Abstract
Influenza virus causes seasonal epidemics and also poses a high risk for pandemics. To develop a broadly cross-protective influenza vaccine we have previously shown that a formulation consisting of the extracellular domain of M2 membrane protein (M2e) immobilized on gold nanoparticles (AuNPs) and soluble CpG as an adjuvant can elicit protective immunity against different influenza A subtypes. The vaccine formulation contains M2e that is immobilized on AuNPs, and an excess amount that is freely dissolved in solution, whose role in inducing protective immunity against virus infection is unclear. Using a mouse model, the current study shows that inclusion of excess soluble M2e antigen along with M2e immobilized on AuNPs is vital for inducing high levels of antibody response, and in providing complete protection against lethal influenza virus challenge. We also show that the vaccine induces long-lasting protection against mortality and morbidity upon lethal challenge with influenza A virus.
Keywords: influenza, long-term protection, nanoparticle-conjugated M2e, soluble M2e, universal vaccine
Background
Influenza virus, as a respiratory pathogen, produces epidemics every year. Due to its rapid transmission it typically causes 3-5 million cases of severe-illness and a quarter to half a million deaths worldwide[1]. Influenza viruses also pose a high pandemic risk. Four pandemics have already resulted from influenza outbreaks in the twentieth century: in 1918, 1957, 1968, and 2009[2, 3].After 2003, the highly pathogenic avian influenza H5N1 virus has caused outbreaks with a high rate of mortality in large parts of Asia and the Middle East[4]. Vaccines are acknowledged as the most useful medical intervention for preventing influenza infection and reducing morbidity and mortality. Current commercial influenza vaccines rely on generating neutralizing antibodies against hemagglutinin (HA) and neuraminidase (NA), which are two dominant membrane glycoproteins expressed on the surface of influenza A virus. Although vaccine manufacturers update vaccine formulations annually[5, 6], seasonal influenza vaccines protect little against new unpredictable pandemic viruses because of mutations in HA and NA[7]. Experts in the field think that the emergence of a new influenza pandemic virus is only a question of “when, not if”[8].
M2 is another integral membrane protein of influenza virus. The 23 amino acid-long extracellular part of M2 (M2e) is considered to be an attractive target to develop a universal influenza vaccine because it is highly conserved in amino acid sequence amongst influenza A viruses[9]. However, M2e is naturally present in very small numbers on the virus surface, it has poor immunogenicity[10, 11], and due to its small size it is sterically-hindered by HA and NA. Overall, very little anti-M2e antibodies are produced in humans even after natural influenza infection[12, 13]. Because of this poor immunogenicity of M2e peptide, we and other researchers have focused on using recombinant and synthetic approaches to link M2e antigen to a carrier to enhance its immunogenicity[14-22].
It has been shown in our previous study that by immobilizing M2e on AuNPs and using soluble CpG (sCpG: CpG is not attached to AuNPs) as an adjuvant, strong M2e-specific antibodies are elicited in serum, and 100% survival rate is achieved in mice against influenza A/PR/8/34 (H1N1) lethal challenge[15]. We also demonstrated that mice vaccinated with only soluble M2e and sCpG but without AuNPs was not able to generate strong anti-M2e specific antibodies, attesting to the vital role of AuNPs as an antigen carrier in the vaccine formulation. Our vaccine formulation contains M2e in excess (~ 500 molar excess) to help fully cover the AuNP surface with M2e. Consequently, not only is M2e present immobilized on AuNPs, but a large number of M2e molecules are also present in a freely soluble form. In our previous study[15], we were motivated to retain excess M2e in the formulation and to not remove it so that a constant dose of M2e could be maintained across different test groups, which were designed to evaluate the effect of AuNPs and sCpG individually and in combination, on protective immunity. However, the role of this free M2e not immobilized on AuNPs towards induction of protective immunity was not investigated. In other words, does the immune response change as the quantity of freely soluble M2e in the formulation is altered while keeping the amount of M2e immobilized on AuNPs constant. To address this question, in our current study, we fixed the amount of AuNPs and varied the amount of M2e at different molar excess ratios. This approach resulted in formulations each with a constant amount of M2e immobilized on the AuNP surface but with a different amount of soluble M2e. We evaluated these formulations with respect to their stability in vitro, and the immune response and protection from lethal influenza A challenge in mice. In addition, the goal of a universal influenza A vaccine is to make annual vaccinations obsolete, while protecting the human population from seasonal and pandemic influenza A infections. Thus, to be an effective influenza A vaccine, the vaccine-induced immunity needs to be long-lived. Accordingly, in a mouse model we further examined the maintenance of immune response and protective efficacy 8 months post-vaccination.
Methods
Chemicals
Gold(III) chloride trihydrate (520918-5G), trisodium citrate dihydrate (S1804-500G) and phosphate-citrate buffer tablet (P4809-50TAB) were purchased from Sigma-Aldrich. Sodium chloride (BDH0286-500G), tween 20 (BP337-100) and dithiothreitol (20289) were purchased from Fisher Scientific. O-Phenylenediamine (OPD) (00-2003) was purchased from Invitrogen. Milli-Q water with a resistance of 18.2 MΩ cm was used in all the experiments.
Peptides, antibodies and oligonucleotides
M2e (acetylated-SLLTEVETPIRNEWGSRSNDSSDC-amidated, MW: 2736 Da) was chemically synthesized by AAPPTec, LLC, USA. CpG oligodeoxynucleotide (ODN) 1826 VacciGrade was purchased from InvivoGen (San Diego, CA, USA). CpG ODN 1826 has a sequence of 5’-TCCATGACGTTCCTGACGTT-3’ (CpG motifs are underlined) and is recognized by toll-like receptor 9 (TLR9). All secondary antibodies were purchased from Southern Biotech, USA.
