Abstract
Background
In the COMFORT-I study, the Janus kinase (JAK)1/JAK2 inhibitor ruxolitinib provided significant reductions in splenomegaly, improvements in myelofibrosis (MF)-related symptoms, and a survival advantage relative to placebo in patients with intermediate-2 or high-risk MF. This post-hoc analysis assessed the effects of ruxolitinib treatment on measures of metabolic and nutritional status.
Patients and Methods
Patients were randomized to receive ruxolitinib (n = 155; 15 or 20 mg twice a day for patients with baseline platelet counts of 100–200 × 109/L or > 200 × 109/L, respectively) or placebo (n = 154). The primary endpoint was the proportion of patients with a ≥ 35% spleen volume reduction from baseline to week 24. A secondary endpoint was the proportion of patients with ≥ 50% improvement in Total Symptom Score (TSS) from baseline to week 24, measured by the modified Myelofibrosis Symptom Assessment Form v2.0. Weight, cholesterol, and albumin were measured at specified time points throughout the study.
Results
Compared with placebo, ruxolitinib treatment was associated with increased weight (mean change: +3.9 kg vs. −1.9 kg), total cholesterol (mean percentage change: +26.4% vs. −3.3%), and albumin levels (mean percentage change: +5.8% vs. −1.7%) at week 24; sustained improvements were observed with longer-term ruxolitinib therapy. Relative to placebo, increases in mean weight, total cholesterol, and albumin levels were observed with ruxolitinib treatment regardless of the degree of spleen volume and TSS reductions at 24 weeks.
Conclusion
Treatment with ruxolitinib improved measures of metabolic and nutritional status of patients with intermediate-2 or high-risk MF.
Keywords: Myelofibrosis, JAK Inhibitor, Weight, Cholesterol, Albumin
Introduction
Myelofibrosis (MF) is a chronic Philadelphia chromosome–negative myeloproliferative neoplasm that primarily affects older individuals1,2 and is characterized by progressive bone marrow fibrosis and ineffective hematopoiesis.3–5 Patients with MF typically experience cytopenias, splenomegaly—attributable to extramedullary hematopoiesis and splenic sequestration—and/or debilitating symptoms, which may severely diminish their quality of life.6–8 Although some symptoms, such as early satiety, abdominal discomfort, and splenic pain, may result from splenomegaly, many symptoms experienced by patients with MF, including fatigue, night sweats, bone pain, fever, and weight loss, appear to be consequences of systemic inflammation and hypercatabolism driven by abnormal levels of circulating cytokines.9,10
Dysregulated signaling of the Janus kinase (JAK)-signal transducers and activators of transcription (STAT) pathway is central to the pathogenesis of MF.11 Various mechanisms of this dysregulated signaling have been identified, including somatic mutations that result in neoplastic myeloproliferation and dysfunctional hematopoiesis.11–13 In addition, aberrant JAK-STAT signaling underlies secondary effects of myeloproliferation, particularly the excess proinflammatory cytokine production responsible for MF-associated symptoms as well as metabolic disturbances and chronic weight loss.9,10
Cachexia is a multifactorial syndrome characterized by the loss of skeletal muscle and fat mass with detrimental consequences on quality of life, morbidity, and mortality in patients with MF.7 The causative factors underlying cachexia in MF are complex and not well understood, but they may include reduced nutritional intake due to massive splenomegaly and metabolic disturbances caused by the systemic inflammatory state. The JAK-STAT pathway is a key regulator of proinflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α), which have been implicated in the modulation of body mass and cachexia.14,15 In addition, C-reactive protein (CRP), a marker of systemic inflammation, is elevated in patients with cancer-related cachexia.14 Moreover, abnormal levels of proinflammatory cytokines have been identified as negative prognostic factors for overall survival in patients with primary MF, along with cachexia and constitutional symptoms.1,9,16 In addition to weight loss, cachexia is often associated with hypoalbuminemia.17 A chronic inflammatory and hypercatabolic state has been shown to inhibit albumin synthesis in the liver, further contributing to cachexia-induced hypoalbuminemia.18
MF is also characterized by abnormally low cholesterol levels, which has been associated with shortened survival.7,19,20 An analysis of lipid data from 207 patients with MF treated at a single center showed that decreased levels of total or high-density lipoprotein cholesterol (HDL-C) were associated with shortened survival independent of the Dynamic International Prognostic Scoring System-Plus.20
Treatment with ruxolitinib, an oral JAK1/JAK2 inhibitor, resulted in significant reductions in spleen volume and improvements in symptoms and quality of life in patients with intermediate-2 or high-risk MF in 2 phase III studies, COMFORT (COntrolled MyeloFibrosis Study with ORal JAK Inhibitor Therapy)-I21 and COMFORT-II.22 In addition, evidence from both trials suggested that ruxolitinib was associated with a survival advantage compared with placebo21,23 and what was previously considered best available therapy.24 Ruxolitinib treatment has been shown to modify plasma markers associated with MF symptomatology.21 For example, in the COMFORT-I study, ruxolitinib-treated patients had decreased plasma levels of inflammatory markers commonly upregulated in MF such as TNF-α, IL-6, and CRP.21
We hypothesized that the clinical benefit of ruxolitinib may be related at least in part to the alleviation of cachexia and improvement of patients’ metabolic/nutritional status as ruxolitinib-treated patients in COMFORT-I generally experienced an increase in body weight whereas placebo-treated patients experienced a decrease in weight. Therefore, we conducted a post-hoc analysis of long-term data from the COMFORT-I study to further investigate the effects of ruxolitinib treatment on body weight, total cholesterol, and albumin, and the association of these changes with reduction in spleen volume and MF-related symptoms.
