Abstract
Individuals with alcohol use disorders (AUDs) have deficits in cognitive control, but how they change with treatment is unclear. Seven patients with AUD and anxiety from an open-label trial of disulfiram plus lorazepam performed a multisensory Stroop task during fMRI (both pre and post initiation of treatment), and were compared to nine healthy controls (HCs) (n = 16; Albuquerque, NM; years 2009–2012). Evoked BOLD signal and resting state functional connectivity were compared (HC vs. AUD; Scan 1 vs. Scan 2). AUD demonstrated hyperactivity and altered connectivity in the cognitive control network compared to HC, but treatment did not normalize function.
Keywords: alcohol use disorder, alcohol dependence, fMRI, connectivity, cognitive control, Stroop, lorazepam, benzodiazepine, treatment
INTRODUCTION
Loss of control of alcohol use and continuing to drink despite negative consequences are characteristics of alcohol use disorder (AUD), an illness with devastating individual and societal costs (American Psychiatric Association, 2000; Ezzati, Lopez, Rodgers, Vander Hoorn, & Murray, 2002). Difficulty maintaining abstinence may be partially mediated by impairments in cognitive control, either as a result of longstanding alcohol use, or as a premorbid trait that contributed to development of the disorder (Kopera et al., 2012; Scheurich, 2005). Individuals with AUD have higher levels of impulsivity, and impaired performance on response inhibition and working memory tasks, which require cognitive control (Bjork, Hommer, Grant, & Danube, 2004; Dao-Castellana et al., 1998; Goudriaan, Oosterlaan, de Beurs, & van den Brink, 2006; Kopera et al., 2012; Li, Luo, Yan, Bergquist, & Sinha, 2009; Pitel et al., 2009; Wilcox, Dekonenko, Mayer, Bogen-chutz, & Turner, 2013). Moreover, impairments in these domains may predict poorer response to treatment and relapse (Charney, Zikos, & Gill, 2010; Evren, Durkaya, Evren, Dalbudak, & Cetin, 2012; Miller, 1991) and are related to increased sensitivity to drug-related cues and cue-induced craving (Noel et al., 2007; Petit, Kornreich, Noel, Verbanck, & Campanella, 2012; Volkow et al., 2009). Identifying the neurobiological basis for deficits in cognitive control and identifying how these markers change with treatment may help guide development of treatments for AUD. In this study, we identified functional brain differences between AUD and healthy controls (HC) during a Stroop-like task and during resting state, using functional connectivity analyses. We also examined functional brain changes in AUD in response to treatment.
The cognitive control network has been extensively studied in HC, and is comprised of the dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), anterior cingulate cortex (ACC), supplementary motor area (SMA), pre-SMA, premotor, and motor cortex, parietal cortex, thalamus, striatum, insula, and cerebellum (Ridderinkhof, van den Wildenberg, Segalowitz, & Carter, 2004; Roberts & Hall, 2008). This network is altered in AUD (Wilcox et al., 2013). However, results of functional neuroimaging studies of AUD using tasks requiring cognitive control (response inhibition, working memory, and Stroop-like tasks) have been mixed, and indicate the story may be more complex than straightforward hypo- or hyperfunction (Desmond et al., 2003; Karch et al., 2008; Li et al., 2009; Park et al., 2011; Pfefferbaum et al., 2001; Schmaal et al., 2013; Schulte, Muller-Oehring, Sullivan, & Pfefferbaum, 2012; Tapert et al., 2001, 2004). Differences between studies are not consistently explained by length of abstinence, task performance differences, or presence of comorbidities/concurrent other substance use or disorder. Perhaps some of the more consistent findings across studies are DLPFC (Karch et al., 2008; Li et al., 2009; Park et al., 2011; Pfefferbaum et al., 2001) and medial prefrontal cortex (mPFC)/ACC hypofunction (Dao-Castellana et al., 1998; Karch et al., 2008; Tapert et al., 2001). Functional changes in VLPFC (Desmond et al., 2003; Li et al., 2009; Park et al., 2011; Pfefferbaum et al., 2001; Tapert et al., 2004), parietal lobes, premotor/motor cortex (Karch et al., 2008; Park et al., 2011; Pfefferbaum et al., 2001; Tapert et al., 2001, 2004), and subcortical regions (thalamus, striatum) (Karch et al., 2008; Park et al., 2011; Schmaal et al., 2013) are less consistent, with some studies demonstrating hyperactivation, and others hypoactivation. Of the tasks testing cognitive control, studies using response inhibition tasks are the most consistent, showing either no significant differences between groups or hypoactivation in AUD (Karch et al., 2008; Li et al., 2009; Schmaal et al., 2013).
Given the lack of consistency across tasks engaging cognitive control, we chose to derive our hypotheses from three studies. The first was the only study performed using a Stroop-like task in AUD, which demonstrated that AUD did not deactivate the posterior cingulate cortex (PCC) whereas HC did (congruent minus incongruent contrast) (Schulte et al., 2012). The second compared AUD with high anxiety to AUD with low anxiety during a response inhibition task, and this study found that high trait anxiety during response inhibition was associated with increased activation in the left DLPFC, right VLPFC, temporal gyri, inferior parietal lobe, precuneus, PCC, and thalamus but also with decreased activation in parietal lobe, precentral gyrus, and superior frontal gyrus (BA10) (Karch et al., 2008). The third study, which was performed in this laboratory, using an identical task to the one used in the present study (a multisensory Stroop task), demonstrated increased activation in PFC, striatum, and thalamus in cocaine users compared to HC (Mayer et al., 2013). We hypothesized that the present sample of AUD participants with anxiety would demonstrate increased brain activation in regions involved in cognitive control during the task.
Functional connectivity analyses provide information about spatially distributed networks by mapping correlations in intrinsic low-frequency brain oscillations in the BOLD response (Fox & Raichle, 2007). Prior investigations of functional connectivity within the cognitive control network, and between this network and other brain regions, demonstrate functional differences between AUD and HC. For example, AUD severity is associated with weaker functional connectivity between putamen and ACC, mPFC, and insula (Courtney, Ghahremani, & Ray, 2013), and between dorsal striatum (caudate) and mOFC (Lee et al., 2013). Moreover, AUDs demonstrate less synchrony between PCC and both cerebellum (Chanraud, Pitel, Pfefferbaum, & Sullivan, 2011) and middle cingulate (Schulte et al., 2012) but increased connectivity between midbrain and both middle cingulate/SMA and putamen (Schulte et al., 2012) compared to HC. Connectivity studies in AUD focused on other networks (stress reactivity, cue reactivity, motor, sensory processing) have also demonstrated decreased connectivity between cortical regions known to be involved in cognitive control [dorsal ACC (dACC)], VLPFC, middle frontal gyrus, parietal lobules, DLPFC, premotor cortex, insula, and other regions in AUD compared to HC and in individuals with more severe AUD (Maurage et al., 2013; O’Daly et al., 2012; Rogers, Parks, Nickel, Katwal, & Martin, 2012). We therefore expected that in our study AUD would demonstrate decreased functional connectivity during resting state among regions involved in top–down cognitive control (corticocortical and corticostriatal circuitry).
