Abstract
Biphasic glucose-stimulated insulin secretion (GSIS) from pancreatic beta cells involves SNARE protein-regulated exocytosis. SNARE complex assembly further requires the regulatory proteins Munc18c, Munc18-1 and Doc2b. Munc18-1 and Munc18c are required for 1st- and 2nd-phase GSIS, respectively. These distinct Munc18-1 and Munc18c roles are related to their transient high-affinity binding with their cognate t-SNAREs; Syntaxin 1A and Syntaxin 4, respectively. Doc2b is essential for both phases of GSIS, yet the molecular basis for this remains unresolved. Because Doc2b binds to Munc18-1 and Munc18c via it’s distinct C2A and C2B domains, respectively, we hypothesized that Doc2b may provide a plasma membrane-localized scaffold/platform for transient docking of these Munc18 isoforms during GSIS. Toward this, macromolecular complexes composed of Munc18c, Doc2b, and Munc18-1 were detected in beta cells. In vitro interaction assays indicated that Doc2b is required to bridge the interaction between Munc18c and Munc18-1 in the macromolecular complex; Munc18c and Munc18-1 failed to associate in the absence of Doc2b. Competition-based GST-Doc2b interaction assays revealed that Doc2b could simultaneously bind both Munc18-1 and Munc18c. Hence, these data support a working model wherein Doc2b functions as a docking platform/scaffold for transient interactions with the multiple Munc18 isoforms operative in insulin release, promoting SNARE assembly.
Keywords: SNARE protein, Munc18c, Munc18-1, insulin exocytosis, islet beta cells, Doc2b
INTRODUCTION
Insulin is secreted from the pancreatic islet beta cells in two distinct phases through the trafficking, docking and fusion of insulin granules with the plasma membrane (PM), in a Soluble N-ethylmaleimide sensitive factor attachment receptor (SNARE) protein-dependent manner [1-3]. SNARE-regulated docking and fusion exocytosis events involve the formation of SNARE core complexes. SNARE core complexes consist of two target membrane (t)-SNARE proteins, syntaxin and SNAP25 (or SNAP23), associated with the vesicle/granule membrane (v)-SNARE protein VAMP [4, 5]. In response to glucose stimulus, insulin granules containing the v-SNARE VAMP2 traffic to the PM to partner with the t-SNAREs, docking the granules for subsequent fusion and insulin release. SNARE complexes in beta cells can form with either Syntaxin 1A or Syntaxin 4 t-SNARE isoforms; an individual beta cell expresses both isoforms [6, 7]. Other than beta cells only neuronal cells are known to utilize both of these syntaxin isoforms, yet in neurons they are localized to different synaptic densities [8, 9]. Syntaxin assembly in SNARE core complexes is further regulated by specific high-affinity binding partners referred to as Munc18 proteins. Syntaxin 1A binds to the isoform Munc18-1, while Syntaxin 4 binds to Munc18c [10]. How a single beta cell coordinates the utilization of these two mutually exclusive pairs of Syntaxin-Munc18 complexes to orchestrate biphasic insulin release remains unresolved.
Reduced levels of Munc18-1, Munc18c, Syntaxin 1A and Syntaxin 4 proteins are correlated with Type 2 Diabetes (T2D) [11-15]. Studies of Munc18-1 in human islets has revealed that Munc18-1 impacts only the first-phase of glucose-stimulated insulin release (GSIS) [16]. In contrast, Munc18c heterozygous knockout mice have defects only in the second phase of GSIS; further Munc18c depletion in these islets using RNAi showed an ablation of second-phase GSIS, again without any impact upon first-phase GSIS [17]. The partitioned roles of Munc18 proteins in the phases of GSIS are consistent with the requirements for their respective Syntaxin partners in GSIS; Syntaxin 1A knockout mouse islets show defective first-phase [6], Syntaxin 4 knockout mice show defective second phase GSIS [7]. Oddly, Syntaxin 4 is also required for first-phase, but recent studies have implicated this role in complexes with cytoskeletal proteins and not Munc18c [18-20]. Numerous and varied attempts at understanding how these Munc18 proteins facilitate interactions of their cognate syntaxin partners in SNARE core complex assembly suggest that while the basic mechanisms are conserved, differences do exist [21, 22]. Cumulative evidence from in vitro studies and beta cells studies supports a model where Munc18-1 binds to the SNARE core complex [16, 23-25]. In contrast, Munc18c is seen to dissociate from Syntaxin 4 concurrent with Syntaxin 4 activation and engagement with the SNARE core complex [26-29]. This dissociation is detected in cells and primary tissues, although in vitro there is lack of consensus [21, 30], and may possibly be related to the involvement of post-translational phosphorylation of Munc18c in response to glucose [27]. Nevertheless, since Munc18 proteins are proposed to exist in various states of dis/association with their cognate Syntaxin partners [31], one could speculate that these Munc18 proteins may require neighboring docking platforms during their interim periods of dissociation from Syntaxins or SNARE complexes.
