Abstract
The SIGFRIED (SIGnal modeling For Real-time Identification and Event Detection) software provides real-time functional mapping (RTFM) of eloquent cortex for epilepsy patients preparing to undergo resective surgery. The current study presents the first application of paradigms used in functional magnetic resonance (fMRI) and electrical cortical stimulation mapping (ESM) studies for shared functional cortical mapping in the context of RTFM. Results from the three modalities are compared.
A left-handed 13-year old male with intractable epilepsy participated in functional mapping for localization of eloquent language cortex with fMRI, ESM, and RTFM. For RTFM, data were acquired over the frontal and temporal cortex. Several paradigms were sequentially presented: passive (listening to stories) and active (picture naming and verb generation.)
For verb generation and story processing, fMRI showed atypical right lateralizing language activation within temporal lobe regions of interest (ROI) and bilateral frontal activation with slight right lateralization. Left hemisphere ESM demonstrated no eloquent language areas. RTFM procedures using story processing and picture naming elicited activity in the right lateral and basal temporal regions. Verb generation elicited strong right lateral temporal lobe activation as well as left frontal lobe activation.
RTFM results confirmed atypical language lateralization evident from fMRI and ESM. We demonstrated the feasibility and usefulness of a new RTFM stimulation paradigm implementation during pre-surgical evaluation. Block design paradigms used in fMRI may be optimal for this purpose. Further development is needed to create age-appropriate RTFM test batteries.
Introduction
Functional cortical mapping is an essential part of epilepsy surgery programs. The gold standard for functional mapping of language and motor function remains electrical cortical stimulation mapping (ESM) to inhibit selected functional processes. 1 Although the method is routinely used during evaluation prior to epilepsy surgery, it has several drawbacks, including the invasive nature of surgery, the risk of inducing seizures, long testing time, unpleasant sensations induced in patients (especially relevant for pediatric population), and the fact that it is inhibiting normal physiological function, rather than measuring it. Many attempts have been made to perform functional mapping with non-invasive methods, such as functional magnetic resonance imaging (fMRI). 2 Although fMRI has proven to be useful, especially in language mapping, and can provide real-time results 3, it is relatively expensive, time intensive, requires substantial technical expertise, and cannot be performed directly at a patient's bedside.
Recently, technologies have been developed that bridge neurophysiology and computational sciences. These technologies include brain-computer interfaces (BCI), which analyze changes in spontaneous cortical activation during execution of particular overt or covert tasks. BCI technologies have enabled real-time functional mapping (RTFM) of cortical function with minimal additional risk to patients. It does not require the inhibition of physiological processes, as with ESM, but rather measures normal physiological activity in response to various stimuli, such as sounds, pictures, or words. This novel method allows assessment of the larger neural network involved in the processing of relevant information instead of testing the inhibition of only a limited portion of the circuitry supporting the function, as in case of ESM.
Whereas both RTFM and ESM procedures are invasive (i.e., they both utilize grids placed directly on the patients' brain), RTFM relies on interpretation on passively recorded brain signals rather than on stimulation that produce temporary lesions to actively disrupt brain function. In other words, RTFM provides an interpretation of physiological brain activation elicited by sound, spoken words or other natural stimuli.
The SIGFRIED (SIGnal modeling For Real-time Identification and Event Detection) software, developed for the general-purpose BCI2000 system, 4 provides real-time functional mapping (RTFM) prior to resective brain surgery. SIGFRIED provides real-time bedside visualization of cortical activation during various passive and active tasks that the patient performs. Results of such mapping for localization of motor function in adults have been shown to be in substantial concordance with those produced using ESM. 5 It has also proven to be useful in intraoperative monitoring. 6
Whether this novel methodology can be applied to pediatric epilepsy surgical patients, in particularly for mapping their language function, was unknown. Moreover, the concordance between functional localization findings with RTFM, fMRI, and ESM has not been previously assessed. In our present study, we provide the first: (1) assessment of the feasibility and methodological advantages of RTFM-based language mapping using BCI2000/SIGFRIED software 7 in pediatric epilepsy surgical patient; (2) application of paradigms currently used in fMRI and ESM studies for functional cortical mapping in the RTFM context; and (3) comparison of the results of functional mapping from three different modalities: RTFM, fMRI, and ESM.
