Abstract
Objective
Pediatricians are encountering body composition information more frequently, with percentage of body fat (%BF) measurement receiving particular attention as a result of the obesity epidemic. One confounding issue is that different methods may yield different %BF results in the same person. The objective of this study was to compare dual-energy X-ray absorptiometry (DXA) with the criterion 4-compartment model (4-CM) for measurement of %BF in a large pediatric cohort and to assist pediatricians in appropriate interpretation of body composition information by recognizing differences between techniques.
Methods
Height, weight, anthropometrics, body density by underwater weighing, total body water by deuterium dilution, and bone mineral content and %BF by DXA (Lunar DPX/DPX-L) were measured in 411 healthy subjects, aged 6 to 18 years. Values for %BF by 4-CM and DXA were compared using regression analysis.
Results
The mean ± standard deviation values for %BF by DXA (22.73% ± 11.23%) and by 4-CM (21.72% ± 9.42%) were different, but there was a strong relationship between the 2 methods (R2 = 0.85). DXA underestimated %BF in subjects with lower %BF and overestimated it in those with higher %BF. The relationship between the 2 methods was not affected by gender, age, ethnicity, pubertal stage, height, weight, or body mass index. The standard error of the estimate was 3.66%.
Conclusion
This analysis demonstrates a predictable relationship between DXA and 4-CM for %BF measurement. Because of its ease of use, consistent relationship with 4-CM, and availability, we propose that DXA has the capacity for clinical application including prediction of metabolic abnormalities associated with excess %BF in pediatrics.
Keywords: body composition, percentage of body fat, obesity, pediatrics, children, adolescents, 4-compartment model, DXA
In the midst of an epidemic of pediatric obesity1-3 and concerns about immediate and long-term complications,3-10 pediatricians are increasingly confronted with body composition information. This information is encountered both in the clinical evaluation of patients and in the pediatric literature. In the past 5 years, at least 27 articles in Pediatrics have included body composition variables from a variety of techniques, the majority from dual-energy X-ray absorptiometry (DXA). The quantity of body composition studies is expected to increase as pediatric investigators use these noninvasive techniques to define characteristics, such as percentage of body fat (%BF), that identify children and adolescents who are at health risk from obesity or abnormal body composition secondary to chronic disease or medication use.11-14
A major issue in the interpretation of body composition analysis is that different methods may yield different results for the same variable in the same person. This is true in both children and adults. In fact, absolute truth is not achievable with any in vivo technique for body composition, because all are indirect and rely on numerous assumptions, never achieving the accuracy of direct actual chemical analysis.15 However, methods vary in their accuracy, defined as their ability to approximate the “true” value for a given body component. A criterion method is one that is accepted as the closest representation of true body composition and is used as a standard against which other methods are compared. The criterion method for body composition is the 4-compartment model (4-CM), combining measurements of total body water (TBW), body density (Db), and total body bone mineral (M) to estimate a fourth component—%BF, fat, or fat-free mass. As pediatricians are well aware, children are not little adults, so the relationship of body composition techniques to the criterion method must be evaluated specifically in children and adolescents. The complex changes in body composition during childhood and adolescence make the interpretation of body composition in children particularly difficult.16-20
In addition to accuracy, an important characteristic of body composition techniques used in children is ease of performance for subjects of all ages. The method should also be reproducible, readily available, and safe. The criterion 4-CM is tedious, time-consuming, and difficult to perform and requires fasting. Underwater weighing (UWW; holding breath repeatedly while completely submerged in a tank of water) was the method most likely to introduce error into the estimate of %BF by 4-CM even in adults.21 It has relatively low precision in young children22 and is difficult and uncomfortable for sick or young children to perform.23 In addition to UWW, 4-CM requires measurement of TBW as well as M by whole-body DXA scanning. Very few centers have all 3 methods available together. However, DXA scans are increasingly available and easily performed by children of all ages, making this method attractive for pediatric body composition measurement. Several papers have compared DXA with the criterion 4-CM for %BF in pediatric subject groups ranging in size from 25 to 141 with the greatest age range in a single study of 9 to 17, but the findings are not consistent.23-27 We recently completed a cross-sectional body composition project during which 411 healthy subjects (aged 6–18 years) performed the criterion 4-CM. The goals of this article are to compare %BF by DXA with %BF by 4-CM in this large and heterogeneous group of children and adolescents, to evaluate factors that may influence the relationship between these 2 body composition methods, and to increase the awareness of pediatricians and pediatric investigators of these factors when analyzing body composition reports or data.
