Abstract
Persistent safety concerns have stalled the development of viable hemoglobin (Hb)-based oxygen carriers (HBOCs). HBOCs have several advantages over human blood, including availability, long-term storage and lack of infectious risk. The basis of HBOC toxicity is poorly understood, however several mechanisms have been suggested, including Hb extravasation across the blood vessel wall, scavenging of endothelial nitric oxide (NO), oversupply of oxygen, and heme-mediated oxidative side reactions. Although there are some in vitro and limited animal studies supporting these mechanisms, heme mediated reactivity appears to provide an alternative path that can explain some of the observed pathophysiological changes. Moreover, recent mechanistic and animal studies support a role for globin and heme scavengers in controlling oxidative toxicity associated with Hb infusion.
The need for HBOCs
Worldwide civilian and military needs have driven the development of hemoglobin (Hb)-based oxygen carriers (HBOCs) or “blood substitutes” over the last three decades. Some of these products have undergone extensive animal and human testing in the United States [1]. The potential benefits of HBOCs include universal compatibility without the need for cross matching of donated blood, availability, lack of infection and long term storage. The term “blood substitute” however, has often been inaccurately used to describe these compounds as they do not perform normal blood functions, such as transport of nutrients, immune response, and coagulation. As an “oxygen bridge” HBOCs can however, complement standard blood transfusions in extreme, life-threatening situations, such as trauma, in some surgical settings and when blood is not an option (e.g., patient refusal due to religious objections and unavailability owing to issues of compatibility or remote location)
What are HBOCs made of?
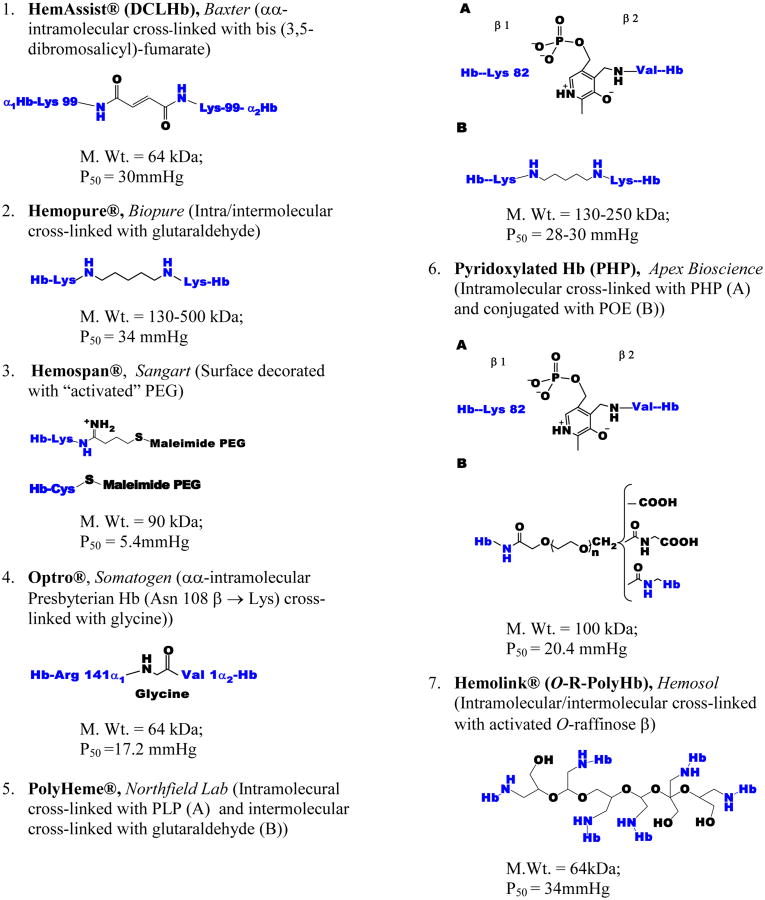
HBOCs are derived from outdated human or bovine blood and are prepared predominantly by chemical modifications of the Hb molecule or in some limited cases Hb is expressed in bacteria or yeast host systems. Chemical modifications aim in general at stabilizing Hb in a tetrameric (as found within red blood cells (RBCs)), or in a polymeric form to increase intravascular retention and to prevent of renal filtration of smaller molecular-size fractions of the protein. The starting material is a stroma free-Hb (SFH), or stroma poor Hb, obtained after RBC lysis followed by filtration and chromatographic procedures. These purification procedures may not eliminate all red cell proteins prior to chemical modifications [2]. Anionic and cationic chromatographic procedures have been used to produce, in some cases, a highly purified human Hb known as (HbA0) with demonstrated purity of approximately 99% [3]. Commonly used formulations that have been tested in animals and human subjects include intra-tetrameric cross-linked Hb, polymers of Hb tetramers (intra-and inter-cross-linked), and Hb tetramers conjugated to non-protein macromolecules, are summarized in Figure 1.
Figure 1. Nature and site(s) of modifications in some HBOCs that undergone clinical development.
Both Optro and HemAssist are site-specifically cross-linked Hb tetramers. In Optro, the C-terminal Arg and the N- terminal Val of the two α subunits of a recombinant mutant Hb are bound together by a fusion junction of the sequence Arg(141α1)GlyVal(1α2). Acylation with bis(3,5 dibromosalicyl)-fumarate occurs between Lys99 of the two α chains to produce HemAssist also known as DCLHb (see Figure 2). Both Hemopure (bovine) and PolyHeme (human) are modified with glutaraldehyde, a five carbon dialdehyde which forms a Schiff base (imine) with lysine (amine) side chains of Hb. PolyHeme is also crosslinked with pyridoxal phosphate (PLP) between Val 1β and lysine β82. Hemospan is conjugated in a two-step procedure; first the surface lysine groups are modified with 2-iminothiolane to add new SH groups, and then maleimide-activated PEG-5000 side arms are coupled to the new sites. In the first step of Pyridoxylated Hb (PHP) preparation, a SFH is cross-linked with pyridoxal 5-phosphate and then conjugated with the bi-functional activated diester of α-carboxymethyl ω-carboxymethyl-polyoxyethylene (POE). In Hemolink, activated O-raffinose (hexaaldehyde) stabilizes the tetramer through three point covalent linkages within the DPG pocket and an intermolecular cross-linking of the stabilized tetramers.
