Abstract
Several genetically engineered mouse (GEM) models of colorectal cancer have been developed and are a mainstay in our efforts to identify means of preventing and treating this disease. Many of these models involve a germline disruption of the adenomatous polyposis coli (Apc) tumor suppressor gene and share the limitation that the great preponderance of tumors appear in the small rather than large intestine. In recent years efforts have been made to increase the similarity of these models to human sporadic colorectal cancer by disrupting Apc in a tissue-specific fashion using the Cre-Lox system so that the genetic aberrations are confined to the colonic epithelium. These models have shown great promise but reproducible and high penetrance colon-specific tumorigenesis has not yet been achieved without invasive techniques to introduce the Cre enzyme. We therefore sought to create a new model with high penetrance colon-specific tumorigenesis but without the need for exogenous Cre administration. We utilized existing mice possessing a conditional knock out for the Apc gene and a latent activated Kras allele and crossed them with mice expressing Cre recombinase solely in the large intestine. Using this approach we generated mice that developed 1–9 colonic adenomas per mouse (average 4.3) but without any tumors in the small intestine or cecum. No invasive tumors were observed. Despite the apparent lack of invasion, the geographical correctness, complete penetrance and intermediate tumor burden make this model a promising addition to our toolkit for the study of colorectal cancer treatment and prevention.
Keywords: Colorectal cancer, Apc, mouse model, tumor, adenoma, Kras
1. Introduction
Five to six percent of individuals will develop colorectal cancer (CRC) their lifetime. In 2013 this will result in approximately 143,000 new cases and 51,000 deaths in the US alone [1]. The heavy burden that CRC imposes on our society emphasizes the need to develop effective strategies to prevent and treat this disease. A mainstay in this effort has been the usage of mouse models of colorectal cancer. Both carcinogen-induced and genetically engineered mouse (GEM) models have exhibited a great deal of utility in this regard.
Dozens of GEM models of colorectal cancer have been developed over the last two decades and have been the topic of several thorough reviews [2–6]. Many of these models involve a germline disruption of the Adenomatous Polyposis Coli (Apc) tumor suppressor gene. Apc is a central inhibitor of the Wnt signaling pathway, a pathway that is aberrantly activated by mutation in approximately 90% of human CRC [7]. The Min (multiple intestinal neoplasia) or ApcMin/+ mouse was the first and perhaps most widely used of Apc mutant mice [8–9]. The ApcMin/+ mouse develops 20–30 intestinal tumors, however like most of its counterparts and derivatives; a key limitation of this model is that the great preponderance of tumors appear in the small intestine rather than colon. This is a key difference to the human situation where the incidence of colorectal tumors around 16 times higher than that seen in the small intestine [1]. Moreover, the genetic defect in the Min mouse is in the germline, which means that the procarcinogenic mutation resides not only in the colonocytes, but in the underlying myoepithelial and other lamina propria cells, with which there is important cross-talk during carcinogenesis [10]. This is also an important factor that distinguishes it from sporadic human colon cancer.
Several other GEM models with mutations in Apc have since been developed, with varying tumor burden and latency. Although all of these GEMS develop tumors primarily in the SI rather than colon, other characteristics of these models have made them useful tools in the study of CRC. For example, we have found the Apc1638N mouse useful in studying nutritional modulation of CRC as it has both an intermediate tumor burden and latency. In our hands 70% on these mice mouse develops 1–3 small intestinal tumors by the age of 7–8 months and tumorigenesis can be modulated by dietary B vitamin intake [11] and maternal B vitamin intake [12]. Furthermore, similar to the human situation others have shown that obesity promotes tumorigenesis in this model [13].
Nevertheless, to more closely model human CRC, GEM models are clearly needed in which tumorigenesis is shifted away from the small intestine and into the large intestine and the genetic mutations are confined to the colonic epithelium. Approaches to achieve this goal have included coupling germline Apc mutations with other germline mutations; exposing Apc mutants to colitic agents and the creation of conditional knockouts using the Cre-Lox system. This latter approach has shown the most promise in achieving greater tumor burden in the colon than small intestine.