Vaccine preparation
AuNPs with a diameter of 12 nm were synthesized by the Turkevich method[23, 24] of reduction of gold (III) chloride trihydrate (HAuCl4·3H2O) with trisodium citrate dihydrate (Na3C6H5O7.2H2O). The detailed protocol is described in our previous work[15]. M2e was immobilized on AuNPs by mixing AuNPs and M2e peptide in four different molar ratios. First, tween 20 at a final concentration of 0.1% was added to 1mL AuNP suspension to improve the stability of AuNPs. The particles were then centrifuged at 17,000 g for 25 min at 4 °C. Cysteine-terminated M2e peptides were added to each AuNP pellet (224 μg) to achieve molar excess ratios of 250, 500, 1000 and 5000; hereafter, these formulations are referred to as 250M2e-AuNP, 500M2e-AuNP, 1000M2e-AuNP, and 5000M2e-AuNP, respectively. The mixture was incubated overnight at 4°C to complete the functionalization process. To obtain washed M2e-AuNP formulation (no free M2e in solution), 500M2e-AuNP was first prepared, then excess free M2e was removed by repeated centrifugation and resuspension of the pellet in water for a total of three times. M2e-AuNP pellets for each formulation were finally suspended in 100 μl water with 80 μg sCpG. This recipe allows immunization of four mice.
Characterization of AuNP-M2e Stability
AuNP-M2e conjugate was observed by transmission election microscopy (TEM) (Hitachi H-8100, MI, USA). TEM samples were prepared by placing a drop of the AuNP-M2e solution on a 300 mesh copper grid coated with carbon film and allowing it to dry. Size distribution of AuNP-M2e was measured using Dynamic Light Scattering (DLS) (Wyatt Technology Corporation, CA, US). Ultra violet-visible (UV-vis) absorbance spectra were also recorded for AuNP-M2e solutions from 400 to 700 nm at room temperature with Cary 300 UV-vis spectrophotometer (Agilent Technologies, Inc., CA, USA).
To compare stability of AuNPs capped with M2e, an aggregation parameter (AP) was used for empirical measurement of the aggregation process. AP is defined as , where A and A0 is the integrated absorbance between 600 and 700 nm for modified AuNPs and original AuNPs, respectively[25].
Quantification of M2e conjugated to AuNPs
To quantify the amount of M2e immobilized on AuNPs, different formulations were first washed to remove excess free M2e peptide. Next 10 μl of 100 mM dithiothreitol (DTT) was added into 50 μl of washed M2e-AuNP pellet to dissociate the covalent bond between gold and thiol[26, 27]. The supernatant containing released M2e peptide was lyophilized and redissolved in 10 μl of water. Protein concentration of M2e in this solution was measured via absorbance at 280 nm using Nanodrop2000 spectrophotometer (Thermo Fisher Scientific Inc, DE, USA). Samples were prepared in triplicate.
Mice immunizations
Female mice (BALB/c, 6-8 weeks) (Charles River Laboratories, MA, USA) were used in experiments following procedures approved by Texas Tech University Animal Care Committee (IACUC). For studying the effect of free M2e on the immune response, five mice per group were anesthetized and each mouse was intranasally immunized with 56 μg AuNPs mixed with either 8.2 μg M2e (500 molar excess), or 4.1 μg M2e (250 molar excess), or with no free M2e (fully washed), and 20 μg sCpG as adjuvant; these formulations are hereafter referred to as 500M2e-AuNP+sCpG, 250M2e-AuNP+sCpG, and washed M2e-AuNP+sCpG, respectively. It was found that about 1.2 - 1.3 μg M2e is immobilized on 56 μg AuNPs while the remaining M2e is present in soluble form. To evaluate long-term protection, another group of mice (six mice per group) was intranasally immunized with 500M2e-AuNP+sCpG. All formulations were administered in a volume of 25 μl per mouse, given drop-wise to the nares at day 1 and was repeated (boosted) at day 21. Blood was collected through retro-orbital sinuses at study days 0, 21, and 42, and in addition at 4 month and 8 month for the long-term protection study. The collected sera were centrifuged at 17000 g for 15 min and stored at -20 °C until analysis.
Measurement of M2e-specific IgG, IgG1 and IgG2a
M2e-specific antibodies generated by immunized mice were measured by ELISA as described before[15]. Briefly, ninety-six well plates (Maxisorp, Nunc) were coated overnight at 4 °C with 50 μl of 5 μg/ml M2e peptide in phosphate buffered saline (PBS). Plates were blocked with 100 μl of 1% bovine serum albumin in PBS for 2 h at room temperature. Serum from individual mice was diluted 1:1600 or 1:400, added to wells, and incubated for 1 h at room temperature. Next plates were incubated with 50 μl of 1:4000 dilution of horseradish peroxidase (HRP)-labeled anti-IgG antibody for 1 h at room temperature. Plates were washed three times with PBST (0.05% tween 20 in PBS) between each step using ELx405 microplate washer (BioTek, VT). Color was developed with OPD as substrate. After 5-10 minutes, 50 μl of 3M phosphoric acid was added to terminate the reaction. Absorbance at 492 nm was recorded using SpectraMax Plus384 microplate reader (Molecular Devices LLC., CA).
For measurement of IgG1 and IgG2a subtypes, the above procedure was repeated with individual mouse serum diluted 1:1600 or 1:400, and by using horseradish peroxidase (HRP)-labeled anti-IgG1 and anti-IgG2a as secondary antibodies.
Influenza A virus challenge
Mice were anesthetized and intranasally inoculated with 30 μl of influenza A virus (H1N1-A/PR/8/34) at approximately 4 × lethal dose 50% (LD50), 42 days after first immunization or 8 months after first immunization. Mice were weighed prior to virus challenge and then weight loss was recorded daily. All mice were also observed for morbidity and mortality through day 14 post-challenge. If mice experienced weight loss of over 25%, they were euthanized and included as an experimental end point.
Statistical analysis
All statistical analyses were performed using GraphPad Prism for windows, version 5.0 (GraphPad Software, Inc., La Jolla, CA). Comparison of antibody titers between groups of mice was performed with two-way analysis of variance (ANOVA) and a Bonferroni test at a value of p<0.05 for statistical significance. Kaplan-Meier survival curves were generated and compared by the Log-rank (Mantel-Cox) test followed by pairwise comparison using the Gehan-Breslow- Wilcoxon test. The mean body weights were analyzed by analysis of variance (ANOVA) followed by Tukey's multiple comparison test.