Materials and Methods
Subject description
The Institutional Review Board of the Cincinnati Children's Hospital Medical Center (CCHMC), Cincinnati, Ohio, USA approved the study protocol. The subject was a left-handed 13-year old male with intractable epilepsy. His typical seizure consisted of right arm stiffening, hand fumbling, and staring. The patient underwent the standard CCHMC preoperative assessment in order to determine the epileptogenic area and eloquent cortex. The evaluation included a neuropsychological exam, seizure characterization by clinical semiology, long term video-electroencephalography (vEEG), MRI with epilepsy surgery protocol, ictal/interictal SPECT (single-photon emission computed tomography) with subsequent SISCOM (subtraction ictal SPECT co-registered with MRI), FDG-PET (positron emission tomography), MEG (magnetoencephalography), and fMRI. 8
Neuropsychological evaluation
A neuropsychologist performed the neuropsychological evaluation. On the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV), the patient scored in the extremely low to average range on the following scales: Verbal Comprehension Index (VCI), Perceptual Reasoning Index (PRI), Processing Speed Index (PSI), Working Memory Index (WMI), and Full Scale IQ (FSIQ).
Invasive EEG monitoring
Invasive EEG monitoring was recommended, because the non-invasive evaluations revealed incongruent predictions of the suspected epileptogenic zones, and also implicated eloquent functional areas. For invasive monitoring, the patient underwent a three-step surgical procedure. The goal of the first step was to better lateralize seizure activity. In this step, six 4-contact subdural electrocorticographic (ECoG) strips were placed bilaterally on the anterior sub-temporal, medial sub-temporal, and posterior sub-temporal cortical surfaces (24 electrodes) (Fig. 1a). After this subdural strip placement, the RTFM procedure was performed. Subsequent to determining that all seizures originated from the left temporal lobe, the second step of the surgery was placement of additional subdural grids (totalling 80 electrodes) that covered the left temporal lobe (Fig. 1b, Left view). The ESM procedure was performed after additional subdural grid placement. ECoG recordings revealed that the patient had the primary ictal onset in the left temporal lobe (left anterior/subtemporal) with the evolution to right temporal lobe.
Figure 1.
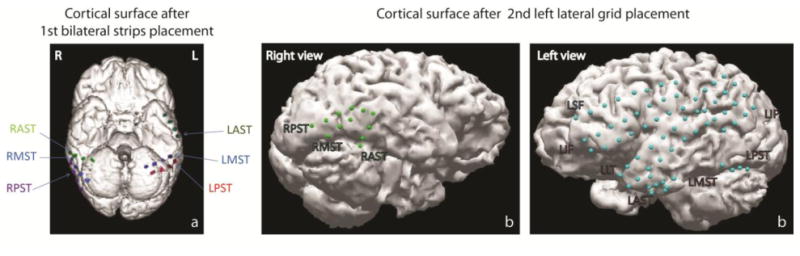
Schematic representation of subdural strip and grid placement for the patient after: (a) 1st surgery with 24 bilateral contact strip placement; and (b): 2nd surgery with additional 56 contact grid placements on the left hemisphere (the total number of placed electrodes on both hemispheres is equal to 80). Strips and grids contacts are presented as colored dots on the 3-D model of the brain surface.
MRI, CT, and ECoG co-registration
A 3D volumetric MRI scan performed at 3T (T1 weighted FFE 3D, isotropic 1mm voxels) and a high resolution CT scan (512 × 512 matrix, 0.5 mm slice thickeness) were carried out to precisely visualize the location of subdural electrodes on the brain surface. The MRI and CT were coregistered and 3D segmentation of the brain surface and grid positions was performed using the Analyze AVW 4.0 platform (Mayo Clinic).