A major advance was made by Shibata et al [14] who inserted loxP sites flanking exon 14 of the Apc gene. Removal of the ‘floxed’ exon from the so-called Apc580S alleles was then achieved by Cre-expressing viruses administered by enema. In mice with two copies of the engineered allele (Apc580S/580S), the AxCANCre adenovirus induced an average of 6.7 tumors/mouse, all within 3 cm of the anus; thus achieving, for the first time, the localization of tumors solely in the large intestine of a GEM. In addition to achieving geographical correctness in tumorigenesis, the conditional nature of gene inactivation more closely models human sporadic CRC, where mutations do not occur in every cell of the body, but occur somatically by chance. We have, however, not been able to reproduce tumorigenesis in this model (unpublished observations).
Hinoi et al [15] then crossed mice with the Apc580S allele with two transgenic mice stains engineered to express Cre in the intestinal tract, one driven by Villin and the other by Cdx2. Villin-Cre Apc+/580S mice developed 30.8 and 4.8 tumors/mouse in the small intestine and colon respectively. The Cdx2-Cre Apc+/580S mice developed 3.0 and 6.4 tumors/mouse in the small intestine and colon respectively.
Hung et al [16] modified the model of Shibata [14], by combining the Apc580S allele with a latent activated (lox-stop-lox; LSL) mutant (G12D) Kras allele [17], and restricted Cre virus exposure to the very distal colon by clamping the intestine. This latter modification was performed in order to limit tumors to the region of the colon distal to the splenic flexure, a region in which their growth could be easily monitored by serial colonoscopy. In Apc580S/580S, Kras+/LSL mice, tumors appeared within 3 weeks of Cre exposure in almost 100% of mice, with an average of 3.6 lesions per mice.
Shortly thereafter, Xue et al [18] reported the colon-specific inactivation of ApcΔ580 allele by tying Cre expression to carbonic anhydrase 1 (CAC), a gene expressed only in the large intestine. The tumor burden in ApcΔ580S/Δ580,CAC+ mice was severe with 100% of mice developing tumors and rectal prolapse by 3 weeks of age. Tumor burden was milder in Apc+/Δ580,CAC+ mice, with 10–20% displaying colon tumors by 10 weeks.
We sought to combine the strengths of these two latter models in order to achieve colon-specific tumorigenesis, without using a viral enema, at an intermediate tumor burden and latency. Thus we utilized the Apc580S [14] and latent activated Kras allele [17] as described by Hung et al [16], but achieved inactivation of Apc and activation of Kras by crossing with CAC+ mice [18]. Importantly, using the CAC+ mouse allowed us for the first time to restrict tumorigenesis to the colorectum without having to physically introduce a Cre-expressing virus. The resulting Apc+/580S,Kras+/LSL,CAC+ mice develop an average of 4.3 macroscopic tumors per mouse in the colorectum but none in the small intestine or cecum. All of the tumors observed during the scope of this experiment were adenomas however, as shown by Hung et al [16], modification of Apc and Kras can result in invasive carcinoma and with liver metastasis. This model adds to our toolkit for the study of the etiology, prevention and treatment of colorectal cancer.
2. Methods
All animal procedures were approved by the institutional review board of the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University.
All of the genetically modified mice used are commercially available and are on the C57BL/6J background: CAC+ [C57BL/6-Tg(Car1-cre)5Flt/J] and KrasLSL [B6.129S4-Krastm4Tyj/J] from Jackson Labs, Bar Harbor ME. Apc580S [B6.Cg-Apctm2Rak] from NCI Mouse Repository, Frederick, MD. CAC+/- mice and double transgenic Apc580S /580S,Kras+/LSL mice were transferred to the HNRCA from colonies at Purdue University (West Lafayette, IN) and Tufts Medical Center (Boston, MA) respectively. Apc580S /580S,Kras+/LSL and CAC+ mice were crossed, with approximately one quarter of offspring being the desired genotype; Apc+/580S,Kras+/LSL,CAC+. Offspring were genotyped for these 3 genes by PCR from tail snip DNA as previously described [16, 18].