Hb tetramers or stabilized tetramers have also been successfully expressed in host systems, such as bacteria using recombinant technology (Figure 2). Correct protein folding and heme insertion and proper orientation of the heme within the protein and their impact on the protein function and long term stability are among the issues that still need to be resolved as this technology evolves [4].
Figure 2. Graphic representations of two commonly tested HBOCs in humans.
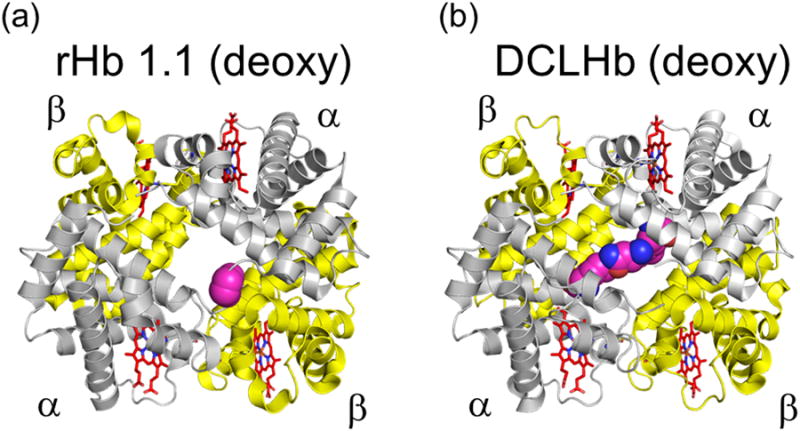
a) Optro™ (rHb 1.1, deoxy). This Hb is expressed as a crossed-linked deoxy form with glycine αα linked genes on expression plasmid (Hb Presbyterian (βN108K)). b) HemAssist (DCLHb™). This Hb is chemically cross-linked at the αα subunits with bis(3.5-dibromosalicyl)fumarate. Figures were constructed using the Adobe Illustrator (Adobe Systems Incorporated, Mountain View, CA) and PyMOL Molecular Graphics System (Schrodinger, LLC, New York, NY).
Do HBOCs carry and deliver oxygen?
Techniques that generate a set of oxygen equilibrium curves (OECs) are the most commonly used in vitro assays that reflect HBOC's own oxygen-binding affinity under well-controlled experimental conditions, such as temperature, pH, and oxygen tension [2]. One question which remains unresolved is whether oxygen binding properties of a given HBOC should by design match that of the RBCs (i.e., low oxygen affinity [large P50] or high oxygen affinity [small P50] (Box 1). No animal or human data are available that can convincingly support either of these options.
Box 1. Oxygen transport by hemoglobin.
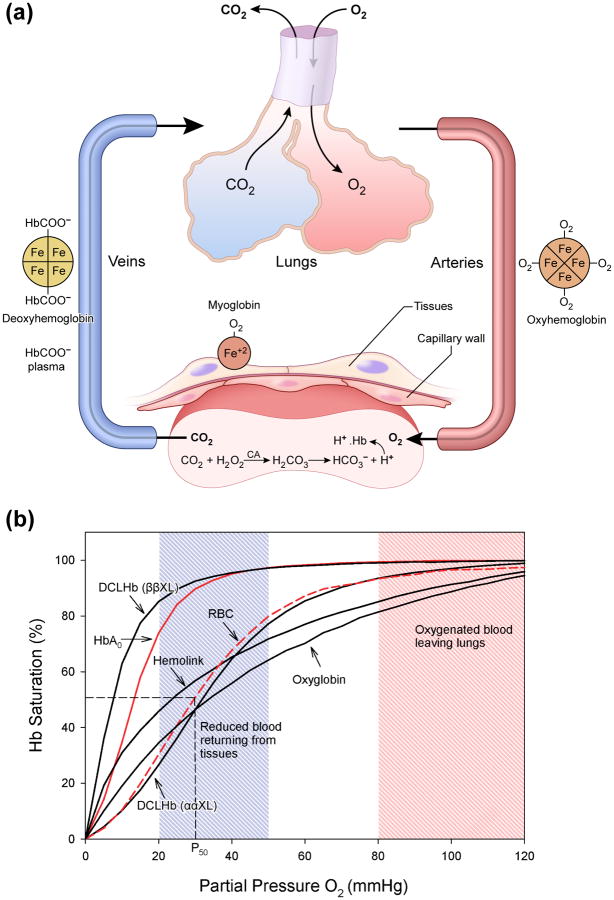
The blood is saturated with O2 at a PO2 of 98.7-99.7% mmHg and when it reaches tissues, where PO2 is about 20 mmHg, O2 is discharged (Figure Ia). The ability to switch from high to low O2 tensions in lungs and tissues respectively is well captured by the classical sigmoidal O2 equilibrium curve (OEC) [5]. Hb binds the first O2 slowly, which is then accelerated proportionally with the binding of the second, third and the fourth molecule (Figure Ib).
| (1) |
| (2) |
Thus, over the range of PO2 values between pulmonary venous blood and peripheral tissues capillary blood, the Hb molecule discharges 25-30% of O2 it carries. The greater the sigmoidal character of the OEC, the greater will be the O2 released per unit drop in PO2. This effect can be described qualitatively by the Hill equation (A = [HbO2]/[Hb][O2]n) (where (n) represents the Hill coefficient, which for a sigmoidal Hb is about 2.8). The oxygen affinity (P50) and cooperativity (n) are greatly influenced by a number of allosteric modulators within the RBC, such, pH, CO2, 2,3-diphospglycerate (DPG) and chloride ions. These allosteric affectors of Hb behave mostly as antagonists. When one binds to Hb the other falls away. In an acidic tissue environment Hb picks protons (H+) in exchange for O2 and the OCE of Hb is shifted to the right. This is known as Bohr effect. Similarly, chloride, DPG, which stabilizes the Hb tetramer in the deoxy form and carbon dioxide (CO2) collectively force the Hb to drop O2 at the tissues
Figure I. Box 1. Gas exchange and performance of oxygen carriers.