Munc18 proteins interact with Double C2 domain proteins, Doc2a and Doc2b. Doc2a expression is limited to islet and neuronal cells, and in neurons Doc2a can bind to Munc18-1 [32]. However, recent evidence shows that islets from Doc2a knockout mice have normal islet function [33]. Contrastingly, Doc2b is ubiquitously expressed and can associate with Munc18-1 or Munc18c in beta cells [16, 26]. Doc2b homozygous knockout mouse islets exhibit defects in both phases of GSIS in perifusion analyses, and conversely, islets from transgenic mice over-expressing Doc2b show amplified GSIS during both phases [34, 35]. Munc18-1 binds to the C2A domain of Doc2b [32], while Munc18c binds to the C2B domain of Doc2b [36]. Syntaxins 1 and 4 compete with Doc2b for binding to their respective Munc18 partners [32, 36], given credence to the notion that Doc2b may serve as an interim binding partner for each Munc18 protein. Given the ability of Doc2b to bind to these Munc18 isoforms via its different C2 domains, we questioned whether Doc2b could accommodate binding of both Munc18 isoforms simultaneously, serving as a docking platform for transiently interacting Munc18 proteins.
In this report, we demonstrate the existence of a novel heterotrimeric complex consisting of Doc2b, Munc18-1 and Munc18c in islet beta cells. Our results demonstrate that Munc18-1 and Munc18c cannot bind directly to each other, but by binding directly to their respective sites on Doc2b, they can associate, both in cells and in vitro. Furthermore, competition studies revealed that Munc18-1 and Munc18c proteins do not compete with each other for occupancy on Doc2b, but instead can bind concurrently. Altogether, there is supportive data for Doc2b serving as a scaffolding platform for transient binding of Munc18 proteins to promote insulin release.
MATERIALS AND METHODS
Materials
Rabbit anti-Munc18c antibody was generated in-house as described [37]. Doc2b antibody was purchased from Abcam (Cambridge, MA). Munc18-1 antibody was acquired from Synaptic Systems (Gottingen, Germany). Goat anti-rabbit-HRP and anti-mouse-HRP secondary antibodies were purchased from Bio-Rad (Hercules, CA). Munc18c antibody for human protein detection and Protein G+ agarose beads were acquired from Santa Cruz (Santa Cruz, CA). Enhanced chemiluminescence (ECL) reagent was purchased from Amersham Biosciences (Pittsburg, PA). Glutathione sepharose 4B beads were purchased from GE Healthcare (Pittsburg, PA). Ni-Nickel NTA agarose beads was purchased from Invitrogen (Grand Island, New York).
Cell culture and Co-immunoprecipitation
MIN6 beta cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Grand new island, NY) supplemented with 15% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, 292 μg/mL L-glutamine, and 50 μM beta-mercaptoethanol. Cells were washed twice with and incubated for 2 h in freshly prepared modified Krebs-Ringer bicarbonate buffer (MKRBB; 5 mM KCl, 120 mM NaCl, 15 mM Hepes pH 7.4, 24 mM NaHCO3, 1 mM MgCl2, 2 mM CaCl2, and 1 mg/mL BSA). Cells were stimulated with 20 mM glucose or 50 mM KCl for 5 minutes and harvested in a 1 % NP40 lysis buffer (25 mM Tris, pH 7.4, 1 % NP40, 10 % glycerol, 50 mM sodium fluoride, 10 mM sodium pyrophosphate, 137 mM sodium chloride, 1 mM sodium vanadate, 1 mM PMSF, 10 μg/ml aprotinin, 1 μg/mL pepstatin and 5 μg/mL leupeptin), and lysates cleared by microcentrifugation (16,000 x g) for 10 min at 4 °C for subsequent use in co-immunoprecipitation experiments. INS-1 derived 832/13 rat beta cells were preincubated in HEPES balanced salt solution as previously described [38]. Cells were harvested in 1 % NP40 lysis buffer. Cleared detergent lysates from MIN6 or INS-1 derived 832/13 beta cells (2.5 mg) or recombinant proteins (2.5 μg) were combined with rabbit anti-Munc18c antibody or mouse anti-Munc18-1 antibody for 2 h at 4°C, followed by a second incubation with protein G Plus-agarose for 2 h. The resultant immunoprecipitates were subjected to 10% SDS-PAGE followed by transfer to PVDF membranes for immunoblotting with respective proteins. Human islet co-immunoprecipitations were conducted similarly, using islets obtained through the Integrated Islet Distribution Program (IIDP) (Supplemental Fig. 1A).
Recombinant proteins
The generation of pET28a (+) His-Munc18c, pGEX-Munc18c, pGEX-Munc18-1 and pGEX-Doc2b plasmids has been previously described [16, 26, 36, 39]. pGEX fusion constructs were transformed into E. coli for expression of GST fusion proteins, which were further purified by glutathione-sepharose affinity chromatography as described previously [40]. Recombinant Doc2b and Munc18-1 were obtained following thrombin cleavage of GST-Doc2b and GST-Munc18-1, respectively. Recombinant His tagged Munc18c was expressed in E. coli and purified using Ni-NTA Nickel chelating resin as previously described [26].
GST interaction assays
The GST tagged proteins linked to Sepharose beads were combined with 2.5 μg of recombinant proteins for 2 h at 4 °C in 1 % NP-40 lysis buffer, followed by three washes with lysis buffer. The associated proteins were resolved on 10% SDS-PAGE followed by their transfer to PVDF membranes for immunoblotting for respective proteins. Competition studies involved pre-binding of GST-Doc2b and Munc18 proteins for 2 h, pelleting and washing to remove excess Munc18, and then addition of the competitor Munc18 isoform to the reaction. Immunoblots were quantified by optical density scanning of at least 4 independent experiments using a minimum of two separate batches of recombinant protein to randomize for batch-to-batch variation in protein preparations. For quantitation of pre-associated:unassociated Munc18 proteins in beta cell lysates, GST-Doc2b on beads was combined with 3 mg of lysate from glucose-stimulated (5 min) MIN6 cells for 2h, followed by washes with lysis buffer. Initial and post-incubation lysates were assessed by SDS-PAGE for immunoblotting with Munc18c or Munc18-1 antibodies. Three independent batches of recombinant protein and lysates prepared from MIN6 cell passages were used.