Functional mapping
Three types of functional mapping were used in order to determine localization of eloquent motor and language cortex: fMRI, ESM, and RTFM.
fMRI
Functional MRI was performed using standardized paradigms for clinical evaluation at CCHMC. 9 The fMRI examination was performed on a Philips 3T Achieva scanner utilizing standard block paradigms for language assessment (Table 1). The paradigms were reviewed with the subject prior to the exam performance. Image processing was performed using Brain Voyager QX (version 2.2.1.1650, Brain Innovations, Maastricht, Netherlands). Pre-processing included 3D motion correction, slice scan time correction, and spatial smoothing using a Gaussian filter (FWHM - 4mm). A general linear model logistic regression approach was utilized for statistical analysis. Co-registered interpolated activation maps were produced and overlaid onto isotropic 1mm T1 weighted anatomic images using a mutual information algorithm, fine-tuned manually. Clinical statistical maps were made by real-time alterations of significance levels. Both verb generation runs were combined for final statistical analysis. Regions of interests (ROIs) for quantitative analysis were based upon prior normative studies in both the frontal and temporal parietal regions. 10 Standard lateralization indices (LI) of activated voxels were calculated between each hemisphere in both the frontal and temporal-parietal regions for verb generation, and the temporal parietal region of interest for story processing. LI of ≤ -0.2 were deemed to represent right hemispheric lateralization and LI ≥ 0.2 left hemispheric lateralization. Median statistical thresholds for LI calculation were p < 0.0007 (qFDR <0.05) for story processing, and p < 0.00001 (qFDR <0.05) for the combined verb generation paradigms.
Table 1. Tasks presented during RTFM.
| Tasks | Mapping methodology | |||
|---|---|---|---|---|
| Task name | Task description | RTFM | ESM | fMRI |
| Story processing | Involved the auditory presentation of five simple stories, each composed of ten sentences with specifically formulated and complex syntactic constructions that engage multiple brain regions. The control tasks were identical periods of temporally reversed speech (for fMRI) and silent intervals (for RTFM). For this subject, two verb generation (finger tap) tasks, and one story processing task (reversed speech) were performed 20 | + (1st grid placement) | - | + |
| Picture naming | Pictures with simple objects (black on a white background) were presented to a subject, and he was asked to name them covertly and overtly 21 | + (1st grid placement) | + | - |
| Verb generation | Involved the auditory presentation of a series of concrete nouns every 5 s. The patient was instructed to covertly (silently) generate as many verbs associated with the noun as possible. The control task was bilateral sequential finger thumb opposition (finger tapping) cued by a target tone played every 5 s 22 (for fMRI) and silent intervals (for RTFM) | + (2nd grid placement) | - | + |
(“+” indicates that the task was performed in the indicated modality; and “-“ indicates that it was not)
ESM
ESM was performed for identification of language and motor cortex. Stimulations consisted of five-second trains of 200 msec square-wave pulses of alternating polarity at 50 Hz were applied to the adjacent electrode pairs using an Ojemann Cortical Stimulator (Integra LifeSciences Corporation, Plainsboro, New Jersey, USA). Stimulation current intensities were progressively increased beginning at 2 mA to a maximum of 10 mA with 2-mA increments at each cortical site unless after-discharge activity was detected on ECoG. ESM of language functions consisted of picture naming (Table 1).
RTFM
ECoG signals recorded from the grids were separated into two streams – for clinical recording and for RTFM research study in order to ensure recording of the ECoG for seizures remained uninterrupted during our study procedure. For clinical purposes, ECoG signal acquisition was performed together with simultaneous video-recording with the 128-channel Stellate EEG system (Stellate, Quebec, Canada) at a sampling rate of 2000 Hz. For RTFM purposes, the data were acquired using g.USBamp devices (24 Bit biosignal amplification unit, g.tec Medical Engineering GmbH, Austria) at a sampling frequency of 1200 Hz. The ground and reference scalp electrode were applied on the mastoid contralateral to the grid placement. BCI2000 with the SIGFIED module were used for signal processing and display visualization. RTFM procedure was repeated twice – after 1st and 2nd grid placements (Fig. 1).