A) Gas exchange and transport by hemoglobin. Oxygen is carried from the lungs to the tissues by Hb, and CO2 is carried back to the lungs. Part of CO2 (∼20%) is carried on the amino termini of Hb as carbamino compounds (R-NH-COO− + H+). The major mechanism for the transport of CO2 to the lungs is carried out in the plasma, i.e in the plasma as HCO3− (∼80%). This reaction is catalyzed by the enzyme carbonic anhydrase (CA). DeoxyHb picks up those protons and aids in the formation of bicarbonate ions from CO2 in the blood plasma. B) Oxygen equilibrium curves for some commonly used HBOCs compared with that of free hemoglobin and fresh red blood cells. OECs for several HBOCs compared with that of fresh RBCs (dotted red line) (P50 = 29-30 mmHg) and an isolated purified Hb (HbA0) (red line) (P50 = 8-12 mmHg). Some maintained their classical sigmoidal shape, whereas others lack such an important property, and are either left or right shifted and some curves do not reach the saturation levels at higher oxygen tension (i.e PO2 =100 mmHg) (see for example OECs of Hemolink and oxyglobin, an FDA approved HBOC for use in Veterinary Medicine).
Several invasive and noninvasive techniques were used to monitor oxygen delivery by HBOCs, which resulted in conflicting data in animals [6]. This includes but is not limited to methods such as phosphorescence quenching by palladium–porphyrin fiberoptic or injected palladium-porphyrin bound albumin techniques, tissue and microcirculation inserted oxygen microelectrodes in the hamster dorsal skin fold and in some cases, Electron Paramagnetic Resonance Oximetry in small animals were also used (for review of these techniques see [6]). Generally HBOCs appear to oxygenate tissue shortly after infusion. However, evaluation of oxygenation of multiple organs and tissue-specific oxygenation after HBOCs infusion remains a challenge and it is the goal of preclinical evaluation of these products. The dynamic balance between HBOCs and tissue oxygen-sensing mechanisms including the oxygen sensing machinery, hypoxia-inducible factor (HIF) was recently explored in an 80% HBOC for blood exchange transfusion rat model. HIF-1α and some of its target genes, such as erythropoietin (EPO) which is involved in adaptation to hypoxia were measured in kidney tissues obtained from these animals. Both the HIF protein and its transcriptional activity were effectively suppressed during the first 4-6 hours of infusion of a bovine polymerized Hb in contrast to the infusion of non-oxygen carrying hetastarch in which tissue HIF-1α remained high. However, as Hb oxidation increases with time, as measured by accumulation of metHb in circulation, both HIF expression as well EPO (both plasma and its gene expression in the kidneys) began to rise dramatically, before these proteins were either cleared and/or rendered largely oxidized [7].
How safe are HBOCs?
The effects of infusion of early preparations of SFH in animals and humans are well documented [8]. Crosslinking and/or polymerization of Hb have succeeded in minimizing renal toxicity associated with these preparations [1,2]. Table 1 summarizes most commonly reported clinical and preclinical side effects associated with current generation HBOCs [9]. Transient hypertension is one of the most reported effects associated with almost all HBOCs tested so far in animals and in humans. Increases in mean blood pressure observed varied from 20 to 30% in small animals [9]. Changes in systemtic and pulmonary blood pressures are usually accompanied by changes in systematic vascular resistance as well as cardiac output. The removal of vascular endothelial nitric oxide (NO), a potent vasodilator molecule has been attributed as the cause of these hemodynamic changes following infusion of HBOCs (see next section on the mechanism of HBOC toxicity). However, endothelin, a powerful vasoconstrictor has also been reported to play a role [10].
Table 1. Preclinical and clinical side effects associated with HBOC infusion.
| 1. | Vasoactivity/hypertension |
| 2. | Gastrointestinal side effects |
| 3. | Pancreatic and liver enzyme elevation |
| 4. | Oxidative stress |
| 5. | Cardiac involvement |
| 6. | Hemostasis |
| 7. | Neurotoxicity |
| 8. | Renal effects |
Biochemical as well histological changes were reported to accompany infusion of most current generation HBOCs in animals [1]. Elevation in aspartate aminotransferase (AST), creatine phosphokinase, amylase and changes in total bilirubin have been observed in humans [9]. Gastrointestinal complications as a result of pancreatic injury, evidence of hepatocellular injury and esophageal spasm were also reported in human clinical trials [9]. Pancreatic changes in particular were attributed to NO scavenging by HBOCs resulting in spasm of the esophageal sphincter of Oddi and/or impairment in pancreatic perfusion due to ischemia [1].
Renal toxicity associated with some HBOCs appears to be mediated through heme-mediated oxidative events although some reports implicate NO pathways as well [11]. The effects of HBOCs on hemostasis were investigated in a number of in vitro models. The effect of polymerized bovine Hb for example in a rabbit model of arterial thrombosis and bleeding model for example was found to reduce arterial thrombosis rate and increases bleeding time [12]. Coagulation defects, thrombocytopenia and thrombosis were also reported in clinical trials of some HBOCs [9].
Several clinical trials have revealed increased frequencies of myocardial infarction in patients who were infused with some HBOCs [9]. Early reports on one of the tetrameric Hbs infused in several animals induced minimal to moderate myocardial lesions in rhesus monkeys or pigs but not in dogs, sheep and rats [13]. Infusion of a polymerized bovine Hb in guinea pigs has been shown to lead to diffuse, punctate myocardial lesions [11].
Neurotoxicity of SFH was demonstrated early in neuronal cells under several experimental conditions [14]. In a subarachnoid hemorrhage model, this Hb induced a decrease in blood flow and neuronal death when injected in the cisterna magna and induced a decrease in blood flow and neuronal death [15]. Using a guinea pigs exchange transfusion model, polymerized bovine Hb induced brain-barrier disruption and oxidative stress [16]. Exchange transfusion with a polymerized bovine Hb induced a considerable expression of heme oxygenase 1 (HO-1) in renal tissues of rats and guinea pigs [11]. Studies in animal models however, do not always correlate well with human disease and in some cases it is very difficult to reproduce conditions that mimic human response.
