Abstract
Background
APRI (aspartate aminotransferase [AST] to platelet ratio index) is widely used to assess fibrosis and cirrhosis risk, especially in HCV-infected patients. Few studies have evaluated APRI and HBV-related hepatocellular carcinoma (HCC) risk. Prospective evidence is needed to assess whether APRI predicts HCC risk in HBV patients.
Method
In a prospectively enrolled clinical cohort of 855 HBV patients with a 1-year exclusion window (followed for >1 year and did not develop HCC within 1 year), the predictive value of APRI in HCC risk was evaluated by Cox proportional hazards model using univariate and multivariate analyses and longitudinal analysis.
Results
Higher APRI prospectively conferred a significantly increased risk of HCC in univariate analysis (quartile analysis, P trend=2.9×10−7). This effect remained highly significant after adjusting for common host characteristics but not cirrhosis (P trend=7.1×10−5), and attenuated when cirrhosis is adjusted (P trend=0.021). The effect remained prominent when the analysis was restricted to patients with a more stringent 2-year exclusion window (P trend=0.008 in quartile analysis adjusting all characteristics including cirrhosis), indicating that the association was unlikely due to including undetected HCC patients in the cohort, thus minimizing the reverse-causation limitation in most retrospective studies. Longitudinal comparison demonstrated a persistently higher APRI value in HBV patients who developed HCC during follow-up than those remaining cancer-free.
Conclusion
APRI might be a marker of HCC risk in HBV patients in cirrhosis-dependent and independent manners. Further studies are warranted to validate this finding and test its clinical applicability in HCC prevention.
Keywords: APRI, HBV, HCC
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most rapidly increasing causes of cancer mortality in the United States 1. The overall age-adjusted HCC incidence rate in the United States tripled between 1975 and 2005, partially accounted for by the increase in hepatitis C virus (HCV) infection and the influx of immigrants from hepatitis B virus (HBV) endemic regions 2. Among patients diagnosed with early stage HCC, tumor resection and/or liver transplantation have been shown to improve the outcome with a five-year survival rate of >30% 3. However, many HCC patients have advanced disease at diagnosis and thus are not eligible for curative treatments, contributing to a low overall five-year survival rate of approximately 10% 2. These facts highlight the importance of risk prediction, active surveillance, and early diagnosis in HCC management.
HCC develops in a multistage process involving chronic liver injury and local inflammation, progressive liver fibrosis and cirrhosis, initiation of neoplastic niches, and malignant transformation. It is well known that advanced fibrosis and cirrhosis are high-risk conditions which prompt intensive surveillance for HCC. Although liver biopsy remains as the golden standard for evaluating chronic liver diseases, its clinical use is limited by its invasive nature, potentially life-threatening complications, sampling errors, and intra- and inter-observer variabilities. These limitations necessitate the development of alternative non-invasive methods 4.
APRI (aspartate aminotransferase [AST] to platelet ratio index) is a widely investigated indirect marker in assessing liver fibrosis and cirrhosis 5, 6. APRI was originally developed for predicting fibrosis and cirrhosis in patients with chronic HCV infection 7, and most of the investigations that followed were focused on HCV-related fibrosis evaluation 6. A few studies have also reported that APRI might be helpful in the assessment of liver fibrosis in HBV patients 8-10. A recent study suggested that APRI was also associated with post-operative prognosis in early stage HBV-related HCC patients 11. Based on these findings, we hypothesized that APRI might be a marker for HCC risk in HBV patients and sought to prospectively test this hypothesis in the present study. To the best of our knowledge, there is no report prospectively evaluating APRI as a risk predictor of HBV-related HCC.
MATERIALS and METHODS
Study Population
The subjects in this study were identified from a clinic-based patient cohort. The cohort was comprised of patients who visited the Liver Disease Prevention Center at Thomas Jefferson University Hospital for the treatment of chronic HBV or HCV infection and liver diseases such as cirrhosis, fibrosis, and/or HCC. There were no restrictions on age, gender, ethnicity, disease stage, and etiology in patient enrollment. The enrollment started in 1988 and is ongoing. All HBV patients in our cohort have been treated with oral antiviral drugs and/or interferons. Treatment strategy stringently followed the guidelines of American Association for Study of Liver Disease (AASLD). The AASLD guidelines evolved over time, along with the approval of new drugs or appearance of new clinical evidence. In our HBV patient cohort, interferon was only briefly used after 1992 for a very small number (<20) of patients. As to the oral anti-HBV drugs, Lamivudine (LAM) was the first drug available in 1998 until 2002 when adefovir dipivoxil (ADV) was approved. Also other drugs were used when they became available; Entecavir (ETV) in 2005, telbivudine (TLV) in 2006, and tenofovir disoproxil fumarate (TDF) in 2008. When patients developed virologic breakthrough (one log increase in HBV DNA level [copies/mL] after reaching the nadir while on antiviral therapy such as LAM), ADV or TDF was added or switched. This was also true for those who started on TLV or ETV. In addition, with the increasing preference of combination therapy over monotherapy, TDF or ADV was also added to LAM. Moreover, combination of ETV and TDF is used frequently. In the current AASLD guideline, either TDF or ETV is used as the first line drug for the newly diagnosed patients due to their extremely low rate of drug resistance. Because >90% of the patients in this cohort were of Korean ancestry, we restricted this study to Korean Americans. For this study, we included those patients who met all the following criteria: (1) patients had only HBV infection but no concomitant infection with HCV, HIV, or other viruses, in order to eliminate the confounding effects from different disease etiologies; (2) patients had both AST and platelet count measured simultaneously at study entry to calculate the baseline APRI index; and (3) patients had a 1-year exclusion window (followed for >1 year and did not develop HCC within 1 year ), which help to minimize the confounding effects of those patients who actually had developed HCC but were not diagnosed at study entry. This study was approved by the Institutional Review Board (IRB) of Thomas Jefferson University and strictly adhered to all guideline related to conducing human subject-based research.