Statistical Analysis
All data were evaluated for statistical significance using two-tailed Student’s t test. Data are expressed as the mean ± S.E.
RESULTS
Doc2b, Munc18c and Munc18-1 associate in MIN6 beta cells
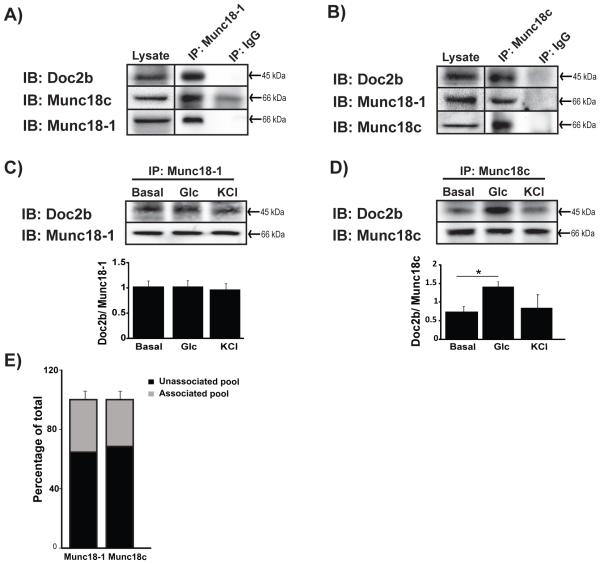
Previous studies show associations of Doc2b with Munc18-1 or Munc18c in MIN6 beta cells [16, 26], although whether these complexes are mutually exclusive has yet to be examined. Toward addressing this, co-immunoprecipitation assays were performed using cleared detergent MIN6 cell lysates with anti-Munc18-1 or anti-Munc18c antibodies and compared with matched IgG controls. The Munc18-1 antibody co-immunoprecipitated both Doc2b and Munc18c (Fig. 1A). Similarly, the Munc18c antibody co-immunoprecipitated both Doc2b and Munc18-1 (Fig. 1B), and both reactions were specific as gauged against paired IgG control reactions. These associations of Doc2b, Munc18-1 and Munc18c were also detected in human islets and rat INS 832/13 beta cell lysates (Supplemental Fig. 1A-B).These data suggest that Munc18c, Munc18-1, and Doc2b might associate to form a heterotrimeric complex through either direct or indirect interactions.
Figure 1. Interaction between Munc18-1, Doc2b and Munc18c in MIN6 beta cells.
Cleared detergent lysates prepared from MIN6 cells that were stimulated with glucose for 5 min were immunoprecipitated (IP) with mouse anti-Munc18-1 (A) or rabbit anti-Munc18c (B) antibodies. Coprecipitated proteins were resolved by 10% SDS-PAGE for immunoblot detection (IB). IgG was used to control for antibody specificity. Input proteins were taken from each reaction to confirm the presence of each protein in the lysates. Data are representative of at least 3 independent sets of beta cell lysates. Vertical lines indicate splicing of lanes from within the same gel exposure. MIN6 cells left unstimulated (basal) or stimulated for 5 min with glucose or KCl were used for immunoprecipation with anti-Munc18-1 (C) or anti-Munc18c (D) and Doc2b co-precipitated was quantified from three independent experiments. Bar graphs show the average ± SE; *p<0.05 versus basal. (E) Optical density quantitation of three independent GST-Doc2b interaction assays using MIN6 cell lysates. Pre, prior to GST-Doc2b addition; Post, flow-through following 2 h incubation and pelleting of GST-Doc2b beads. The unassociated pool was determined by comparing the quantity of Munc18 proteins in the flow-through to that in the input (prior to GST-Doc2b addition).
Interestingly, Munc18-1 co-immunoprecipitated similar quantities of Doc2b under basal, glucose and KCl conditions (Fig. 1C). Consistent with previous findings [26], Munc18c association with Doc2b was enhanced by glucose- but not KCl-stimulation (Fig. 1D). This is consistent with prior work showing Munc18c to be affiliated with the glucose-dependent second phase of insulin release [17]. Using GST-Doc2b as ‘bait’ to capture that percentage of Munc18c or Munc18-1 found to be ‘free/unassociated’ and not pre-complexed with other proteins in MIN6 cells, we calculated ~30-35% of each to be ‘free’ to our bait (Fig. 1E), suggesting that ~65-70% of cellular Munc18-1 and Munc18c proteins participate in protein complexes in MIN6 cells. This indicates that while the majority of the Munc18 proteins are in complexes, with Doc2b and other binding partners, that there exists a sufficient pool of unbound Munc18 proteins that are available for transient interaction with the Doc2b scaffold, with the potential to associate/dissociate in response to glucose.
Munc18c and Munc18-1 do not directly interact in vitro
To test whether Munc18 proteins can bind directly to each other, independent of Doc2b, soluble recombinant proteins were used in co-immunoprecipitation reactions with anti-Munc18-1 or anti-Munc18c antibodies. A total of 5 μg of soluble recombinant Munc18-1 and Munc18c proteins were combined for 2 h in 1% NP-40 lysis buffer and immunoprecipitated with anti-Munc18-1 antibody. Though Munc18-1 did not co-immunoprecipitate Munc18c, the presence of each protein in the reaction was confirmed by sampling of the input reaction (Fig. 2A). Parallel reactions using IgG in place of Munc18-1 antibody confirmed the specificity of the interaction. Similarly, reciprocal parallel co-immunoprecipitation reactions were conducted using anti-Munc18c antibody with the same batches of recombinant proteins, and again, failed to show any interaction between Munc18c and Munc18-1 (Fig. 2B). These data indicate that Munc18 proteins do not bind directly, and suggested that their association in MIN6 cell lysate complexes was linked to the presence of Doc2b or another cellular factor(s).