Patients and Methods
Patients and Study Design
Detailed methods for the COMFORT-I study have been previously reported.21 Briefly, eligible patients from the United States, Canada, or Australia were ≥ 18 years of age with primary MF, post–polycythemia vera MF, or post–essential thrombocythemia MF; disease that was classified as intermediate-2 or high risk according to the International Prognostic Scoring System; a platelet count of ≥ 100 × 109/L; and a palpable spleen (≥ 5 cm below the left costal margin). All patients had disease requiring treatment and were refractory to or intolerant of available therapies.
Eligible patients were randomized to receive placebo (n = 154) or ruxolitinib (n = 155) at 2 different starting dosages depending on baseline platelet count. Patients with a platelet count of 100 to 200 × 109/L received a 15-mg twice-a-day (BID) starting dosage of ruxolitinib, and those with a platelet count > 200 × 109/L received a 20-mg BID starting dosage of ruxolitinib; dosages were adjusted for lack of efficacy or excess toxicity. Patients randomized to placebo crossed over to ruxolitinib or discontinued within 3 months of the primary data analysis (when all patients completed 24 weeks and half of the patients remaining on study completed 36 weeks of treatment). The primary endpoint was the proportion of patients achieving ≥ 35% reduction in spleen volume (assessed by abdominal imaging) from baseline to week 24. A secondary endpoint was the proportion of patients who achieved ≥ 50% reduction in Total Symptom Score (TSS) from baseline to week 24 using the modified Myelofibrosis Symptom Assessment Form v2.0.21 The study was conducted in accordance with the International Conference on Harmonization guidelines for Good Clinical Practice. The protocol was approved by the institutional review board at each participating site, and all patients provided written informed consent.
Evaluations
All patients originally randomized to ruxolitinib or placebo were included in this post-hoc analysis. For patients in the placebo group who crossed over to ruxolitinib, data were only included for time points prior to crossover. Body weight was measured as part of the routine assessment of patients during study visits at baseline, weeks 4, 8, 12, 16, 24, 36, and every 12 weeks thereafter. The plasma lipid profile was assessed at baseline, weeks 4, 12, 24, 48, and every 24 weeks thereafter. Serum albumin levels were assessed at baseline, weeks 2, 4, 6, 8, 12, 16, 20, 24, and every 6 weeks thereafter. For patients receiving placebo, the above mentioned parameters were assessed up to week 48, as most patients receiving placebo either discontinued from the study or crossed over to ruxolitinib by this time point. All patients were instructed to fast for at least 8 hours before each study visit. Lipid and albumin concentrations were assessed at a central laboratory. Spleen volume was assessed by magnetic resonance imaging or computed tomography at baseline and every 12 weeks thereafter. The TSS was assessed daily by use of electronic diaries through week 24.