For a variety of reasons, the benefit of benzodiazepines in individuals with co-occurring anxiety and AUD is unclear and controversial (Lader, 2011). Benzodiazepines were recently explored (in combination with disulfiram) for the treatment of AUD in individuals with co-occurring anxiety in a 16-week open-label trial, at the end of which the benzodiazepine was tapered off (clinicaltrials.gov identifier: NCT00721526; manuscript under preparation). Benzodiazepines are commonly used to lower anxiety levels during treatment of alcohol withdrawal, and they may decrease craving, but there are few studies of their longterm effects on alcohol consumption (Mayo-Smith, 1997; Posternak & Mueller, 2001). The rationales for combining a benzodiazepine with disulfiram in this clinical trial were that the benzodiazepine could serve as a positive reinforcer for taking the disulfiram, and could decrease anxiety and insomnia symptoms (possible relapse triggers) (Brower, 2003; Posternak & Mueller, 2001). Moreover, in addition to preventing drinking via its usual mechanism (expectation of the alcohol-disulfiram reaction), disulfiram could minimize one of the primary risks of benzodiazepines (sedation in combination with other sedating drugs) by discouraging concurrent heavy drinking.
The effect of benzodiazepines on cognitive performance and networks mediating cognitive control is not clear. On the one hand, benzodiazepines are associated with decrements in performance on cognitive tasks (Rush, Higgins, Bickel, & Hughes, 1993, 1994). On the other hand, because anxiety or stress may affect performance on tasks of cognitive control (Schoofs, Preuss, & Wolf, 2008) and related PFC function (Porcelli et al., 2008; Qin, Hermans, van Marle, Luo, & Fernandez, 2009), benzodiazepines could, theoretically, improve cognitive control. Effects of benzodiazepines on brain networks in AUD or individuals with anxiety have not been investigated. However, benzodiazepines are associated with decreased brain activation in PFC and other brain regions during cognitive tasks in HC (Coull, Frith, & Dolan 1999; Sperling et al., 2002; van Ruitenbeek, Vermeeren, Mehta, Drexler, & Riedel, 2013). Treating anxiety in AUD with concurrent anxiety could, theoretically, either improve or worsen cognitive control, but we hypothesized that, given their anxiety, treatment with benzodiazepines in these AUD would be associated with normalization (decreased activation) in the cognitive control network.
In summary, the current study had two primary aims. The first aim was to compare functional activation during a multisensory Stroop task (Mayer et al., 2011, 2013) and intrinsic resting state functional connectivity between AUD with co-occurring anxiety relative to HC. The second aim was to investigate effects of benzodiazepine treatment on these metrics. We hypothesized that the present sample of AUD participants would demonstrate increased brain activation in cognitive control networks compared to HC during the task and decreased functional connectivity during resting state within PFC and within corticostriatal circuitry, and that treatment with a benzodiazepine would result in normalization of brain function.
METHODS
Participants: AUD
Seven subjects with a confirmed diagnosis of alcohol dependence (43.6 +/− 14.8 years old, 13.6 +/− 2.1 years of education, 71% male) were recruited from an open label treatment study targeting individuals with co-occurring alcohol dependence and anxiety disorder (clinicaltrials.gov identifier: NCT00721526). The treatment study was designed to investigate the efficacy of a treatment combining lorazepam, disulfiram, and a standard psychosocial intervention. AUD participants were required to meet criteria for a primary or secondary (alcohol-induced) anxiety disorder, to have a goal of abstinence, and to have at least four heavy drinking days in the last 30 days. Participants were excluded from the study if they had a history of significant medical conditions (e.g., seizure disorder, sleep apnea, symptomatic coronary artery disease, kidney disease, liver disease), severe alcohol withdrawal, psychiatric conditions (e.g., schizophrenia, schizoaffective disorder, bipolar disorder), or contraindications for MRI. At screening, over a 90 day reporting period, AUD (n = 7) reported consuming an average of 23.1 standard drinks per drinking day (SD 23.6) and had an average of 32.1% days abstinent (SD 38.6).
Per SCID assessment, all AUD participants met criteria for active alcohol dependence, five for a current episode of MDD of moderate severity, four for panic disorder, four for social phobia, six for generalized anxiety disorder, and two for PTSD. One participant met criteria for cocaine dependence in remission for at least one month, and one for stimulant dependence current. The former had not used cocaine for over 3 months. The latter had used methamphetamine 12 out of the prior 90 days, but had not used methamphetamine for 20 days prior to Scan 1, and did not use methamphetamine between Scan 1 and Scan 2.
Participants: HC
Nine HC (37.6 +/− 9.0 years old, 14.2 +/− 1.6 years of education, 33% male) were selected from a pool of 16 participants who had been recruited as controls for an unrelated study but who had undergone similar tasks during fMRI (Mayer et al., 2013; Wilcox Teshiba, Merideth, Ling, & Mayer, 2011). HCs were initially excluded based on similar criteria as the participants with AUD with the additional criteria of any history of diagnosed psychiatric disorder, major medical condition, learning disorder, attention deficit hyperactive disorder, or any major neurological condition. None of these participants met criteria for current alcohol or other drug dependence or abuse, or past drug or alcohol dependence. Seven participants were eliminated from the original group of 16 (Wilcox et al., 2011) for remote past hallucinogen, cannabis, and alcohol abuse, or for taking progesterone/natural supplements. None of the HC met criteria for alcohol or other substance use disorder.
Procedures
Informed consent for all participants was obtained according to institutional guidelines at the University of New Mexico. After consent, all individuals with AUD underwent an MRI scan (Scan 1) prior to initiation of the lorazepam and disulfiram combination treatment. Six of the seven AUD participants underwent a second identical scan session 5 to 7 days after initiating medication treatment (Scan 2). HC only underwent a single MRI scan.
All AUD participants stated they had been abstinent from alcohol for a minimum of 24 h (which was confirmed by breathalyzer), had not used recreational drugs within the prior 3 days, were not in acute withdrawal based on a score on the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) (Sullivan, Sykora, Schneiderman, Naranjo, & Sellers, 1989) of ≤ 8, and had urine toxicology screens negative for opiates, cocaine, and amphetamines prior to all MRI scans. Two AUD participants were positive for benzodiazepines prior to the first scan, and reported recently receiving oxazepam for the treatment of alcohol withdrawal. However, we kept them in the study because, for both participants, their last dose had been >24 h prior to the scan, and, given the pharmacokinetics of oxazepam, would have had blood levels significantly lower than the levels were at Scan 2. Five were taking other medications at both Scan 1 and Scan 2 [diphenhydramine n = 2, cough syrup n = 1, quetiapine n = 1, citalopram n = 1, melatonin n = 2, loratadine n = 1, pseudoephedrine n = 1] but none were taken within 2 h prior to the scan.
For HC, urine toxicology screens were negative for all drugs prior to MRI scans except for one, in which a urine screen was not collected. No HC were on medications at the time of the scan.
All AUD vs. HC comparisons were done with the AUD data acquired during the initial scan for AUD (Scan 1). All Scan 1 vs. Scan 2 comparisons were done with the AUD data alone (as only AUD underwent two scan sessions). For AUD, mean days between Scan 1 and Scan 2 was 12.7 (SD 11.0), mean days between the first lorazepam dose and Scan 2 was 6.50 (SD 0.6), the lorazepam dose at Scan 2 was 0.5 mg by mouth three times daily, and mean hours between last lorazepam dose and Scan 2 was 2.5 (SD 0.6). All AUD participants who returned for the second scan (n = 6) reported being abstinent at least 8 days before Scan 2, and, besides the participant for whom the time between Scan 1 and Scan 2 was 36 days, all participants reported being completely abstinent between Scan 1 and Scan 2. For the week prior to Scan 1, participants (n = 6) reported consuming an average of 3.0 standard drinks per drinking day (SD 4.8) and had an average of 66.7% days abstinent (SD 51.6). For the week prior to Scan 2, all participants were completely abstinent. The average days since last drink (n = 6) at Scan 1 were 9.5 (SD 7.1) whereas average days since last drink at Scan 2 were 22.0 (SD 9.1).