The SIGFRIED method is described in detail in Schalk et al 7 and Brunner et al. 5. Briefly, the SIGFRIED procedure first estimates the statistical properties of the brain signals recorded during the resting condition. Using the resulting statistical model of the resting condition, it then estimates the likelihood that a new data point is produced by the resting signal distribution. The output of SIGFRIED is the negative log of that probability (so that resulting values are scaled to facilitate visualization). Thus, this output can be expected to be small for samples that are similar to those in the resting signal distribution, and large for samples that are different than those in the resting state distribution.
Practically, the following actions were performed during the real-time SIGFRIED procedure: First, the signal from each grid contact was re-referenced using a common average reference (CAR) filters. Then, for each grid contact and 500-ms period, the time series ECoG signal was converted into the frequency domain using an autoregressive model with a model order of 120. Frequencies between 70 and 100 Hz (10 bins at 4-Hz bandwidth) were submitted to SIGFRIED. During online processing, SIGFRIED then used the established baseline model to calculate for each grid contact the likelihood that the signal at that grid contact was statistically different from the modelled baseline signals. This likelihood was calculated every 100 ms. Finally, for each grid contact and task, the distribution of the negative log-transformed likelihood values was further re-referenced to those values calculated during the resting period between the tasks by calculating the value of r2, that is, the proportion of values that was accounted for by the task. This resulted in a value between 0 (not different) and 1 (very different) for each grid contact and task.
The results from the signal analyses described above were visualized in real-time using a topographic interface. The interface contained, for each task (i.e., story processing), a display of the r2 values at each location. Each display contained one circle at each electrode's location. The size of each circle and its tint was proportional to the r2 value. Thus, a large red circle represented a large statistical difference between the corresponding task and rest, whereas a small black circle indicated a small statistical difference. The display corresponding to each task was autoscaled to the minimum and maximum r2 value.
The subject sat in front of a screen and was instructed to relax and remain as still as possible. Baseline cortical activity was first recorded for 6 minutes. After that, several paradigms were consequently presented to the subject (Table 1). These included simple tasks that were presented passively, such as story processing (performed after 1st grid placement), and complex tasks that required the subject's active participation and response, such as picture naming (performed after 1st grid placement) and verb generation (performed after 2nd grid placement procedure).
Results
fMRI
fMRI revealed atypical language lateralization for both story processing and verb generation paradigms. For verb generation, at median thresholds, there was bilateral, symmetric inferior frontal activation, and more pronounced right lateralization of activation in the temporal parietal regions. LI in the inferior frontal ROI was -0.03, and within the temporal – parietal ROI, -0.94. For story processing, there was bilateral activation in the frontal lobes and more localized right lateralizing activation in the right temporal lobe along the superior temporal sulcus. Temporal-parietal ROI analysis at median statistical threshold resulted in a LI of -0.86. Overall fMRI results were concordant for right hemispheric lateralization for temporal-parietal language ROI with both paradigms, and indeterminate (but slightly right lateralizing) for frontal lobe language ROI with verb generation.
ESM
ESM in the left hemisphere demonstrated no eloquent language areas. We observed no significant arrest or interruption of speech responses with ESM during the picture-naming task on either left temporal or frontal expected language areas (Fig. 2).
Figure 2.
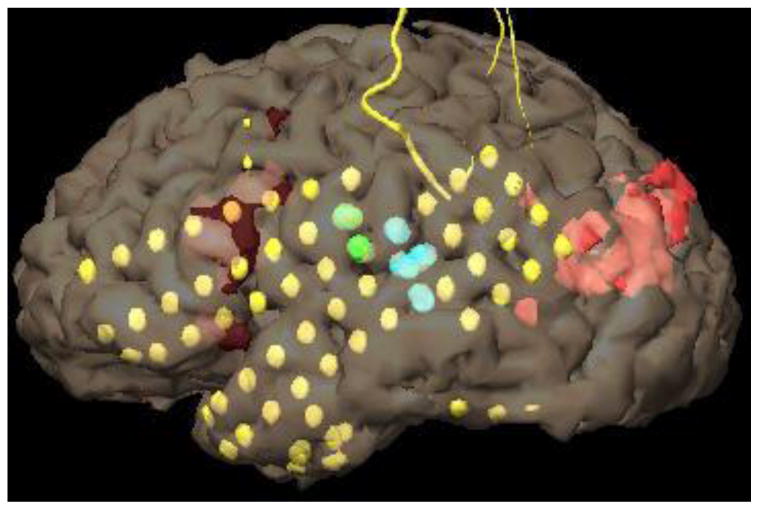
Results of left hemispheric ESM after 2nd grid placement. Green: Tongue motor. Blue: sensation of tongue thickness (tongue sensory), no aphasia. Testing of all other grid pairs including all of the remaining LIF and LLT grid pairs did not produce significant speech deficits. Yellow dots represent subdural electrodes.