The primers used for genotyping were as follows: Apc-FWD 5’-GAGAAACCCTGTCTCGAAAAAA-3’, Apc-RVS 5’-AGTGCTGTTTCTATGAGTCAAC-3’ (Wildtype= 320bp, Mutant= 430bp); KRAS-FWD 5’-ATATGTCTTTCCCCAGCACAG-3’, KrasG12D-FWD 5’-ACCATGGCTTGAGTAAGTCTG-3’, Kras-RVS 5’-ATTAGCTGTATCGTCAAGGCG-3’ (Wildtype= 650 bp, Mutant= 517bp); CAC-FWD 5’-ACCAGCCAGCTATCAACTCG-3’, CAC-RVS 5’-TTACATTGGTCCAGCCACC-3’ (Positive= 199bp).
Mice were housed on a 12-hr light-dark cycle and provided unrestricted access to water and chow (Mouse/Rat Sterilizable Diet, Harlan. Madison, WI). We aimed to euthanize mice at between 6 and 25 weeks of age in order to gain a preliminary understanding of the time course of tumor development in these mice. Mice were monitored daily for any outward signs of lethargy or distress and weighed weekly. Mice exhibiting >15% loss of body weight (from maximum), appearing lethargic or unresponsive or with anal prolapse were euthanized.
Mice were euthanized by CO2 asphyxiation followed by cervical dislocation and exsanguination by cardiac puncture. The abdomen of mice was opened and the small intestine, cecum and colon removed. Each intestinal section was rinsed through with PBS, opened longitudinally, rinsed in PBS then PBS with protease inhibitors (Roche, Indianapolis, IN) and laid flat on an ice-cold glass plate. Each section was carefully examined for the presence of tumors under a dissecting microscope. Tumors were photographed, measured, location noted and then excised and fixed in 10% buffered formalin. “Swiss-roll” preparations were then made for the remaining tissue of the small intestine and colon and fixed in formalin, as previously described [19]. After embedding in paraffin a mid-longitudinal section of these tissues was created. A section was cut across the center of the cecum and fixed in formalin. H&E-stained slides were then examined by an expert rodent pathologist (RTB).
As downstream read-outs of Apc inactivation and Kras activation, we assessed the abundance of β–catenin and phospho-Erk proteins, respectively, by immunohistochemistry. Briefly, heat mediated antigen retrieval was performed on deparaffinized and rehydrated slides. After blocking with goat serum, slides were then probed with antibodies towards bcatenin and phopho-ERK (Cell Signaling, Danvers, MA) and detections was achieved using the Vectastain ABC peroxidase system and DAB peroxidase substrate (Vector laboratories, Burlingame, CA).