Data collection
Demographic and clinical data were obtained for each patient through medical chart review and/or consultation with the treating physicians. The details on data collection and patient diagnosis were described previously 12, 13. Demographic variables collected for this study included age; gender; ethnicity; smoking status; drinking status; and family history of cancer. For each patient, hepatitis viral infection status was clinically determined before or at enrollment. After their first visit, patients were followed-up at the center at different time intervals as determined by the treating clinicians. Liver cirrhosis was diagnosed mainly through imaging studies, which showed medial segment atrophy of the left lobe, caudate lobe hypertrophy, or liver nodularity for early stage disease, and signs of portal hypertension such as varices, splenomegaly, patent paraumbilical vein, or ascites in advanced stage disease. Other criteria were used to complement to imaging studies, including liver biopsy, laboratory tests such as thrombocytopenia, serum albumin, and prolonged prothrombin time, and clinical presentations such as ascites, encephalopathy, and gastrointestinal bleeding, etc. AST values and platelet counts were collected at initial and follow-up visits. The endpoint of this study was HCC development. Time to HCC development was defined as the date from the study entry to the date of HCC diagnosis during follow-up, or the date of last follow-up if the patients were alive without HCC diagnosis at the time of this analysis. Patients free of HCC at death or at the end of last follow-up were censored for analysis.
APRI index calculation
The standard established protocols of liver function tests and complete blood panel test were used to measure the AST and platelet count, respectively. The upper limit of normal range (ULN) of AST is ≤ 37 U/L for males and ≤ 31 for females 14. The APRI index was calculated as follows: [(AST value/ULN) /platelet count (109/L)] × 100 7.
Statistical analysis
Statistical analyses were performed using the SAS software version 9.3 (SAS Institute, Cary, NC). To include the largest possible sample size, we performed the primary prospective analysis with a 1-year exclusion window. To minimize the confounding effects of those patients who actually had HCC, but were not diagnosed at initial sample collection, we further restricted the analyses to sub-cohorts of patients with a 2-year exclusion window. The distributions of host variables were analyzed by chi-square test. In the prospective analyses, the Cox proportional hazards regression model was used to estimate the association between APRI and HCC risk, as represented by hazard ratio (HR) and 95% confidence interval (CI). Age, gender, smoking status, drinking status, family history of cancer, and cirrhosis status were adjusted in the multivariate analyses. We did not include treatment in the multivariate analysis because all patients received standard-of-care treatment (antiviral therapy for the vast majority of patients) based on the AASLD guidelines and thus adjustment of treatment do not affect the analysis result. The cumulative incidence of HCC over time was derived using the Nelson-Aalen method 15. Interactions between variables were assessed by adding a product term into the Cox regression model. The longitudinal trends of temporal changes in average APRI values for HCC patients and cancer-free HBV patients were plotted by fitting a smoothing spline over time. All statistical tests were two-sided, and a P value of <0.05 was considered statistically significant.
RESULTS
Characteristics of the study population
There were a total of 855 patients included in the primary cohort with a 1-year exclusion window. The median follow-up time among all patients was 52.4 months (range, 12.2-255.8). Over the entire follow-up duration, 82 HBV patients developed HCC with an incidence rate of 9.59%.The average age of the subjects was 42.6 years old (standard deviation, ±11.7). As summarized in Table 1, the majority of patients were male (65.5 %); never smokers (67.8%); never drinkers (62.5%); without family history of cancer (64.9%) and without cirrhosis (66.7%). As expected, older age (HR=4.59, 95% CI 2.68-7.89) and cirrhosis (HR=4.22, 95% CI 2.42-7.36) were the most significant risk factors for HCC development. Additionally, HCC risk was significantly increased in male patients (HR=2.40, 95% CI 1.18-4.87) and ever smokers (HR=2.01, 95% CI 1.15-3.54). Although significantly increased HCC risks were also observed in ever drinkers and patients with family history of cancer in the univariate analysis, the associations became non-significant in the multivariate analysis.
Table 1.