RTFM
The RTFM procedure was well tolerated by the patient. Most stimuli paradigms elicited some degree of cortical activation. With the 1st grid coverage, story processing as well as picture naming elicited activity in the right lateral and basal temporal regions (Fig. 3). After the 2nd grid coverage, verb generation task demonstrated atypical language activation, evident in strong right temporal lobe activation together with left frontal lobe activation (Fig. 4). The RTFM results indicating atypical right hemisphere dominance for language correlated well with right-lateralizing fMRI results (as well as indirect ESM results indicating possible right-hemisphere dominance for language). The RTFM results for verb generation after 2nd grid placement procedure also were in line with the RTFM results for story processing and picture naming obtained after 1st grid placement procedure.
Figure 3.
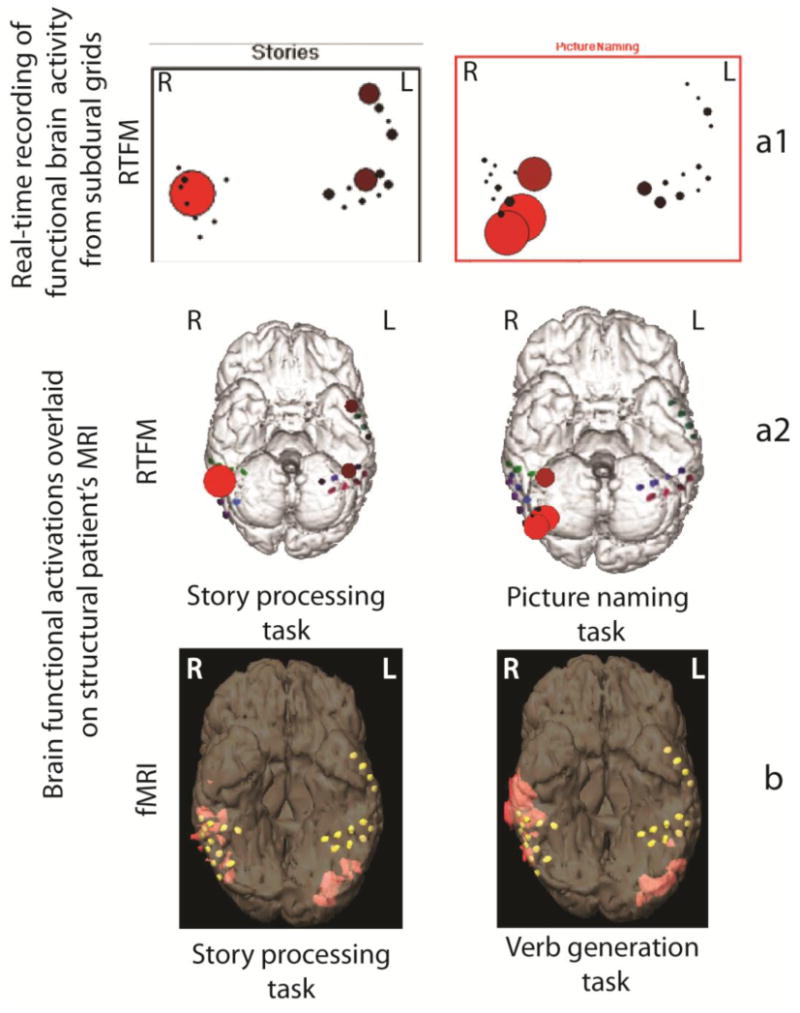
Cortical language activation in epilepsy patient: (a) – results of RTFM recording performed after 1st grid placement procedure. Cortical RTFM activation maps indicate right-sided language laterlization elicited during picture naming and story processing task. The RTFM responses are maximal in the right lateral and basal temporal regions (locations with significant levels of activation are presented as large red circles; grid placement locations are indicated as red, green and blue dots); (a1) – indicates the response recorded from the grids in real-time; (a2) – indicates the same response overlaid off-line on the 3-D model of the patient's brain; (b) – fMRI activation maps with verb generation and story processing tasks (locations with significant levels of activation are indicated in orange; grid placement locations are indicated as yellow dots). With “L” is indicated left hemisphere, with “R” is indicated right hemisphere.