3. Results
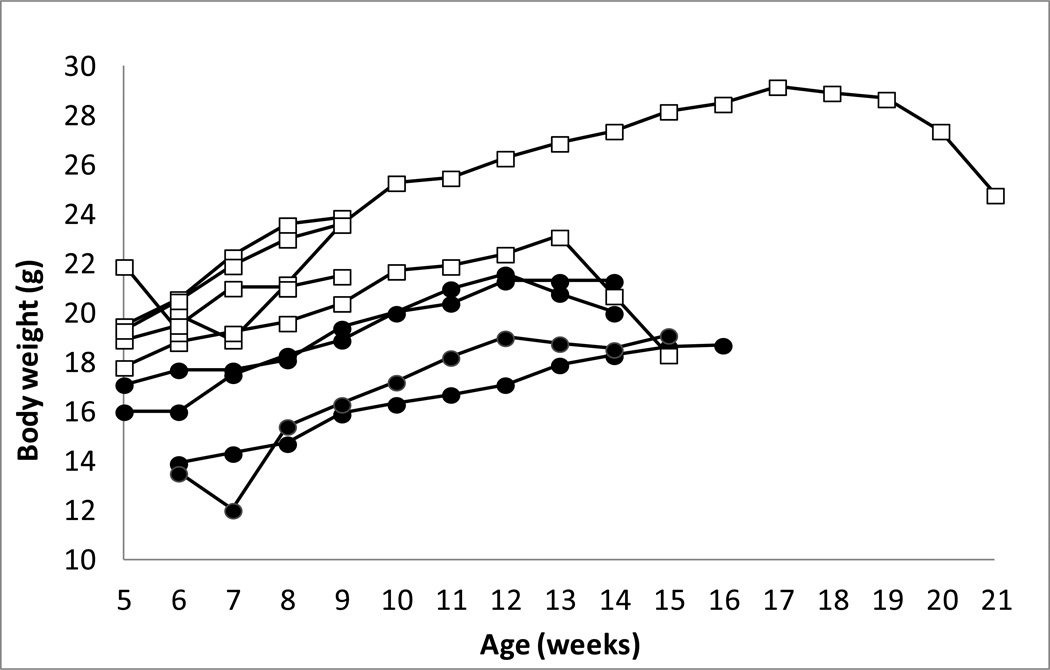
Of the 11 Apc+/580S,Kras+/LSL, CAC+ (AKC) mice examined, 1 died unexpectedly and 8 mice were euthanized due to various indications including weight loss (1), anal prolapse (4), distended anal region likely to prolapse (2) or lethargy and unresponsiveness (1). Two mice were euthanized at a specific time point with any negative health indication (Table 1). Growth of mice is shown in Figure 1.
Table 1.
Tumor number in Apc+/580S,Kras+/LSL, CAC+ mice.
| Mouse ID |
Sex | Age at Death (wk) |
Cause of death | Number Macroscopic tumors in entire tissue |
Number Microscopic tumors in one tissue section |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| SI | Cecum | CR | SI | Cecum | CR | |||||
| 460 | F | 6 | Euthanized- 6 wk time point | 0 | 0 | 1 | 0 | 0 | 0 | |
| 281 | M | 7.5 | Euthanized- anal prolapse | 0 | 0 | 1 | 0 | 0 | 1 | |
| 235 | M | 11 | Euthanized-distended anal region | 0 | 0 | 5 | 0 | 0 | 4 | |
| 236 | M | 11 | Euthanized-distended anal region | 0 | 0 | 9 | 0 | 0 | 2 | |
| 158 | F | 14.5 | Euthanized- anal prolapse | 0 | 0 | 3 | 0 | 0 | 3 | |
| 154 | M | 15 | Found dead | - | 0 | 3 | - | - | - | |
| 159 | F | 15 | Euthanized- 15 wk time point | 0 | 0 | 3 | 0 | 0 | 1 | |
| 180 | F | 16 | Euthanized- lethargy | 0 | 0 | 4 | 0 | 2 | 1 | |
| 189 | F | 16 | Euthanized- anal prolapse | 0 | 0 | 5 | 0 | 0 | 1 | |
| 147 | M | 21 | Euthanized- weight loss | 0 | 0 | 5 | 0 | 1 | 2 | |
| 228 | M | 15 | Euthanized -anal prolapse | 0 | 0 | 8 | 0 | 0 | 5 | |
| Average | 4.6 | 2.2 | ||||||||
SI, small intestine; CR, colorectum; F, female; M, male. Macroscopic tumors were detected from the entire tissue under a dissecting microscope. Microscopic tissues were detected in one cross-section cut from a SI or CR swiss roll or a section from the center of the cecum.
Figure 1. Growth curves for Apc+/580S,Kras+/LSL, CAC+ mice.
Body weight (g) over time for individual male (white squares) and female (black circles) Apc+/580S,Kras+/LSL, CAC+ mice.