These studies have however, focused our research efforts on important pathways that are potentially responsible for the unexplained toxicities associated with HBOCs.
Mechanisms of HBOC toxicity: Current hypotheses
Nitric oxide depletion and hypertension
The NO “revolution” that began in early 1980s dramatically impacted the field of blood substitutes. Free Hb undergoes a rapid (∼107 M-1 s-1) and irreversible reaction with NO to produce metHb, in which Hb kinetically behaves as a dioxygenase enzyme [17]. Much slower processes that follow in which metHb reacts with NO to produce several iron-NO complexes which may further deplete NO have been also reported [18,19]. Endogenous NO is an important signaling molecule for the autoregulation of systemic and pulmonary vascular tone, and if removed by Hb, this will result in a vasoconstriction, systemic and pulmonary hypertension and decreased cardiac output [1].
The blood substitute community quickly embraced this newly discovered molecule which by all accounts was seen to be responsible for all of the HBOC's ills [19]. Several attempts designed to control the interaction between NO with HBOCs were made by preventing extravasation of Hb across the endothelial lining of vascular wall, the source of NO. Several research groups and manufacturers of HBOCs began to increase the HBOCs molecular size or sequester it with bulky surface modifications in order to prevent extravasation. Alternatively, removal of smaller molecular species from the HBOC cocktails was another strategy aimed at eliminating the smaller molecular size species which were believed to be responsible for the reaction with and depletion of NO [20]. However, the extravasation hypothesis was specifically tested in rats infused with either a tetrameric Hb (DCLHb) or its highly polymerized version (Poly-DCLHb™) (∼1-2% tetramer) [21]. Results clearly showed that both hemodynamic and renal changes were equal in both set of animals [22]. Moreover, a polymerized human Hb (Polyheme™) in which the tetramers were purposefully reduced to less than 1% was also found to be vasoactive in humans [9]. This shows that extravasation of the Hb through the vessel wall is not a prerequisite for its scavenging of NO. Indeed, extensive kinetic diffusion calculations [23] on the vessel wall and across the unstirred layer surrounding the RBC showed that NO can in its short half-life reach the vessel lumen and react with free and intraerythrocytic Hb [24,25]. The use of inhalational NO, NO donors, the inhibition of NO synthetic pathways, or the re-engineering and the encapsulation of the Hb molecule to inhibit its reaction with NO seemed to have blunted these responses, but with little or no long-term tangible effects on organ toxicities [1].
Equally disappointing are some very imaginative strategies aimed at blood pressure control which have been widely promoted in the literature. These include the transformation of the Hb molecule into an NO carrier through S-nitosylation of cysteine 93 residue of the β subunits or enzymatically transforming Hb in the presence of nitrite into a source for NO (nitrite reductase) that require in both cases the well-known allosteric transition of Hb molecule. These two hypotheses were built in large part on in vitro experimental results with little or no validated animal studies to support them. Exporting NO from the erythrocyte or from free Hb would not only be mechanistically difficult without intermediates [26] particularly in the view of the fact that Hb will rapidly and irreversibly consume NO [24]. In studies where large animals (swine) experiencing hemorrhagic shock that mimic battlefield conditions, nitrite infusion with HBOCs resulted in transient drop in blood pressure, but increased the risk of pulmonary complications including pulmonary edema and congestion [27].
Auto-regulation and the oversupply of oxygen
An alternative concept to the direct NO scavenging by Hb and subsequent vasoconstriction of blood vessels is the theory of oversupply of oxygen (a known vasoconstrictor) that was put forward to explain the hypertensive effects of first generation HBOCs. A fundamental rethinking of the design of HBOCs with reduced ability to oversupply oxygen (higher oxygen affinity) became a research priority to resolve Hb toxicity [28]. The premise of this theory is based on the assumption that the introduction of cell-free Hb, even at a low concentration, greatly augments oxygen supply, engaging protective mechanism that include vasoconstriction to counteract the “poisonous” oxygen, possibly through reactive oxygen species (ROS) formation and local destruction of NO [29]. The early reports that surface modification of bovine Hb with polyethylene glycol resulted in non-hypertensive product [30], spurred the modification of a newer generation of PEGylated Hbs both by academia [31] and industry [28,29]. Several HBOCs with low Hb concentration (4-6 g/dl), large molecular weight (attachment of 4-6 molecules of 500 kd PEG) and higher oxygen affinities ranging from P50 of 3-5 mmHg were therefore designed that included for example, Hemospan (MP4), Prolong (a new version of Hemo-life, developed originally by Enzon Inc [30] and a zero-linked polymerized bovine Hb, OxyVita (a large polymer held together by pseudopeptide bonds on the surface of adjacent tetramers) [32]. There are a number of animal studies published on the role of MP4 in the microcirculation which show improved blood flow and tissue oxygenation, and improved functional density [33] as well as in uncontrolled hemorrhage [34]. As with all HBOCs, early clinical trials with MP4 were promising. However, in a more recently published study with patients undergoing primary hip arthroplasty, MP4 normalized blood pressure changes, but was associated with higher risks of adverse events, including elevations of liver enzymes and troponin concentration, hallmarks of adverse events associated with first generation HBOCs [35].
Heme-mediated reactions: the poison is in the heme
In recent years several lines of evidence emerged from in vitro and animal studies support the dominance of Hb oxidative reactions in the overall toxicology of HBOCs [19,24]. In a non-compartmentalized environment the heme iron, a transition metal undergoes redox transformation into higher more reactive oxidation states of Hb. Hb, outside the reducing red cell environment undergoes uncontrollable spontaneous oxidation of the ferrous heme iron (HbFe2+-O2) to non-oxygen carrying ferric heme (HbFe3+) (metHb). In addition, Hb generates its own oxidants, such as superoxide ion (O2•-) which dismutates rapidly to form hydrogen peroxide (H2O2). This can be contrasted with the RBCs where Hb, is continuously reduced back to the ferrous functional form by efficient enzymatic machinery. Ferrous and ferric forms of Hb react with H2O2 to produce ferryl (HbFe4+) and ferryl radical (•HbFe4+) intermediates as part of a well-known pseudoperoxidative cycle (Figure 3): The reaction of Hb with oxidants such H2O2 can be described by Eq 1-2:
Figure 3. Redox cycles of hemoglobin and its transition into higher oxidation state (pseudoperoxidase) and subsequent oxidative changes.