Clinical Assessment
All participants completed a battery of measures, including the Fagerstrom Test for Nicotine Dependence (FTND) (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991), the Structured Clinical Interview for DSM Disorders I Module E (SCID-I-E) for substance abuse and dependence (Kranzler, Kadden, Babor, Tennen, & Rounsaville, 1996), and the Edinburgh handedness inventory (Oldfield, 1971). All participants completed a measure that estimates intelligence (Weschsler Test of Adult Reading; WTAR) (Wechsler, 1999) and performed a Stroop color and word test (Golden, 1978). For AUD, the Timeline Followback calendar was used to determine alcohol and other drug usage during the previous 30 days (Sobell, Sobell, Litten, & Allen, 1992). Self-rated anxiety using a five-point Likert scale was acquired verbally just before and just after performing the multisensory Stroop task, and the Alcohol Urge Questionnaire (AUQ) (Bohn, 1995) was administered before and after each scan in all AUD. A State-Trait Anxiety Index (STAI) (Spielberger, Gorsuch, & Suchene, 1970) and a Beck Depression Inventory-Second Edition (BDI) (Richter, Werner, Heerlein, Kraus, & Sauer 1998) were performed in HC and Hamilton Anxiety (HAM-A) (Maier, Buller, Philipp, & Heuser, 1988) and Depression Scales (HAM-D) (Williams, 1988) were performed in AUD. All scales were subsequently transformed to Z-scores to facilitate group comparisons using published data (Blanchard, Scharff, Schwarz, Suls, & Barlow, 1990; Spielberger, 1983; van Hemert, van de Vijver, & Poortinga, 2002; Zimmerman, Chelminski, & Posternak, 2004).
Tasks
A multisensory numeric Stroop task (Mayer et al., 2011, 2013) was presented to all participants during fMRI scanning (Figure 1). HCs underwent a longer variation of the task relative to AUD. For HC, each 10 s block consisted of simultaneously presented multisensory (visual and auditory) congruent or incongruent numeric stimuli (targets) occurring at a low (0.33 Hz) or a high (0.66 Hz) frequency. Each block started with the cue word (exemplary visual angle = 7.69°) “LOOK,” “HEAR,” or “NONE” followed by a stream of target numbers (one, two, or three; exemplary visual angle = 9.73°). If the cue word was “LOOK,” participants were instructed to press a button corresponding to the visual stimuli and ignore the number that was simultaneously presented aurally. If the cue word was “HEAR,” subjects attended to the aural number stream while ignoring visual targets. An additional passive condition (cue word “NONE”) was included (data not presented), during which participants were instructed not to respond to the targets. In contrast, AUD participants only completed the high frequency (0.66 Hz) trials with active attentional manipulations (i.e., “LOOK” and “HEAR” trials). There was a 1325 ms delay between the presentation of the cue (175 ms duration) and the presentation of the first target number (200 ms duration) to maximize attentional focus. Three or six trials were presented within each low- or high-frequency block, respectively. The inter-block interval was varied between 8, 10, and 12 s to decrease temporal expectations and permit modeling of the baseline (visual fixation plus baseline gradient noise). A total of 48 trials per trial type (192 total trials), and 8 blocks of each type (32 total blocks), were presented across two separate imaging runs for AUD. HC underwent the same number of trials and blocks as AUD for the trials-of-interest for this study, but this was spread over six imaging runs (to accommodate the other trial types). Before being placed in the scanner, participants practiced the behavioral task until demonstrating competency.
FIGURE 1.
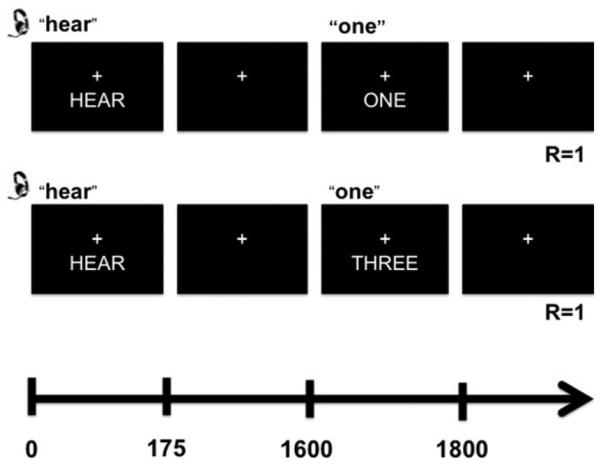
This figure depicts the trial structure of the multimodal Stroop task. “R” indicates the correct response for each condition illustrated. The upper trial is a congruent trial, and the lower trial is an incongruent trial. Both trials are auditory trials; the participant is asked to press the response button according to what they hear and not what they see.
A 2 × 2 mixed-measures analysis of variance (ANOVA) [group (AUD vs. HC) × condition (congruent vs. incongruent)] and a 2 × 2 repeated measures ANOVA [time (Scan 1 vs Scan 2) × condition (congruent vs. incongruent)] were conducted on median response time data for the task. No participants were found to be behavioral outliers based on reaction time.
For the resting state scan (RST), participants maintained visual fixation on a white cross (visual angle = 0.92°) on the center of the screen for 5.5 min. The resting state scan occurred after the multisensory Stroop task and our previously described tasks (Wilcox et al., 2011) in HC, and it took place at the beginning of the scan (prior to the multisensory Stroop task) in AUD participants.
MR Imaging and Analyses
High-resolution anatomic images T1 (voxel size = 1 mm3) and whole-brain echo-planar images (temporal resolution = 2,000 ms; echo time = 29 ms; flip angle = 75°; FOV = 240 mm; matrix size = 64 × 64; voxel size = 3.75 × 3.75 × 4.55 mm; slices = 33) were collected on a 3 Tesla Siemens Trio scanner. All participants were analyzed for excessive frame-wise head motion (greater than three times the interquartile range above 75th percentile on two or more of six parameters) compared to the rest of their cohort based on previously published algorithms (Mayer, Franco, Ling, & Canive, 2007) and none were determined to have excessive head motion using these criteria. Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996) was used to generate functional images using standard pre-processing techniques (time-slice correction, motion correction, 6 mm Gaussian full-width half-maximum spatial filter, and spatial normalization to Talairach space). A voxel-wise deconvolution analysis was then performed to generate a single hemodynamic response function (HRF) that spanned the first 22 s post-stimulus onset for each trial-type with error trials modeled separately (Mayer et al., 2011). Percent signal change (PSC) estimates were calculated based on the beta coefficients for the images occurring 6 to 14 s post-cue onset for each relevant trial type, and then divided by the average model intercept.
Resting state pre-processing was identical to the functional images acquired during the selective attention task. A regression analysis was then conducted on individual subjects’ time-series to remove potential sources of noise (physiological and machine-based) from the data (Wilcox et al., 2011). We chose to focus on three regions that are known to be recruited during cognitive control (Ridderinkhof et al., 2004; Roberts & Hall, 2008), and which also demonstrated group differences in evoked activation during the multisensory Stroop task: bilateral caudate, left DLPFC, and left ACC. In particular, we chose caudate because it is activated during Stroop tasks (Ali, 2010; Ghahremani et al., 2012; Mayer et al., 2013), it plays a significant role in control of motor behavior during decision making, and it signals anticipated reward and action reward contingencies, which is particularly relevant for studies of addictive disorders (Ridderinkhof et al., 2004). Regions of interest (ROI) were defined using either anatomical masks (bilateral caudate) from FSL Harvard Oxford Atlas or 16 mm diameter spheres (left DLPFC, left ACC) empirically derived from group comparisons on the multisensory Stroop task. The center of mass coordinates in Talairach space were as follows: left DLPFC (−35, 37, 28) and left ACC (−8, 14, 35).