The association between patient characteristics and HBV-related HCC risk
| Variables | Total number of HBV patients (%) |
HCC patients (%) | Univariate |
Multivariate* |
|||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age | |||||||
| ≤ Median | 432 (50.5) | 18 (22.0) | 1.00 | 1.00 | |||
| > Median | 423 (49.5) | 64 (78.0) | 5.29(3.12-8.98) | 6.7 × 10−10 | 4.59(2.68-7.89) | 3.2 × 10−8 | |
| Gender | |||||||
| Female | 295 (34.5) | 11 (13.4) | 1.00 | 1.00 | |||
| Male | 560 (65.5) | 71 (86.6) | 2.83(1.50-5.34) | 0.001 | 2.40(1.18-4.87) | 0.015 | |
| Smoking status | |||||||
| Never smokers | 580 (67.8) | 38 (46.3) | 1.00 | 1.00 | |||
| Ever smokers | 275 (32.2) | 44 (53.7) | 2.60(1.67-4.03) | 2.0 × 10−5 | 2.01(1.15-3.54) | 0.015 | |
| Drinking status | |||||||
| Never drinkers | 534 (62.5) | 41 (50.0) | 1.00 | 1.00 | |||
| Ever drinkers | 321 (37.5) | 41 (50.0) | 2.01(1.29-3.12) | 0.002 | 0.94(0.54-1.64) | 0.831 | |
| Family history of cancer | |||||||
| No | 555 (64.9) | 46 (56.1) | 1.00 | 1.00 | |||
| Yes | 300 (35.1) | 36 (43.9) | 1.66(1.07-2.57) | 0.024 | 1.21(0.77-1.90) | 0.417 | |
| Cirrhosis | |||||||
| No | 570 (66.7) | 16 (19.5) | 1.00 | 1.00 | |||
| Yes | 285 (33.3) | 66 (80.5) | 6.99(4.04-12.09) | 3.4 × 10−12 | 4.22(2.42-7.36) | 4.0 × 10−7 | |
Adjusted for age, gender, smoking and drinking status, family history of cancer, and cirrhosis, where appropriate.
Prospective association of APRI with HCC risk in HBV patients
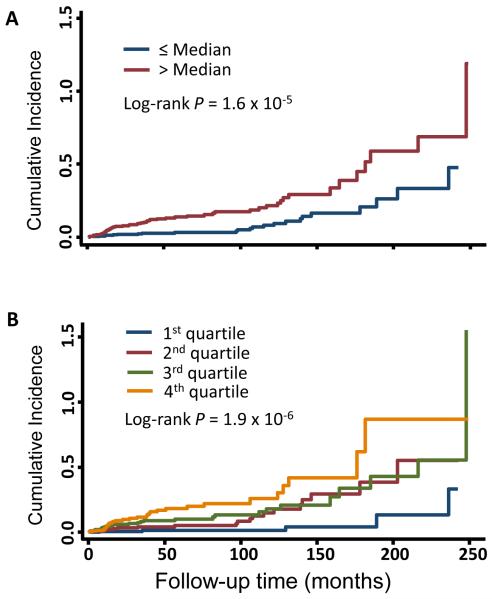
We analyzed the association between baseline APRI and HCC risk using univariate and multivariate Cox proportional hazard model. We categorized APRI into two levels (dichotomization analysis using the median APRI value in all subjects), and four levels (quartile cutoffs), and used the lowest APRI level as reference. In the primary prospective HBV patient cohort with a 1-year exclusion window, univariate analysis of dichotomized APRI levels showed a significant association between higher APRI and HCC risk (HR=2.75, 95%CI 1.70-4.43, P=3.4×10−5). This effect attenuated to a borderline significant level in the multivariate analysis adjusted for all host variables (HR=1.60, 95%CI 0.95-2.70, P=0.076) (Table 2). Similarly, significant association as well as the attenuation were also observed in the quartile analyses, which in addition exhibited a significant dose-dependent effect of baseline APRI and HCC risk in both univariate (Pfor trend=2.9×10−7) and multivariate (Pfor trend=0.021) analyses. Consistent with the Cox analyses, cumulative incidences of HCC assessed by the Nelson-Aalen method were significantly higher in patients with higher APRI in both dichotomization and quartile analyses (Figure 1). Next, we further restricted the prospective analyses to patients with a 2-year exclusion window (686 patients). The longer exclusion window helped minimize the confounding effect of undiagnosed HCC patients at the time of baseline APRI measurement. The results of analyses with the 2-year exclusion windows were highly consistent with that of the 1-year exclusion window, either in the dichotomization or the quartile analysis (Table 2). Because we suspected that the attenuated association between APRI and HCC risk was due to a strong confounding from cirrhosis, we conducted another multivariate analysis adjusting for all other host variables but cirrhosis. The results of this analysis were similar to that of the univariate analysis in both cohorts of 1-year and 2-year exclusion windows. The results of similar multivariate analysis without adjusting for each of other host characteristics including age, gender, smoking status, drinking status and family history of cancer yielded similar results to those of the multivariate analysis adjusting for all the variables (data not shown).
Table 2.