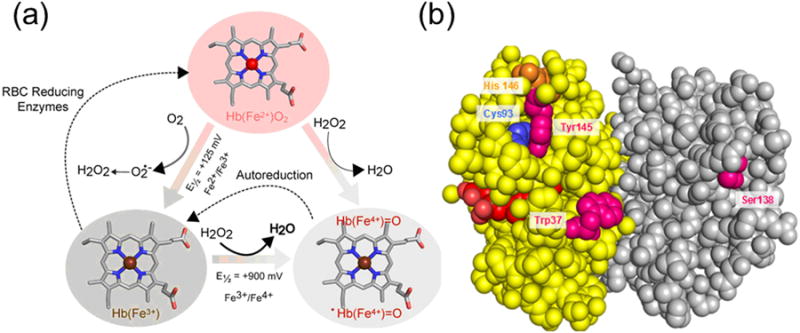
A) Pseudoperoxidase cycle Heme iron, a transition metal within the heme prosthetic group of Hb undergoes spontaneous oxidation from ferrous to ferric oxidation states. This process indirectly produces H2O2, which can further react with ferric and ferrous Hb to produce ferryl species and its protein cationic radical. Thermodynamically, these reactions are driven by the redox potentials of each pair. B) Protein-heme oxidative modifications and oxidation of amino acids. Hb Beta subunits (yellow) bear the burden of the radical chemistry as a handful of amino acids in the “hotspots” are targeted and are irreversibly oxidized. In addition, heme-to-protein linkages are formed which will ultimately result in heme loss.
| (1) |
| (2) |
These reactions if remained unchecked will affect both the Hb molecule and nearby tissues. This is largely due to the fact that ferryl and its associated protein radical exhibit high redox potential (∼1.0V) and are thus thermodynamically very reactive towards biological targets [36]. First, at the protein level, several key amino acids located in β subunits in what is known as the “hotspots” are targeted by the radical chemistry emanated from heme which results in an irreversible oxidation of these amino acids [37] (Figure 3). In addition to the destabilization of the oxidatively damaged β subunits, altered heme-protein linkages are formed which eventually lead to heme and/or modified heme product release [36].
Recent studies from our laboratory show that a considerable amount of ferric Hb accumulates in animals from which blood was exchange transfused with HBOCs. These reactions occur at much higher rates in animals such as guinea pigs that lack endogenous reductive mechanisms, such as ascorbic acid. Rats on the other hand were able to enzymatically produce ascorbate to control free Hb oxidation in their circulation [38]. Indirect EPR measurements of ferryl radicals in rabbits infused with HBOCs were also reported recently [39]. Subtle oxidative changes at the amino acid levels in proteins after infusion of HBOCs or stroma-free Hb were recently identified by more sensitive mass spectrometric methods [40]. Intramolecular Hb cross-links, porphyrin-globin covalent adducts modifications have been found in Hb recovered from the spinal fluid after subarachnoid hemorrhage [41] and from the urine [42], suggesting that peroxidative reactions may contribute to Hb toxicity in vivo. A notable case of a Jehovah's Witness patient who refused blood transfusion on religious grounds was successfully treated with an HBOC. Infusion of one of the bovine polymerized HBOCs in this patient who was involved in a car accident led to reversal of cardiac hypoxia secondary to sever anemia following trauma. Total of 6 units that were infused slowly over an 8 hour period together with 1g of ascorbic acid twice daily over a 6 day period have according to the authors minimized the adverse event related to volume overloads, vasoactivity and methemoglobin formation [43].
Released heme triggers inflammatory responses
Autoxidation and oxidative modification as described above will ultimately lead to unfolding of the molecule and heme loss. Heme can selectively bind to several receptors, transcription factors, enzymes that may alter gene transcription and metabolism including the transcriptional activity of heme oxygenase (HO-1) and other antioxidant enzymes as a countermeasure against free heme. Heme due to its hydrophobicity enters cell membrane for example, heme can activate the toll-like receptors (TLRs), particularly, TLR-4 which triggers a cascade of inflammatory responses [44]. In a murine mouse model of sickle cell disease infused with free Hb to mimic hemolysis TLR-4 was clearly activated which led to pro inflammatory and prothrombotic responses, thereby promoting blood cell adhesion and vaso-occlusion [45].
Can heme-mediated toxicity be controlled? Role of protein and heme scavengers
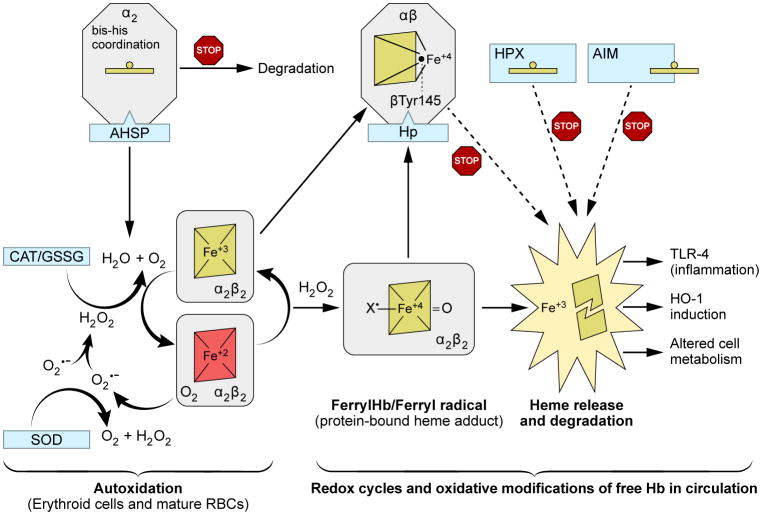
In mammals, several pathways exist to control oxidative reactions of free Hb as a result of hemolysis of red blood cells due to aging or diseases (Figure 4). These control mechanisms span from early erythropoiesis in erythroid precursors, heme degradation in macrophages, and several metabolic pathways controlling iron transport and storage [46]. Some of the key globin binding proteins that appear to inhibit oxidative damage and degradation are: (a) Alpha hemoglobin-stabilizing protein (AHSP), which provides protection against oxidative damage to α subunits and surrounding proteins during erythropoiesis [47] and (b) Haptoglobin (Hp) and its CD163 receptor on macrophages, which coordinate Hb dimer clearance when Hb is released from aging erythrocytes or during mild chemical-or disease-induced hemolysis [48] and (c) Hemopexin (Hpx) an effective heme-binding proteins in plasma [49] (Figure 4). In healthy animals in which endogenous Hp was induced or when given in complex with Hb in an exchange transfusion model, there was total reversal of Hb organ oxidative toxicity [50]. Free Hb toxicity in murine sickle cell mouse models was also inhibited by Hp or hemopexin or the combination of the two proteins [45].