For BOLD analyses, voxel-wise 2 × 2 [group (AUD vs. HC) × condition (congruent vs. incongruent)] mixedmeasures ANOVA were performed on the spatially normalized percent signal change measure for the highfrequency task. In addition, voxel-wise 2 × 2 [time (Scan 1 vs. Scan 2) × condition (congruent vs. incongruent)] repeated measures ANOVA were performed on the PSC estimates for the high-frequency task for AUD only. For connectivity analyses, paired samples t-tests were then performed to examine the group and longitudinal differences (Scan 1 to Scan 2) in intrinsic activity for each of these seeds. For all evoked task-associated findings, false positives were corrected at z > 2.3 (input Z-stat volume voxel-wise thresh-old) and p < .05 (p threshold for clusters) based on the Gaussian Random Fields theory as implemented in FSL (http://www.fmrib.ox.ac.uk/fsl/feat5/programs.html) using a whole brain mask. For all resting state connectivity results, false positives were corrected at z > 2.6 (input Z-stat volume voxel-wise threshold) and p < .05 (p threshold for clusters); a higher z threshold was used for connectivity results, as multiple seeds were tested.
RESULTS
Clinical Data
There were no significant differences (p > .10) between groups on major demographic variables or cognitive tests (Table 1). AUD had significantly greater self-reported anxiety than HC (p ≤ .01) (Table 1). There were also significant longitudinal differences within AUD participants on the anxiety Likert scale (post multisensory Stroop task; p = .04), craving measures (AUQ post-scan; p = .03), and CIWA scale (p = .04) consistent with the known beneficial effects of benzodiazepine administration on anxiety and alcohol withdrawal (Table 2).
TABLE 1.
Demographic and clinical characteristics of individuals with alcohol use disorders (AUD) and healthy controls (HC)
| HC | AUD | p Value | Cohen’s da | |||
|---|---|---|---|---|---|---|
| Gender (% F) | 67 | 29 | .31b | |||
| Ethnicity (% hispanic) | 67 | 43 | .61b | |||
| Mean | SD | Mean | SD | |||
|
| ||||||
| Age (yrs) | 37.56 | 8.99 | 43.57 | 14.80 | .33 | 0.49 |
| Education (yrs)c | 14.22 | 1.56 | 13.57 | 2.15 | .30 | 0.35 |
| HQc | 87.67 | 20.32 | 88.14 | 14.85 | .86 | 0.03 |
| Depression rawd | 4.67 | 4.44 | 19.67 | 6.35 | ||
| Depression Zd | −0.59 | 0.67 | 5.15 | 1.98 | <.01 | 3.88 |
| Anxiety rawe | Tr = 30.00 | Tr = 6.58 | 17.17 | 8.30 | ||
| St = 27.67 | St = 6.82 | |||||
| Anxiety Ze | Tr = −0.50 | Tr = 0.69 | 2.21 | 1.85 | Tr = .01 | 1.94 2.11 |
| St = −0.78 | St = 0.77 | St = <.01 | ||||
| FTND | No smokers | 1 smoker; score 9 | ||||
| WTAR T | 53.70 | 8.56 | 57.86 | 4.14 | .23 | 0.62 |
| Stroop Color T | 45.11 | 8.59 | 43.57 | 9.14 | .73 | 0.17 |
| Stroop Word T | 50.22 | 15.16 | 49.14 | 9.81 | .87 | 0.08 |
| Stroop Color-Word T | 50.56 | 7.04 | 48.86 | 7.29 | .64 | 0.24 |
| Stroop interference T | 50.89 | 5.37 | 48.71 | 6.82 | .49 | 0.36 |
Note: HQ = handedness quotient, BDI = Beck Depression Inventory, HAM-D = Hamilton Depression Scale, HAM-A = Hamilton Anxiety Scale, FTND = Fagerstrom Test for Nicotine Dependence, WTAR = Weschler Test of Adult Reading. Z scores and T scores derived from population means and standard deviations (see methods for details). Only data from 6 AUD were available for Hamilton Depression and Anxiety scales. Data from all AUD and HC for the remainder of the scales. T tests performed in Excel, and unequal/equal variances accounted for.
Derived from means and SD; http://ncalculators.com/statistics/effect-of-size-calculator.htm
Fisher’s exact test; http://www.graphpad.com/quickcalcs/contingency2/
Non-parametric tests (Mann–Whitney) performed when significance met (p < .05) on the Shapiro–Wilk test of normality in SPSS.
BDI-II Scale performed in HC and Hamilton Depression Scale performed in AUD.
STAI Trait and State performed in HC (Tr = Trait, St = State) and Hamilton Anxiety Scale performed in AUD.
TABLE 2.
Clinical characteristics of AUD at two timepoints: Scan 1 (before treatment) and Scan 2 (5–7 days after initiation of lorazepam/disulfiram combination)
| Scan 1 |
Scan 2 |
|||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | Cohen’s da | |
| Anxiety Likert pre Stroopb | 3.00 | 1.10 | 2.17 | 0.98 | .06 | 1.11 |
| Anxiety Likert post Stroop | 3.00 | 1.10 | 2.17 | 1.17 | .04 | 1.10 |
| AUQ pre | 19.67 | 8.69 | 19.17 | 6.31 | .89 | 0.06 |
| AUQ post | 26.83 | 10.13 | 17.00 | 5.06 | .03 | 1.53 |
| CIWA-ARb | 2.83 | 2.04 | 1.50 | 1.38 | .04 | 2.74 |
| SBP | 145.67 | 23.69 | 140.50 | 20.07 | .61 | 0.22 |
| DBP | 93.33 | 16.03 | 89.17 | 10.67 | .50 | 0.31 |
| HR | 81.33 | 24.46 | 77.83 | 8.45 | .70 | 0.23 |
| WTAR T | 57.67 | 4.50 | 58.17 | 3.49 | .62 | 0.24 |
| Stroop Color T | 41.50 | 8.02 | 43.50 | 9.01 | .36 | 0.42 |
| Stroop Word Tb | 47.00 | 8.76 | 45.83 | 8.35 | .60 | 0.31 |
| Stroop Color-Word T | 47.83 | 7.41 | 50.5 | 6.35 | .14 | 0.73 |
| Stroop interference T | 48.83 | 7.47 | 50.83 | 4.92 | .38 | 0.45 |
Note: Pre/post Stroop = during scan, just before/after multisensory Stroop task, AUQ = Alcohol Urge Questionnaire, CIWA = revised clinical institute withdrawal assessment for alcohol scale, SBP = systolic blood pressure, DBP = diastolic blood pressure, HR = heart rate, WTAR = Weschler Test of Adult Reading. T indicates t score used. All t tests (two-tailed, paired) performed in Microsoft Excel.
Derived from means, SD, and correlation between samples at http://cognitiveflexibility.org/effectsize/ using recommended approaches for within subjects effects [Morris, S. B., & DeShon, R. P. (2002). Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological Methods, 7, 105–125.]
Non-parametric tests (Wilcoxon Signed Rank) performed when significance met (p < .05) on the Shapiro–Wilk test of normality in SPSS.
Selective Attention Response Time Data
Accuracy data for all subject groups was quite high (AUD: 94.94 ± 5.24%; HC: 97.28 ± 2.03%) and non-parametric tests revealed no significant differences between groups when all trials were lumped together, or when trial types were analyzed separately (incongruent or congruent). Similarly non-parametric tests revealed no significant longitudinal differences within the AUD sample (Scan 1: 94.27 ± 5.40%; Scan 2: 96.09 ± 4.28%).
