diagnosed with MNGIE [1]. The cause of such a mutation has not yet been reported.
For 2 years, our patient had been receiving EN and intermittent PN treatments. B-Ultrasonography performed 1 year prior to the present admission indicated no fatty liver, and as the patient reported, his previous blood lipid test results were within the normal limits. Consequently, current severe fatty liver and TG hyperlipidemia were considered to be associated with long-term nutrition therapy. It is known that PN often causes hepatic steatosis and cholestasis complications, and TG hyperlipidemia affected 25-50% of patients receiving PN therapy [4]. Cavicchi et al. [5] conducted a 10-year observation of patients receiving long-term PN and found that 65% of the patients developed chronic cholestasis after a median of 6 months. Among these patients, the 2-, 4-, and 8-year incidence rates of chronic cholestasis were 55%, 64%, and 72%, respectively. Garone et al. [1] reviewed 102 MNGIE cases and found that among the patients enrolled, 5 had fatty liver, 1 had hepatomegaly, 1 had liver cirrhosis, 1 had abnormal liver function, and 2 had TG hyperlipidemia. Although the alanine aminotransferase level of our patient was mildly elevated (Table 1), liver damage and clinical manifestation were not necessarily in synchronism. In a study by Cavicchi et al. [5], 11 of 57 liver biopsy samples presented varying severity of pathological changes, ranging from portal area inflammatory infiltration to necrosis, even though they had normal liver function.
One week before admission, the TPN solution administered to our patient included energy at a dose of 35.63-46.06 kcal/kg/day, glucose at a dose of 4.91-5.86 g/kg, and soybean fat emulsion at a dose of 1.46-1.94 g/kg (Table 1). For patients with low body weights, 20-25 kcal/kg/day is generally sufficient to meet their energy needs [6]. Fat utilization ability reduces excessive nutrient substrates in the body, and glucose promotes insulin secretion and fat synthesis, and inhibits fatty acid oxidation, all of which are contributors to fatty acid accumulation in liver cells [7]. Cavicchi et al. [5] reported that a soybean fat emulsion dosage of > 1 g/kg/day provided to patients who were receiving home PN was associated with chronic cholestasis and liver diseases. The 2-year incidence of severe liver damage was 50%; however, it was only 20% for patients who were administered lower doses. Soybean fat emulsion is rich in ω-6 polyunsaturated fatty acids, phytosterols, and arachidonic acid, all of which are considered related to liver damage.
Medium chain fatty acids (MCFAs) in a structured triacylglycerol emulsion are carnitine independent, and they can directly enter the mitochondria without a transporter, for use in oxidation. Consequently, it contributes to less TG accumulation in blood, as well as less interference to liver function [8]. TPN formulation does not include carnitine; therefore, long-term TPN directly affects fatty acid oxidation due to carnitine deficiency. Low levels of creatine, carnitine, and folic acid are observed among patients with mitochondrial dysfunction [9,10]; therefore, they are often used together as a "mitochondrial cocktail" therapy for mitochondrial diseases. The recommended dosage for adults was 1 g/day [11], but we increased it to 3 g/day to further reduce the TG accumulation and liver damage.
In conclusion, from this case, we learned that the total energy and fat doses in long-term PN should be decreased for patients with low weight, and metabolic parameters and organ functions should be monitored intensively. Individualized PN formulations, rather than standardized ones, should be adopted and adjusted [12]. For patients who already have liver damage, glucose and fat intakes should be limited to 2-3 and 1 g/kg/day, respectively. Besides, parenteral administration of MCFA emulsion and branched amino acid-enriched amino acid solution as nitrogen sources are highly recommended [4,13]. Furthermore, adding fish oil, making full use of the gastrointestinal tract, glutamine supplementation, and probiotic administration are beneficial to promote bile acid enterohepatic circulation and recover partial intestinal microflora, as well as to reduce bacterial translocation and improve intestinal barrier function [7]. Coenzyme Q10, vitamin E, vitamin B2, creatine, carnitine, and folic acid supplements may help improve respiratory chain function in mitochondria [9,14,15]. However, further research is needed to confirm the clinical significance of these drugs for patients with MNGIE. Patients with mirror tongue and large-cell anemia should be provided with B vitamins, particularly vitamin B1, to prevent PN-related Wernicke encephalopathy.
Unfortunately, when our patient was diagnosed, his disease was already in a severe stage, and the nutrition therapy was not sufficiently appropriate. Although his situation improved after the therapy was adjusted, the effect was not significant. Therefore, early and rational nutrition support is crucial to provide patients with the opportunity to get further treatment.
Acknowledgments
We appreciate the willingness and cooperation of our patient and his parents, who have given their consent for the publication of this report.
Footnotes
Conflict of Interests: None of the authors has any conflicts of interest.