Statistical Analyses
Body weight, cholesterol, and albumin parameters at baseline were compared between treatment groups using the t test for continuous variables and the chi-square test for categorical variables. Changes from baseline to week 24 in body weight, total cholesterol, and albumin were compared between treatment groups using the t test. Additionally, ruxolitinib-treated patients were stratified according to spleen volume reduction status at week 24 (≥ 35%, 10%–< 35%, < 10% reduction from baseline) and the change from baseline to week 24 in body weight, cholesterol, and albumin were compared with all patients receiving placebo using analysis of variance. A similar analysis was conducted stratifying ruxolitinib-treated patients by TSS status at week 24 (≥ 50% or < 50% reduction in TSS from baseline at week 24). All P values reported are descriptive and not intended for statistical inferences.
For the purpose of these analyses, upper limits for total cholesterol and low-density lipoprotein cholesterol (LDL-C) were defined according to the National Cholesterol Education Program Adult Treatment Panel III guidelines for adults without cardiovascular risk (the active guidelines at the time of initiation of COMFORT-I) as 240 mg/dL and 160 mg/dL, respectively; the lower limit for HDL was defined as 40 mg/dL.25 In the absence of official guidelines defining a lower limit for total cholesterol, 150 mg/dL was used, which is consistent with data suggesting that total cholesterol < 150 mg/dL is associated with poorer prognosis in patients with MF.7
Plasma Marker Analysis
Plasma samples were collected at baseline and week 24 from placebo- and ruxolitinib-treated patients, and levels of a broad panel of plasma markers were determined using the Myriad RBM Human Multi-Analyte Profiling panel. These data, along with levels of total cholesterol, body weight, and albumin at baseline and week 24, were imported into OmicSoft Array Studio Version 6.0 for analysis. Patients lacking both baseline and week 24 measurements for total cholesterol, body weight, and albumin were excluded. Changes at week 24 relative to baseline were determined and converted to LOG2 scale, with heat maps generated using hierarchical clustering. Owing to differences in the magnitude of the changes in plasma proteins relative to weight change, heat maps colors were normalized using Robust Center Scaling (OmicSoft Array Studio 6.0), which subtracts the median value and then centers the result by dividing it by the median absolute deviation.
Results
Baseline demographics and disease characteristics were generally similar between ruxolitinib and placebo groups.21 Patients in the ruxolitinib and the placebo groups had similar baseline values for body weight and lipid levels, and the mean albumin level was higher in the ruxolitinib group (Table 1).
Table 1.
Baseline Body Weight, Body Mass Index, Total Cholesterol, and Albumin
| Characteristic | Ruxolitinib (n = 155) | Placebo (n = 154) |
|---|---|---|
| Mean (SD) body weight, kg | 72.2 (16.2) | 72.2 (13.7) |
| Mean BMI (SD), kg/m2 | 25.1 (5.0) | 24.8 (4.0) |
| BMI < 22 kg/m2, % of patients | 23 | 28 |
| Mean (SD) total cholesterol, mg/dL | 117.3 (34.5) | 114.3 (35.6) |
| Total cholesterol < 150 mg/dL, % of patients | 82 | 82 |
| Mean (SD) LDL-C, mg/dL | 55.4 (26.2) | 53.8 (26.5) |
| Mean (SD) HDL-C, mg/dL | 29.3 (10.9) | 29.1 (10.7) |
| Mean (SD) albumin, g/L | 42.8 (3.7) | 41.8 (4.1) |
There were no significant differences in baseline characteristics between treatment groups with the exception of albumin (P < .05). P values were calculated using the t test for continuous variables and the Chi-square test for categorical variables.
Abbreviations: BMI = body mass index; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; SD = standard deviation.
Treatment Effects on Body Weight, Total Cholesterol, and Albumin Levels
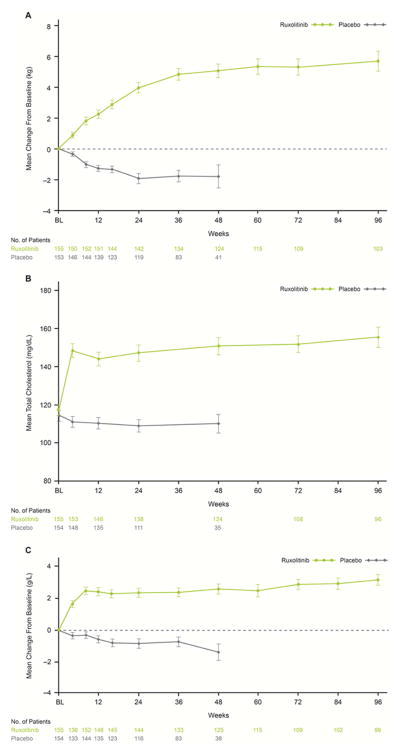
Patients receiving ruxolitinib experienced gradual increases in body weight, whereas patients receiving placebo experienced decreases in body weight over time (Fig. 1A). At week 24, mean weight increase was 3.9 kg in the ruxolitinib group, whereas a mean weight decrease of 1.9 kg was observed in the placebo group (P < .0001). Weight appeared to plateau at approximately 36 weeks in the ruxolitinib group; the mean weight increase from baseline in ruxolitinib-treated patients was 5.7 kg at week 96. Overall, 96.1% of ruxolitinib-treated patients experienced any weight increase at some time during this study. Consistent with weight increase, the mean body mass index of ruxolitinib-treated patients increased by approximately 1.4 kg/m2 compared with a decrease of 0.7 kg/m2 in placebo-treated patients at week 24 (P < .0001), and stabilized after approximately 36 weeks of treatment.