Results from the first ANOVA (group × condition; mixed-measures) for median response time data indicated significant main effects of group (F1, 14 = 14.16, p = .002) and condition (F1, 14 = 25.20, p < .001), but no significant condition × group (F1, 14 = 3.16, p = .097) interaction. Response times for HC (mean = 572.52 ms +/− 49.08) were faster than those for AUD (679.69 ms +/− 65.13) overall. Response times for congruent (mean = 584.95 ms +/− 75.67) trials were faster than incongruent (mean = 653.87 ms +/− 90.15) trials.
Results for the second ANOVA (condition × time; repeated measures in AUD; n = 6) demonstrated a significant effect of condition (F1, 5 = 12.99, p = .015; incongruent > congruent), but no significant time (F1, 14 = 0.60, p = .48) or condition × time (F1, 14 = 0.93, p = .38) interactions.
Functional Results
Multisensory Stroop Task: Group, Condition, Group × Condition
AUD participants demonstrated greater activation than HC in a variety of brain regions (main effect of group; Figure 2) consistent with our main hypothesis. Two patterns were observed. In the first pattern, regions were activated in both groups but to a greater degree in AUD. In the second pattern, AUD demonstrated increased activation whereas HC exhibited deactivation. Regions involved in the first pattern included the bilateral posterior insula and superior temporal gyri (BAs 13,22,41,42), and the left DLPFC (BA 9), SMA, dACC (BAs 6,24,31), motor cortex, inferior and superior parietal lobules, and precuneus (BAs 1,2,3,4,6,9,7,19,37,39,40,44). Regions involved in the second pattern included the bilateral inferior frontal gyrus (BA 47), orbitofrontal cortex (BAs 11,25), anterior insula (BA 13), superior temporal gyri (BAs 21,22,38), motor and pre-motor cortex (BAs 4,5,6), basal ganglia, thalamus and globus pallidus and the right motor cortex (BAs 3,4), dACC and cingulate gyrus, middle and superior frontal gyri (BAs 6,8,24,32), uncus, and parahippocampal gyrus (BAs 28,34,35) extending into amygdala.
FIGURE 2.
This figure depicts the regions showing differences in activation between individuals with alcohol use disorder plus anxiety (AUD) and healthy controls (HC) during a multisensory numeric Stroop task (main effect of group). Activation maps are color-coded (cold colors: AUD > HC; warm colors: AUD < HC) according to the magnitude and the direction of the z score. Axial (Z) slice location is provided according to the Talairach atlas. AUD exhibited increased activation compared to HC in a variety of regions including the bilateral insula, inferior frontal gyrus (IFG), basal ganglia, thalamus and left dorsolateral prefrontal cortex (DLPFC), and dorsal anterior cingulate cortex (dACC).
For all participants, increased activation during incongruent compared to congruent trials was observed in the cognitive control network including the bilateral rostral ACC (rACC), medial frontal gyrus (BAs 6,9,10,24,32), dACC, SMA, insula (BA 13), cerebellum, thalamus, basal ganglia, globus pallidus, red nucleus, mamillary bodies, and pons extending into left hypothalamus, as well as in left DLPFC (BAs 9,46), VLPFC, and ventromedial PFC (VMPC) (BAs 44,45,47), inferior parietal lobule, posterior insula, and middle and superior temporal gyri (BAs13,21,22,39,40,42), extending into motor cortex (BAs 4,6).
The group by condition interaction was not significant.
Multisensory Stroop Task: Time, Condition, Time × Condition
There were significant differences in brain activation from Scan 1 to Scan 2 for AUD when all trials (both conditions) were combined (main effect of time) but these findings appeared to track white matter and, therefore, were likely artifact or secondary to registration errors. There were no significant main effects of condition.
There was a significant time by condition interaction for AUD within one cluster, with simple effects testing (paired t-tests) indicating significantly greater deactivation during congruent trials compared to incongruent trials within the right inferior parietal lobule, insula, and middle, and superior temporal gyri (BAs 13,22,39,40) during Scan 1, whereas during Scan 2 there was slight activation rather than deactivation during both incongruent and congruent trials (congruent greater than incongruent; non-significant).
Seed-Based Connectivity Analysis: Group
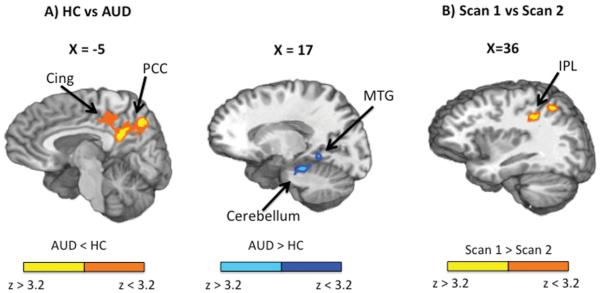
Increased connectivity between the bilateral caudate seed and left fusiform gyrus, parahippocampal gyrus, middle temporal gyrus (BAs 19,20,21,36,37), amygdala, hippocampus, cerebellum, and pons, was observed for AUD relative to HC (in which the caudate seed was anticorrelated with these regions). Increased connectivity between the bilateral caudate seed and bilateral cingulate gyrus, PCC, precuneus, and right paracentral lobule (BAs 7,23,31) was observed for HC relative to AUD (in which caudate seed was anticorrelated with these regions) (Figure 3(A)).
FIGURE 3.
This figure depicts the regions showing differences in intrinsic resting state functional connectivity between AUD and HC (panel A) and differences in intrinsic resting state functional connectivity in AUD between Scan 1 and Scan 2 (panel B). Panel A: Activation maps are color-coded according to the magnitude and the direction of the z score (cold colors: AUD > HC; warm colors: AUD < HC). Saggital (X) slice locations are provided according to the Talairach atlas. Increased connectivity between the bilateral caudate seed and bilateral posterior cingulate cortex (PCC) and cingulate gyrus (Cing) was observed for HC compared to AUD. Increased connectivity between the bilateral caudate seed and left cerebellum, and middle temporal gyrus (MTG) was observed for AUD relative to HC. Panel B: Activation maps are color-coded according to the magnitude and direction of the z score (warm colors: Scan 1 > Scan 2). Sagittal (X) slice location is provided according to the Talairach atlas. Increased anticorrelation between the bilateral caudate seed and left inferior parietal lobe (IPL) was observed for Scan 2 relative to Scan 1.
There were no significant Group effects for the left ACC or left DLPFC seeds.
Seed-Based Connectivity Analysis: Time
Finally, increased anticorrelation between caudate seed and left inferior and superior parietal lobule and post central gyrus (BAs 2,7,40) was observed in Scan 2 relative to Scan 1 (in which correlation between these regions was negligible) (Figure 3(B)).
There were no significant time effects for the left ACC or left DLPFC seeds.
DISCUSSION
Similar to prior work in AUD (Desmond et al., 2003; Schulte et al., 2012; Tapert et al., 2004) and work from our laboratory in individuals with cocaine use disorders (Mayer et al., 2013), AUD demonstrated hyperactivation in a variety of areas including the bilateral VLPFC, insula, premotor cortex, parietal lobes, precuneus, thalamus, striatum, and left DLPFC and ACC during a multimodal Stroop task, as well as alterations in resting state functional connectivity compared to HC. Although less extensive, there were also functional changes in resting state connectivity during treatment with a combination of lorazepam and disulfiram. Similar to our study in individuals with cocaine use disorders (Mayer et al., 2013), we also observed greater activation during incongruent relative to congruent trials in the cognitive control network, indicating that the task was operating on the brain in the expected fashion.