Results
Characterization of AuNP-M2e stability at different molar ratios
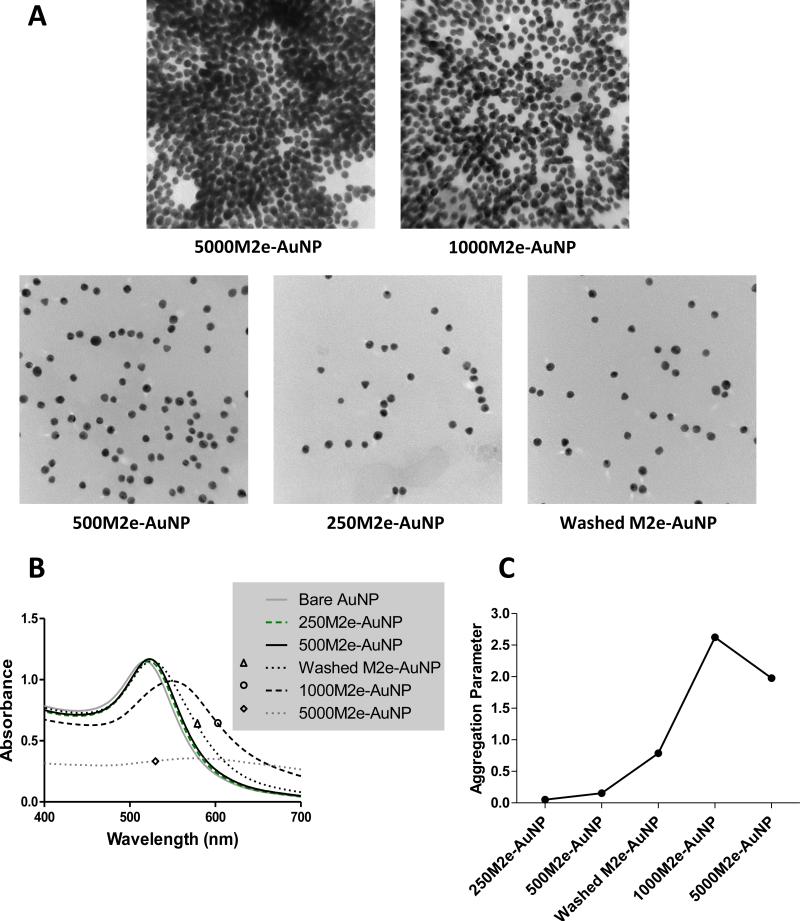
We have previously shown that AuNPs with 12 nm diameter can be chemically synthesized with low polydispersity, and can be stably conjugated with M2e (at 500 molar excess ratio) through the thiol-gold interaction arising from the cysteine residue in M2e[15]. As shown in Figure 1, this vaccine formulation comprises of three components M2e, AuNPs, and CpG, of which, M2e is present both in an immobilized form and a freely soluble form, while CpG is present only in a soluble form. Our previous study shows that immobilization of M2e to AuNPs is critical to achieve protection against lethal influenza infection. In the current study we sought to understand the importance of soluble M2e on the immune response and protection against influenza virus challenge. Thus, we first evaluated whether stable formulations can be prepared by increasing and decreasing M2e to AuNP molar ratio with respect to 500, the molar ratio we had used previously. Accordingly, bare AuNP pellets after centrifugation (224 μg in 48 μl) were mixed with cysteine-terminated M2e peptides to achieve M2e to AuNP molar ratios of 250, 500, 1000 and 5000. It was observed that AuNP colloids aggregated immediately after M2e was added at high molar ratios of 1000 and 5000 to the AuNP solution. The aggregation of AuNPs was initiated by a color change from red to purple, and to colorless in the end. This is due to clusters of AuNP colloids settling down to the bottom of the tube. The AuNP colloidal clusters were further observed using TEM to monitor their aggregation condition. From Figure 2A it is seen that at high M2e to AuNP molar ratios of 1000 and 5000, AuNPs aggregate, while at molar ratios of 250 and 500 the M2e-conjugated AuNPs remain stable in solution, as indicated by discretely-spaced individual AuNPs. Washed M2e-AuNPs also remained stable after removing excess soluble M2e from the vaccine formulation. Aggregation was also confirmed by examining size distribution, which showed a considerably broader size distribution for 5000M2e-AuNP and 1000M2e-AuNP as compared to 500M2e-AuNP, 250M2e-AuNP, and washed M2e-AuNP (Supplementary Figure 1).
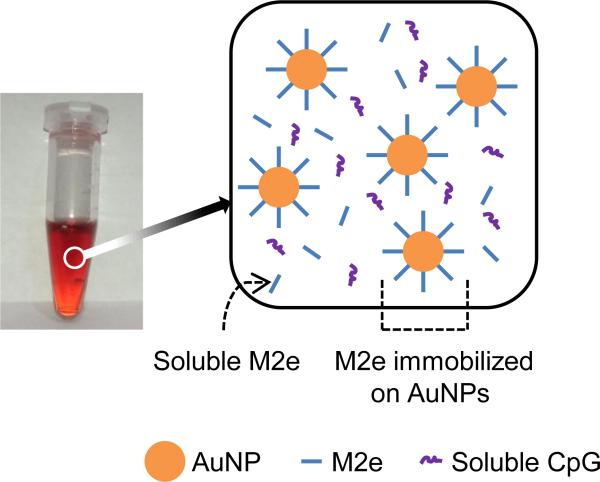
Fig 1. Vaccine formulation.
(Left) Photograph of the vaccine formulation. (Right) Schematic showing vaccine components, which consist of M2e immobilized on gold nanoparticles (AuNPs), soluble excess M2e, and soluble CpG (sCpG) as adjuvant.
Fig 2. Stability characterization of AuNPs with different molar ratios of M2e.
(A) Transmission electron micrographs of AuNPs conjugated with different molar ratios of M2e. (B) Absorption spectrum of vaccine formulations containing AuNPs and different molar ratios of M2e. (C) Aggregation parameter of vaccine formulations containing AuNPs and different molar ratios of M2e.
We further analyzed formulation stability using UV-vis spectroscopy. Negatively charged bare AuNPs with 12 nm diameter were stable and observed to display maximum absorbance peak at a wavelength of 518 nm (Figure 2B). As seen in Figure 2B, M2e-conjugated AuNPs were stable when M2e was added at molar ratios of 250 and 500. Additionally, washed M2e-AuNPs also remained stable in solution. In contrast, when M2e was added in higher molar ratios of 1000 and 5000, it induced aggregation as shown by decrease in absorbance at 518 nm and an increase in absorbance at extended wavelengths with sharp increase in aggregation parameter (Figure 2C). These data suggest that when the molar ratio of M2e to AuNP reaches 1000, it exceeds the critical protein concentration for the ‘M2e-AuNP’ colloidal system. Such a protein concentration-dependent aggregation of AuNPs has been previously noted and reported in literature[28, 29]. Since 1000M2e-AuNP and 5000M2e-AuNP were unstable, we chose the stable formulations of AuNPs functionalized with M2e peptide in molar ratios of 1:250 (250M2e-AuNP) and 1:500 (500M2e-AuNP), and washed M2e-AuNPs (no free M2e in solution) to further characterize the contribution of freely-soluble M2e in the vaccine formulation towards protective immunity.