Figure 1.
Mean Change in Body Weight (A), Total Cholesterol (B), and Albumin (C) Over Time in Patients Receiving Ruxolitinib or Placebo
Vertical lines indicate the standard error of the mean.
As with body weight and body mass index, total cholesterol increased in patients receiving ruxolitinib and decreased in patients receiving placebo (Fig. 1B). At week 24, the mean percentage increase from baseline in total cholesterol among ruxolitinib-treated patients was 26.4% (observed mean increase: 29.5 mg/dL) versus a mean decrease of 3.3% (observed mean decrease: 4.98 mg/dL) in the placebo group (P < .0001). At 96 weeks, the mean percentage increase in total cholesterol among ruxolitinib-treated patients was 35.8% (observed mean increase: 38.0 mg/dL). A total of 96.8% of patients in the ruxolitinib group experienced any degree of increase in total cholesterol. For the majority of ruxolitinib-treated patients, the levels of total cholesterol and LDL-C did not exceed the upper limits defined for this analysis (240 mg/dL and 160 mg/dL, respectively) (Supplemental Fig. 1A and 1B). Median total cholesterol levels of approximately 150 mg/dL and median HDL-C levels of approximately 40 mg/dL were achieved by week 4 and maintained with long-term therapy (Supplemental Fig. 1A and 1C).
Albumin levels increased in response to ruxolitinib treatment (Fig. 1C). After 24 weeks of treatment, ruxolitinib-treated patients experienced a mean increase in albumin levels of 5.8% (observed mean increase: 2.3 g/L) compared with a mean decrease of 1.7% (observed mean decrease: 0.8 g/L) in the placebo arm (P < .0001). In the ruxolitinib group, albumin increases stabilized at approximately 10 weeks and were maintained throughout the study. At 96 weeks, the mean percentage increase in albumin among ruxolitinib-treated patients was 7.6% (observed mean increase: 3.1 g/L). Overall, 94.8% of ruxolitinib-treated patients experienced any increase in albumin levels during the course of the study.
Association of Increases in Body Weight, Total Cholesterol, and Albumin With Spleen Volume Reduction
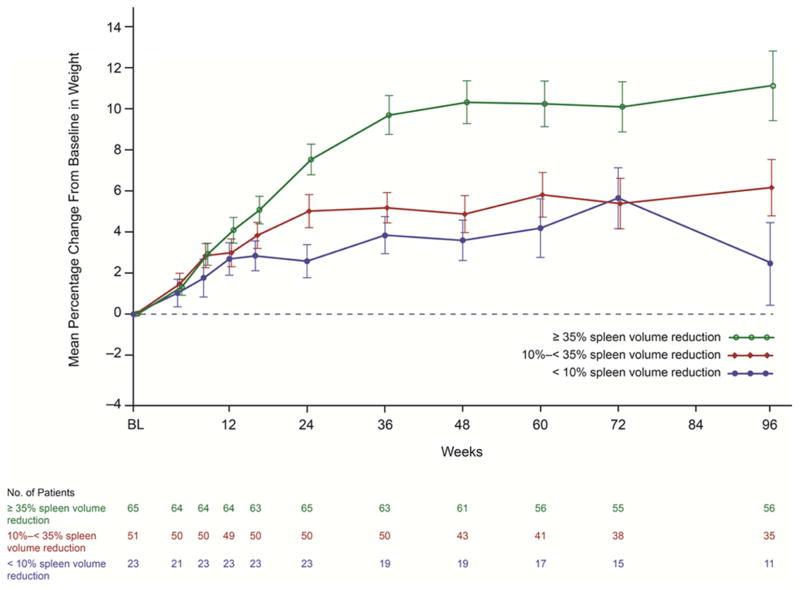
Analysis of weight change stratified by the degree of spleen volume reduction from baseline at week 24 showed that relative to placebo, ruxolitinib-treated patients experienced weight increases regardless of the degree of spleen volume reduction. Ruxolitinib-treated patients with a spleen volume reduction of ≥ 35% experienced a greater mean percentage increase in body weight (7.5%) compared with patients with a spleen volume reduction of 10% to < 35% (5.0% increase) and < 10% (2.5% increase). In patients receiving placebo, a 2.3% decrease in body weight was observed (P < .001 for the 3 spleen volume reduction group comparisons to placebo). This pattern was maintained beyond week 24, and a higher reduction in spleen volume in ruxolitinib-treated patients continued to be associated with greater weight increase up to week 96 (Fig. 2).