Although the observed hyperactivation was consistent with our hypothesis and findings from some prior studies, it is still not clear why other work has demonstrated hypometabolism (Dao-Castellana et al., 1998) or hypoactivation (Li et al., 2009; Park et al., 2011; Schmaal et al., 2013; Tapert et al., 2001) in brain regions mediating cognitive control in AUD. Response inhibition tasks such as the Go No-Go and Stop Signal paradigms may be associated with hypoactivation in AUD (Li et al., 2009; Schmaal et al., 2013), and the more complicated Stroop and working memory paradigms with hyperactivation (Desmond et al., 2003; Mayer et al., 2013; Schulte et al., 2012) in individuals with AUD and other substance use disorders (SUD), although not all studies of working memory tasks have been found to be associated with hyperactivation in AUD (Park et al., 2011; Tapert et al., 2001). Alternatively, hyperactivation, in some cases, may represent compensatory activation due to deficiencies in other brain regions mediating cognitive control (Desmond et al., 2003; Mayer et al., 2013). Our relatively small sample size may have limited power for the group × condition interactions.
Also consistent with our initial hypotheses, AUD demonstrated decreased corticostriatal connectivity, which is an important circuit in the cognitive control network (Ridderinkhof et al., 2004). Specifically AUD demonstrated decreased connectivity between caudate and cingulate, PCC and precuneus, compared to HC. These findings replicate previous work showing impaired connectivity between dorsal striatum and a variety of cortical areas involved in cognitive control in more severe AUD (Courtney et al., 2013; Lee et al., 2013) and in other addictions (Hong, 2013). Our findings are also in support of studies in HC and individuals with attention deficit hyperactivity disorder, which indicate that increased corticostriatal functional connectivity may mediate improvements in performance on cognitive tasks (Cubillo et al., 2010; Hwang, Velanova, & Luna, 2010).
In addition, AUD demonstrated increased connectivity compared to HC between caudate and brainstem, consistent with other work demonstrating increased connectivity between lower brain regions (midbrain) and striatum in AUD (Beck et al., 2012; Schulte et al., 2012), which may be related to the increased ability of alcohol cues to capture the attention of AUD (bottom up processing). The increased connectivity in AUD between caudate and limbic regions (temporal gyri, parahippocampal gyrus, hippocampus, and amygdala) could be theorized to increase the influence of affective responses on motor planning and decision-making pathways, thereby providing a possible mechanism by which craving networks may out-compete networks mediating behavioral restraint. In addition, AUD demonstrated increased connectivity between cerebellum and caudate compared to HC. Prior work has demonstrated impairments in connectivity between cortical regions (BA 6 and 9) and cerebellum in AUD compared to HC (Rogers et al., 2012). The increased connectivity between cerebellum and caudate may be a reflection of some compensatory response to the loss of this fronto-cerebellar circuit function in AUD (Chanraud, Zahr, Sullivan, & Pfefferbaum, 2010).
After treatment with disulfiram and lorazepam, and at least 5–7 days of abstinence from alcohol, despite significant improvements in measures of anxiety and alcohol withdrawal symptoms, AUD demonstrated no significant task performance and minimal task-related activation changes. However, increased anticorrelation between caudate seed and left inferior and superior parietal lobule and post central gyrus (BAs 2,7,40) was observed during Scan 2 relative to Scan 1 (in which correlation between these regions was negligible). None of the changes from Scan 1 to Scan 2 occurred in areas where we had seen differences between HC and AUD for either evoked or intrinsic analyses, providing no evidence of normalization of brain function.
There were some notable potential confounds that, ultimately, we do not believe extensively drove the results. For one, AUD had greater anxiety and depression levels than HC. Similarly, AUD had a slower reaction time than HC during the multimodal Stroop task. When testing the effect of group on some outcome variable, the use of covariates for which there is a difference between groups on that covariate is questionable, especially when the covariate is closely linked to the independent variable of interest (e.g. depression and anxiety commonly co-occur with alcohol dependence status) (Miller, 2001). Because it is impossible to statistically disentangle the effects of depression, anxiety, or reaction time from that of diagnosis in this sample, we chose not to enter depression, anxiety, or reaction time as covariates into our models. Ultimately, it is unlikely that these findings were solely related to group differences in anxiety. Although anxiety has been shown to be associated with increased brain activation during cognitive control in AUD (Karch et al., 2008), we would have also expected the BOLD signal to decrease with significant decreases in subjective anxiety levels from Scan 1 to Scan 2. Nor do we believe it is likely that reaction time alone drove results. Longer reaction times may be associated with an increase in the BOLD signal due to a “time on task” effect in lateral and medial frontal areas, but it also delays the onset of the BOLD signal (Yarkoni, Barch, Gray, Conturo, & Braver, 2009). That our neuroimaging findings in the present study were very similar to findings from prior work comparing CCA to HC with equivalent tasks and matched reaction times (Mayer et al., 2013) is reassuring, and implies that activation differences in the present study were unlikely to be due to group reaction time differences. However, results still have limited generalizability, and may potentially be only relevant to AUD with co-occurring anxiety or depression.
There were a few other notable limitations. For one, more AUD than HC in the present study had recently taken psychoactive medications. However, quetiapine, citalopram, diphenhydramine, or oxazepam, for example, would have been expected to decrease activation, if anything, and therefore was unlikely to be driving the group task-related effects (Abbott, Jaramillo, Wilcox, & Hamilton, 2013; Coull et al., 1999; McCabe, Mishor, Cowen, & Harmer, 2010; Sperling, 2002; van Ruitenbeek et al., 2013). Second, the fMRI tasks themselves were different (e.g., HC underwent a longer, more involved task than AUD). However, since we isolated the variance associated with the trials of interest from those of the non-analyzed trials, it is unlikely that effects of the non-analyzed trials influenced our estimation of the PSC. Third, the two samples (AUD and HC) were small. However, that we were able to see any differences between groups at all is notable (and speaks to a large effect size). In fact, posthoc calculations of Cohen’s d for percent signal change (task) or average z (connectivity) within significant regions for the effects of group showed a range of 1.7 to 2.8, which is a large effect (Cohen, 1988). Fourth, there was no placebo group, so we cannot be sure that any behavioral or clinical changes from Scan 1 to Scan 2 were more related to treatment (e.g., disulfiram and lorazepam), increased time since last drink, or resolution of (subclinical) alcohol withdrawal symptoms. Fifth, HC did not undergo a second MRI scan, so we cannot be sure that either task-evoked or connectivity changes are just changes that would have occurred over the course of time. Sixth, one of the AUD also met criteria for current methamphetamine dependence, which could have theoretically accounted for some of the effects seen in our study. Finally, with such a small sample size, there always is a greater risk that an outlier could be driving effects. For these reasons, results should be interpreted with caution.
In summary, similar to findings in other SUD (Mayer et al., 2013), in this work, AUD demonstrated a variety of functional brain changes in the cognitive control network during a multisensory numeric Stroop task. Treatment with a combination of disulfiram and lorazepam neither resulted in a significant normalization of these brain changes, nor did it appear to worsen either performance on a cognitive control task, or related brain function.
GLOSSARY
- Cognitive control
Cognitive control refers to the set of executive functions that update context information in the service of exerting control over thoughts and behavior, inhibiting habitual acts, and optimizing adaptive decision making. Tasks that test cognitive control include inhibitory control tasks (e.g., Stop Signal tasks), distractor interference control tasks (e.g. Stroop tasks), and working memory tasks (e.g., Sternberg task) (Wilcox et al., 2013).
- fMRI
This is a functional neuroimaging procedure using MRI technology that measures brain activity by detecting associated changes in blood flow.
- Stroop task
This task measures executive function and tests cognitive control. A Stroop task specifically measures the effects of interference on reaction time during a task, with increased reaction times usually seen during incongruent (presence of distractor stimuli) relative to congruent (absence of distractor stimuli) trials (Wilcox et al., 2013).