Amount of M2e conjugated to AuNPs
After verifying the stability of different vaccine formulations, we next quantified the amount of M2e immobilized on them. Based on M2e quantification procedure as described in methods section, we found that for the 500M2e-AuNP formulation, out of the total 8.2 μg M2e added, 1.3 ± 0.25 μg of M2e is immobilized on the AuNP surface, while for 250M2e-AuNP formulation, out of the total 4.1 μg of M2e, 1.2 ± 0.22 μg of M2e is immobilized on the AuNP surface. Because the washed M2e-AuNP formulation is prepared by first preparing the 500M2e-AuNP formulation and then washing it, it also contained the same amount of M2e conjugated to AuNP surfaces as that in the 500M2e-AuNP formulation. It is important to note that this result is in close agreement with our previous study in which we determined that the amount of M2e immobilized on AuNPs in the 500 molar excess formulation was 1.25±0.25 μg, even though a different detection technique based on ELISA was used previously[15]. This demonstrates that once the number of AuNPs of a certain size is fixed, the amount of M2e immobilized to these AuNPs does not change (p=0.63, student t test) despite a change in the quantity of excess M2e added. The amounts of total M2e and AuNP-immobilized M2e for each stable formulation are summarized in Table 1.
Table 1.
Vaccine formulations - dose per animal
| Group No. | Experimental group | Soluble M2e (μg) | M2e immobilized on 56 μg AuNPs (μg) | Soluble CpG-ODN(μg) |
|---|---|---|---|---|
| 1 | 500M2e-AuNP+sCpG | 6.9 | 1.3 | 20 |
| 2 | 250M2e-AuNP+sCpG | 2.9 | 1.2 | 20 |
| 3 | Washed M2e-AuNP+sCpG | 0 | 1.3 | 20 |
| 4 | Naive | ----- | ----- | ----- |
Effect of soluble M2e quantity on the immune response
To investigate the role of soluble M2e, i.e., M2e that is not immobilized on AuNPs, we vaccinated mice intranasally with M2e-AuNP+sCpG vaccine formulations with different amounts of excess soluble M2e as shown in Table 1. An ELISA was performed on pooled sera for each group to obtain a dilution curve of M2e-specific IgG antibodies for day 21 and day 42 (Supplementary Figure 2). Immune sera of individual mice at 1:1600 dilution were then examined further for M2e-specific IgG and IgG subtypes (Figure 3).
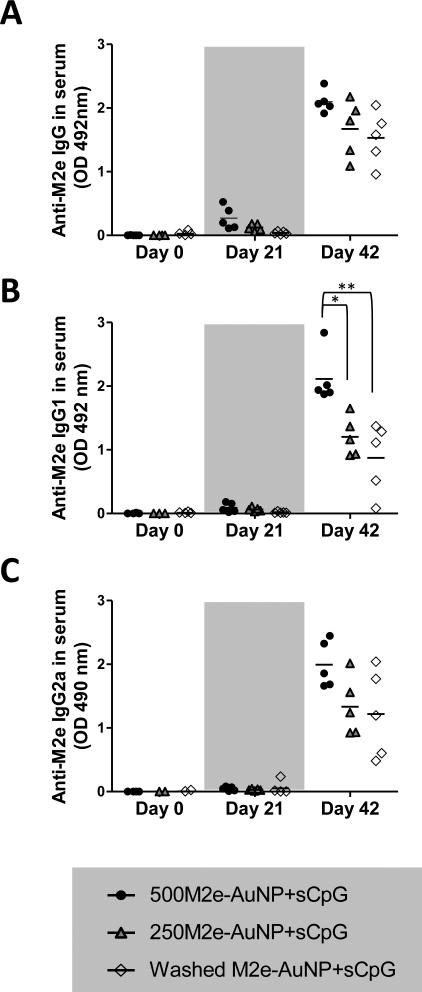
Fig 3. Effect of free M2e quantity on M2e-specific IgG and IgG subtype antibody response in mice.
Groups of mice (n=5 per group) were vaccinated on days 0 and 21 with different formulations each containing the same amount of M2e conjugated to AuNPs and sCpG, but different amounts of free M2e (6.9 μg in 500M2e-AuNP+sCpG, 2.9 μg in 250M2e-AuNP+sCpG, and 0 μg in washed M2e-AuNP+sCpG). Serum was collected on day 0, 21, and 42 for analysis. (A) M2e-specific IgG antibody in 1:1600 diluted serum of individual mice. (B) IgG1 antibody in 1:1600 diluted serum of individual mice. (C) IgG2a antibody in 1:1600 diluted serum of individual mice. Each symbol represents an animal and the horizontal bar represents the mean. Optical density (OD) of the ELISA reaction was measured at 492 nm wavelength. * represents p<0.05, ** represents p<0.01.
The vaccine formulation 500M2e-AuNP+sCpG, which contains 6.9 μg of soluble M2e and 1.3 μg of M2e immobilized on AuNPs elicited the highest anti-M2e serum IgG response (Figure 3A). Upon reducing the amount of soluble M2e to 2.9 μg but maintaining the immobilized M2e at the same level (250M2e-AuNP+sCpG formulation), a little lower amount of anti-M2e serum IgG antibody response was observed (Figure 3A). Upon complete exclusion of soluble M2e from the formulation while still maintaining 1.3 μg of M2e immobilized to AuNPs (washed M2e-AuNP+sCpG formulation), an even lower antibody response was observed (Figure 3A). Similar trend was seen for M2e-specific IgG1 and IgG2a antibody responses (Figures 3B and 3C). Especially, IgG1 antibody was significantly enhanced by increasing the amount of soluble M2e from 0 to 6.9 μg (p<0.01). The decreasing trend in anti-M2e IgG and IgG2a response is notable between 500M2e-AuNP+sCpG and washed M2e-AuNP+sCpG groups, but it is only marginally significant (p=0.073 for IgG and p=0.088 for IgG2a). These results indicate that soluble M2e is a critical component of the vaccine formulation, and that its quantity in the vaccine formulation plays a vital role in inducing enhanced anti-M2e antibody response.