METHODS
The study sample included 411 healthy children and adolescents who were participants in the Pediatric Rosetta Study at St Luke’s-Roosevelt Hospital Center in New York, a cross-sectional study of pediatric body composition. There were 236 boys and 175 girls between the ages of 6 and 18 years. A medical history from a parent or a guardian and a physical examination at the time of the body composition evaluation confirmed normal health status. Pubertal stage was assessed by the criteria of Tanner by the pediatric endocrinologist or nurse in younger subjects and by self-assessment in subjects 11 to 12 years and older.28,29 Subjects with current or previous medical conditions or medications that would affect body composition were excluded. There were no height or weight criteria for entry into the study. We used a questionnaire to establish ethnicity; the criterion was consistent Asian, black, white, or Hispanic background of both parents and all 4 grandparents. Subjects with ethnic backgrounds that did not fit these criteria were classified as “other.” The study was approved by the Institutional Review Board of St Luke’s–Roosevelt Hospital. Informed consent was obtained from a parent or a guardian for subjects under the age of 18, and assent was obtained from each subject over the age of 7. Informed consent was obtained from subjects who were 18 years of age.
Studies were performed at least 1 hour after food intake, with all subjects wearing a hospital gown and foam slippers. Bathing suits were worn for UWW. Weight was measured to the nearest 0.1 kg on a balance-beam scale (Weight Tronix, New York, NY), and height to the nearest 0.1 cm using a wall-mounted stadiometer (Holtain, Crosswell, Wales).
DXA
Whole-body DXA scans were performed using Lunar models DPX with pediatric software version 3.8G and DPX-L with pediatric software 1.5G (GE Lunar Corporation, General Electric, Madison, WI).30 Each scan provided estimates of M in kilograms and %BF. Subjects who weighed <35 kg were scanned in the pediatric large mode, and those who weighed >35 kg were scanned in the adult medium mode. The coefficient of variation (CV) for repeated measures of %BF in adult subjects by whole-body DXA is 3.3% in our laboratory.31 The CV for repeated M measurements by whole-body DXA is 1.5% in adults30 and is 0.6% in our laboratory phantom.
An anthropomorphic spine phantom made up of calcium hydroxyapatite embedded in a 17.5 × 15 × 17.5-cm Lucite block was scanned with both DXA instruments for quality control each morning before subject evaluation. The phantom was also scanned immediately before and after all DXA system manufacturer maintenance visits. The measured phantom bone mineral density was stable throughout the study period at 1.166 to 1.196 g/cm2. Ethanol and water bottles (8-L volume), simulating fat and fat-free soft tissues, respectively, were scanned as soft-tissue quality control markers monthly. The range in measured R values during the study period was 1.255 to 1.258 (CV: 0.127%) and 1.367 to 1.371 (CV: 0.103%) for ethanol and water, respectively.
TBW
TBW in liters was measured by dilution of deuterium (2H2O) given orally. Saliva samples of 3 mL were collected at 0 and 120 minutes. The second sample was collected at 150 minutes if the subject weighed >91 kg.32-34 The subject drank a dose (0.1 g/kg body weight) of 99.8 atom % excess 2H2O (Icon Corp, Summit, NJ) after the first saliva sample was collected. The subject then drank 30 mL of spring water, which was used to rinse the dosing cup. Subjects were reminded not to drink or eat anything until after the second saliva sample was collected. The dose concentration in the collected specimen was measured on a single-frequency infrared spectrophotometer after the specimen was lyophilized. The TBW volume was calculated by dividing the dose by the net 2H2O concentration in the specimen. The measured TBW was not corrected for nonaqueous exchange. In our laboratory, the CV for the TBW measurement by this method is 2.1% in adults.35
UWW
Db was determined using a 4-point platform scale system36 (Precision Biomedical System, Inc, University Park, PA). Residual lung volume was determined before UWW using the nitrogen washout technique.37 Subsequently, subjects entered the hydrodensitometry tank and were asked to exhale as much air as possible from their lungs during complete submersion. After between 5 and 10 trials were performed, an underwater weight was recorded as the average of the highest 3 values.38 The subjects wore bathing suits for all measurements. The between-day CV for measurement of Db by UWW corrected for residual lung volume in adults in our laboratory is 0.33%.38
4-CM Method
4-CM was used as the criterion method.39 The 4-CM equation is %BW = (2.747/Db − 0.714 W + 1.146 M − 2.0503)100, where Db is in kg/L, W is TBW (kg) as a fraction of weight (kg), and M is bone mineral content (kg) as a fraction of weight (kg).