Figure 4. Mechanisms of hemoglobin oxidation and control.
The scheme begins with the process of autoxidation that occurs inside and outside the RBCs. This process proceeds at much higher rates in circulation with minimum antioxidant and reductive mechanisms. Fueled by H2O2 free Hb will undergo a series of redox events, generating higher more reactive oxidative species, such ferryl and ferryl radicals. Oxidative changes that follow will ultimately led to unfolding of the protein and heme loss. Heme stimulates endothelial TLR4 singling and downstream oxidative responses which ultimately lead to altered cell metabolism. The scheme also outlines and contrasts the role of globin and heme scavengers at different stages to intercept the process of Hb oxidation. Starting within the erythroid, AHSP which locks newly synthesized α subunits in a stable hexacoordinate configuration unable to react with oxidants thus preventing subunits degradation [51]. This will enable α and β subunits to form first Hb tetramer in these cells. Hp on the other hand allows the αβ dimer to consume oxidants while in the meantime the Hb-Hp complex diffuses the emerging and damaging radicals and likely inhibiting heme loss from the complex. Hxp, a heme-binding scavenger forms the second line of defense against excess heme and when first line of scavengers of Hb such as Hp is overwhelmed. Hpx sequester heme in a hexacoordinate conformation in a complex with a 1:1 stoichiometry. Multiple other proteins such albumins, lipoproteins and A1M can bind and effectively remove some of the released heme [52].
Concluding remarks and future perspectives
In spite of 30 years of active research and development, no clinically viable product has been approved in the United States. Oxidative toxicity of Hb in a free environment and the consequences of these events are difficult to study in living systems and have unfortunately been ignored by the research community. The focus was instead on strategies that can prevent Hb extravasation through the blood vessel wall and on the reaction of Hb with NO. The reaction with NO is particularly problematic in some researcher's minds because the removal of NO by Hb raises the blood pressure in patients infused with Hb. Recent in vitro and animal studies showed that one can successfully design against Hb oxidative side reactions by inclusion of reducing agents, antioxidants, anti-inflammatory molecules or by the re-engineering of Hb into and oxidatively stable molecule (see Box 2 for unresolved questions).
Box 2. Outstanding questions.
The most significant questions that need to be addressed in order to move the field of blood substitutes forward:
Can we explain the observed histopathological changes solely on the basis of the reaction between cell-free Hb and NO? Should more definitive and unambiguous evidence be presented?
What is the more effective strategy to resolve Hb oxidative toxicities: re-engineering of the Hb molecule by chemical or recombinant means, or exploring an alternative cheaper protective mechanism such as the re-introduction of endogenous scavenger proteins in complex with Hb or free form in circulation?
When blood is not available, due incompatibility or religious objection, should the ratio of relative risk to benefit using current generation HBOCs be re-considered?
Would nanoparticles or stem cell derived-RBCs be viable alternative technologies in blood transfusion to replace Hb-based manufacturing of blood substitutes?
Highlights.
HBOC therapeutics are intended to correct oxygen deficit
Safety concerns slowed the development of these life-saving products
Evidence is presented to show the dominance of oxidative toxicity
protein re-engineering and/or the use of antioxidants can control toxicity
Acknowledgments
The Author wishes to acknowledge the contribution of past and present members of the Laboratory of Biochemistry and Vascular Biology (LBVB) to this work. This work was supported by National Institutes of Health (NIH) grant HL110900, and the U.S. Food and Drug Administration (MODSCI)
Glossary
- Oxygen equilibrium curve (OECs)
the oxygen equilibrium curve is a plot that shows the percent saturation of Hb at various partial pressures of oxygen. The purpose of an oxygen dissociation curve is to show the equilibrium of oxyHb and unbound hemoglobin at various partial pressures. P50 reflects the partial pressure at which the Hb/erythrocytes are half-saturated with oxygen
- Stroma-free or Stroma poor hemoglobin (SFH)
Hb that has been stripped of most of the red cell proteins found in hemolyzed blood by filtration and chromatographic procedures. SFH is then used by manufacturers as staring material for further chemical modifications producing HBOCs
- Erythropoiesis
is the process by which erythrocytes are produced. It is stimulated by decreased oxygen in circulation, which is detected by the kidneys, which then secrete the hormone erythropoietin (EPO)
- Vasoconstriction
is the narrowing of the blood vessels resulting from contraction of the muscular wall of the vessels, in particular the large arteries and small arterioles. The process is the opposite of vasodilation, the widening of blood vessels
- Thrombocytopenia
thrombocytopenia refers to a relative decrease of platelets in blood. A normal human platelet count ranges from 150,000 to 450,000 platelets per microlitre of blood
- Liposomes
is an artificially-prepared vesicle composed of a lipid bilayer. The liposome can be used as a vehicle for administration of nutrients and pharmaceutical drugs including Hb
- S-nitosylation
involves the covalent incorporation of a nitric oxide moiety into thiol groups, to form S-nitrosothiol (SNO). Where the thiol group belongs cysteine-93 residues in Hb also referred to as SNOHb
- Nitrite reductase
refers to the role of Hb in catalyzing the reduction of nitrite to produce nitric oxide