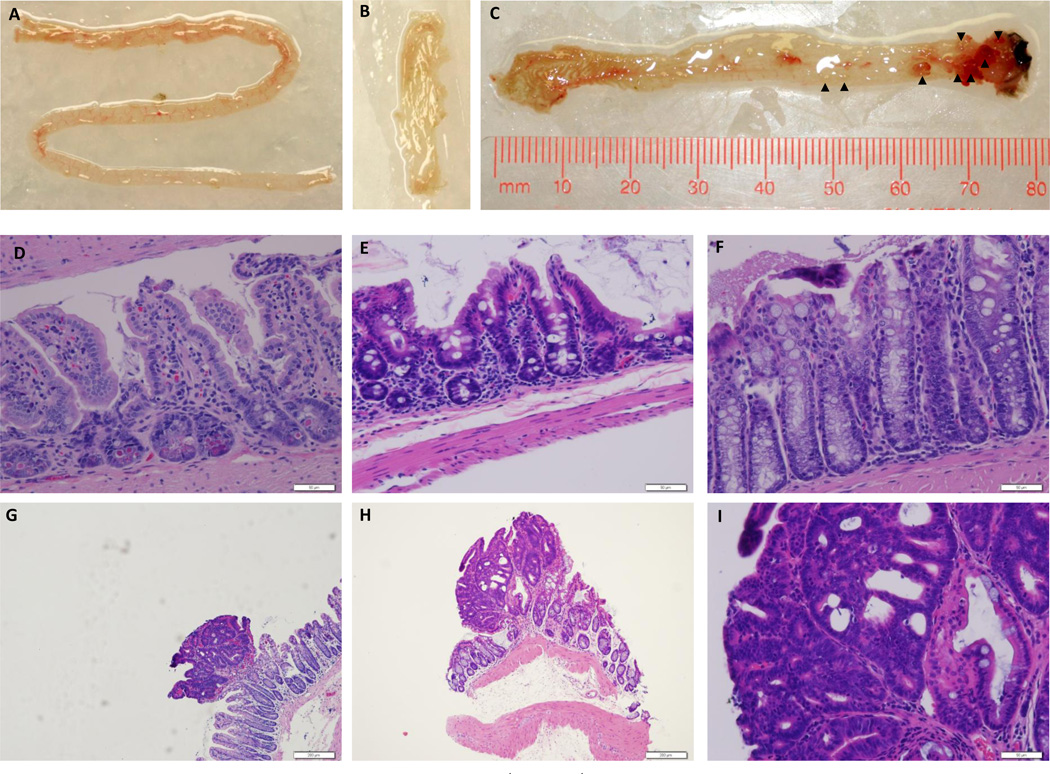
At necropsy intestines were scrutinized for the presence of tumors under a dissecting microscope. All AKC mice displayed macroscopic tumors in the large intestine but no macroscopic tumors were observed in the cecum or small intestine (Table 1, Figure 2 A–C). Histological examination of the colorectal tumors revealed that all tumors were adenomas, with none penetrating through the muscularis (Figure 2G, H). We did not perform an exhaustive screen for metastases, but none were observed.
Figure 2. Representative tissue photographs and micrographs from a Apc+/580S,Kras+/LSL, CAC+ mouse.
Small intestine (A), cecum (B) and colon (C) from a 15 week old Apc+/580S,Kras+/LSL, CAC+ mouse euthanized for anal prolapse. Arrow heads indicate histologically-confirmed tumors. (D,E,F) H&E-stained sections from normal small intestine, cecum and colon respectively (400×). Scale bars are 50µM. Representative colonic adenomatous polyps (G, H) taken under 100× magnification. Scale bar = 200 µM. Representative colonic adenomatous polyp (I) taken under 400× magnification. Scale bar = 50 µM.