Effect of soluble M2e quantity on protection against influenza virus
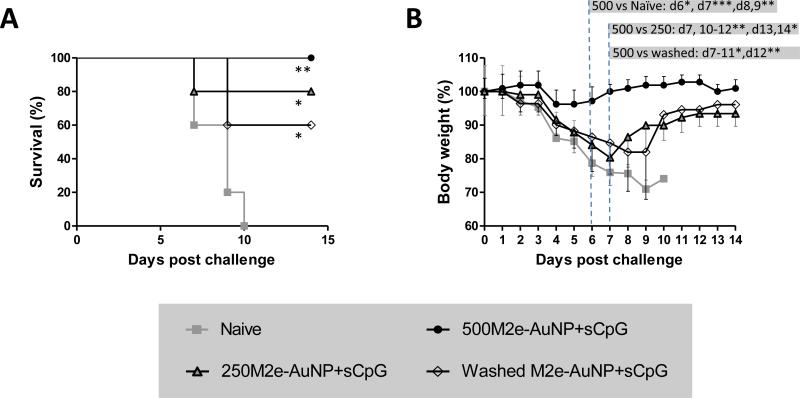
Results from ELISA demonstrated that presence of soluble M2e in higher amounts in the vaccine formulation improves its ability to elicit M2e-specific IgG antibody and its subtypes. We next tested whether the quantity of soluble M2e in the vaccine formulation also affects protection of mice from lethal influenza A challenge. Accordingly, vaccinated mice were challenged using a lethal dose of influenza A/PR/8/34 (H1N1) virus (~4× LD50). Mice receiving M2e immobilized on AuNPs but without soluble M2e (washed M2e-AuNPP+sCpG) had the lowest level of M2e-specific serum antibodies compared to other groups, and also demonstrated a relatively low survival rate of 60% (Figure 4A). Incorporation of soluble M2e in the formulation and increasing its dose from 2.9 μg to 6.9 μg correspondingly improved the survival rate to 80% and 100%. It was found that weight loss occurred in all groups (Figure 4B). However, only mice receiving the vaccine formulation of 500M2e-AuNP+sCpG exhibited significantly less weight loss compared to the naïve group (p<0.05 at day 6; p<0.001 at day 7; p<0.01 at days 8 and 9). Further, 500M2e-AuNP+sCpG group showed significantly less weight loss compared to 250M2e-AuNP+sCpG (p<0.01 at day 7, 10-12; p<0.05 at days 13-14) and washed M2e-AuNP+sCpG (p<0.05 at days 7-11; p<0.01 at day 12) vaccine formulation groups. Thus, the quantity of free M2e in the vaccine formulation is critical to induce better survival and to reduce sickness in mice (reflected by lower weight loss) upon influenza A infection.
Fig 4. Effect of free M2e quantity on protective efficacy against influenza A/PR/8/34 (H1N1) virus.
Groups of mice (n=5 per group) were vaccinated on days 0 and 21 with different formulations each containing the same amount of M2e conjugated to AuNPs and sCpG, but different amounts of free M2e (6.9 μg in 500M2e-AuNP+sCpG, 2.9 μg in 250M2e-AuNP+sCpG, and 0 μg in washed M2e-AuNP+sCpG), and challenged on day 42 with about 4xLD50 of influenza A/PR/8/34 (H1N1) virus. (A) Survival rate after virus infection (* represents p<0.05, ** represents p<0.01, both with respect to naïve mice), and (B) body weight after virus infection (* represents p<0.05, ** represents p<0.01, *** represents p<0.001).
Long-term immune response and protection against A/PR/8/34 (H1N1) virus
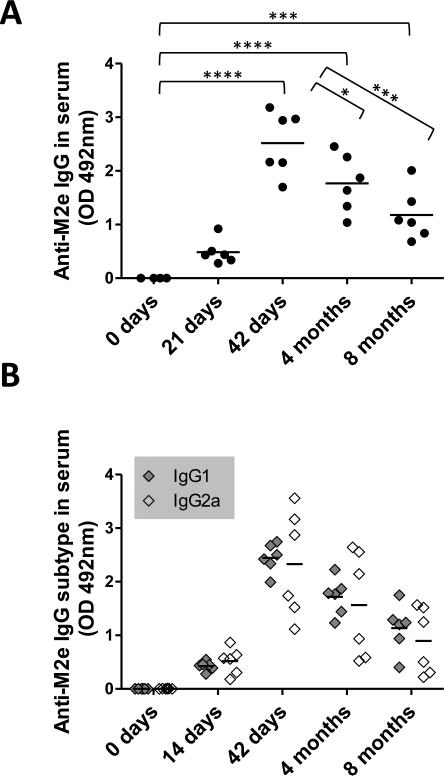
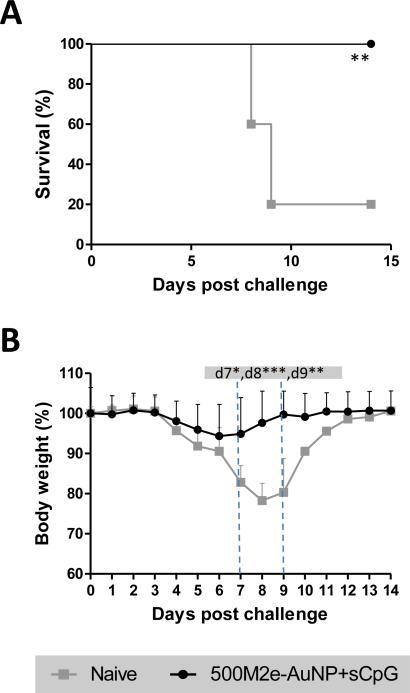
Aforementioned results demonstrate that intranasal vaccination with the vaccine formulation comprising of about 1.3 μg of M2e immobilized on AuNPs and an additional 6.9 μg of free M2e (500M2e-AuNP+sCpG) was able to elicit a significantly strong M2e-specific antibody response, and protected mice from lethal influenza infection with significantly low weight loss. We were further motivated to investigate the long-term antibody response from this vaccine formulation. Accordingly, mice were intranasally immunized with 500M2e-AuNP+sCpG and kept for 8 months post vaccination. Levels of total M2e-specific serum IgG and IgG subtypes at 0 day (pre-immune sera), 21 day, 42 day, 4 months, and 8 months (before challenge) were determined using ELISA. As observed from Figure 5A and 5B, at early time points, i.e., day 21 and day 42, anti-M2e IgG and IgG subtype antibodies were all detected at increased levels which is consistent with Figure 3, and our prior work[15]. Over time, at months 4 and months 8, M2e-specific antibody levels exhibited a decreasing trend but were still maintained at significantly high levels compared to day 0 (p<0.001). To evaluate the long-term protective efficacy induced by 500M2e-AuNP+sCpG vaccine formulation, mice were challenged 8 months after vaccination with a lethal dose of A/PR/8/34 (H1N1) influenza virus (~4 × LD50). From Figure 6A it can be seen that mice immunized with 500M2e-AuNP+sCpG vaccine were 100% protected (6/6). Further from Figure 6B it can be observed that vaccinated mice do not show significant weight loss compared to their post-challenge weight. In contrast, naïve mice exhibit significant weight loss compared to their post-challenge weight, which at days 7 to 9 is also significantly lower compared to vaccinated mice (p<0.05). These results demonstrate that 500M2e-AuNP+sCpG vaccine is able to induce long-lasting immunity and protection against influenza virus after intranasal immunization.