Figure 2.
Mean Percentage Change From Baseline in Body Weight in Ruxolitinib-Treated Patients by Week 24 Spleen Volume Reduction Group
Vertical lines indicate the standard error of the mean.
Similarly, analysis of changes in total cholesterol levels stratified by the degree of spleen volume reduction from baseline at week 24 showed that compared with placebo, ruxolitinib treatment was associated with increased total cholesterol levels regardless of the degree of spleen volume reduction. The greatest reduction in spleen volume (≥ 35%) at week 24 was associated with the highest mean percentage increase in total cholesterol (41.6%) compared with the increases in total cholesterol in patients with spleen volume reductions of 10% to < 35% (17.0%) and < 10% (6.8%). In the placebo group, a mean decrease in total cholesterol of 2.8% was observed (P ≤ .047 for the 3 spleen volume reduction group comparisons to placebo).
Relative to placebo, improvements in serum albumin levels were also observed in all ruxolitinib-treated patients regardless of the degree of spleen volume reduction from baseline at week 24. Ruxolitinib-treated patients with the largest degree of spleen volume reduction (≥ 35%) at week 24 experienced the largest increase in albumin levels from baseline (8.6%) compared with patients with a spleen volume reduction of 10% to < 35% (5.6%) and < 10% (1.0%). Patients randomized to placebo experienced a mean decrease in albumin levels from baseline of 1.2% (P < .001 for the ≥ 35% and 10% to < 35% spleen volume reduction group comparisons to placebo; P = .234 for the < 10% spleen volume reduction group comparison to placebo).
Association of Increases in Body Weight, Total Cholesterol, and Albumin With Total Symptom Score
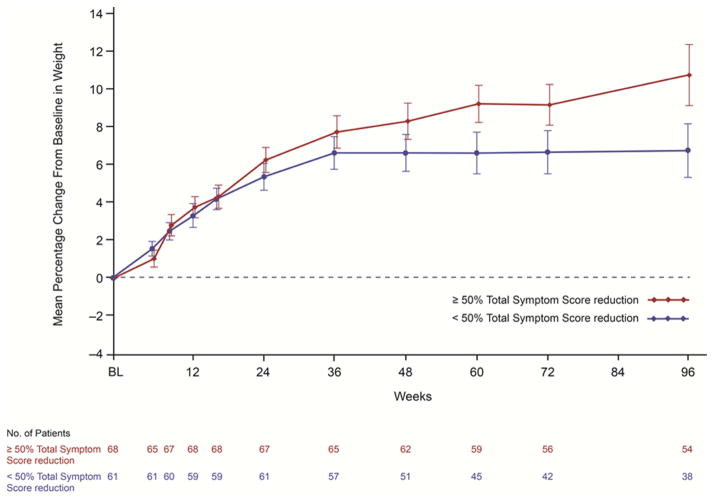
In the ruxolitinib arm, weight increase occurred regardless of reduction in TSS at week 24. Patients with ≥ 50% reduction in TSS had a mean weight increase of 6.3% and those with TSS reduction < 50% had a mean weight increase of 5.3% at week 24. In the placebo arm, patients experienced a 2.5% decrease in body weight (P < .001 for both TSS reduction group comparisons to placebo). With longer follow-up, weight continued to improve in ruxolitinib-treated patients who had a ≥ 50% reduction in TSS, with mean weight increase approximately 10% above baseline at week 96 (Fig. 3).
Figure 3.