The association between APRI and HCC risk
| APRI values | Total number of HBV patients |
HCC cases |
Univariate analysis |
Multivariate analysis* |
Multivariate analysis† |
Log-rank P | |||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| In patients with a 1-year exclusion window (n=855) | |||||||||
| By median | |||||||||
| ≤ Median | 429 | 24 | 1.00 | 1.00 | 1.00 | 1.6 × 10−5 | |||
| > Median | 426 | 58 | 2.75(1.70-4.43) | 3.4 × 10−5 | 1.60(0.95-2.70) | 0.076 | 2.27 (1.39-3.72) | 0.001 | |
| By quartile | |||||||||
| 1st quartile | 211 | 5 | 1.00 | 1.00 | 1.00 | 1.9 × 10−6 | |||
| 2nd quartile | 218 | 19 | 4.05(1.51-10.85) | 0.005 | 1.92(0.70-5.27) | 0.203 | 2.31 (0.85-6.30) | 0.102 | |
| 3rd quartile | 212 | 26 | 5.31(2.04-13.84) | 0.001 | 2.26(0.84-6.12) | 0.108 | 3.29 (1.24-8.70) | 0.017 | |
| 4th quartile | 214 | 32 | 8.93(3.46-23.04) | 5.9 × 10−6 | 3.00(1.11-8.09) | 0.03 | 5.21 (1.98-13.71) | 8.2 × 10−4 | |
| P trend | 2.9 × 10−7 | 0.021 | 7.1 × 10−5 | ||||||
| In patients with a 2-year exclusion window (n=686) | |||||||||
| By median | |||||||||
| ≤ Median | 342 | 16 | 1.00 | 1.00 | 1.00 | 3.1 × 10-5 | |||
| > Median | 344 | 44 | 3.18(1.79-5.66) | 8.0 x10−5 | 2.04(1.10-3.79) | 0.023 | 2.79 (1.54-5.03) | 6.8 × 10−4 | |
| By quartile | |||||||||
| 1st quartile | 174 | 4 | 1.00 | 1.00 | 1.00 | 1.9 × 10−5 | |||
| 2nd quartile | 168 | 12 | 3.38(1.09-10.51) | 0.035 | 1.72(0.54-5.46) | 0.359 | 2.09 (0.66-6.60) | 0.210 | |
| 3rd quartile | 172 | 20 | 5.26(1.79-15.41) | 0.002 | 2.54(0.83-7.77) | 0.102 | 3.54 (1.18-10.58) | 0.024 | |
| 4th quartile | 172 | 24 | 8.93(3.08-25.89) | 5.5 × 10−5 | 3.55(1.17-10.78) | 0.026 | 6.05 (2.04-17.92) | 0.001 | |
| P trend | 2.8 × 10−6 | 0.008 | 6.3 × 10−5 | ||||||
Adjusted for age, gender, smoking and drinking status, family history of cancer, and cirrhosis.
Adjusted for age, gender, smoking and drinking status, and family history of cancer.
Figure 1. Cumulative incidence of HBV-related HCC.
The cumulative incidence of HCC risk was derived using the Nelson-Aalen method by analyzing APRI as categorical variable by median (A), and quartile (B) cutoff. The P values were calculated by log-rank test.
Stratified and joint effects analyses of APRI on HBV-related HCC risk
We stratified the main effects analysis by different host characteristics (Table 3). In the univariate analysis, there was no significant association between APRI and HCC risk in younger patients (P=0.166) and non-cirrhotic patients (P=0.422). Univariate analyses in both strata of all other variables showed significant associations between higher APRI and HBV-related HCC risk. In multivariate analyses, the significant association between APRI and HCC risk remained prominent in younger patients (P=0.050), ever smokers (P=0.040), and ever drinkers (P=0.029) (Table 3). We further assessed the joint and interaction effects of APRI and major host variables on HCC risk. Although significant joint effects were observed between APRI and the majority of host variables, no interaction was identified (Table 4).
Table 3.