Fig 5. Long-term M2e-specific IgG and IgG subtype antibody response in mice.
Mice (n=6) were vaccinated with 500M2e-AuNP+sCpG on days 0 and 21, and serum was collected on day 0, day 21, day 42, 4 months, and 8 months for analysis. (A) M2e-specific IgG antibody in 1:400 diluted serum of individual mice. Each circle represents an animal and the horizontal bar represents the mean. (B) IgG1 and IgG2a antibodies in 1:400 diluted serum of individual mice. Each diamond represents an animal and the horizontal bar represents the mean. Optical density (OD) of the ELISA reaction was measured at 492 nm wavelength. * represents p<0.05, *** represents p<0.001, **** represents p<0.0001.
Fig 6. Long-term immunity evaluation against influenza A/PR/8/34 (H1N1) virus challenge.
Mice (n=6) were vaccinated with 500M2e-AuNP+sCpG on days 0 and 21, and challenged with about 4xLD50 of influenza A/PR/8/34 (H1N1) virus 8 months after first immunization dose. (A) Survival rate and (B) body weight after virus infection. n=6 in each group. * represents p<0.05, ** represents p<0.01, *** represents p<0.001.
Discussion
Our previous work demonstrated that presence of M2e immobilized onto AuNPs plays a critical role in improving the immunogenicity and efficacy of M2e compared with its purely soluble form[15]. However, because we had added M2e in excess, only a fraction of M2e was conjugated to AuNPs and a large part remained freely soluble. The function of this soluble fraction of M2e in inducing immune response remained unclear. To investigate this further, in the current study, we designed and constructed vaccine formulations comprising of AuNPs mixed with M2e at different molar excess ratios, and from amongst these we selected the stable formulations to further examine their immunogenicity in mice. In all investigated formulations sCpG was included as an adjuvant, which stimulates TLR-9.
In the current study, the effect of soluble M2e in the formulation was demonstrated. Addition of M2e in molar excess ratios of 1000 and 5000 produced unstable AuNPs, and were thus not evaluated in vivo. Formulations with molar excess ratios of 250 and 500 were found to be stable. For formulations containing 250 and 500 molar excess M2e, and the washed formulation, the amount of M2e immobilized on AuNPs was found to be similar (1.2 to 1.3 μg; p=0.63). This result is expected because at both 250 and 500 molar excess ratios sufficiently high number of M2e molecules are available, which can help completely cover the available AuNP surface. On the other hand, the amount of freely soluble M2e in the vaccine formulation with 250 molar excess M2e was about 2.5 fold lower than in the 500 molar excess formulation. Using these two formulations, we could thus change the dose of freely soluble M2e by about 2.5 fold but were able to maintain the dose of M2e immobilized on AuNPs constant, allowing us to exclusively study the effect of free M2e on the immune response. Additionally, a washed M2e-AuNP formulation was included, in which there is no soluble M2e, and all of M2e is present only in AuNP-immobilized form.
Upon changing the amount of free M2e in the formulation but keeping the amount of M2e immobilized on AuNPs fixed, a significant effect on immune response and protection against lethal influenza challenge was observed. Specifically, as the amount of free M2e was increased from 0 μg to 2.9 μg to 6.9 μg, an increasing trend in serum anti-M2e IgG levels was observed. Further, the levels of M2e-specfic IgG1 and IgG2a antibodies were also enhanced with an increasing dose of soluble M2e. Upon challenge, the group vaccinated with the largest amount of free M2e (6.9 μg in 500M2e-AuNP+sCpG formulation) exhibited 100% protection against influenza A/PR/8/34 (H1N1) virus and was accompanied with the least weight loss (~4%). In contrast, as the amount of free M2e in the vaccine formulation was reduced to 2.9 μg and 0 μg, the survival rates were reduced to 80% and 60%, respectively, and the mice exhibited significantly higher weight loss. Thus, it is clear that the quantity of soluble M2e in the vaccine formulation is critical to induce high levels of anti-M2e antibodies and in protecting mice against lethal influenza virus challenge.
Our previous study has demonstrated that AuNPs, onto which M2e is immobilized are also critical for achieving protection against lethal influenza virus challenge[15]. Together, these findings suggest that to design vaccines based on antigen-carrier systems the combination of the correct dose of soluble antigen and carrier-immobilized antigen is critical. Indeed, using silica nanoparticles, Wibowo et al have demonstrated the importance of simply mixing a nanoparticle with a soluble antigen to increase immunogenicity[30]. They have shown that upon mixing silica nanoparticles 50 nm in diameter with a soluble sub-unit capsomere protein antigen of M2e (CapM2e), the immune response increased to levels comparable to the use of allydrogel as an adjuvant. Immunogenicity-enhancement observed by them occurred despite adsorption of just 7.2 % of M2e (as part of CapM2e) antigen on to the silica nanoparticles. In contrast, in our study, the fraction of M2e adsorbed on to AuNPs was almost 18.8 % for the 500M2e-AuNP group (1.3 μg/6.9 μg x 100). The importance of soluble antigen in the vaccine formulation has also been suggested for other antigens. Balaji et al, showed that a nanovaccine composed of soluble and particle-encapsulated fusion protein F1-V (a plague antigen) stimulated enhanced antibody production compared with encapsulated F1-V alone[31, 32]. They proposed that the enhanced efficacy arising from inclusion of soluble antigen with encapsulated antigen may be attributable to generation of immune complexes, which correlate to production of more avid antibodies. This hypothesis suggests that as a future direction, it is important to examine the avidity of antibody induced by our vaccine formulations. Overall, data presented by us, and others[33] points towards a previously unrecognized role of the enhancement of vaccine immunogenicity by the addition of a sizable amount of a soluble antigen into a nanoparticle formulation, where only a small fraction of the antigen is adsorbed/attached to the nanoparticle surface. Additional studies are required to study this effect in greater detail; especially to assess whether antigen attachment to the nanoparticles is required at all?