Anthropometric Measurements
The following anthropometric measures were made as previously described40: chest, biceps, thorax, umbilicus, suprailiac, abdomen, thigh, subscapular, triceps, calf, and suprascapular skinfolds; upper arm, wrist, upper chest, chest, waist, iliac crest, thigh, and calf circumferences; and arm and thigh lengths.
Statistical Analysis
The mean values of %BF by DXA and 4-CM were compared by a paired 2-tailed t test. Regression analysis was used to assess agreement and bias between determinations of %BF. In the regression analysis, %BF by 4-CM was the dependent variable and %BF by DXA was the independent variable. The null hypothesis that the relationship was consistent with the line of identity was tested using the F distribution. Regression analysis was also used to determine whether the relationship between the 2 methods for %BF was affected by gender, pubertal stage, ethnicity, weight, height, body mass index (BMI), and anthropometric measurements. The method of backward elimination was used to identify a subset of anthropometric variables that had a significant effect on the relationship. The 95% limits of agreement, defined as the mean bias ± 2 standard deviations, were determined by the method of Bland and Altman.41
All statistical calculations were performed using the STATA version 7.0 statistical software package for personal computers (College Station, TX). The level of significance was .05 for all statistical tests of hypothesis.
RESULTS
The descriptive statistics of the study population are presented in Table 1. There were 89 black, 47 Asian American, 153 white, 74 Hispanic, and 48 “other” participants. Children with a wide range of body size for age and gender were included; the mean z scores for height, weight, and BMI ranged from 0.3 to 0.6.
TABLE 1.
Descriptive Statistics for Subjects (n = 411; 236 Boys, 175 Girls)
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| Age, y | 12.2 | 3.1 | 6 | 18 |
| Weight, kg | 51.3 | 17.6 | 20.9 | 128.4 |
| Weight z score* | 0.6 | 1.0 | −2.2 | 3.4 |
| Height, cm | 154.4 | 15.9 | 120 | 188 |
| Height z score* | 0.3 | 1.0 | −2.6 | 5.4 |
| BMI, kg/m2 | 21.0 | 4.6 | 13.1 | 40.7 |
| BMI z score* | 0.5 | 1.0 | −2.8 | 2.7 |
Z score for age and gender from Centers for Disease Control and Prevention 2000 growth charts.42
The descriptive statistics for the body composition variables used in 4-CM (TBW by 2H2O dilution, Db by UWW, and M by DXA) are presented in Table 2. The mean values for %BF by 4-CM and DXA for the study group as a whole are presented in Table 3. The %BF means by 4-CM and DXA were significantly different (P < .0001; mean difference: −1.012 with limits of agreement ± 8.89). Mean %BF was significantly greater for girls than boys by each method (4-CM: 25.9% vs 18.6%; DXA: 28.0% vs 18.8%; P < .0001 for each).
TABLE 2.
Descriptive Statistics for Body Composition Measurements (n = 411)
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| M, kg* | 1.948 | 0.720 | 0.797 | 4.118 |
| TBW, L† | 28.1 | 9.1 | 11.9 | 56.2 |
| Db, kg/L‡ | 1.049 | 0.022 | 0.984 | 1.094 |
By DXA (Lunar DPX/DPX-L).
By deuterium dilution.
By UWW.
TABLE 3.
Descriptive Statistics for %BF Measurements by DXA (Lunar DPX/DPX-L) and by the Criterion 4-CM
| Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|
| 4-CM (%BF) | 21.72 | 9.42 | 0.11 | 49.42 |
| DXA (%BF) | 22.73* | 11.23 | 4.50 | 52.10 |
Significantly different from mean %BF by 4-CM (P < .0001).