The association between APRI and HCC risk stratified by patients’ characteristics
| Variables | APRI values | Number of patients |
HCC cases |
Univariate |
Multivariate* |
||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age | |||||||
| Younger (≤ Median) | |||||||
| ≤ Median | 256 | 8 | 1.00 | 1.00 | |||
| > Median | 176 | 10 | 1.93(0.76-4.92) | 0.166 | 2.87(1.00-8.27) | 0.050 | |
| Older (> Median) | |||||||
| ≤ Median | 173 | 16 | 1.00 | 1.00 | |||
| > Median | 250 | 48 | 2.37(1.34-4.19) | 0.003 | 1.46(0.78-2.75) | 0.239 | |
| Gender | |||||||
| Female | |||||||
| ≤ Median | 160 | 2 | 1.00 | 1.00 | |||
| > Median | 135 | 9 | 6.27(1.35-29.17) | 0.019 | 2.74(0.53-14.22) | 0.232 | |
| Male | |||||||
| ≤ Median | 269 | 22 | 1.00 | 1.00 | |||
| > Median | 291 | 49 | 2.35(1.42-3.90) | 0.001 | 1.56(0.90-2.7) | 0.117 | |
| Smoking status | |||||||
| Never smokers | |||||||
| ≤ Median | 303 | 10 | 1.00 | 1.00 | |||
| > Median | 277 | 28 | 3.03(1.46-6.26) | 0.003 | 1.19(0.54-2.66) | 0.664 | |
| Ever smokers | |||||||
| ≤ Median | 126 | 14 | 1.00 | 1.00 | |||
| > Median | 149 | 30 | 2.79(1.44-5.38) | 0.002 | 2.09(1.04-4.24) | 0.040 | |
| Drinking status | |||||||
| Never drinkers | |||||||
| ≤ Median | 271 | 10 | 1.00 | 1.00 | |||
| > Median | 263 | 31 | 3.28(1.60-6.73) | 0.001 | 1.3(0.58-2.90) | 0.518 | |
| Ever drinkers | |||||||
| ≤ Median | 158 | 14 | 1.00 | 1.00 | |||
| > Median | 163 | 27 | 2.49(1.27-4.85) | 0.008 | 2.24(1.08-4.63) | 0.029 | |
| Family history of cancer | |||||||
| No | |||||||
| ≤ Median | 271 | 14 | 1.00 | 1.00 | |||
| > Median | 284 | 32 | 2.46(1.31-4.63) | 0.005 | 1.57(0.79-3.12) | 0.194 | |
| Yes | |||||||
| ≤ Median | 158 | 10 | 1.00 | 1.00 | |||
| > Median | 142 | 26 | 3.53(1.65-7.55) | 0.001 | 2.19(0.93-5.14) | 0.073 | |
| Cirrhosis | |||||||
| No | |||||||
| ≤ Median | 355 | 8 | 1.00 | 1.00 | |||
| > Median | 215 | 8 | 1.52(0.55-4.19) | 0.422 | 1.53(0.5-4.68) | 0.453 | |
| Yes | |||||||
| ≤ Median | 74 | 16 | 1.00 | 1.00 | |||
| > Median | 211 | 50 | 1.97(1.11-3.5) | 0.020 | 1.76(0.95-3.26) | 0.074 | |
Adjusted for age, gender, smoking and drinking status, family history of cancer, and cirrhosis, where appropriate.
Table 4.
Joint effects of APRI and host variables on HCC risk
| Variables | APRI | Strata | Number of patients |
HCC cases |
Univariate | Multivariate* | ||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Age | ||||||||
| Lower | Younger | 256 | 8 | 1.00 | 1.00 | |||
| Lower | Older | 173 | 16 | 4.06(1.73-9.52) | 0.001 | 5.01(2.12-11.85) | 2.4 × 10−4 | |
| Higher | Younger | 176 | 10 | 1.86(0.74-4.73) | 0.190 | 1.93(0.75-4.96) | 0.171 | |
| Higher | Older | 250 | 48 | 9.7(4.55-20.65) | 3.9 × 10−9 | 7.02(3.22-15.28) | 9.3 × 10−7 | |
| P for interaction | 0.657 | 0.350 | ||||||
| Gender | ||||||||
| Lower | Female | 160 | 2 | 1.00 | 1.00 | |||
| Lower | Male | 269 | 22 | 5.28(1.24-22.45) | 0.024 | 2.76(0.62-12.25) | 0.182 | |
| Higher | Female | 135 | 9 | 5.88(1.27-27.21) | 0.024 | 1.88(0.39-9.04) | 0.432 | |
| Higher | Male | 291 | 49 | 12.59(3.06-51.77) | 4.5 × 10−4 | 4.34(1.01-18.71) | 0.049 | |
| P for interaction | 0.273 | 0.833 | ||||||
| Smoking status | ||||||||
| Lower | Never smokers | 303 | 10 | 1.00 | 1.00 | |||
| Lower | Ever smokers | 126 | 14 | 2.88(1.28-6.48) | 0.011 | 1.62(0.66-3.99) | 0.290 | |
| Higher | Never smokers | 277 | 28 | 3.00(1.45-6.20) | 0.003 | 1.35(0.63-2.91) | 0.444 | |
| Higher | Ever smokers | 149 | 30 | 7.92(3.85-16.29) | 1.8 × 10−8 | 2.93(1.32-6.49) | 0.008 | |
| P for interaction | 0.862 | 0.563 | ||||||
| Drinking status | ||||||||
| Lower | Never drinkers | 271 | 10 | 1.00 | 1.00 | |||
| Lower | Ever drinkers | 158 | 14 | 2.55(1.13-5.75) | 0.024 | 0.77(0.31-1.92) | 0.581 | |
| Higher | Never drinkers | 263 | 31 | 3.29(1.61-6.73) | 0.001 | 1.26(0.59-2.70) | 0.557 | |
| Higher | Ever drinkers | 163 | 27 | 6.51(3.11-13.59) | 6.3 × 10−7 | 1.47(0.62-3.48) | 0.382 | |
| P for interaction | 0.607 | 0.414 | ||||||
| Family history of cancer | ||||||||
| Lower | No | 271 | 14 | 1.00 | 1.00 | |||
| Lower | Yes | 158 | 10 | 1.41(0.62-3.17) | 0.410 | 1.06(0.46-2.43) | 0.891 | |
| Higher | No | 284 | 32 | 2.47(1.31-4.65) | 0.005 | 1.45(0.74-2.83) | 0.277 | |
| Higher | Yes | 142 | 26 | 4.63(2.40-8.90) | 4.5 × 10−6 | 1.93(0.94-3.94) | 0.072 | |
| P for interaction | 0.560 | 0.648 | ||||||
| Cirrhosis | ||||||||
| Lower | No | 355 | 8 | 1.00 | 1.00 | |||
| Lower | Yes | 74 | 16 | 5.44(2.32-12.78) | 1.0 × 10−4 | 3.56(1.50-8.48) | 0.004 | |
| Higher | No | 215 | 8 | 1.62(0.61-4.31) | 0.336 | 1.53(0.57-4.13) | 0.399 | |
| Higher | Yes | 211 | 50 | 10.52(4.98-22.21) | 6.8 × 10−10 | 5.80(2.72-12.35) | 5.2 × 10−6 | |
| P for interaction | 0.758 | 0.918 | ||||||
Adjusted for age, gender, smoking and drinking status, family history of cancer, and cirrhosis, where appropriate.