Figure 2. Munc18-1 and Munc18c do not directly interact.
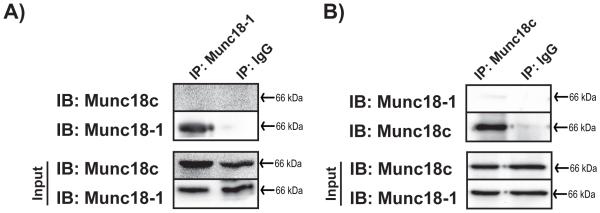
Purified untagged recombinant proteins (2.5 μg of each) were combined and immunoprecipitated (IP) with mouse anti-Munc18-1 (A) or rabbit anti-Munc18c (B) antibodies. Co-immunoprecipitated proteins were resolved by 10% SDS-PAGE for immunoblot detection (IB). IgG was used to control for antibody specificity. Input proteins were taken from each reaction to confirm the presence of each protein in the reaction. Data are representative of at least three independent experiments using two or more independent batches of recombinant proteins.
Munc18-1 interacts with Munc18c through Doc2b
To determine whether Doc2b might facilitate the association of the Munc18-1 and Munc18c proteins, in vitro mixing assays using recombinantly expressed and purified GST-Munc18-1 or GST-Munc18c were performed. Fig. 3A shows that in reactions containing GST-Munc18-1 (linked to sepharose beads), Doc2b and Munc18c proteins were both co-precipitated; no binding of Munc18c to GST-Munc18-1 was detected in the absence of Doc2b protein. Neither Doc2b nor Munc18c proteins bound to the GST control (Fig. 3A). In reciprocal binding assays, GST-Munc18c coupled to beads precipitated Munc18-1 only in the presence of Doc2b (Fig. 3B), indicating that the presence of Doc2b is necessary for the detection of Munc18-1 and Munc18c co-precipitation.
Figure 3. Associations amongst Munc18-1, Doc2b and Munc18c in GST interaction assays.
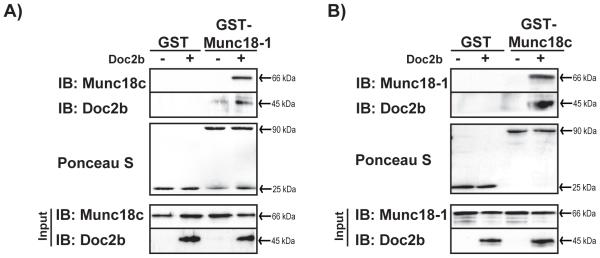
Bacterially-expressed GST-tagged-Munc18-1 and -Munc18c proteins were purified and linked to sepharose beads. GST-Munc18-1 (A) or GST-Munc18c (B) were used in interaction assays using reactions containing equimolar amounts of soluble untagged recombinant proteins in the presence or absence of Doc2b, and were compared against binding to the GST protein alone. Coprecipitated proteins were resolved by 10% SDS-PAGE for immunoblot detection (IB). Ponceau S staining was used to gauge equivalent loading of GST-tagged proteins. Input proteins were sampled from reactions to confirm the presence of each protein in the reaction. Data are representative of at least three independent experiments using two or more independent batches of recombinant proteins.
Co-immunoprecipitation analyses were employed as an independent approach to GST interaction assays to test the concept of Doc2b scaffolding both Munc18-1 and Munc18c proteins. Equal molar quantities of the three recombinant proteins (with GST and epitope tags removed) were combined in co-immunoprecipitation reactions including either Munc18-1 antibody (Fig. 4A) or Munc18c antibody (Fig. 4B). In the presence of Doc2b, each Munc18 isoform was co-precipitated. The inclusion of Doc2b was confirmed by its presence in input samples taken from each reaction, since its presence in co-immunoprecipitation reactions was obscured by interference from the heavy chain in immunoblots. IgG failed to precipitate proteins, demonstrating specificity of the interactions. Again, neither Munc18 isoform co-precipitated the other in the absence of Doc2b in the reaction. Input samples collected just after protein mixing show the existence of the proteins in each reaction. Data gained from this second experimental approach confirm that Doc2b forms a macromolecular complex with Munc18-1 and Munc18c proteins.
Figure 4. Munc18-1 and Munc18c co-immunoprecipitate only in the presence of Doc2b.
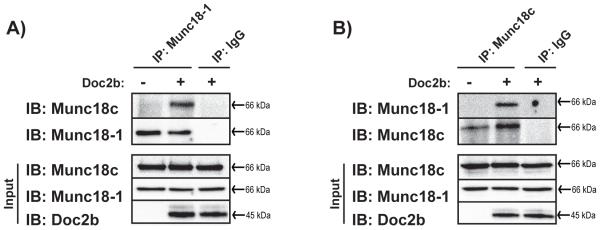
Purified untagged recombinant proteins were added in equimolar amounts as in Fig. 2, and in the presence or absence of Doc2b for subsequent immunoprecipitation (IP) with mouse anti-Munc18-1 (A) or rabbit anti-Munc18c (B) antibodies. Coprecipitated proteins were resolved by 10% SDS-PAGE for immunoblot detection (IB). IgG was used to control for antibody specificity. Input proteins were sampled from reactions to confirm the presence of each protein. Data are representative of at least three independent experiments using two or more independent batches of recombinant proteins.