Figure 4.
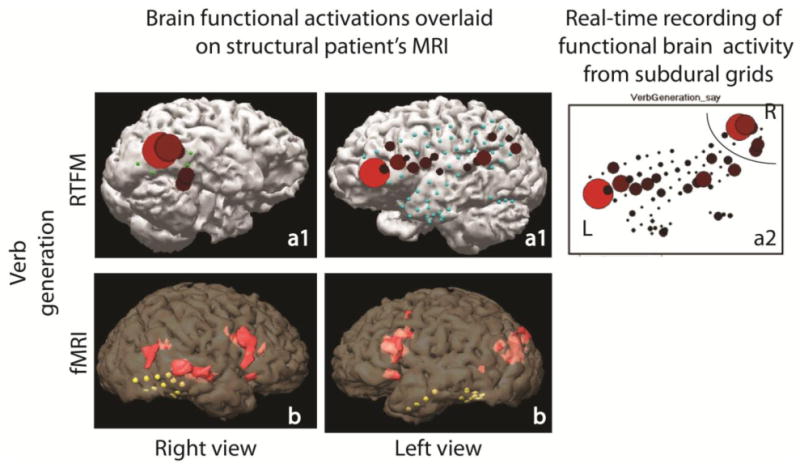
Cortical language activation elicited during verb generation task in epilepsy patient: (a) – RTFM activation maps recorded after 2nd grid placement procedure; (a1) – represents 3D model of the brain with off-line overlaid activation maps, indicated in picture a2; (b) – fMRI activation maps for right and left cerebral hemispheres.
Discussion
We observed right-hemisphere lateralizing language function as directly evidenced from fMRI and RTFM results and indirectly from ESM results. Such interhemispheric language function reorganization observed in our studied patient is a common phenomenon in patients with left lateralized epilepsy focus. 11 The transfer of language function occurs both intra- and inter-hemispherically in chronic epilepsy patients, especially when left hemisphere epilepsy develops at an early age. 12 This leads to impairment in non-verbal functions (“crowding” phenomenon), 13-16 as well as deficits in verbal intellectual abilities 17 and academic skills (both reading and spelling). 18 Identifying these changes is of highest priority for epilepsy surgery, as this identification may guide the surgery and consequently improve the likelihood of favourable language functioning after the surgery. In our study, RTFM confirmed abnormal language lateralization evident directly from fMRI data and indirectly from ESM. This case shows that RTFM may correctly identify atypical language lateralization with language transfer from the left to the right hemisphere in patients with chronic epilepsy, which can be due to the presence of pathologic tissue in the language dominant hemisphere. The results of our study indicate a potential for the RTFM technique to be applied in intra-operative surgical practice in pediatric patients for fast and reliable language mapping complimentary to intraoperative ESM.
While the use of RTFM has many advantages, its potential future clinical role, in particular with respect to ESM mapping is still somewhat unclear. In its emerging clinical use, RTFM is usually employed prior to ESM mapping. Its results are then used to inform and optimize ESM mapping. More specifically, at present the final clinical determinations are still made using ESM mapping, but RTFM testing is typically useful for choosing cortical sites of lower priority for ESM mapping. This approach may reduce the time of ESM stimulation and, consequently, the possible number of seizures elicited during ESM. This is particularly important for children, who have low tolerance for ESM procedure.