Longitudinal change of APRI values over time
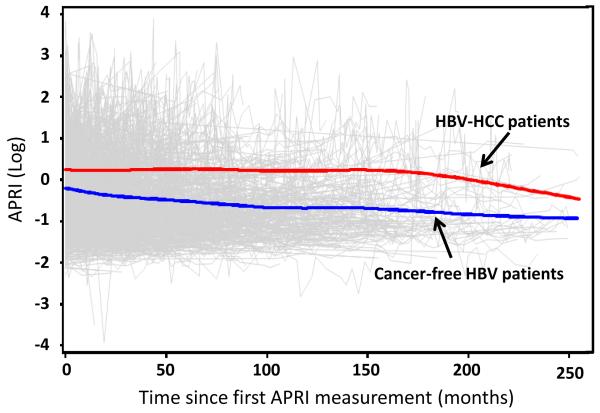
In the primary cohort of 1-year exclusion window, the longitudinal trends of average APRI values were plotted by fitting a smoothing spline over time (Figure 2). The average APRI values in HBV patients who developed HCC (n=82) were persistently higher than those remaining cancer-free (n=773) over the complete follow-up duration. An almost identical profile of this longitudinal plotting was also observed in the analysis of the sub-cohort with 2-year exclusion window (data not shown).
Figure 2. Longitudinal trends of average APRI values.
Blue line, trend of average APRI in HBV patients who remained cancer-free; red line, trend of average APRI values in HBV patients who developed HCC in the end; thin grey lines, individual profiles for all subjects.
Sub-cohort analyses of the effect of Alpha-fetoprotein (AFP) and viral DNA load on the association between APRI and HCC risk
Important variables such as AFP, viral DNA load, and viral HBs antigen level have been associated with HCC risk in HBV patients. However, the data for these variables are not completely available in all patients in this analysis. To help elucidate the effects of these variables on the APRI-HCC association, we analyzed the association of AFP or viral DNA load with HCC risk, and also performed a spearman’s rank correlation analysis between APRI and AFP or viral DNA load in sub-cohorts of patients that have available data of either of these two variables. We did not conduct the correlation analysis between APRI and HBs antigen because we didn’t have detailed HBs antigen values. The HBs antigen variable was recorded as either positive or negative in our database and 98% of the patients with available HBs antigen status were HBs antigen positive. We found that a higher AFP level was significantly associated with an increased HCC risk in both univariate analysis, and multivariate analysis adjusting for all major variables except cirrhosis (Supplementary Table S1). This result was consistent with our previous study reporting AFP as a strong independent prospective predictor of long-term HCC risk in HBV patients 16. However, we did not observe an association between HCC risk and viral DNA load, probably due to the limited sample size in the sub-cohort. In the correlation analysis, we noticed a moderate correlation between APRI and AFP (ρ=0.569) but the correlation between APRI and viral DNA load was much weaker (ρ=0.248). We further analyzed the association between APRI and HCC risk in sub-cohorts adjusting the common variables plus AFP or viral DNA load. The results were summarized in Supplementary Table S2. In the AFP sub-cohort, APRI still independently predicted higher HCC risk after AFP was adjusted (HR 2.12, 95% CI 1.06-4.26, P=0.03). In the viral DNA load sub-cohort, the analysis was not feasible because all the HCC cases had a higher-than-median APRI level. Taken together, these data indicated that APRI added additional clinical value in HCC risk prediction independent of AFP or viral DNA load.
Sub-cohort analyses of the associations of major demographic and clinical variables with HCC risk in cirrhotic patients
To access which factors other than APRI are associated with HCC risk in cirrhotic patients, we conducted sub-cohort analyses to analyze the association of each of the major demographic (age, gender, smoking status, drinking status, family history of cancer) and clinical (AFP and viral DNA load) variables with HCC risk in cirrhotic patients (Supplementary Table S3). We found that compared to younger patients, older patients had an increased HCC risk by univariate (HR=2.54, 95%CI 1.52-4.25, P=4.0×10−4) and multivariate (HR=2.95, 95%CI 1.75-4.96, P=5.0×10−05) analyses. Other important factors such as AFP and HBV DNA load did not exhibit a significant association. However, it should be noted that due to the limited number of cirrhotic patients in our cohort (33.3%), these sub-cohort analyses in cirrhotic patients for most variables were not adequately powered and the results should be interpreted with caution (Supplementary Table S3).