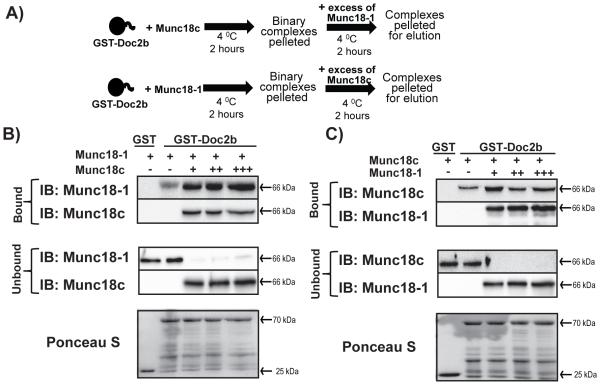
We next tested the notion that Doc2b could act as a scaffold for binding both Munc18-1 and Munc18c, concurrently. Using a competition-based approach (modeled in Fig. 5A), GST-Doc2b (coupled to beads) was pre-incubated with an excess of soluble recombinant Munc18-1 for 2 h to achieve maximal occupancy, with GST-Doc2b-Munc18-1 complexes subsequently pelleted by centrifugation to remove the unbound excess Munc18-1 (Fig. 5B). Increasing molar equivalents, starting with one molar equivalent (designated as ‘+’), to two (++) or three molar equivalents (+++), of soluble Munc18c protein were subsequently added, and the reaction incubated for additional 2 h. The presence of unbound Munc18c in the supernatant indicated that Munc18c is present in saturation and not limiting. Even a three-fold molar excess of Munc18c did not cause dissociation of Munc18-1 from GST-Doc2b beads (Fig. 5B). Reciprocal reactions starting with GST-Doc2b pre-loaded with Munc18c followed by subsequent addition of excess of Munc18-1 did not result in the dissociation of Munc18c from GST-Doc2b (Fig. 5C). These data suggest that no competition exists for Doc2b binding between the Munc18 isoforms.
Figure 5. Munc18-1 and Munc18c bind non-competitively to the Doc2b scaffold.
(A) Schematic representation of the GST-Doc2b competition assay. GST-Doc2b-linked beads were pre-incubated with one of the two Munc18 isoforms for 2h at 4°C. Binary complexes were pelleted to eliminate unbound protein for subsequent addition of the other Munc18 isoform. (B) GST-Doc2b was preincubated with Munc18-1 followed by addition of Munc18c in excess of 1 (+), 2 (++) or 3 (+++) molar equivalents to the binding reaction. (C) GST-Doc2b was preincubated with Munc18c followed by addition of Munc18-1, as described in panel (B). Both bound and unbound proteins were captured and resolved by 10% SDS-PAGE for immunoblot detection (IB). Data are representative of at least four independent experiments using three or more independent batches of recombinant proteins.
DISCUSSION
In this study, we demonstrate the existence of a novel heterotrimeric complex formed between Doc2b, Munc18-1 and Munc18c in human islet and mouse- and rat-beta cell lines. In vitro studies using purified recombinant proteins demonstrated that Munc18-1 and Munc18c proteins did not bind directly to each other in the absence of Doc2b. Furthermore, competition studies showed that Doc2b could accommodate interactions with both Munc18-1 and Munc18c proteins, simultaneously. These data are consistent with prior reports of distinct binding motif specificities of each Munc18 isoform for Doc2b, and of concept that Doc2b might serve as a scaffolding platform for transiently interacting Munc18 proteins as they transit to aid in SNARE complex formation during docking and fusion in beta cells.
What is the advantage of Doc2b providing this scaffolding function? Scaffolds are known designated hubs for catalytic events, e.g. active zones in neurons cluster SNARE and accessory machinery [41]. Although islets don’t have well characterized active zones, a scaffold such as Doc2b may carry out this function. For proteins like Munc18c that seem to transiently interact with Syntaxin 4 for cycles of granule docking and fusion during second phase GSIS, which can occur over a period of several hours, there would be an advantage to keep Munc18c in vicinity of the PM-localized Syntaxin 4. Since it is unclear whether Munc18-1 needs to dissociate from Syntaxin 1A, its need for the scaffold is less clear. Munc18-1 was shown to undergo phosphorylation in neurons [42], and this reduced its affinity for Syntaxin 1A, In addition, more recent literature supports the notion that Munc18-1 joins and facilitates SNARE core complex assembly in neurons and in beta cells [16, 25]. In addition to providing a scaffold for Munc18 proteins, Doc2b also contains a far N-terminal domain through which it binds Munc13-1 to facilitate synaptic vesicle exocytosis [43, 44]. Munc13-1 has also been implicated in second phase GSIS [45]. Hence, by virtue of its ability to provide docking sites for Munc13-1, Munc18-1 and Munc18c, Doc2b scaffolding provides a new model for how the beta cell might organize a hub of exocytosis capabilities.