If used alone, one of the most obvious benefits of RTFM procedure would be reduction of time and concomitant increase in cost effectiveness of the pre-surgical work-up. The ESM procedure utilizes two electrodes at a time to create a “lesion” between these two locations and observe possible change in function in this particular cortical area. From our personal experience working with children, we can tell that that “mapping” the cortical area located under 128 contacts (pretty standard coverage for children undergoing evaluation for epilepsy surgery in Florida Hospital for Children) may take up to 6-8 hours. At the same time, the full evaluation of complete language, motor and sensory function while using RTFM with several repetitions rarely exceeds two hours. In general, the results of RTFM mapping are provided within just a few minutes. Importantly, activation under electrodes localized with RTFM is seen as a whole network, whereas ESM provides only single location results after each consecutive stimulation.
RTFM also has more flexibility for presenting language tasks for both receptive and expressive language function than ESM. This is because during RTFM, patients can provide responses within a broader time frame than during ESM, which is restricted in time by electric pulse duration. As a result, additional areas of the brain might be found to be involved in language function than those determined by ESM. Flexibility to present stimuli in time are especially important for mapping language function in children and low-functioning individuals, who need more time to process information and do not have capacity to respond within stringent time required by ESM.
As in other imaging modalities (fMRI, MEG), it is of great importance for RTFM testing to use appropriate stimulation batteries. In a previous study by Schalk et al., 7 which concentrated on methodological developments of RTFM technique with SIGFRIED software, vertical cursor movement controlled with motor cortical ECoG activity towards the vertical position of a target was used to identify and assess motor cortex. In another study, Brunner et al. 5 employed alternating sequences of repetitive movements of the tongue (protrusion and retraction) and movements of the hand (opening and closing) and resting – similar to those used in ESM protocols. In their case study, Roland et al. 19 used recitation of the alphabet for one of the patients and a part of the Pledge of Allegiance for another one. However, no studies using specific controlled tasks, investigating different aspects of language functions necessary for pre-surgical evaluation were performed yet. Moreover, none of the paradigms targeted pediatric population specifically. In our case study, we utilized specific test batteries to localize expressive (picture naming, verb generation) and receptive (story processing) language functions. Block design allowed us a close comparison with fMRI findings.
In the future, specific language stimuli corresponding to cognitive functioning level of study participant must be developed for RTFM purposes. Future studies may choose to address other important functions, e.g., executive functions, cognitive and emotion-related circuitry, with appropriate, validated, behavioural paradigms, such as recognition of emotions in faces.
Conclusions
Our case study suggests that RTFM appears to be feasible for functional cortical mapping, especially for determining language lateralization in pediatric epilepsy surgery patients. RTFM results confirmed abnormal language lateralization evident from fMRI and ESM. Compared to ESM, RTFM is less likely to induce a seizure, does not interrupt ECoG recording of spontaneous seizures, and provides: (1) real-time (bedside) visualization of cortical functional activation; (2) broad selection of stimulation paradigms; (3) opportunity to study patients without requiring active participation (for example, story processing task); (4) functional testing soon after grid placement while antiepileptic drugs lowered.
We demonstrated possibilities for new stimulation paradigm implementation for RTFM used in pre-surgical evaluation. A number of different stimuli can be used, and block design paradigms may be optimal for this purpose. Further development is needed to create age-appropriate paradigm batteries for RTFM.