Additionally, the goal of flu vaccination is the generation of long-term immunity that will enable prompt immune response when subjects encounter an influenza virus. Thus, we evaluated longevity and efficacy of the generated immunity by intranasal administration of our vaccine in mice. In the current study, we found that 500M2e-AuNP+sCpG vaccine formulation was able to induce long-lasting immunity and provided 100% protection against mortality and morbidity following influenza virus A/PR/8/34 (H1N1) challenge, 8 months post first-vaccine-dose. This protection was achieved despite decrease in serum anti-M2e antibodies over time. A similar result has also been reported previously where with a virus-like particle-based vaccine expressing tandem repeats of M2e, it was shown that while the level of M2e-specific antibody decreased long-term post immunization, still the mice were protected against challenge with influenza A/Philippines/82 virus[34]. The exact mechanism of this long-term immunity stimulated via 500M2e-AuNP+sCpG in not clear. It has previously been shown that stimulation of B cells via ligation of the B cell receptor (BCR) in the presence of CpG can induce differentiation of B cells into plasma cells[35]. Similarly, because our formulation contains M2e immobilized in high density on AuNPs, which can potentially stimulate BCR[36], and also contains sCpG, it is possible that the vaccine can stimulate plasma cell development that can maintain antibody levels for long-term. However, more experiments are needed to study this hypothesis.
AuNPs have more than 70 years of history of use in humans to treat extreme cases of rheumatoid arthritis[37]. Various bioavailability and toxicity studies have been done and the conclusions suggest that gold nanoparticle size, route of administration, and properties of the molecule conjugated to its surface can affect its biodistribution and safety profile[38-43]. However, no studies exist that have examined biodistribution of AuNPs upon intranasal administration. Thus, it is important to specifically study the biodistribution and safety of our vaccine formulation administered intranasally. Studies to this regard are ongoing in our laboratory and they will be presented when results are available. However, our preliminary histological studies of lung and brain sections of vaccinated animals 3 months post vaccination show no differences when compared to naïve controls (unpublished observation). We also could not find any gold in whole brains of these animals using inductively coupled plasma mass spectrometry (ICP-MS) as the measurement technique, which can allow sensitive quantitation (pg/ml levels) of gold [44]. We have also not observed any adverse health effects in the vaccinated mice that were monitored for up to 8 months post vaccination. Specifically, vaccinated mice did not display any signs of illness, such as weight loss, inactivity, hunched or unkempt appearance, lack of grooming, or any ectopic signs of inflammation, such as hair loss, redness or swelling.
In conclusion, we show that the presence of free M2e in the vaccine formulation is critical to stimulate a good immune response and to provide 100% protection against influenza A virus infection. The vaccine formulation also provides long-term term protection in mice upto 8 months. An important aspect of our vaccine formulation is that both AuNPs and M2e can be chemically synthesized. So it can potentially allow for a fully synthetic approach of vaccine production, which can be implemented in weeks rather than in months. Also, this approach is amenable for rapid manufacture of influenza vaccines that contain a new protective sequence of M2e to adapt to the antigenic shift and drift of emergent strains. Thus, collectively, our results support further evaluation of M2e-AuNP+sCpG as a universal influenza A vaccine.
Supplementary Material
Acknowledgements
Authors WT and HSG have submitted a provisional patent to the United States Patent and Trademark Office on the use of gold nanoparticles for influenza vaccination through the Texas Tech University System Office of Research, Commercialization & Federal Relations. Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R21AI099575.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosure
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.WHO Influenza (Seasonal) 2014 Website: http://wwwwhoint/mediacentre/factsheets/fs211/en/
- 2.Neumann G, Kawaoka Y. The first influenza pandemic of the new millennium. Influenza Other Respir Viruses. 2011;5:157–66. doi: 10.1111/j.1750-2659.2011.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis. 2006;12:9. doi: 10.3201/eid1201.051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel J, Farrar J, Han A, Hayden F, Hyer R, De Jong M. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353 doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 5.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21:1776–9. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 6.Cox R, Brokstad K, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59:1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 7.Subbarao K, Murphy BR, Fauci AS. Development of effective vaccines against pandemic influenza. Immunity. 2006;24:5–9. doi: 10.1016/j.immuni.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Allen PJ. Avian influenza pandemic: not if, but when. Pediatric Nurs. 2005;32:76–81. [PubMed] [Google Scholar]
- 9.Reid AH, Fanning TG, Janczewski TA, McCall S, Taubenberger JK. Characterization of the 1918 “Spanish” influenza virus matrix gene segment. J Virol. 2002;76:10717–23. doi: 10.1128/JVI.76.21.10717-10723.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–33. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 11.Holsinger LJ, Alams R. Influenza virus M2 integral membrane protein is a homotetramer stabilized by formation of disulfide bonds. Virology. 1991;183:32–43. doi: 10.1016/0042-6822(91)90115-r. [DOI] [PubMed] [Google Scholar]
- 12.De Filette M, Fiers W, Martens W, Birkett A, Ramne A, Löwenadler B, et al. Improved design and intranasal delivery of an M2e-based human influenza A vaccine. Vaccine. 2006;24:6597–601. doi: 10.1016/j.vaccine.2006.05.082. [DOI] [PubMed] [Google Scholar]