In this Doc2b scaffolding model, it might be envisioned that Doc2b hovers in the vicinity of the PM. While some studies demonstrate the persistent PM localization of Doc2b [35, 36], one study in beta cells showed that ectopically expressed myc-Doc2b translocated to the PM in response to high levels of calcium [46]. In neurons Doc2b can also translocate to the PM upon calcium stimulation [47], although contradicting this, Doc2b has been shown to regulate neurotransmitter release in a manner independent of calcium [48]. These data might be reconciled if Doc2b were found to exist in two pools in the beta cell, one as a PM scaffold, the other as a mobile calcium sensor. Further complicating the matter of calcium responsiveness and subcellular localization in beta cells is the observation that high calcium drives ectopically expressed Doc2b to bind with Syntaxin rather than with Munc18 proteins [46]. Hence, studies evaluating the localization of the endogenous Doc2b scaffold in the subcellular space of beta cells will be required to determine if the scaffold exhibits sensitivity to changes in [Ca2+]i during the phases of GSIS.
Prior studies of human diabetic islets reveal reduced levels in Munc18-1 and Munc18c proteins [11, 49]. Given that attenuations in first- and second-phase insulin secretion are observed in individuals with T2D, and that each of the Munc18 proteins regulates a specific phase of insulin secretion, the regulation of their interactions by Doc2b is important. While our studies provide the first in vitro characterization of the Doc2b scaffold, attempts to characterize the stoichiometry of the macromolecular complexes were complicated by the absence of incorporation of the known post-translational modification(s) of Munc18c as well as potential others of Munc18-1 (data not shown), as these are known to impact binding affinity of Doc2b and might also impact binding stoichiometry. Future studies of the stoichiometry of binding of Munc18 and Munc13 proteins to the Doc2b scaffold will require integration of these post-translational modifications. Nevertheless, since Doc2b was recently shown to be limiting for GSIS and its over-expression to be of benefit to enhancing glucose homeostasis in vivo [33-35], Doc2b has emerged as an exciting potential therapeutic target for diabetes remediation and/or prevention.
Supplementary Material
Summary Statement.
Herein we describe a novel macromolecular complex composed of Munc18c, Doc2b and Munc18-1 in beta cells. These data provide the basis for a new model wherein Doc2b scaffolding may facilitate the transient Munc18-SNARE associations occurring during biphasic insulin exocytosis.
ACKNOWLEDGEMENTS
We are grateful to Dr. Sander Groffen for the pGEX-Doc2b plasmid and to Dr. Patrick Fueger for the INS-1 832/13 cells. We would also like to thank Kimberly Fong and Jamie Gendron for technical assistance.
FUNDING
This study was supported by grants from the National Institutes of Health (DK067912 to D.C.T.), the Juvenile Diabetes Research Foundation (17-2013-454 and 1-INO-2014-165-A-V to D.C.T.) and the American Heart Association (12PRE11890042 to L.R.).
REFERENCES
- 1.Regazzi R, Wollheim CB, Lang J, Theler JM, Rossetto O, Montecucco C, Sadoul K, Weller U, Palmer M, Thorens B. VAMP-2 and cellubrevin are expressed in pancreatic beta-cells and are essential for Ca(2+)-but not for GTP gamma S-induced insulin secretion. EMBO J. 1995;14:2723–2730. doi: 10.1002/j.1460-2075.1995.tb07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadoul K, Berger A, Niemann H, Weller U, Roche PA, Klip A, Trimble WS, Regazzi R, Catsicas S, Halban PA. SNAP-23 is not cleaved by botulinum neurotoxin E and can replace SNAP-25 in the process of insulin secretion. J. Biol. Chem. 1997;272:33023–33027. doi: 10.1074/jbc.272.52.33023. [DOI] [PubMed] [Google Scholar]
- 3.Kiraly-Borri CE, Morgan A, Burgoyne RD, Weller U, Wollheim CB, Lang J. Soluble N-ethylmaleimide-sensitive-factor attachment protein and N-ethylmaleimide-insensitive factors are required for Ca2+-stimulated exocytosis of insulin. Biochem. J. 1996;314:199–203. doi: 10.1042/bj3140199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490:201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 6.Ohara-Imaizumi M, Fujiwara T, Nakamichi Y, Okamura T, Akimoto Y, Kawai J, Matsushima S, Kawakami H, Watanabe T, Akagawa K, Nagamatsu S. Imaging analysis reveals mechanistic differences between first- and second-phase insulin exocytosis. J. Cell. Biol. 2007;177:695–705. doi: 10.1083/jcb.200608132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spurlin BA, Thurmond DC. Syntaxin 4 Facilitates Biphasic Glucose-Stimulated Insulin Secretion from Pancreatic Beta Cells. Mol. Endocrinol. 2006;20:183–193. doi: 10.1210/me.2005-0157. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jewell JL, Oh E, Thurmond DC. Exocytosis mechanisms underlying insulin release and glucose uptake: conserved roles for Munc18c and syntaxin 4. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R517–531. doi: 10.1152/ajpregu.00597.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices--guilty as charged? Ann. Rev. Cell. Dev. Biol. 2012;28:279–308. doi: 10.1146/annurev-cellbio-101011-155818. [DOI] [PubMed] [Google Scholar]