References
- 1.Gallentine WB, Mikati MA. Intraoperative electrocorticography and cortical stimulation in children. J Clin Neurophysiol. 2009;26:95–108. doi: 10.1097/WNP.0b013e3181a0339d. [DOI] [PubMed] [Google Scholar]
- 2.Mehta AD, Klein G. Clinical utility of functional magnetic resonance imaging for brain mapping in epilepsy surgery. Epilepsy Res. 2011;89:126–32. doi: 10.1016/j.eplepsyres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Kesavadas C, et al. Real-time functional MR imaging (fMRI) for presurgical evaluation of paediatric epilepsy. Pediatr Radiol. 2007;37:964–74. doi: 10.1007/s00247-007-0556-4. [DOI] [PubMed] [Google Scholar]
- 4.Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Trans Biomed Eng. 2004;51:1034–43. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- 5.Brunner P, et al. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav. 2009;15:278–86. doi: 10.1016/j.yebeh.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leuthardt EC, et al. Electrocorticographic frequency alteration mapping: a clinical technique for mapping the motor cortex. Neurosurgery. 2007;60:260–70. doi: 10.1227/01.NEU.0000255413.70807.6E. discussion 270-1. [DOI] [PubMed] [Google Scholar]
- 7.Schalk G, et al. Real-time detection of event-related brain activity. Neuroimage. 2008;43:245–9. doi: 10.1016/j.neuroimage.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo JH, et al. Multimodality imaging in the surgical treatment of children with nonlesional epilepsy. Neurology. 2011;76:41–8. doi: 10.1212/WNL.0b013e318204a380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leach JL, Holland SK. Functional MRI in children: clinical and research applications. Pediatr Radiol. 2010;40:31–49. doi: 10.1007/s00247-009-1452-x. [DOI] [PubMed] [Google Scholar]
- 10.Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27:202–12. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kadis DS, et al. Intrahemispheric reorganization of language in children with medically intractable epilepsy of the left hemisphere. J Int Neuropsychol Soc. 2007;13:505–16. doi: 10.1017/S1355617707070397. [DOI] [PubMed] [Google Scholar]
- 12.Liegeois F, et al. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127:1229–36. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- 13.Gleissner U, et al. Clinical and neuropsychological characteristics of pediatric epilepsy patients with atypical language dominance. Epilepsy Behav. 2003;4:746–52. doi: 10.1016/j.yebeh.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Strauss E, Satz P, Wada J. An examination of the crowding hypothesis in epileptic patients who have undergone the carotid amytal test. Neuropsychologia. 1990;28:1221–7. doi: 10.1016/0028-3932(90)90057-u. [DOI] [PubMed] [Google Scholar]
- 15.Orsini DL, Satz P. A syndrome of pathological left-handedness. Correlates of early left hemisphere injury. Arch Neurol. 1986;43:333–7. doi: 10.1001/archneur.1986.00520040021012. [DOI] [PubMed] [Google Scholar]
- 16.Satz P, Orsini DL, Saslow E, Henry R. The pathological left-handedness syndrome. Brain Cogn. 1985;4:27–46. doi: 10.1016/0278-2626(85)90052-1. [DOI] [PubMed] [Google Scholar]
- 17.Billingsley R, Smith ML. Intelligence profiles in children and adolescents with left temporal lobe epilepsy: relationship to language laterality. Brain Cogn. 2000;43:44–9. [PubMed] [Google Scholar]
- 18.Breier JI, et al. Atypical language representation in patients with chronic seizure disorder and achievement deficits with magnetoencephalography. Epilepsia. 2005;46:540–8. doi: 10.1111/j.0013-9580.2005.48904.x. [DOI] [PubMed] [Google Scholar]
- 19.Roland J, Brunner P, Johnston J, Schalk G, Leuthardt EC. Passive real-time identification of speech and motor cortex during an awake craniotomy. Epilepsy Behav. 2010;18:123–8. doi: 10.1016/j.yebeh.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Karunanayaka PR, et al. Age-related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34:349–60. doi: 10.1016/j.neuroimage.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Kunii N, Kamada K, Ota T, Kawai K, Saito N. A detailed analysis of functional magnetic resonance imaging in the frontal language area - a comparative study with extraoperative electrocortical stimulation. Neurosurgery. 2011 doi: 10.1227/NEU.0b013e3182181be1. [DOI] [PubMed] [Google Scholar]
- 22.Vannest J, et al. FMRI activation in language areas correlates with verb generation performance in children. Neuropediatrics. 2010;41:235–9. doi: 10.1055/s-0030-1267982. [DOI] [PubMed] [Google Scholar]