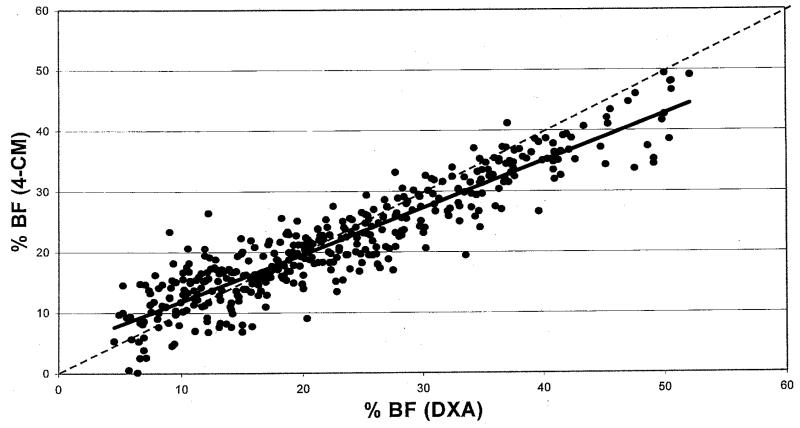
The results of linear regression analysis comparing %BF by DXA with %BF by 4-CM are shown in Fig 1. The R2 for this relationship was 0.85, and the standard error of the estimate was 3.66%. The equation was %BF 4-CM = 0.7739(%BF DXA) + 4.128
Fig 1.
Results of linear regression analysis comparing %BF by DXA (Lunar DPX/DPX-L) with %BF by the 4-CM in 411 children and adolescents aged 6 to 18. The dotted line represents the line of identity (slope = 1; intercept = 0), and the bold line represents the regression line given by the model. The regression line was significantly different from the line of identity (P < .0001); the slope was significantly different from 1, and the intercept was significantly different from 0 (P < .0001 for each). R2 = 0.85; SEE = 3.66%; %BF 4-CM = 0.7739 (%BF DXA) + 4.128.
The regression line deviated significantly from the line of identity (P < .0001). The slope of the regression line was significantly different from 1, and the intercept was significantly different from 0 (P < .0001 for each). The pattern of this relationship indicates that DXA underestimates 4-CM %BF in individuals with lower values of %BF and overestimates 4-CM %BF in individuals with higher values. DXA and 4-CM %BF are equivalent at 18.26%, where the regression line crosses the line of identity. Below this point of agreement, DXA values are lower, and above it, DXA values are higher than 4-CM %BF. The relationship between %BF by DXA and 4-CM was not affected by gender (P = .81), age (P = .25), ethnicity (P = .86), pubertal stage (P = .14), height (P = .33), weight (P = .33), or BMI (P = .21). Five skinfold and circumference measures were identified as a subset of variables that significantly affected the relationship between DXA and 4-CM (P = .0015); however, including them in the equation increased the R2 by only 0.007. The anthropometric measures were 3 circumferences (wrist, chest, and iliac crest) and 2 skinfolds (chest and calf). When these variables were tested individually, none had a significant effect on the relationship (Circumferences: wrist [P = .08], chest [P = .77], iliac crest [P = .25]; skinfolds: chest [P = .10], calf [P = .08]).
The relationships of %BF by DXA to 2 other 4-CMs (one specifically recommended for pediatric studies16,19 and the other for adults43) were very similar in slope, intercept, R2, and SEE (results not shown). The mean values for %BF for the study group by all methods (DXA and 4-CMs) were very close, ranging from 21.72% to 22.98%.
DISCUSSION
This comparison of %BF measured by DXA with a criterion 4-CM is the largest and most ethnically diverse pediatric study to date. Analysis of body composition results in 411 pediatric subjects demonstrated a predictable consistent relationship between DXA and 4-CM measurements of %BF. The relationship was characterized by overestimation of %BF by DXA (Lunar DPX and DPX-L models) in subjects with higher %BF and underestimation in those with lower %BF. The amount of overestimation or underestimation by DXA varies with fatness. Our findings suggest that %BF by DXA is not the same as the criterion 4-CM. This finding does not preclude its use for clinical and research measurement of %BF in children and adolescents.