- 11.Andersson SA, Olsson AH, Esguerra JL, Heimann E, Ladenvall C, Edlund A, Salehi A, Taneera J, Degerman E, Groop L, Ling C, Eliasson L. Reduced insulin secretion correlates with decreased expression of exocytotic genes in pancreatic islets from patients with type 2 diabetes. Mol. Cell. Endocrinol. 2012;364:36–45. doi: 10.1016/j.mce.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Ostenson CG, Gaisano H, Sheu L, Tibell A, Bartfai T. Impaired gene and protein expression of exocytotic soluble N-ethylmaleimide attachment protein receptor complex proteins in pancreatic islets of type 2 diabetic patients. Diabetes. 2006;55:435–440. doi: 10.2337/diabetes.55.02.06.db04-1575. [DOI] [PubMed] [Google Scholar]
- 13.Bergman BC, Cornier MA, Horton TJ, Bessesen DH, Eckel RH. Skeletal muscle munc18c and syntaxin 4 in human obesity. Nutr. Metab. (Lond) 2008;5:21. doi: 10.1186/1743-7075-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bostrom P, Andersson L, Vind B, Haversen L, Rutberg M, Wickstrom Y, Larsson E, Jansson PA, Svensson MK, Branemark R, Ling C, Beck-Nielsen H, Boren J, Hojlund K, Olofsson SO. The SNARE protein SNAP23 and the SNARE-interacting protein Munc18c in human skeletal muscle are implicated in insulin resistance/type 2 diabetes. Diabetes. 2010;59:1870–1878. doi: 10.2337/db09-1503. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Oh E, Stull ND, Mirmira RG, Thurmond DC. Syntaxin 4 up-regulation increases efficiency of insulin release in pancreatic islets from humans with and without type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2014;99:866–870. doi: 10.1210/jc.2013-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh E, Kalwat MA, Kim MJ, Verhage M, Thurmond DC. Munc18-1 regulates first-phase insulin release by promoting granule docking to multiple syntaxin isoforms. J. Biol. Chem. 2012;287:25821–25833. doi: 10.1074/jbc.M112.361501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh E, Thurmond DC. Munc18c Depletion Selectively Impairs the Sustained Phase of Insulin Release. Diabetes. 2009;58:1165–1174. doi: 10.2337/db08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalwat MA, Wiseman DA, Luo W, Wang Z, Thurmond DC. Gelsolin Associates with the N-terminus of Syntaxin 4 to Regulate Insulin Granule Exocytosis. Mol. Endocrinol. 2012;26:128–141. doi: 10.1210/me.2011-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jewell JL, Luo W, Oh E, Wang Z, Thurmond DC. Filamentous actin regulates insulin exocytosis through direct interaction with Syntaxin 4. J. Biol. Chem. 2008;283:10716–10726. doi: 10.1074/jbc.M709876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porat-Shliom N, Milberg O, Masedunskas A, Weigert R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell. Mol. Life. Sci. 2013;70:2099–2121. doi: 10.1007/s00018-012-1156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Rathore SS, Lopez JA, Davis EM, James DE, Martin JL, Shen J. Comparative studies of Munc18c and Munc18-1 reveal conserved and divergent mechanisms of Sec1/Munc18 proteins. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E3271–3280. doi: 10.1073/pnas.1311232110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu SH, Latham CF, Gee CL, James DE, Martin JL. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8773–8778. doi: 10.1073/pnas.0701124104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dulubova I, Khvotchev M, Liu S, Huryeva I, Sudhof TC, Rizo J. Munc18-1 binds directly to the neuronal SNARE complex. Proc. Natl. Acad. Sci. U. S. A. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulyas-Kovacs A, de Wit H, Milosevic I, Kochubey O, Toonen R, Klingauf J, Verhage M, Sorensen JB. Munc18-1: sequential interactions with the fusion machinery stimulate vesicle docking and priming. J. Neurosci. 2007;27:8676–8686. doi: 10.1523/JNEUROSCI.0658-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zilly FE, Sorensen JB, Jahn R, Lang T. Munc18-bound syntaxin readily forms SNARE complexes with synaptobrevin in native plasma membranes. PLoS. Biol. 2006;4:e330. doi: 10.1371/journal.pbio.0040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jewell JL, Oh E, Bennett SM, Meroueh SO, Thurmond DC. The tyrosine phosphorylation of Munc18c induces a switch in binding specificity from syntaxin 4 to Doc2beta. J. Biol. Chem. 2008;283:21734–21746. doi: 10.1074/jbc.M710445200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh E, Thurmond DC. The stimulus-induced tyrosine phosphorylation of Munc18c facilitates vesicle exocytosis. J. Biol. Chem. 2006;281:17624–17634. doi: 10.1074/jbc.M601581200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umahara M, Okada S, Yamada E, Saito T, Ohshima K, Hashimoto K, Yamada M, Shimizu H, Pessin JE, Mori M. Tyrosine phosphorylation of Munc18c regulates platelet-derived growth factor-stimulated glucose transporter 4 translocation in 3T3L1 adipocytes. Endocrinology. 2008;149:40–49. doi: 10.1210/en.2006-1549. [DOI] [PubMed] [Google Scholar]