DISCUSSION
In the current study, we used a prospective approach to evaluate the role of APRI in the risk prediction of HBV-related HCC, and identified a significant dose-dependent association between baseline APRI and the risk of developing HCC in HBV patients. Our data suggested that APRI, an index calculated from two routinely available laboratory tests, might be a potential prospective risk factor of HBV-HCC.
APRI was initially developed as a predictive marker of liver fibrosis and cirrhosis in HCV-infected patients by Wai et al 7. Subsequently, various studies have attempted to externally validate this marker for liver disease assessment 6. Although the findings of these studies were controversial, APRI continues to be used in the assessment of liver diseases due to its noninvasiveness and cost effectiveness 8, 9, 17-20. The evaluation of APRI in HBV patients was not as extensively conducted as in HCV patients. APRI has been evaluated as a prognostic biomarker in HCC patients after radiofrequency ablation therapy 21 and surgical resection 11. A recent study reported that APRI might predict HCC risk in a cohort of Japanese patients with non-alcoholic fatty liver disease 22. Despite the inconsistent results, these studies substantiated the potential usefulness of APRI in evaluation of liver diseases. However, to the best of our knowledge, no study has directly assessed the predictive role of APRI in the risk of HBV-related HCC, especially using a prospective and longitudinal approach. In the present study, using prospectively collected data, we reported a significant correlation between baseline APRI and risk of developing HCC in dose-dependent and longitudinally persistent manners.
Accurate risk prediction and early detection remain major challenges in HCC management. Diagnosis after apparent symptoms usually reflects an advanced tumor stage. Moreover, the use of small nodules (<1 cm) does not provide a confident diagnosis as they are frequently observed in non-cancerous liver tissues 23-25. Therefore, it is possible that our primary cohort with a 1-year exclusion window may include some undetected HCC cases. To minimize the confounding effect of those patients who actually had HCC but were not diagnosed at study entry, we further restricted the analysis to a sub-cohort of patients with a 2-year exclusion window. As shown in Table 2, marked increases in the magnitude and significance of the APRIHCC association were observed in the more stringent analysis with the 2-year exclusion window compared to the primary analysis with a 1-year exclusion window. For instance, in the multivariate analysis by median APRI, the association between higher APRI and HCC risk strengthened from borderline (HR=1.60, P=0.076) to significant (HR=2.04, P=0.023). Quartile analysis also exhibited a more significant dose-dependent trend (P for trend decreased from 0.021 to 0.008). These data strongly substantiated the prospective value of APRI in the prediction of HCC risk.
A notable finding in our study was the influence of cirrhosis on the results of multivariate Cox model analysis. A much more prominent association between APRI and HCC risk was observed in the multivariate analysis without adjusting cirrhosis as compared to the one adjusting for all host variables (Table 2). Similar observations were reported in our previous studies 13. This observation suggests that the APRI-HCC association in our study was partly mediated by cirrhosis, which is consistent with the well-recognized role of APRI in the prediction of cirrhosis and fibrosis. Nonetheless, the effect of APRI on HCC risk remained significant even after adjusting cirrhosis, especially in the cohort of the more stringent 2-year exclusion window (Table 2), indicating that the predictive role of APRI was also independent of cirrhosis. This was consistent with the result of stratified analysis that showed a borderline significant association between APRI and HCC risk in cirrhotic patients (Table 3). On the other hand, the modest association between APRI and HCC risk was not likely to be overshadowed by the strong effect of cirrhosis, because the association was non-significant in non-cirrhotic patients as observed in the stratified analysis (Table 3).
A major strength of our study is the unique and homogenous Asian American HBV patient cohort enrolled in a single institute, which eliminated the confounding effects of patient ethnicity and disease etiology. Another notable strength is the restriction of the prospective analyses to a sub-cohort with a 2-year exclusion window that minimized the confounding resulting from the possible inclusion of undetected HCC cases at study entry. Moreover, the non-invasive and cost-effective nature of obtaining APRI through routine laboratory testes makes it possible for repetitive monitoring during patient follow-up and treatment.
Our study also has limitations. First, the generalizability of our findings to patients of other ethnicities or disease etiologies remains to be assessed. Second, the retrospective clinic-based chart review might result in incomplete collection of important demographic variables that were not recorded in our medical charts, thus limiting our ability to adjust a more inclusive panel of confounders in the multivariate analyses. Third, our multivariate main effects and stratified analyses indicated the APRI-HCC association is both mediated by and independent of cirrhosis (Tables 2 and 3). However, the proportion contributed by each of the two mechanisms needs to be evaluated in larger studies. Moreover, although we analyzed the sub-cohort with a 2-year exclusion window to address the reverse causation issue, it was possible that HCC might have initiated in some patients even prior to 2-years before their clinical diagnosis. Well-powered subcohorts with even more stringent exclusion criteria are needed in the future to further address this issue. Finally, additional well-powered prospective trials are warranted to determine the clinical benefit of APRI to the performance of the current strategies for HCC screening that are commonly based on bi-annual abdominal imaging studies supplemented by other major clinical variables.