Our findings are similar to those of other studies that compare Lunar DXA with 4-CM for measurement of %BF.24,44 Pediatric body composition studies using Hologic systems demonstrated a different relationship between the 2 methods.23,25,26 One report (n = 30) did not find a significant difference between %BF by Hologic DXA (model QDR 1000W) compared with 4-CM.23 However, 2 other reports found that Hologic DXA (models QDR 2000 and 2000W) systematically overpredicted %BF compared with the criterion.25,26 Unlike the current and previous small pediatric study using Lunar scanners, the over-prediction of %BF was independent of subject %BF.25,26 Of interest, the SEE and limits of agreement were similar to our results. Although statistical modeling to create “translation” equations between DXA and the criterion are possible, the variability between methods persists.45 The 2 important issues for pediatricians are to recognize 1) that all DXA systems differ from the criterion 4-CM and 2) that this difference does not obviate the use of DXA for measurement of %BF in children and adolescents.
Relationship of DXA Systems to 4-CM for Measurement of %BF
The relationships between %BF by DXA and 4-CM differ by DXA manufacturer but have similar SEE and limits of agreement. The 2 major manufacturers are GE Lunar Corp and Hologic, Inc, and each has its own measurement algorithm. The different pattern of the relationship to %BF by 4-CM for each manufacturer but similar statistical characteristics suggests calibration differences associated with system-specific algorithms rather than a flaw in DXA technology. Factors that may differ between manufacturers’ algorithms include corrections for body thickness, body proportions, bone maturation, and bone edge characteristics and concerns about fat-free mass hydration.25,26,46,47
Like all indirect in vivo body composition methods, DXA technology relies on numerous assumptions of constancy that may not always be correct. For example, R values are theoretical constants related to photon attenuation for specific substances, but R values measured by DXA for homogeneous material may systematically change as thickness or depth varies.48 In an in vitro study that used a Lunar system to measure phantoms of varying depths, all fat values were close to chemical calculations, but percentage of fat of the phantoms was overestimated when phantom depth was greater and was underestimated when lower.49 These findings are similar to the relationship that we observed between our Lunar DXA system and 4-CM. The modest but significant effect of anthropometric measures on our model may represent an effect of body thickness or fatness. In a study of healthy adults, correction for anthropometric dimensions did not improve the relationship between DXA and the criterion 4-CM, emphasizing the importance of specific pediatric studies.50
Utility of DXA for the Measurement of %BF in Pediatrics
This study assessed the use of a Lunar DXA system (models DPX and DPX-L) for the measurement of %BF in the pediatric population by comparing it with the criterion 4-CM. Although the results differ, our findings suggest that there is a strong and predictable relationship. The 4-CM is not practical for large-scale projects or for young or sick children and is available in only a few centers. In addition, the criterion 4-CM is not perfect as illustrated by the minimum %BF of 0.11% in 1 of our subjects (Table 3). However, DXA is easily and quickly performed, safe, and increasingly available.
Demonstration of DXA precision in pediatrics would strengthen its role as a measure of %BF, particularly its value in longitudinal studies. DXA has been shown to be a precise measure of %BF in adults.31 One pediatric study showed adequate reproducibility of DXA in prepubertal girls who had 2 DXA scans 6 weeks apart.51 However, assessment of same-day intraindividual precision for %BF by DXA would be an important addition. Preliminary same-day intraindividual data in our laboratory indicate that DXA is precise for children and adolescents.
We propose that if each DXA system’s specific characteristics are recognized, then they all have great potential as pediatric research and clinical tools. An example of a research use of DXA that may lead to clinical application is the prediction of the risk of comorbidities in obese children and adolescents.5
CONCLUSION
Measurement of body composition in children and adolescents is becoming more widespread. Determination of normal values for these measurements and of their relationships to health risk has clinical implications. The current obesity epidemic influenced our decision to focus on %BF. This was based on the pressing need to establish definitions of obesity based on metabolic consequences of increased adiposity, rather than relying on weight/height indices. Because DXA is widely available and well tolerated by pediatric subjects, it is important that pediatricians understand the meaning of its results.
Recognition that DXA differs from the criterion measure and that not all DXA systems are the same will lead to better interpretation of research and clinical results. Future areas of investigation include pediatric DXA precision studies and comparisons between DXA systems. Results from these will add to the findings of this report and will enhance the use of DXA for defining the relationship between body composition and health outcome. Because the prevention of adult disease is a central goal of pediatrics, practicing pediatricians should be knowledgeable about this body composition technique.