- Pseudoperoxidase
the reaction between hydrogen peroxide and ferrous or ferric Hb is considered a classic one, due to the slow kinetics compared with true peroxidases, which allowed the reaction to be followed with spectroscopic equipment
- Reduction potential
is a measure of the tendency of a chemical species to acquire electrons and thereby be reduced. Reduction potential is measured in volts (V), or millivolts (mV). Each species has its own intrinsic reduction potential; the more positive the potential, the greater the species' affinity for electrons and tendency to be reduced
- Toll-like receptors (TLRs)
mammalian TLRs play an important role in versatile recognition of pathogen-associated molecular patterns. They are the first identified and best characterized receptors among the signaling pattern recognition receptors (PRRs). They initiate key inflammatory responses and also shape adaptive immunity
- Extravasation
leakage of infused substances into the vasculature into the subcutaneous tissue
- Thrombosis
is the formation of a blood clot inside a blood vessel, obstructing the flow of blood through the circulatory system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim HW, Greenburg AG. Artificial oxygen carriers as red blood cell substitutes: a selected review and current status. Art Organs. 2004;28:813–827. doi: 10.1111/j.1525-1594.2004.07345.x. [DOI] [PubMed] [Google Scholar]
- 2.Alayash AI. Setbacks in blood substitutes research and development: a biochemical perspective. Clin Lab Med. 2010;106:76–85. doi: 10.1016/j.cll.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Adamson JG, Moore C. Hemolink, an O-Raffinose crosslinked hemoglobin-based oxygen carrier. In: Chang TMS, editor. In blood substitutes, principles, methods, products and clinical trials. 1998. pp. 62–79. [Google Scholar]
- 4.Varnado CL, et al. Development of recombinant hemoglobin-based oxygen carriers. Antioxid Redox Signal. 2013;18:2314–28. doi: 10.1089/ars.2012.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickerson RE, Geis I, editors. Hemoglobin:strcture, function, evolution, and pathophysiology. Benjamin/Cummings publishing Company; 1983. [Google Scholar]
- 6.Buehler PW, Alayash AI. Toxicities of hemoglobin solutions: in search of in-vitro and in-vivo model systems. Transfusion. 2004;44:1516–1530. doi: 10.1111/j.1537-2995.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 7.Manalo DJ, et al. Acellular haemoglobin attenuates hypoxia-inducible factor-1alpha (HIF-1alpha) and its target genes in haemodiluted rats. Biochem J. 2008;414:461–469. doi: 10.1042/BJ20080313. [DOI] [PubMed] [Google Scholar]
- 8.Chen JY, et al. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics. 2009;64:803–813. doi: 10.1590/S1807-59322009000800016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverman TA, Weiskopf RB. Hemoglobin-based oxygen carriers: current status and future directions. Anesthesiology. 2009;111:946–963. doi: 10.1097/ALN.0b013e3181ba3c2c. [DOI] [PubMed] [Google Scholar]
- 10.Creteure J, Vincent JL. Potential uses of hemoglobin-based oxygen carriers in critical care medicine. Crit Care Clin. 2009;25:311–324. doi: 10.1016/j.ccc.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Buehler PW, et al. Hemoglobin-based oxygen carriers: From mechanisms of toxicity and clearance to rational drug design. Trends Mol Med. 2010;16:447–57. doi: 10.1016/j.molmed.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Marret E, et al. The effects of a polymerized bovine-derived hemoglobin solution in a rabbit model of arterial thrombosis and bleeding. Anesth Analg. 2004;98:604–610. doi: 10.1213/01.ane.0000099366.73625.dd. [DOI] [PubMed] [Google Scholar]
- 13.Burhop K, et al. Review of hemoglobin-induced myocardial lesions. Arit Cells Blood Substit Immobol Biotechnol. 2004;32:353–374. doi: 10.1081/bio-200027429. [DOI] [PubMed] [Google Scholar]
- 14.Regan RF, et al. Hemoglobin potentiates excitotoxic injury in cortical cell culture. J Neurotrauma. 1996;13:233–231. doi: 10.1089/neu.1996.13.223. [DOI] [PubMed] [Google Scholar]
- 15.Turner CP, et al. Heme-oxygenase-1 is induced in glia throughout brain by subarachnoid hemoglobin. J Cerab Blood Flow Metab. 1998;18:257–273. doi: 10.1097/00004647-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Butt O, et al. Blood-brain barrier disruption and oxidative stress in guinea pig after systemic exposure to modified cell-free hemoglobin. Am J Pathol. 2011;178:1316–1328. doi: 10.1016/j.ajpath.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eich RF, et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 18.Alayash AI, et al. Nitric oxide binding to human ferrihemoglobins cross-linked between either alpha or beta subunits. Arch Biochem Biophys. 1993;303:332–338. doi: 10.1006/abbi.1993.1292. [DOI] [PubMed] [Google Scholar]
- 19.Alayash AI. Hemoglobin-based blood substitutes: oxygen carriers, pressor agents, or oxidants? Nat Biotechnol. 1999;17:545–549. doi: 10.1038/9849. [DOI] [PubMed] [Google Scholar]
- 20.Gould SA, Moss GS. Clinical development of human polymerized hemoglobin as a blood substitute. World J Surg. 1996;20:1200–1207. doi: 10.1007/s002689900183. [DOI] [PubMed] [Google Scholar]
- 21.Buehler PW, et al. Chemical characterization of diaspirin cross-linked hemoglobin polymerized with poly(ethylene glycol) Anal Chem. 2006;78:4634–4641. doi: 10.1021/ac060188q. [DOI] [PubMed] [Google Scholar]
- 22.Abassi Z, et al. Effects of polymerization on the hypertensive action of diaspirin crosslinked hemoglobin in rats. J Lab Clin Med. 1997;29:603–610. doi: 10.1016/s0022-2143(97)90194-3. [DOI] [PubMed] [Google Scholar]
- 23.Liu X. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J Biol Chem. 2002;277:26194–26199. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 24.Alayash AI. oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 25.Robinson JM, Lancaster JR. Hemoglobin-mediated hypoxia-induced vasodilation via nitric oxide. Am J Res Cell Mol Biol. 2005;32:257–261. doi: 10.1165/rcmb.F292. [DOI] [PubMed] [Google Scholar]