Mean Percentage Change From Baseline in Body Weight in Ruxolitinib-Treated Patients by Week 24 Total Symptom Score Reduction Group
Vertical lines indicate the standard error of the mean.
The mean percentage increase from baseline to week 24 in total cholesterol was similar between the 2 TSS reduction groups (mean percentage increases of 27.5% with TSS reduction ≥ 50% and 26.4% with TSS reduction < 50%). In the placebo group, a mean decrease in total cholesterol levels of 3.5% was observed (P < .001 for both TSS reduction group comparisons to placebo).
In ruxolitinib-treated patients, changes in albumin levels were slightly higher in patients who had a ≥ 50% reduction in TSS (7.0%) at week 24 compared with patients with TSS reduction < 50% (5.2%). Patients receiving placebo experienced a mean reduction in albumin levels of 1.6% (P < .001 for both TSS reduction group comparisons to placebo).
Plasma Marker Expression
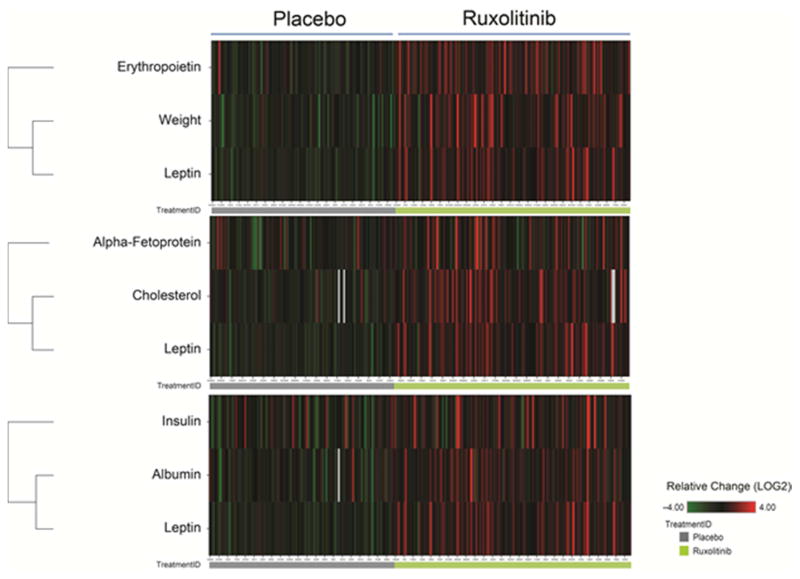
Hierarchical clustering analysis was used to compare changes in body weight, total cholesterol, or albumin for each patient with changes in a broad panel of plasma markers. Weight changes were most closely associated with changes in leptin and erythropoietin, total cholesterol changes were closely associated with changes in leptin and alpha-fetoprotein, and albumin changes were most closely associated with changes in insulin and leptin (Fig. 4).
Figure 4.
Hierarchical Clustering of Plasma Markers With Changes in Weight Alone (top), Changes in Total Cholesterol Alone (middle), or Changes in Albumin Alone (bottom)
Red denotes increases at week 24 relative to baseline. Green denotes decreases at week 24 relative to baseline. Owing to differences in the magnitude of changes between markers, the heat map color intensities were normalized.
Discussion
The results of this post-hoc analysis of the COMFORT-I study showed that compared with placebo, ruxolitinib was associated with clinically meaningful improvements in body weight, total cholesterol, and serum albumin in patients with intermediate-2 or high-risk MF. The weight increase associated with ruxolitinib therapy had been previously reported in the phase I/II study of ruxolitinib in MF and in the primary analyses of the phase III COMFORT studies for a period up to 48 weeks;12,21,22 however, this analysis showed that the weight gain observed with ruxolitinib therapy in the COMFORT-I study at the primary analysis was maintained with long-term therapy. Gain in lean body mass in patients treated with ruxolitinib in the COMFORT-I study may be even more substantive than that indicated by weight gain considering the decrease in spleen mass and potential decrease in fluid retention from edema. Of note, the weight increase in ruxolitinib-treated patients was accompanied by other metabolic improvements, indicative of the overall improvement in the hypercatabolic state associated with MF.