Biographies

Claire E. Wilcox, MD, is an Assistant Professor at the University of New Mexico in the Department of Psychiatry. She is board certified in both general psychiatry and addiction psychiatry, and is an attending in the dual diagnosis clinic. Her main research interests are the neural circuitry of decision making in substance use disorders, behavioral and pharmacological treatments for substance use disorders, and the use of functional MRI to predict treatment outcomes and to define mechanisms of effect during treatment of substance use disorders.

Andrew R. Mayer, PhD, is an Associate Professor of Translational Neuroscience, The Mind Research Network and an Adjunct Assistant Professor of Neurology and Psychology, University of New Mexico Health Science Center. Dr. Mayer’s research systematically examines how the brain uses auditory and visual information dependent on task demands. For example, auditory signals excel at producing rapid bottom-up shifts of attention (orienting responses) whereas the visual modality is superior for making fine-grain discriminations about object location and type. He also examines how the brain processes complementary versus conflicting (selective attention) auditory and visual information. Our work suggests that unisensory cortex can be up- (increased signal) or down- (decreased signal) regulated during bimodal stimulation during demanding attentional tasks.

Michael Bogenschutz, MD, is a Professor at the University of New Mexico in the Department of Psychiatry. He is board certified in both general psychiatry and addiction psychiatry, and is an attending in the dual diagnosis clinic. He is also the Vice Chair for Addictions and Clinical Research in Psychiatry. His main research interests are behavioral and pharmacologic treatments for drug and alcohol dependence, clinical trials, co-occurring psychiatric disorders, mutual help, and development of clinical and educational programs in addiction psychiatry.

Josef Ling, B.A., has worked for 10 years as an imaging analyst and bioinformatics software engineer at the MIND Research Network (MRN). Mr. Ling worked for a decade as software engineer before joining the Mind Research Network.

Charlene Dekonenko, BA, is a fourth-year medical student at the University of New Mexico School of Medicine. She received her undergraduate degree in Biology at St. Mary’s University in San Antonio, Texas in 2004. As an undergraduate, she worked as a laboratory technician in an HIV/AIDS epidemiology lab at the University of Texas at San Antonio Health Science Center. She then moved to New Mexico in 2004 where she continued research as a research assistant in the Department of Infectious Disease at the University of New Mexico until entering medical school in 2010. Her current research involves work within the Department of Psychiatry and the Department of Neurology at the University of New Mexico.

Heather Cumbo, MD, is a resident in psychiatry at the University of New Mexico.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article
REFERENCES
- Abbott CC, Jaramillo A, Wilcox CE, Hamilton DA. Antipsychotic drug effects in schizophrenia: A review of longitudinal FMRI investigations and neural interpretations. Current Medicinal Chemistry. 2013;20(3):428–437. doi: 10.2174/0929867311320030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N, Green DW, Kherif F, Devlin JT, Price CJ. The role of the left head of caudate in suppressing irrelevant words. Journal of Cognitive Neuroscience. 2010;22(10):2369–2386. doi: 10.1162/jocn.2009.21352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . DSM-IV-TROR. fourth American Psychiatric Association; Washington, DC: 2000. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Beck A, Wustenberg T, Genauck A, Wrase J, Schlagenhauf F, Smolka MN, Heinz A. Effect of brain structure, brain function, and brain connectivity on relapse in alcohol-dependent patients. Archives of General Psychiatry. 2012;69(8):842–852. doi: 10.1001/archgenpsychiatry.2011.2026. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Hommer DW, Grant SJ, Danube C. Impulsivity in abstinent alcohol-dependent patients: relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34(2–3):133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Scharff L, Schwarz SP, Suls JM, Barlow DH. The role of anxiety and depression in the irritable bowel syndrome. Behaviour Research and Therapy. 1990;28(5):401–405. doi: 10.1016/0005-7967(90)90159-g. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism Clinical and Experimental Research. 1995;19(3):600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Medicine Reviews. 2003;7(6):523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cerebral Cortex. 2011;21(10):2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: A window into white matter integrity of the working brain. Neuropsychology Review. 2010;20(2):209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DA, Zikos E, Gill KJ. Early recovery from alcohol dependence: Factors that promote or impede abstinence. Journal of Substance Abuse Treatment. 2010;38(1):42–50. doi: 10.1016/j.jsat.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Lawrence Erlbaum Associates, Hove and London; Hillsdale, NJ: 1988. [Google Scholar]
- Coull JT, Frith CD, Dolan RJ. Dissociating neuromodulatory effects of diazepam on episodic memory encoding and executive function. Psychopharmacology. 1999;145(2):213–222. doi: 10.1007/s002130051051. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, Ray LA. Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addiction Biology. 2013;18(3):593–604. doi: 10.1111/adb.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Halari R, Ecker C, Giampietro V, Taylor E, Rubia K. Reduced activation and inter-regional functional connectivity of fronto-striatal networks in adults with childhood attention-deficit hyperactivity disorder (ADHD) and persisting symptoms during tasks of motor inhibition and cognitive switching. Journal of Psychiatric Research. 2010;44(10):629–639. doi: 10.1016/j.jpsychires.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: Metabolic and neuropsychological findings. Psychological Medicine. 1998;28(5):1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19(4):1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Evren C, Durkaya M, Evren B, Dalbudak E, Cetin R. Relationship of relapse with impulsivity, novelty seeking and craving in male alcohol-dependent inpatients. Drug and Alcohol Review. 2012;31(1):81–90. doi: 10.1111/j.1465-3362.2011.00303.x. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews: Neuroscience. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, London ED. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. Journal of Neuroscience. 2012;32(21):7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden CJ. Stroop color and word test: A manual for clinical and experimental uses. Skoelting; Chicago, IL: 1978. [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Neurocognitive functions in pathological gambling: A comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101(4):534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hong SB, Zalesky A, Cocchi L, Fornito A, Choi EJ, Kim HH, Yi SH. Decreased functional brain connectivity in adolescents with internet addiction. PloS One. 2013;8(2):e57831. doi: 10.1371/journal.pone.0057831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Velanova K, Luna B. Strengthening of topdown frontal cognitive control networks underlying the development of inhibitory control: A functional magnetic resonance imaging effective connectivity study. Journal of Neuroscience. 2010;30(46):15535–15545. doi: 10.1523/JNEUROSCI.2825-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch S, Jager L, Karamatskos E, Graz C, Stammel A, Flatz W, Mulert C. Influence of trait anxiety on inhibitory control in alcohol-dependent patients: Simultaneous acquisition of ERPs and BOLD responses. Journal of Psychiatric Research. 2008;42(9):734–745. doi: 10.1016/j.jpsychires.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Kopera M, Wojnar M, Brower K, Glass J, Nowosad I, Gmaj B, Szelenberger W. Cognitive functions in abstinent alcohol-dependent patients. Alcohol. 2012;46(7):665–671. doi: 10.1016/j.alcohol.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Kadden RM, Babor TF, Tennen H, Rounsaville BJ. Validity of the SCID in substance abuse patients. Addiction. 1996;91(6):859–868. [PubMed] [Google Scholar]