- 13.Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza M2e to the TLR5 ligand flagellin. Vaccine. 2008;26:201–14. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- 14.Tompkins SM, Zhao Z-S, Lo C-Y, Misplon JA, Liu T, Ye Z, et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis. 2007;13 doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingrole RS, Tao W, Tripathy JN, Gill HS. Synthesis and Immunogenicity Assessment of Elastin-Like Polypeptide-M2e Construct as an Influenza Antigen. Nano LIFE. 2014;4 doi: 10.1142/s1793984414500044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibañez LI, Roose K, De Filette M, Schotsaert M, De Sloovere J, Roels S, et al. M2e-displaying virus- like particles with associated RNA promote T helper 1 type adaptive immunity against influenza A. PloS one. 2013;8:e59081. doi: 10.1371/journal.pone.0059081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–63. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 18.Fan J, Liang X, Horton MS, Perry HC, Citron MP, Heidecker GJ, et al. Preclinical study of influenza virus A M2 peptide conjugate vaccines in mice, ferrets, and rhesus monkeys. Vaccine. 2004;22:2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Fu T-M, Grimm KM, Citron MP, Freed DC, Fan J, Keller PM, et al. Comparative immunogenicity evaluations of influenza A virus M2 peptide as recombinant virus like particle or conjugate vaccines in mice and monkeys. Vaccine. 2009;27:1440–7. doi: 10.1016/j.vaccine.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Wibowo N, Chuan YP, Lua LH, Middelberg AP. Modular engineering of a microbially-produced viral capsomere vaccine for influenza. Chem Eng Sci. 2013;103:12–20. [Google Scholar]
- 21.Seth A, Ritchie FK, Wibowo N, Lua LH, Middelberg AP. Non-Carrier Nanoparticles Adjuvant Modular Protein Vaccine in a Particle-Dependent Manner. PloS one. 2014;10:e0117203. doi: 10.1371/journal.pone.0117203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhury MY, Li R, Kim J-H, Park M-E, Kim T-H, Pathinayake P, et al. Mucosal Vaccination with Recombinant Lactobacillus casei-Displayed CTA1-Conjugated Consensus Matrix Protein-2 (sM2) Induces Broad Protection against Divergent Influenza Subtypes in BALB/c Mice. PloS one. 2014;9:e94051. doi: 10.1371/journal.pone.0094051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110:15700–7. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- 24.Tkachenko A, Xie H, Franzen S, Feldheim DL. In: Assembly and Characterization of Biomolecule-Gold Nanoparticle Conjugates and Their Use in Intracellular Imaging NanoBiotechnology Protocols. Rosenthal SJ, Wright DW, editors. Humana Press; 2005. pp. 85–99. [DOI] [PubMed] [Google Scholar]
- 25.Lévy R, Thanh NT, Doty RC, Hussain I, Nichols RJ, Schiffrin DJ, et al. Rational and combinatorial design of peptide capping ligands for gold nanoparticles. J Am Chem Soc. 2004;126:10076–84. doi: 10.1021/ja0487269. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Jin R, Mirkin CA, Letsinger RL. Multiple thiol-anchor capped DNA–gold nanoparticle conjugates. Nucleic Acids Res. 2002;30:1558–62. doi: 10.1093/nar/30.7.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacDairmid A, Gallagher M, Banks J. Structure of dithiothreitol monolayers on Au (111). J Phys Chem B. 2003;107:9789–92. [Google Scholar]
- 28.Zhang F, Skoda MW, Jacobs RM, Zorn S, Martin RA, Martin CM, et al. Gold nanoparticles decorated with oligo (ethylene glycol) thiols: protein resistance and colloidal stability. J Phys Chem A. 2007;111:12229–37. doi: 10.1021/jp074293v. [DOI] [PubMed] [Google Scholar]
- 29.Schollbach M, Zhang F, Roosen-Runge F, Skoda MW, Jacobs RM, Schreiber F. Gold nanoparticles decorated with oligo (ethylene glycol) thiols: Surface charges and interactions with proteins in solution. J Colloid Interface Sci. 2014;426:31–8. doi: 10.1016/j.jcis.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 30.Wibowo N, Chuan YP, Seth A, Cordoba Y, Lua LH, Middelberg AP. Co-administration of non-carrier nanoparticles boosts antigen immune response without requiring protein conjugation. Vaccine. 2014;32:3664–9. doi: 10.1016/j.vaccine.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Ulery BD, Kumar D, Ramer-Tait AE, Metzger DW, Wannemuehler MJ, Narasimhan B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLoS One. 2011;6:e17642. doi: 10.1371/journal.pone.0017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phanse Y, Carrillo-Conde BR, Ramer-Tait AE, Broderick S, Kong CS, Rajan K, et al. A systems approach to designing next generation vaccines: combining α-galactose modified antigens with nanoparticle platforms. Sci Rep. 2014:4. doi: 10.1038/srep03775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L, Seth A, Wibowo N, Zhao C-X, Mitter N, Yu C, et al. Nanoparticle vaccines. Vaccine. 2014;32:327–37. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 34.Kim M-C, Song J-M, Eunju O, Kwon Y-M, Lee Y-J, Compans RW, et al. Virus-like particles containing multiple M2 extracellular domains confer improved cross-protection against various subtypes of influenza virus. Mol Ther. 2012;21:485–92. doi: 10.1038/mt.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poeck H, Wagner M, Battiany J, Rothenfusser S, Wellisch D, Hornung V, et al. Plasmacytoid dendritic cells, antigen, and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood. 2004;103:3058–64. doi: 10.1182/blood-2003-08-2972. [DOI] [PubMed] [Google Scholar]
- 36.Zabel F, Kündig TM, Bachmann MF. Virus-induced humoral immunity: on how B cell responses are initiated. Curr Opin Virol. 2013;3:357–62. doi: 10.1016/j.coviro.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 37.E. Abraham Peter B, Himmel G. Management of rheumatoid arthritis: rationale for the use of colloidal metallic gold. J Nutr Environ Med. 1997;7:295–305. [Google Scholar]
- 38.Chen Y-S, Hung Y-C, Liau I, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4:858–64. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho W-S, Cho M, Jeong J, Choi M, Cho H-Y, Han BS, et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2009;236:16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 40.Khlebtsov N, Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem Soc Rev. 2011;40:1647–71. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 41.Lasagna-Reeves C, Gonzalez-Romero D, Barria M, Olmedo I, Clos A, Ramanujam VS, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun. 2010;393:649–55. doi: 10.1016/j.bbrc.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 42.Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, et al. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res. 2008;41:1721–30. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 43.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloids Surf B Biointerfaces. 2008;66:274–80. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Allabashi R, Stach W, de la Escosura-Muñiz A, Liste-Calleja L, Merkoçi A. ICP-MS: a powerful technique for quantitative determination of gold nanoparticles without previous dissolving. J Nanopart Res. 2009;11:2003–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.