In summary, our study indicated that APRI, an index derived from two routine laboratory blood tests, was prospectively associated with the risk of HCC in HBV patients. Given the importance of risk prediction and early detection in HCC management, it is worthwhile to conduct additional studies to validate our finding and test the clinical applicability of APRI in HCC prevention.
Supplementary Material
Acknowledgments
The work was supported by a Tobacco Grant from the Pennsylvania Department of Health, National Cancer Institute Grant CA159047, American Cancer Society Research Scholar Grant 123741-RSG-13-003-01-CCE, and a V Scholar Grant from the V Foundation for Cancer Research.
Footnotes
Conflict of interest: None declared
REFERENCES
- [1].El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- [2].Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1485–91. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Blum HE. Hepatocellular carcinoma: therapy and prevention. World journal of gastroenterology : WJG. 2005;11:7391–400. doi: 10.3748/wjg.v11.i47.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- [5].Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. doi: 10.1186/1471-230X-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology. 2007;46:912–21. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- [7].Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- [8].Lebensztejn DM, Skiba E, Sobaniec-Lotowska M, Kaczmarski M. A simple noninvasive index (APRI) predicts advanced liver fibrosis in children with chronic hepatitis B. Hepatology. 2005;41:1434–5. doi: 10.1002/hep.20736. [DOI] [PubMed] [Google Scholar]
- [9].Shin WG, Park SH, Jang MK, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. 2008;40:267–74. doi: 10.1016/j.dld.2007.10.011. [DOI] [PubMed] [Google Scholar]
- [10].Lin CS, Chang CS, Yang SS, Yeh HZ, Lin CW. Retrospective evaluation of serum markers APRI and AST/ALT for assessing liver fibrosis and cirrhosis in chronic hepatitis B and C patients with hepatocellular carcinoma. Intern Med. 2008;47:569–75. doi: 10.2169/internalmedicine.47.0595. [DOI] [PubMed] [Google Scholar]
- [11].Hung HH, Su CW, Lai CR, et al. Fibrosis and AST to platelet ratio index predict postoperative prognosis for solitary small hepatitis B-related hepatocellular carcinoma. Hepatol Int. 2010;4:691–9. doi: 10.1007/s12072-010-9213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wan S, Hann HW, Myers RE, et al. Telomere length in circulating serum DNA as a novel non-invasive biomarker for cirrhosis: a nested case-control analysis. Liver international : official journal of the International Association for the Study of the Liver. 2012;32:1233–41. doi: 10.1111/j.1478-3231.2012.02801.x. [DOI] [PubMed] [Google Scholar]
- [13].Fu X, Wan S, Hann HW, et al. Relative telomere length: a novel non-invasive biomarker for the risk of non-cirrhotic hepatocellular carcinoma in patients with chronic hepatitis B infection. Eur J Cancer. 2012;48:1014–22. doi: 10.1016/j.ejca.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–85. e11. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- [15].Hann HW, Wan S, Myers RE, et al. Comprehensive analysis of common serum liver enzymes as prospective predictors of hepatocellular carcinoma in HBV patients. PloS one. 2012;7:e47687. doi: 10.1371/journal.pone.0047687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hann HW, Fu X, Myers RE, et al. Predictive value of alpha-fetoprotein in the long-term risk of developing hepatocellular carcinoma in patients with hepatitis B virus infection - Results from a clinic-based longitudinal cohort. Eur J Cancer. 2012 doi: 10.1016/j.ejca.2012.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yu ML, Lin SM, Lee CM, et al. A simple noninvasive index for predicting long-term outcome of chronic hepatitis C after interferon-based therapy. Hepatology. 2006;44:1086–97. doi: 10.1002/hep.21363. [DOI] [PubMed] [Google Scholar]
- [18].Lieber CS, Weiss DG, Morgan TR, Paronetto F. Aspartate aminotransferase to platelet ratio index in patients with alcoholic liver fibrosis. Am J Gastroenterol. 2006;101:1500–8. doi: 10.1111/j.1572-0241.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- [19].Wai CT, Cheng CL, Wee A, et al. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int. 2006;26:666–72. doi: 10.1111/j.1478-3231.2006.01287.x. [DOI] [PubMed] [Google Scholar]
- [20].Cales P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–81. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- [21].Kao WY, Chiou YY, Hung HH, et al. Risk factors for long-term prognosis in hepatocellular carcinoma after radiofrequency ablation therapy: the clinical implication of aspartate aminotransferase-platelet ratio index. Eur J Gastroenterol Hepatol. 2011;23:528–36. doi: 10.1097/MEG.0b013e328346d529. [DOI] [PubMed] [Google Scholar]
- [22].Kawamura Y, Arase Y, Ikeda K, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–61. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- [23].de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(Suppl 1):S75–87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- [24].Kojiro M, Roskams T. Early hepatocellular carcinoma and dysplastic nodules. Semin Liver Dis. 2005;25:133–42. doi: 10.1055/s-2005-871193. [DOI] [PubMed] [Google Scholar]
- [25].Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.