ACKNOWLEDGMENTS
This work was supported in part by grants from the National Institutes of Health (DK37352 and DK42618).
We thank all of the pediatric volunteers, the Body Composition Unit staff, and especially Barbara Fedun, RN, for maintaining everybody’s enthusiasm during the course of this project.
ABBREVIATIONS
- DXA
dual-energy X-ray absorptiometry
- %BF
percentage of body fat
- 4-CM
4-compartment model
- TBW
total body water
- Db
body density
- M
total body bone mineral content
- UWW
underwater weighing
- CV
coefficient of variation
- BMI
body mass index
REFERENCES
- 1.Dwyer JT, Stone EJ, Yang M, et al. Prevalence of marked overweight and obesity in a multiethnic pediatric population: findings from the Child and Adolescent Trial for Cardiovascular Health (CATCH) study. J Am Diet Assoc. 2000;100:1149–1156. doi: 10.1016/s0002-8223(00)00337-0. [DOI] [PubMed] [Google Scholar]
- 2.Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med. 1995;149:1085–1091. doi: 10.1001/archpedi.1995.02170230039005. [DOI] [PubMed] [Google Scholar]
- 3.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360:473–482. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 4.Faith MS, Pietrobelli A, Allison DB, Heymsfield SB. Prevention of pediatric obesity. In: Bendich A, Deckelbaum RJ, editors. Preventive Nutrition: The Comprehensive Guide for Health Professionals. Humana Press; Totowa, NJ: 1997. pp. 471–486. [Google Scholar]
- 5.Higgins PB, Gower BA, Hunter GR, Goran MI. Defining health related obesity in prepubertal children. Obes Res. 2001;9:233–240. doi: 10.1038/oby.2001.27. [DOI] [PubMed] [Google Scholar]
- 6.Mahoney LT, Burns TL, Stanford W. Coronary risk factors measured in childhood and young adult life are associated with coronary artery calcification in young adults: the Muscatine Study. J Am Coll Cardiol. 1996;27:277–284. doi: 10.1016/0735-1097(95)00461-0. [DOI] [PubMed] [Google Scholar]
- 7.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 8.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 9.Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents: a follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327:1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- 10.Freedman DS, Dietz WH, Srinivasan SR, Berenson G. The relationship of overweight to cardiovascular risk factors among children and adolescents: the Bogalusa Heart Study. Pediatrics. 1999;103:1175–1182. doi: 10.1542/peds.103.6.1175. [DOI] [PubMed] [Google Scholar]
- 11.Sentongo TA, Semeao EJ, Piccoli DA, Stallings VA, Zemel BS. Growth, body composition, and nutritional status in children and adolescents with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2000;31:33–40. doi: 10.1097/00005176-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Nysom K, Holm K, Michaelsen KF, Hertz H, Muller J, Molgaard C. Degree of fatness after treatment for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 1999;84:4591–4596. doi: 10.1210/jcem.84.12.6205. [DOI] [PubMed] [Google Scholar]
- 13.Stettler N, Kawchak DZ, Boyle LL, et al. Prospective evaluation of growth, nutritional status, and body composition in children with cystic fibrosis. Am J Clin Nutr. 2000;72:407–413. doi: 10.1093/ajcn/72.2.407. [DOI] [PubMed] [Google Scholar]
- 14.Arpadi SM, Horlick MN, Wang J, Cuff P, Bamji M, Kotler DP. Body composition in prepubertal children with human immunodeficiency virus type 1 infection. Arch Pediatr Adolesc Med. 1998;152:688–693. doi: 10.1001/archpedi.152.7.688. [DOI] [PubMed] [Google Scholar]
- 15.Goran MI. Measurement issues related to studies of childhood obesity: assessment of body composition, body fat distribution, physical activity, and food intake. Pediatrics. 1998;101:505–518. [PubMed] [Google Scholar]