Total cholesterol, which often decreases with MF, was low (<150 mg/dL) in approximately 80% of patients in the COMFORT-I study at baseline. Although hypocholesterolemia has been well documented in MF, typical total cholesterol levels in patients with MF have not been extensively reported in the literature. Median total cholesterol values ranging from 132 mg/dL to 153 mg/dL in patients with primary MF have been reported previously,20,7 and these values are comparable with the baseline total cholesterol values observed in the COMFORT-I cohorts. In this analysis, ruxolitinib treatment was associated with increases in the levels of total cholesterol, LDL-C, and HDL-C. These increased levels were maintained with long-term therapy, and importantly, the total cholesterol and LDL-C values in ruxolitinib-treated patients in general did not exceed 240 mg/dL and 160 mg/dL, respectively, indicating that ruxolitinib therapy did not increase the risk of hypercholesterolemia.
Given that hypocholesterolemia (total cholesterol < 150 mg/dL) and weight loss have previously been associated with poorer prognosis in patients with MF,7,19,20 the improvements in total cholesterol and weight seen with ruxolitinib in the COMFORT-I study may represent disease-modifying effects that contribute to the prolonged survival advantage associated with ruxolitinib therapy relative to placebo.23
Albumin is a one of many parameters used to assess a patient’s nutritional status. Low albumin levels have been shown to be a predictor of morbidity and mortality in various disease states.26 Albumin synthesis has been shown to be decreased by proinflammatory cytokines such as IL-6 and TNF-α.27 Proinflammatory cytokines are thought to drive hypercatabolism in MF,28 and increased expression levels of proinflammatory cytokines, particularly IL-6 and TNF-α and of markers of systemic inflammation such as CRP,29 have been observed in patients with cancer-related cachexia.15,30 In clinical trials, ruxolitinib treatment was associated with reductions in the plasma levels of proinflammatory cytokines, including IL-6 and TNF-α.12,21,22 In contrast, patients in the placebo group of the COMFORT-I study maintained abnormally high levels of these cytokines at week 24.21 Thus, reduction in levels of proinflammatory cytokines with ruxolitinib treatment may be a mechanism by which albumin levels increase in patients with MF.
Conclusion
Although the assessments of differences between treatment groups in metabolic and nutritional parameters were only exploratory endpoints in this study, the considerable improvements in body weight, cholesterol, and albumin seen in these post-hoc analyses of COMFORT-I may help in the understanding of why ruxolitinib was associated with an improvement in overall survival relative to placebo in COMFORT-I or best available therapy in COMFORT-II.23,24 Collectively, these results provide additional evidence for the disease-modifying effects of ruxolitinib in patients with MF.
Supplementary Material
Clinical Practice Points.
Cachexia is a common manifestation of myelofibrosis and is prognostic of poor survival.
In 2 recent phase III studies, ruxolitinib treatment provided significant spleen volume reductions, improvement in myelofibrosis-related symptoms, and was associated with increased overall survival compared with controls.
This post-hoc analysis of the COMFORT-I study was conducted to assess the effects of ruxolitinib treatment on measures of metabolic and nutritional status.
Compared with placebo, ruxolitinib treatment was associated with increases in body weight, total cholesterol, and albumin levels that were maintained with longer-term treatment.
Ruxolitinib provided improvements in measures of metabolic and nutritional status relative to placebo regardless of the degree of reduction of splenomegaly or symptom burden.
This analysis showed that ruxolitinib treatment may ameliorate the metabolic and nutritional abnormalities that are commonly seen in patients with myelofibrosis.
Acknowledgments
The COMFORT-I study (NCT00952289) was sponsored by Incyte Corporation. This research is supported in part by the MD Anderson Cancer Center Support Grant CA016672. Medical writing support was provided by Alfredo Toschi, PhD, and Susan Sutch, PharmD, CMPP, of Evidence Scientific Solutions, and was funded by Incyte Corporation.
Footnotes
This research was presented, in part, at the 54th American Society of Hematology Annual Meeting and Exposition; December 8–11, 2012; Atlanta, GA, USA.