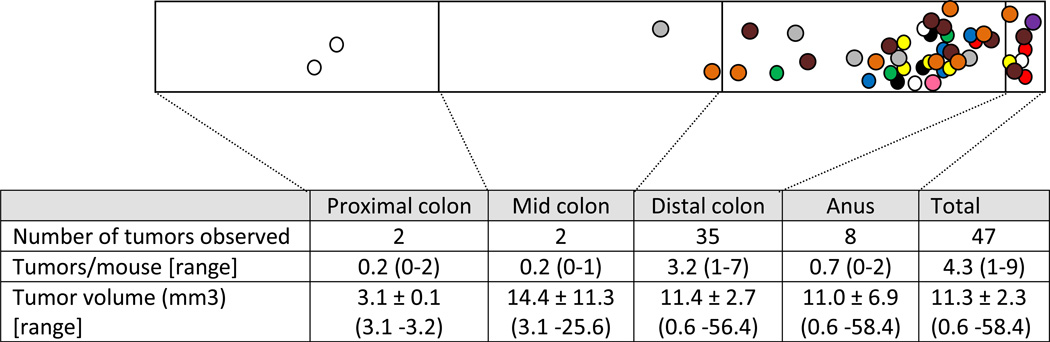
Mice presented with an average of 4.3 macroscopic colon tumors per (range 1–9/mouse), with an average tumor diameter of 1.87 ± 0.09 mm and volume of 11.1 ± 2.3 mm3 (Table 1). The majority of large intestine tumors (91%) appeared in the distal third of this tissue (Figure 3).
Figure 3. Distribution of macroscopic tumors in the colon of Apc+/580S, Kras+/LSL, CAC+ mice.
Different colors indicate tumors from different mice. Mouse #147, 5 tumors (white); #180, 4 tumors (blue); #154, 3 tumors (red); #158, 3 tumors (green); #159, 3 tumors (black); #189, 5 tumors (yellow); #228, 8 tumors (orange); #235, 5 tumors (gray); #236, 9 tumors (brown); #281, 1 tumor (pink); #460, 1 tumor (purple). Tumor location marked by overlaying dots on actual tissue photographs. Tumors not drawn to scale. All tumors were pathologically confirmed adenomatous polyps. Data = mean ± SEM.
In addition to the detection of macroscopic tumors at necropsy, 1–5 microscopic adenomas per mouse were observed in H&E-stained full-length longitudinal sections of the colon (swiss-roll). In contrast no microadenomas were observed in sections of the small intestine. In one mouse, two microadenomas were observed in the cecum section (Table 1).
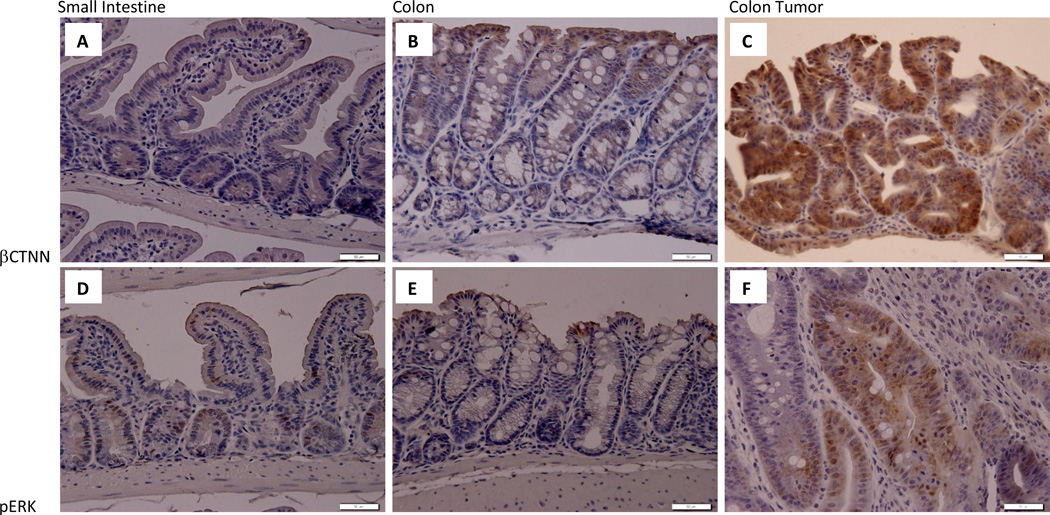
As described above, we sought to drive tumorigenesis in this mod via colon-specific Cre-mediated inactivation of Apc and activation of Kras. In order to assess the activation of pathways that these proteins regulate, we stained for the presence of β-catenin and phosphor-Erk with IHC. As shown in Figure 4, we saw very little staining for these antigens in both small intestine and colon sections. In contrast we saw moderate pERK staining and strong β-catenin staining in colon tumors or AKC mice, indicating that the activation of these pathways is associated with tumorigenesis.