- 16.Lohman TG. Applicability of body composition techniques and constants for children and youths. Exerc Sport Sci Rev. 1986;14:325–357. [PubMed] [Google Scholar]
- 17.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35:1169–1175. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 18.Dietz WH, Robinson TN. Use of body mass index (BMI) as a measure of overweight in children and adolescents. J Pediatr. 1998;132:191–193. doi: 10.1016/s0022-3476(98)70426-3. [DOI] [PubMed] [Google Scholar]
- 19.Roche AF. Methodological considerations in the assessment of childhood obesity. Ann N Y Acad Sci. 1993;699:6–17. doi: 10.1111/j.1749-6632.1993.tb18832.x. [DOI] [PubMed] [Google Scholar]
- 20.Horlick M. Body mass index in childhood—measuring a moving target. J Clin Endocrinol Metab. 2001;86:4059–4060. doi: 10.1210/jcem.86.9.7948. [DOI] [PubMed] [Google Scholar]
- 21.Friedl KE, DeLuca JP, Marchitelli LJ, Vogel JA. Reliability of body-fat estimations from a four-compartment model by using density, body water, and bone mineral measurements. Am J Clin Nutr. 1992;55:764–770. doi: 10.1093/ajcn/55.4.764. [DOI] [PubMed] [Google Scholar]
- 22.Dewit O, Fuller NJ, Fewtrell MS, Elia M, Wells JCK. Whole body air displacement plethysmography compared with hydrodensitometry for body composition analysis. Arch Dis Child. 2000;82:159–164. doi: 10.1136/adc.82.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells JCK, Fuller NJ, Dewit O, Fewtrell MS, Elia M, Cole TJ. Four-component model of body composition in children: density and hydration of fat-free mass and comparison with simpler models. Am J Clin Nutr. 1999;69:904–912. doi: 10.1093/ajcn/69.5.904. [DOI] [PubMed] [Google Scholar]
- 24.Fields DA, Goran MI. Body composition techniques and the four-compartment model in children. J Appl Physiol. 2000;89:613–620. doi: 10.1152/jappl.2000.89.2.613. [DOI] [PubMed] [Google Scholar]
- 25.Roemmich JN, Clark PA, Weltman A, Rogol AD. Alterations in growth and body composition during puberty. I. Comparing multicompartment body composition models. J Appl Physiol. 1997;83:927–935. doi: 10.1152/jappl.1997.83.3.927. [DOI] [PubMed] [Google Scholar]
- 26.Wong WW, Hergenroeder AC, Stuff JE, Butte NF, Smith EO, Ellis KJ. Evaluating body fat in girls and female adolescents: advantages and disadvantages of dual-energy X-ray absorptiometry. Am J Clin Nutr. 2002;76:384–389. doi: 10.1093/ajcn/76.2.384. [DOI] [PubMed] [Google Scholar]
- 27.Bray GA, DeLaney JP, Volaufova J, Harsha DW, Champaigne C. Prediction of body fat in 12-year-old African American and white children: evaluation of methods. Am J Clin Nutr. 2002;76:980–990. doi: 10.1093/ajcn/76.5.980. [DOI] [PubMed] [Google Scholar]
- 28.Tanner JM. Growth and Adolescence. 2nd ed. Blackwell; Oxford, UK: 1962. [Google Scholar]
- 29.Duke PM, Litt IF, Gross RT. Adolescents’ self-assessment of sexual maturation. Pediatrics. 1980;66:918–920. [PubMed] [Google Scholar]
- 30.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy X-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 31.Russell-Aulet MJ, Wang J, Thornton JC, Pierson RN., Jr. Comparison of dual-photon absorptiometry systems for total-body and soft tissue measurements: dual-energy x-rays versus gadolinium 153. J Bone Miner Res. 1991;6:411–415. doi: 10.1002/jbmr.5650060413. [DOI] [PubMed] [Google Scholar]
- 32.Lukaski H, Johnson PE. A simple inexpensive method for determining total body water using a tracer dose of D2O and infrared absorption of biological fluids. Am J Clin Nutr. 1985;41:363–370. doi: 10.1093/ajcn/41.2.363. [DOI] [PubMed] [Google Scholar]
- 33.Mendez J, Procop E, Picon-Reategui E, Aders R, Buskirk ER. Total body water by D2O dilution using saliva samples and gas chromatography. J Appl Physiol. 1970;28:354–357. doi: 10.1152/jappl.1970.28.3.354. [DOI] [PubMed] [Google Scholar]