Conflicts of Interest
Dr Mesa has received research funding from Genentech, Gilead, Incyte, Lilly, NS Pharma, Sanofi-Aventis, and YM Biosciences. Dr Verstovsek has received research funding from AstraZeneca, Bristol Myers Squibb, Celgene, Cell Therapeutics, Inc., Geron, Gilead, Incyte, Infinity Pharmaceuticals, Lilly Oncology, NS Pharma, Promedior, Roche, Seattle Genetics, and Galena. Dr Gupta has served as a consultant/advisory board member for Incyte and Novartis and has received research funding from Incyte, and Novartis through his institution. Dr Mascarenhas has served as a consultant for Incyte and has received clinical trial support from Incyte, Novartis, and Roche. Dr Atallah has served as a consultant for and has received research funding from Incyte Corporation. Drs Burn, Sun, and Sandor are employees of Incyte Corporation and have equity ownership. Dr Gotlib has served on an advisory committee and has received research funding and travel support from Incyte Corporation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Lasho TL, Jimma T, et al. One thousand patients with primary myelofibrosis: the Mayo Clinic experience. Mayo Clin Proc. 2012;87:25–33. doi: 10.1016/j.mayocp.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342:1255–1265. doi: 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- 4.Gregory SA, Mesa RA, Hoffman R, Shammo JM. Clinical and laboratory features of myelofibrosis and limitations of current therapies. Clin Adv Hematol Oncol. 2011;9:1–16. [PubMed] [Google Scholar]
- 5.Mesa RA, Green A, Barosi G, et al. MPN-associated myelofibrosis (MPN-MF) Leuk Res. 2011;35:12–13. doi: 10.1016/j.leukres.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109:68–76. doi: 10.1002/cncr.22365. [DOI] [PubMed] [Google Scholar]
- 7.Mesa RA, Schwager S, Huang J, et al. Weight loss, splenomegaly, and hypocholesterolemia in myeloproliferative neoplasms: patterns and relevance from the pre JAK2 inhibitor era [abstract] Blood. 2009;114:3918. [Google Scholar]
- 8.Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2013;31:1285–1292. doi: 10.1200/JCO.2012.44.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gangat N, Caramazza D, Vaidya R, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. J Clin Oncol. 2011;29:392–397. doi: 10.1200/JCO.2010.32.2446. [DOI] [PubMed] [Google Scholar]
- 10.Mesa RA. The evolving treatment paradigm in myelofibrosis. Leuk Lymphoma. 2013;54:242–251. doi: 10.3109/10428194.2012.710905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintás-Cardama A, Verstovsek S. Molecular pathways: JAK/STAT pathway: mutations, inhibitors, and resistance. Clin Cancer Res. 2013;19:1933–1940. doi: 10.1158/1078-0432.CCR-12-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross NC. Genetic and epigenetic complexity in myeloproliferative neoplasms. Hematology Am Soc Hematol Educ Program. 2011;2011:208–214. doi: 10.1182/asheducation-2011.1.208. [DOI] [PubMed] [Google Scholar]
- 14.Argiles JM, Lopez-Soriano FJ, Busquets S. Counteracting inflammation: a promising therapy in cachexia. Crit Rev Oncog. 2012;17:253–262. doi: 10.1615/critrevoncog.v17.i3.30. [DOI] [PubMed] [Google Scholar]
- 15.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Tefferi A, Vaidya R, Caramazza D, et al. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29:1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Kim HJ, Yun J, et al. Pathophysiological role of hormones and cytokines in cancer cachexia. J Korean Med Sci. 2012;27:128–134. doi: 10.3346/jkms.2012.27.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol. 2005;39:S143–146. doi: 10.1097/01.mcg.0000155514.17715.39. [DOI] [PubMed] [Google Scholar]
- 19.Mesa RA, Huang J, Schwager S, et al. Hypocholesterolemia is independently associated with decreased survival in patients with primary myelofibrosis: an analysis of lipid profiles in 558 myeloproliferative patients [abstract] Blood. 2007;110:2548. [Google Scholar]
- 20.Sulai N, Mengistu B, Gangat N, et al. Decreased levels of total or HDL cholesterol in primary myelofibrosis are associated with shortened survival: DIPSS-Plus independent prognostic value [abstract] Blood. 2012;120:2851. [Google Scholar]
- 21.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787–798. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 23.Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib treatment in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98:1865–1871. doi: 10.3324/haematol.2013.092155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122:4047–4053. doi: 10.1182/blood-2013-02-485888. [DOI] [PubMed] [Google Scholar]
- 25.Grundy SM, Cleeman JI, Merz CN, et al. for the Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 26.Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin Nutr. 2001;20:265–269. doi: 10.1054/clnu.2001.0438. [DOI] [PubMed] [Google Scholar]
- 27.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85:599–610. doi: 10.1093/bja/85.4.599. [DOI] [PubMed] [Google Scholar]
- 28.Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33:1199–1203. doi: 10.1016/j.leukres.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 30.Fearon KC, Voss AC, Hustead DS for the Cancer Cachexia Study Group. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. Am J Clin Nutr. 2006;83:1345–1350. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.