Figure 4. Immunohistochemical analysis of β–catenin and phosphor-ERK in Apc+/580S, Kras+/LSL, CAC+ mice.
Immunohistochemical analysis of β-catenin (A,B,C) and phospho-Erk (D,E,F) abundance in small intestine (A,D), colon (B,E) and colon tumor (C,F) tissues. Images taken under 400× magnification. Scale bars are 50µM.
Although not specifically designed or powered to detect differences in tumorigenesis according to sex, we did not observe differences in the number (P=0.39) or volume (P=0.33) of macroscopic tumors between males and females. Similarly, we did not detect a difference in the incidence in micoradenomas between sexes (P= 0.21) (Data not shown).
4. Discussion
We have combined desirable features from existing GEM models [14, 16–18] to induce colon-specific tumorigenesis by the inactivation of Apc and activation of mutant Kras via colon-specific expression of Cre recombinase. These “AKC” mice develop an average of 4.3 macroscopic adenomatous lesions primary in the distal 3rd of the large intestine and are observed as early as 6 weeks of age. Importantly, we did not observe any macroscopic lesions in the small intestine of these mice. As discussed, this has been a major limitation of several existing models of CRC including the commonly-used Apcmin and Apc1638N models.
We also prepared slides from a section cut from the center of the cecum and from “swiss-rolls” of the small intestine and colorectum. Slides were reviewed (1 per tissue) and we observed 1–5 microadenomas in the colon roll but none in the small intestine roll. For one mouse we observed 2 microadenoms in the cecum section (Table 1). Although we did not perform a comprehensive screen for metastases, the fact that we did not observe any invasive lesions suggests that metastases are unlikely to occur during the time course that we studied the AKC animal.
As discussed above, there are several GEM models of CRC currently available- many of which involve inactivation of one Apc allele. This AKC model has advantages over many existing models of CRC because tumors appear solely in the large intestine. Although some recent models have achieved this goal, they require invasive techniques to introduce Cre-expressing virus to the colon [14–16] or tumor incidence is very low [18], thus impeding the ability to discern differences due to various interventions. In the latter model with only one floxed Apc allele, and wildtype Kras, the tumor incidence is 20% or less. By adding the activated Kras mutation to this model we have increased tumor penetrance to 100% while maintaining an intermediate tumor burden of 4.3 tumors per mouse. This increase in tumor burden is a desirable trait for prevention and therapeutic trials as it will translate into reduced sample size requirements and study costs.
The tumor incidence we have observed in the current AKC mice is similar to that reported by Hung et al [16] (4.3 v.s. 3.6 tumors/mouse) who used a Cre-expressing virus introduced by enema to facilitate inactivation and activation of the same Apc and Kras alleles respectively. When the efficiency of infection by their technique was evaluated it was apparent that infection of superficial epithelial cells, i.e. those not capable of forming tumors, was almost complete; however infection of cells in the basal crypt occurred only occasionally. This is consistent with previous analyses of transgenic CAC+, which mice indicate that Cre is expressed in approximately 15% of epithelial cells in the lower bowel [18]. Thus despite different routes of Cre expression, both methods achieve roughly similar numbers of Cre positive cells and intestinal tumors.
One limitation of the current model is that we did not observe any invasive tumors during the time course that was examined. Previously when Apc inactivation and Kras activation was achieved by adenovirus expressing Cre enemas, invasive tumors were detected in mice 20 weeks after Cre exposure and liver metastasis 4 weeks later [16]. Allowing more time for an invasive phenotype to be acquired may not be possible in the current model as many of the AKC mice (70%) have an indication for euthanasia by 16 weeks of age, including weight loss, poor disposition and anal prolapse.
As with all other mouse models of CRC, the current model has inherent strengths and weaknesses. The geographical correctness of tumorigenesis, full penetrance and intermediate tumor burden offer substantial advances over prior models, thus making the AKC model a useful addition to our toolkit for studying the prevention and treatment of CRC.