- 26.Roche CJ, et al. Generating s-nitrosothiols from Hemoglobin: mechanisms, conformational dependence, and physiological relevance. J Biol Chem. 2013;288:22408–22425. doi: 10.1074/jbc.M113.482679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon-Massat P, et al. The effect of HBOC-201 and sodium nitrite resuscitation after uncontrolled haemorrhagic shock in swine. Injury. 2012;43:638–647. doi: 10.1016/j.injury.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Winslow RM. Cell-free oxygen carriers: scientific foundations, clinical development, and new directions. Biochim Biophys Acta. 2008;1784:1382–1386. doi: 10.1016/j.bbapap.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 29.Winslow RM. Oxygen: the poison is in the dose. Transfusion. 2013;53:424–437. doi: 10.1111/j.1537-2995.2012.03774.x. [DOI] [PubMed] [Google Scholar]
- 30.Conover CD, et al. The ability of polyethylene glycol conjugated bovine hemoglobin (PEG-Hb) to adequately deliver oxygen in both exchange transfusion and top-loaded rat models. Artif Cells Blood Substit Immobil Biotechnol. 1999;27:93–107. doi: 10.3109/10731199909117685. [DOI] [PubMed] [Google Scholar]
- 31.Manjula BN, et al. Conjugation of multiple copies of polyethylene glycol to hemoglobin facilitated through thiolation: influence on hemoglobin structure and function. Protein J. 2005;3:133–146. doi: 10.1007/s10930-005-7837-2. [DOI] [PubMed] [Google Scholar]
- 32.Harrington JP, et al. Structural and redox behavior of OxyVita, a zero-linked polymeric hemoglobin: comparison with natural acellular polymeric hemoglobins. Artif Cells Blood Substit Immobil Biotechnol. 2010;38:64–68. doi: 10.3109/10731191003634562. [DOI] [PubMed] [Google Scholar]
- 33.Wettstein R, et al. Resuscitation with polyethylene glycol-modified human hemoglobin improves microcirculatory blood flow and tissue oxygenation after hemorrhagic shock in awake hamsters. Crit Care Med. 2003;31:1824–1830. doi: 10.1097/01.CCM.0000069340.16319.F2. [DOI] [PubMed] [Google Scholar]
- 34.Winslow RM, et al. Vascular resistance and the efficacy of red cell substitutes in a rat hemorrhage model. J Appl Physiol. 1998;85:993–1003. doi: 10.1152/jappl.1998.85.3.993. [DOI] [PubMed] [Google Scholar]
- 35.Elmer J, et al. hemoglobin-based oxygen carrier for hemorrhagic shock. Resuscitation. 2012;83:1824–1830. doi: 10.1016/j.resuscitation.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Reeder BJ. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid Redox Signal. 2010;13:1087–123. doi: 10.1089/ars.2009.2974. [DOI] [PubMed] [Google Scholar]
- 37.Jia Y, et al. Structural basis of peroxide-mediated changes in human hemoglobin: a novel oxidative pathway. J Biol Chem. 2007;282:4894–907. doi: 10.1074/jbc.M609955200. [DOI] [PubMed] [Google Scholar]
- 38.Buehler PW, et al. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther. 2007;323:49–60. doi: 10.1124/jpet.107.126409. [DOI] [PubMed] [Google Scholar]
- 39.Dunne J, et al. Ascorbate removes key precursors to oxidative damage by cell-free haemoglobin in vitro and in vivo. Biochem J. 2006;399:513–24. doi: 10.1042/BJ20060341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buehler PW, Alayash AI. Oxidation of hemoglobin: mechanism of control in vitro and in vivo. Tran Alt Tran Med. 2007;9:204–212. [Google Scholar]
- 41.Reeder BJ. Toxicity of myoglobin and haemoglobin: oxidative stress in patients with rhabdomyolysis and subarachnoid haemorrhage. Biochem Soc Trans. 2002;30:745–7481. doi: 10.1042/bst0300745. [DOI] [PubMed] [Google Scholar]
- 42.Buehler PW, et al. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 2009;113:2578–2586. doi: 10.1182/blood-2008-08-174466. [DOI] [PubMed] [Google Scholar]
- 43.Fitzgerald MC, et al. A synthetic haemoglobin-based oxygen carrier and the reversal of cardiac hypoxia secondary to severe anemia following trauma. Med J Aus. 2011;194:471–473. doi: 10.5694/j.1326-5377.2011.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 44.Figueiredo RT. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- 45.Belcher J, et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2013 doi: 10.1182/blood-2013-04-495887. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buehler PW, et al. Blood aging, safety, and transfusion: capturing the "radical" menace. Antioxid Redox Signal. 2011;14:1713–1728. doi: 10.1089/ars.2010.3447. [DOI] [PubMed] [Google Scholar]
- 47.Feng L, et al. Structure of oxidized alpha-haemoglobin bound to AHSP reveals a protective mechanism for haem. Nature. 2005;435:697–701. doi: 10.1038/nature03609. [DOI] [PubMed] [Google Scholar]
- 48.Schaer D, et al. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121:1276–1284. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mauk MR, et al. An alternative view of the proposed alternative activities of hemopexin. Protein Sci. 2011;20:791–805. doi: 10.1002/pro.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boretti FS, et al. Intravascular sequestration within the haptoglobin complex is effective in treating hypertensive and oxidative effects of plasma free hemoglobin. J Clin Inves. 2009;119:2271–80. doi: 10.1172/JCI39115. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mollan TL, et al. Alpha-hemoglobin stabilizing protein (AHSP) markedly decreases the redox potential and reactivity of α subunits of human HbA with peroxide. J Biol Chem. 2013;288:4288–4898. doi: 10.1074/jbc.M112.412064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olsson MG, et al. Pathological conditions involving extracellular hemoglobin: molecular mechanisms, clinical significance, and novel therapeutic opportunities for α(1)-microglobulin. Antioxid Redox Signal. 2012;17:813–846. doi: 10.1089/ars.2011.4282. [DOI] [PubMed] [Google Scholar]