- 34.Olson KE. Determination of total body water and its turnover rate. Acta Chir Scand. 1970;136:647–656. [PubMed] [Google Scholar]
- 35.Ma K, Kotler DP, Wang J, Thornton JC, Ma R, Pierson RN., Jr. Reliability of in vivo neutron activation analysis for measuring body composition: comparisons with tracer dilution and dual-energy x-ray absorptiometry. J Lab Clin Med. 1996;127:420–427. doi: 10.1016/s0022-2143(96)90058-x. [DOI] [PubMed] [Google Scholar]
- 36.Akers R, Buskirk ER. An underwater weighing system utilizing ‘force cube’ transducers. J Appl Physiol. 1969;26:649–652. doi: 10.1152/jappl.1969.26.5.649. [DOI] [PubMed] [Google Scholar]
- 37.Wilmore JA. A simplified method for determination of residual lung volume. J Appl Physiol. 1969;27:96–100. doi: 10.1152/jappl.1969.27.1.96. [DOI] [PubMed] [Google Scholar]
- 38.Heymsfield SB, Lichtman S, Baumgartner RN, et al. Body composition of humans: comparison of two improved four-compartment models that differ in expense, technical complexity, and radiation exposure. Am J Clin Nutr. 1990;52:52–58. doi: 10.1093/ajcn/52.1.52. [DOI] [PubMed] [Google Scholar]
- 39.Lohman TG. Advances in Body Composition Assessment. Human Kinetics; Champaign, IL: 1992. [Google Scholar]
- 40.Lohman TG, Roche AF, Martorell R, editors. Anthropometric Standardization Reference Manual. Human Kinetics; Champaign, IL: 1988. [Google Scholar]
- 41.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 42.Center for Disease Control and Prevention [Accessed May 30. 2001];National Center for Health Statistics 2000 CDC growth charts: United States. Available at: www.cdc.gov/nchs/about/major/nhanes/growthcharts/charts.htm.
- 43.Heymsfield SB, Wang Z, Withers RT. Multicomponent molecular level models of body composition analysis. In: Roche AF, Heymsfield SB, Lohman TG, editors. Human Body Composition. Human Kinetics; Champaign, IL: 1996. pp. 129–148. [Google Scholar]
- 44.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 45.Pintauro SJ, Nagy TR, Duthie CM, Goran MI. Cross-calibration of fat and lean measurements by dual-energy x-ray absorptiometry to pig carcass analysis in the pediatric body weight range. Am J Clin Nutr. 1996;63:293–298. doi: 10.1093/ajcn/63.3.293. [DOI] [PubMed] [Google Scholar]
- 46.Testolin CG, Gore R, Rivkin T, et al. Dual-energy X-ray absorptiometry: analysis of pediatric fat estimate errors due to tissue hydration effect. J Appl Physiol. 2000;89:2365–2372. doi: 10.1152/jappl.2000.89.6.2365. [DOI] [PubMed] [Google Scholar]
- 47.Pietrobelli A, Wang Z, Formica C, Heymsfield SB. Dual-energy X-ray absorptiometry: fat estimation errors due to variation in soft tissue hydration. Am J Physiol. 1998;274:E808–E816. doi: 10.1152/ajpendo.1998.274.5.E808. [DOI] [PubMed] [Google Scholar]
- 48.Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol. 1996;271:E941–E951. doi: 10.1152/ajpendo.1996.271.6.E941. [DOI] [PubMed] [Google Scholar]
- 49.Laskey MA, Lyttle KD, Flaxman ME, Barber RW. The influence of tissue depth and composition on the performance of the Lunar dual-energy X-ray absorptiometer and whole-body scanning mode. Eur J Clin Nutr. 1991;46:39–45. [PubMed] [Google Scholar]
- 50.Marcus MA, Wang J, Thornton JC, Ma R, Burastero S, Pierson RN., Jr. Anthropometrics do not influence dual X-ray absorptiometry (DXA) measurement of fat in normal to obese adults: a comparison with in vivo neutron activation analysis (IVNA) Obes Res. 1997;5:122–130. doi: 10.1002/j.1550-8528.1997.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 51.Figueroa-Colon R, Mayo MS, Treuth MS, Aldridge RA, Weinsier RL. Reproducibility of dual-energy X-ray absorptiometry measurements in prepubertal girls. Obes Res. 1998;6:262–267. doi: 10.1002/j.1550-8528.1998.tb00348.x. [DOI] [PubMed] [Google Scholar]