Acknowledgements
This material is based upon work supported by the U.S. Department of Agriculture, under agreement No. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S.Dept of Agriculture.
Abbreviations
- Apc
Adenomatous polyposis coli
- CRC
colorectal cancer
- GEM
genetically engineered mouse
- CAC
carbonic anhydrase 1-coupled Cre transgene
- SI
small intestine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Taketo MM. Mouse models of gastrointestinal tumors. Cancer Sci. 2006;97(5):355–361. doi: 10.1111/j.1349-7006.2006.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tammariello AE, Milner JA. Mouse models for unraveling the importance of diet in colon cancer prevention. J Nutr Biochem. 2010;21(2):77–88. doi: 10.1016/j.jnutbio.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30(2):183–196. doi: 10.1093/carcin/bgn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roper J, Hung KE. Priceless GEMMs: genetically engineered mouse models for colorectal cancer drug development. Trends Pharmacol Sci. 2012;33(8):449–455. doi: 10.1016/j.tips.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Johnson RL, Fleet JC. Animal models of colorectal cancer. Cancer Metastasis Rev. 2013;32(1–2):39–61. doi: 10.1007/s10555-012-9404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653(1):1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 8.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247(4940):322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 9.Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, Gould KA, Dove WF. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256(4940):668–670. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 10.Taketo MM. Roles of stromal microenvironment in colon cancer progression. J Biochem. 2012;151(5):477–481. doi: 10.1093/jb/mvs035. [DOI] [PubMed] [Google Scholar]
- 11.Liu Z, Ciappio ED, Crott JW, Brooks RS, Nesvet J, Smith DE, Choi SW, Mason JB. Combined inadequacies of multiple B vitamins amplify colonic Wnt signaling and promote intestinal tumorigenesis in BAT-LacZxApc1638N mice. FASEB J. 2011;25(9):3136–3145. doi: 10.1096/fj.11-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciappio ED, Liu Z, Brooks RS, Mason JB, Bronson RT, Crott JW. Maternal B vitamin supplementation from preconception through weaning suppresses intestinal tumorigenesis in Apc1638N mouse offspring. Gut. 2011;60(12):1695–1702. doi: 10.1136/gut.2011.240291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravaghi C, Bo J, Laperle KM, Quimby F, Kucherlapati R, Edelmann W, Lamprecht SA. Obesity enhances gastrointestinal tumorigenesis in Apc-mutant mice. Int J Obes (Lond) 2008;32(11):1716–1719. doi: 10.1038/ijo.2008.149. [DOI] [PubMed] [Google Scholar]
- 14.Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, Matsumoto H, Takano H, Akiyama T, Toyoshima K, Kanamaru R, Kanegae Y, Saito I, Nakamura Y, Shiba K, Noda T. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278(5335):120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- 15.Hinoi T, Akyol A, Theisen BK, Ferguson DO, Greenson JK, Williams BO, Cho KR, Fearon ER. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 2007;67(20):9721–9730. doi: 10.1158/0008-5472.CAN-07-2735. [DOI] [PubMed] [Google Scholar]
- 16.Hung KE, Maricevich MA, Richard LG, Chen WY, Richardson MP, Kunin A, Bronson RT, Mahmood U, Kucherlapati R. Development of a mouse model for sporadic and metastatic colon tumors and its use in assessing drug treatment. Proc Natl Acad Sci U S A. 2010;107(4):1565–1570. doi: 10.1073/pnas.0908682107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15(24):3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue Y, Johnson R, Desmet M, Snyder PW, Fleet JC. Generation of a transgenic mouse for colorectal cancer research with intestinal cre expression limited to the large intestine. Mol Cancer Res. 2010;8(8):1095–1104. doi: 10.1158/1541-7786.MCR-10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filipe MI. Mucous secretion in rat colonic mucosa during carcinogenesis induced by dimethylhydrazine. A morphological and histochemical study. Br J Cancer. 1975;32(1):60–77. doi: 10.1038/bjc.1975.134. [DOI] [PMC free article] [PubMed] [Google Scholar]