References
- 1.Garone C, Tadesse S, Hirano M. Clinical and genetic spectrum of mitochondrial neurogastrointestinal encephalomyopathy. Brain. 2011;134:3326–3332. doi: 10.1093/brain/awr245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xieng Y, Gu WY, Hou XL, Yang YL, Jiang Y, Zhou CL. Mitochondrial hyneurogastrointestinal encephalomyopathy of neonatal onset: a case report and literature review. Chin J Evid Based Pediatr. 2013;8:126–130. [Google Scholar]
- 3.Yavuz H, Ozel A, Christensen M, Christensen E, Schwartz M, Elmaci M, Vissing J. Treatment of mitochondrial hyneurogastrointestinal encephalomyopathy with dialysis. Arch Neurol. 2007;64:435–438. doi: 10.1001/archneur.64.3.435. [DOI] [PubMed] [Google Scholar]
- 4.Hartl WH, Jauch KW, Parhofer K, Rittler P Working group for developing the guidelines for parenteral nutrition of the German Association for Nutritional Medicine. Complications and monitoring - Guidelines on Parenteral Nutrition, Chapter 11. Ger Med Sci. 2009;7:Doc17. doi: 10.3205/000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavicchi M, Beau P, Crenn P, Degott C, Messing B. Prevalence of liver disease and contributing factors in patients receiving home parenteral nutrition for permanent intestinal failure. Ann Intern Med. 2000;132:525–532. doi: 10.7326/0003-4819-132-7-200004040-00003. [DOI] [PubMed] [Google Scholar]
- 6.Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C Espen. ESPEN Guidelines on Parenteral Nutrition: intensive care. Clin Nutr. 2009;28:387–400. doi: 10.1016/j.clnu.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Xu ZW, Li YS. Pathogenesis and treatment of parenteral nutrition-associated liver disease. Hepatobiliary Pancreat Dis Int. 2012;11:586–593. doi: 10.1016/s1499-3872(12)60229-x. [DOI] [PubMed] [Google Scholar]
- 8.Rubin M, Moser A, Vaserberg N, Greig F, Levy Y, Spivak H, Ziv Y, Lelcuk S. Structured triacylglycerol emulsion, containing both medium- and long-chain fatty acids, in long-term home parenteral nutrition: a double-blind randomized cross-over study. Nutrition. 2000;16:95–100. doi: 10.1016/s0899-9007(99)00249-x. [DOI] [PubMed] [Google Scholar]
- 9.Allen RJ, DiMauro S, Coulter DL, Papadimitriou A, Rothenberg SP. Kearns-Sayre syndrome with reduced plasma and cerebrospinal fluid folate. Ann Neurol. 1983;13:679–682. doi: 10.1002/ana.410130620. [DOI] [PubMed] [Google Scholar]
- 10.Melegh B, Seress L, Bedekovics T, Kispál G, Sümegi B, Trombitás K, Méhes K. Muscle carnitine acetyltransferase and carnitine deficiency in a case of mitochondrial encephalomyopathy. J Inherit Metab Dis. 1999;22:827–838. doi: 10.1023/a:1005562209034. [DOI] [PubMed] [Google Scholar]
- 11.Artuch R, Vilaseca MA, Pineda M. Biochemical monitoring of the treatment in paediatric patients with mitochondrial disease. J Inherit Metab Dis. 1998;21:837–845. doi: 10.1023/a:1005470702369. [DOI] [PubMed] [Google Scholar]
- 12.Pichard C, Mühlebach S, Maisonneuve N, Sierro C. Prospective survey of parenteral nutrition in Switzerland: a three-year nation-wide survey. Clin Nutr. 2001;20:345–350. doi: 10.1054/clnu.2001.0428. [DOI] [PubMed] [Google Scholar]
- 13.Staun M, Pironi L, Bozzetti F, Baxter J, Forbes A, Joly F, Jeppesen P, Moreno J, Hébuterne X, Pertkiewicz M, Mühlebach S, Shenkin A, Van Gossum A ESPEN. ESPEN Guidelines on Parenteral Nutrition: home parenteral nutrition (HPN) in adult patients. Clin Nutr. 2009;28:467–479. doi: 10.1016/j.clnu.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Napolitano A, Salvetti S, Vista M, Lombardi V, Siciliano G, Giraldi C. Long-term treatment with idebenone and riboflavin in a patient with MELAS. Neurol Sci. 2000;21:S981–S982. doi: 10.1007/s100720070015. [DOI] [PubMed] [Google Scholar]
- 15.Bakker HD, Scholte HR, Jeneson JA. Vitamin E in a mitochondrial myopathy with proliferating mitochondria. Lancet. 1993;342:175–176. doi: 10.1016/0140-6736(93)91379-z. [DOI] [PubMed] [Google Scholar]