- Lader M. Benzodiazepines revisited–will we ever learn? Addiction. 2011;106(12):2086–2109. doi: 10.1111/j.1360-0443.2011.03563.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee E, Ku J, Yoon KJ, Namkoong K, Jung YC. Disruption of orbitofronto-striatal functional connectivity underlies maladaptive persistent behaviors in alcoholdependent patients. Psychiatry Investigation. 2013;10(3):266–272. doi: 10.4306/pi.2013.10.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered impulse control in alcohol dependence: Neural measures of stop signal performance. Alcoholism, Clinical and Experimental Research. 2009;33(4):740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: Reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of Affective Disorders. 1988;14(1):61–68. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Pesenti M, Grandin C, Heeren A, Philippot P, de Timary P. The neural network sustaining crossmodal integration is impaired in alcoholdependence: An fMRI study. Cortex. 2013;49(6):1610–1626. doi: 10.1016/j.cortex.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Franco AR, Ling J, Canive JM. Assessment and quantification of head motion in neuropsychiatric functional imaging research as applied to schizophrenia. Journal of the International Neuropsychological Society. 2007;13(5):839–845. doi: 10.1017/S1355617707071081. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Teshiba TM, Franco AR, Ling J, Shane M, Stephen JM, Jung RE. Modelling conflict and error in the medial frontal cortex. Human Brain Mapping. 2011;33(12):2843–2855. doi: 10.1002/hbm.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Wilcox CE, Teshiba TM, Ling JM, Yang Z. Hyperactivation of the cognitive control network in cocaine use disorders during a multisensory Stroop task. Drug and Alcohol Dependence. 2013;133(1):235–241. doi: 10.1016/j.drugalcdep.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278(2):144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biological Psychiatry. 2010;67(5):439–445. doi: 10.1016/j.biopsych.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GM, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110(1):40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Miller L. Predicting relapse and recovery in alcoholism and addiction: Neuropsychology, personality, and cognitive style. Journal of Substance Abuse Treatment. 1991;8(4):277–291. doi: 10.1016/0740-5472(91)90051-b. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, d’Acremont M, Bechara A, Dan B, Hanak C, Verbanck P. Alcohol cues increase cognitive impulsivity in individuals with alcoholism. Psychopharmacology. 2007;192(2):291–298. doi: 10.1007/s00213-006-0695-6. [DOI] [PubMed] [Google Scholar]
- O’Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Duka T. Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology. 2012;37(10):2267–2276. doi: 10.1038/npp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park MS, Sohn S, Park JE, Kim SH, Yu IK, Sohn JH. Brain functions associated with verbal working memory tasks among young males with alcohol use disorders. Scandinavian Journal of Psychology. 2011;52(1):1–7. doi: 10.1111/j.1467-9450.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- Petit G, Kornreich C, Noel X, Verbanck P, Campanella S. Alcohol-related context modulates performance of social drinkers in a visual Go/No-Go task: A preliminary assessment of event-related potentials. PloS One. 2012;7(5):e37466. doi: 10.1371/journal.pone.0037466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond J, Galloway C, Menon V, Glover G, Sullivan E. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14(1):7–20. doi: 10.1006/nimg.2001.0785. Pt 1. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Rivier J, Beaunieux H, Vabret F, Desgranges B, Eustache F. Changes in the episodic memory and executive functions of abstinent and relapsed alcoholics over a 6-month period. Alcoholism: Clinical and Experimental Research. 2009;33(3):490–498. doi: 10.1111/j.1530-0277.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- Porcelli AJ, Cruz D, Wenberg K, Patterson MD, Biswal BB, Rypma B. The effects of acute stress on human prefrontal working memory systems. Physiology and Behavior. 2008;95(3):282–289. doi: 10.1016/j.physbeh.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Posternak MA, Mueller TI. Assessing the risks and benefits of benzodiazepines for anxiety disorders in patients with a history of substance abuse or dependence. American Journal on Addictions. 2001;10(1):48–68. doi: 10.1080/105504901750160484. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biological Psychiatry. 2009;66(1):25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory. A review. Psychopathology. 1998;31(3):160–168. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K, van den Wildenberg W, Segalowitz S, Carter C. Neurocognitive mechanisms of cognitive control: The role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Roberts KL, Hall DA. Examining a supramodal network for conflict processing: A systematic review and novel functional magnetic resonance imaging data for related visual and auditory stroop tasks. Journal of Cognitive Neuroscience. 2008;20(6):1063–1078. doi: 10.1162/jocn.2008.20074. [DOI] [PubMed] [Google Scholar]
- Rogers BP, Parks MH, Nickel MK, Katwal SB, Martin PR. Reduced fronto-cerebellar functional connectivity in chronic alcoholic patients. Alcoholism, Clinical and Experimental Research. 2012;36(2):294–301. doi: 10.1111/j.1530-0277.2011.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Bickel WK, Hughes JR. Acute effects of triazolam and lorazepam on human learning, performance and subject ratings. Journal of Pharmacology and Experimental Therapeutics. 1993;264(3):1218–1226. [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Bickel WK, Hughes JR. Acute behavioral effects of lorazepam and caffeine, alone and in combination, in humans. Behavioural Pharmacology. 1994;5(3):245–254. doi: 10.1097/00008877-199406000-00003. [DOI] [PubMed] [Google Scholar]
- Scheurich A. Neuropsychological functioning and alcohol dependence. Current Opinion Psychiatry. 2005;18(3):319–323. doi: 10.1097/01.yco.0000165602.36671.de. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Joos L, Koeleman M, Veltman DJ, van den Brink W, Goudriaan AE. Effects of modafinil on neural correlates of response inhibition in alcohol-dependent patients. Biological Psychiatry. 2013;73(3):211–218. doi: 10.1016/j.biopsych.2012.06.032. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Preuss D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33(5):643–653. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Sullivan EV, Pfefferbaum A. Synchrony of corticostriatal-midbrain activation enables normal inhibitory control and conflict processing in recovering alcoholic men. Biological Psychiatry. 2012;71(3):269–278. doi: 10.1016/j.biopsych.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Litten RZ, Allen JP. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen J, Litten RZ, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Sperling R, Greve D, Dale A, Killiany R, Holmes J, Rosas HD, Albert M. Functional MRI detection of pharmacologically induced memory impairment. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Spielberger CD, Gorsuch RL, Suchene RE. Consulting Psychologists Press; Palo Alto, CA: 1970. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Sullivan J, Sykora K, Schneiderman J, Naranjo C, Sellers E. Assessment of alcohol withdrawal: The revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) British Journal of Addiction. 1989;84(11):1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Brown G, Kindermann S, Cheung E, Frank L, Brown S. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcoholism-Clinical and Experimental Research. 2001;25(2):236–245. [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism, Clinical and Experimental Research. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- van Hemert DA, van de Vijver FJR, Poortinga YH. The Beck Depression Inventory as a measure of subjective wellbeing: A cross-national study. Journal of Happiness Studies. 2002;3(3):257–286. [Google Scholar]
- van Ruitenbeek P, Vermeeren A, Mehta MA, Drexler EI, Riedel WJ. Antihistamine induced blood oxygenation level dependent response changes related to visual processes during sensori-motor performance. Human Brain Mapping. 2013;35(7):3095–3106. doi: 10.1002/hbm.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2009;49(3):2536–2543. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Psychological Corporation.
- Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA. Cognitive control in alcohol use disorder: Deficits and clinical relevance. Review Neuroscience. 2013;25(1):1–24. doi: 10.1515/revneuro-2013-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug and Alcohol Dependence. 2011;115(1–2):137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Archives of General Psychiatry. 1988;45(8):742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Barch DM, Gray JR, Conturo TE, Braver TS. BOLD correlates of trial-by-trial reaction time variability in gray and white matter: A multi-study fMRI analysis. PloS One. 2009;4(1):e4257. doi: 10.1371/journal.pone.0004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Chelminski I, Posternak M. A review of studies of the Hamilton depression rating scale in healthy controls: Implications for the definition of remission in treatment studies of depression. Journal of Nervous and Mental Disease. 2004;192(9):595–601. doi: 10.1097/01.nmd.0000138226.22761.39. [DOI] [PubMed] [Google Scholar]