- 29.Bakke J, Bettaieb A, Nagata N, Matsuo K, Haj FG. Regulation of the SNARE-interacting protein Munc18c tyrosine phosphorylation in adipocytes by protein-tyrosine phosphatase 1B. Cell. Commun. Signal. 2013;11:57. doi: 10.1186/1478-811X-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christie MP, Whitten AE, King GJ, Hu SH, Jarrott RJ, Chen KE, Duff AP, Callow P, Collins BM, James DE, Martin JL. Low-resolution solution structures of Munc18:Syntaxin protein complexes indicate an open binding mode driven by the Syntaxin N-peptide. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9816–9821. doi: 10.1073/pnas.1116975109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shi L, Kummel D, Coleman J, Melia TJ, Giraudo CG. Dual roles of Munc18-1 rely on distinct binding modes of the central cavity with Stx1A and SNARE complex. Mol. Biol. Cell. 2011;22:4150–4160. doi: 10.1091/mbc.E11-02-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verhage M, de Vries KJ, Roshol H, Burbach JP, Gispen WH, Sudhof TC. DOC2 proteins in rat brain: complementary distribution and proposed function as vesicular adapter proteins in early stages of secretion. Neuron. 1997;18:453–461. doi: 10.1016/s0896-6273(00)81245-3. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Cantley J, Burchfield JG, Meoli CC, Stockli J, Whitworth PT, Pant H, Chaudhuri R, Groffen AJ, Verhage M, James DE. DOC2 isoforms play dual roles in insulin secretion and insulin-stimulated glucose uptake. Diabetologia. 2014 doi: 10.1007/s00125-014-3312-y. in the press. doi: 10.1007/s00125-014-3312-y. [DOI] [PubMed] [Google Scholar]
- 34.Ramalingam L, Oh E, Thurmond DC. Doc2b enrichment enhances glucose homeostasis in mice via potentiation of insulin secretion and peripheral insulin sensitivity. Diabetologia. 2014;57:1476–1484. doi: 10.1007/s00125-014-3227-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramalingam L, Oh E, Yoder SM, Brozinick JT, Kalwat MA, Groffen AJ, Verhage M, Thurmond DC. Doc2b is a key effector of insulin secretion and skeletal muscle insulin sensitivity. Diabetes. 2012;61:2424–2432. doi: 10.2337/db11-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ke B, Oh E, Thurmond DC. Doc2beta is a novel Munc18c-interacting partner and positive effector of syntaxin 4-mediated exocytosis. J. Biol. Chem. 2007;282:21786–21797. doi: 10.1074/jbc.M701661200. [DOI] [PubMed] [Google Scholar]
- 37.Thurmond DC, Ceresa BP, Okada S, Elmendorf JS, Coker K, Pessin JE. Regulation of insulin-stimulated GLUT4 translocation by munc18c in 3T3L1 adipocytes. J. Biol. Chem. 1998;273:33876–33883. doi: 10.1074/jbc.273.50.33876. [DOI] [PubMed] [Google Scholar]
- 38.Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 39.Oh E, Heise CJ, English JM, Cobb MH, Thurmond DC. WNK1 is a novel regulator of Munc18c-syntaxin 4 complex formation in soluble NSF attachment protein receptor (SNARE)-mediated vesicle exocytosis. J. Biol. Chem. 2007;282:32613–32622. doi: 10.1074/jbc.M706591200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiseman DA, Kalwat MA, Thurmond DC. Stimulus-induced S-nitrosylation of syntaxin 4 impacts insulin granule exocytosis. J. Biol. Chem. 2011;286:16344–16354. doi: 10.1074/jbc.M110.214031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pielage J, Fetter RD, Davis GW. A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J. Cell. Biol. 2006;175:491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Vries KJ, Geijtenbeek A, Brian EC, de Graan PN, Ghijsen WE, Verhage M. Dynamics of munc18-1 phosphorylation/dephosphorylation in rat brain nerve terminals. Eur. J. Neurosci. 2000;12:385–390. doi: 10.1046/j.1460-9568.2000.00931.x. [DOI] [PubMed] [Google Scholar]
- 43.Orita S, Naito A, Sakaguchi G, Maeda M, Igarashi H, Sasaki T, Takai Y. Physical and functional interactions of Doc2 and Munc13 in Ca2+-dependent exocytotic machinery. J. Biol. Chem. 1997;272:16081–16084. doi: 10.1074/jbc.272.26.16081. [DOI] [PubMed] [Google Scholar]
- 44.Ma C, Li W, Xu Y, Rizo J. Munc13 mediates the transition from the closed syntaxin-Munc18 complex to the SNARE complex. Nat. Struct. Mol. Biol. 2011;18:542–549. doi: 10.1038/nsmb.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang L, He Z, Xu P, Fan J, Betz A, Brose N, Xu T. Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell. Metab. 2006;3:463–468. doi: 10.1016/j.cmet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki M, Emoto M, Fukuda N, Hatanaka M, Taguchi A, Miyamoto S, Tanizawa Y. DOC2b is a SNARE regulator of glucose-stimulated delayed insulin secretion. Biochem. Biophys. Res. Comm. 2009;384:461–465. doi: 10.1016/j.bbrc.2009.04.133. [DOI] [PubMed] [Google Scholar]
- 47.Groffen AJ, Brian EC, Dudok JJ, Kampmeijer J, Toonen RF, Verhage M. Ca(2+)-induced recruitment of the secretory vesicle protein DOC2B to the target membrane. J. Biol. Chem. 2004;279:23740–23747. doi: 10.1074/jbc.M400731200. [DOI] [PubMed] [Google Scholar]
- 48.Pang ZP, Bacaj T, Yang X, Zhou P, Xu W, Sudhof TC. Doc2 Supports spontaneous synaptic transmission by a ca(2+)-independent mechanism. Neuron. 2011;70:244–251. doi: 10.1016/j.neuron.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keller MP, Choi Y, Wang P, Davis DB, Rabaglia ME, Oler AT, Stapleton DS, Argmann C, Schueler KL, Edwards S, Steinberg HA, Chaibub Neto E, Kleinhanz R, Turner S, Hellerstein MK, Schadt EE, Yandell BS, Kendziorski C, Attie AD. A gene expression network model of type 2 diabetes links cell cycle regulation in islets with diabetes susceptibility. Genome. Res. 2008;18:706–716. doi: 10.1101/gr.074914.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.