Abstract
Over the past decade or so, CART (cocaine- and amphetamine-regulated transcript) peptides have emerged as major neurotransmitters and hormones. CART peptides are widely distributed in the CNS and are involved in regulating many processes, including food intake and the maintenance of body weight, reward and endocrine functions. Recent studies have produced a wealth of information about the location, regulation, processing and functions of CART peptides, but additional studies aimed at elucidating the physiological effects of the peptides and at characterizing the CART receptor(s) are needed to take advantage of possible therapeutic applications.
In 1995, Douglass et al.1 found that a particular mRNA was upregulated by acute administration of cocaine or amphetamine. They named this transcript ‘cocaine- and amphetamine-regulated transcript’ (CART). The transcript is usually referred to as CART mRNA, and the encoded peptides are referred to as CART peptides. Interestingly, Spiess et al.2 had sequenced a peptide in 1981 that was purified from the ovine hypothalamus, and the sequence of this peptide was found to match a portion of the amino-acid sequence that was specified by the CART mRNA discovered by Douglass et al., indicating that the CART transcript is translated (something that was later confirmed). Moreover, the sequence identified by Spiess et al. immediately followed a pair of basic amino acids that often indicate the site of cleavage of a propeptide amino-acid chain, and it was therefore suggested (and later confirmed) that CART mRNA is translated into CART propeptides that, like other known propeptides, are processed into smaller, active forms. In 1999, Thim et al.3 extracted CART peptides from rat tissues, sequenced them, and showed that two different CART peptides, namely CART 55–102 and CART 62–102, were present. Many studies subsequently showed that both of these peptides are active.
There have been nearly 200 publications on CART peptides in just the past few years, reflecting the findings that these peptides are involved in many physiological functions. Therefore, the CART system is becoming an opportune target for therapeutic drug development. In this Review, we discuss the recent evidence of a role for CART peptides in the regulation of food intake, reward and addiction, the stress response, and anxiety and depression; in addition, we touch on the roles of CART peptides in several other physiological processes.
Gene regulation and processing
The human CART gene spans approximately 2.5 kb and has been mapped to chromosome 5q13–q14 (REF. 4). It comprises an approximately 340-nucleotide proximal promoter region, two introns and three exons. Transcription of the gene results in two alternatively spliced mRNAs that are of different length and that produce propeptides of different lengths, called proCART 1–89 and proCART 1–102 (REF. 1). Interestingly, both of these propeptides are found in rats, whereas only proCART 1–89 is found in humans. The mRNA splicing has no effect on activity, because the active parts of the CART peptides are encoded by regions that lie downstream of the spliced region, and they are intact in both propeptides. However, a change in the numbering of the amino acids does result (see below). The amino-acid sequence contains a leader sequence that facilitates both the entry of the proCART peptides into vesicles and their subsequent processing1.
The amino-acid sequences of the long and short forms of proCART are shown in FIG. 1. The proCART peptides contain several cleavage sites that allow post-translational processing by prohormone convertases. This processing results in at least two biologically active CART peptides. The names of the peptides, CART 55–102 and CART 62–102, derive from the long form of proCART5-9 (FIG. 1). In humans (who produce only the short form of proCART) the equivalent peptides are called CART 42–89 and CART 49–89. In any species that expresses both the long and the short forms of proCART, the amino acid sequences of CART 42–89 and CART 49–89 are identical to those of CART 55–102 and CART 62–102, respectively (see also below). There is some evidence that other fragments of proCART are active as well10, but most animal studies involve CART 55–102 and CART 62–102, and so this article focuses on those peptides.
Figure 1. Amino-acid sequences of rat proCART and CART peptides.
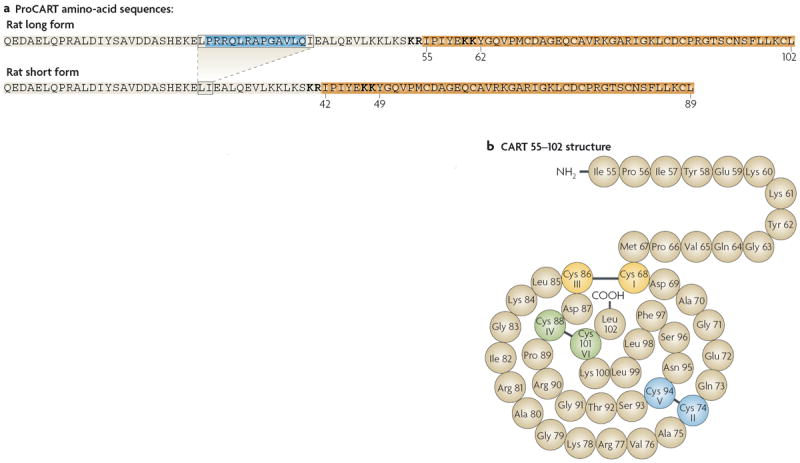
a ∣ In the rat, CART (cocaine- and amphetamine-regulated transcript) mRNA has two splice variants (not shown): one that encodes a long form of proCART and one that encodes a short form. The mRNA that encodes the long form is translated into a 102-amino-acid sequence (top). In the other variant, the section that encodes amino acids 27–39 of the long form (shown in blue) is spliced out, and the resulting short-form CART mRNA is therefore translated into an 89-amino-acid sequence (bottom). The fragments of the long form of proCART that have been reliably shown to be active are amino acids 55–102 and 62–102. In the short form of proCART the numbers of the active amino acids are 42–89 and 49–89, but these amino acids are identical to those of the long form in a given species; this has led to some confusion in the literature because different numbers refer to the same amino-acid sequences. Only the 89-amino-acid form (the short form) of proCART has been found in humans. The amino-acid sequence of this human peptide is slightly different from the amino-acid sequence of the rat peptide. Amino-acid 42, which lies in the active fragment, is isoleucine in the rat peptide but is valine in the human peptide. Pairs of basic amino acids shown in bold are the sites of processing by prohormone convertases. b ∣ The structure of CART 55–102, with the disulphide bridges that are required for activity. The other major active peptide is CART 62–102, which has the same general structure. Part a modified, with permission, from REF. 170 © (2008) Elsevier Science. Part b reproduced, with permission, from REF. 5 © Elsevier Science B. V.
Sequence-alignment studies have shown that CART peptides are evolutionarily conserved. For example, the human and rat gene sequences share 91% nucleotide identity in their coding regions, and the coding-region sequences in the rat and mouse genes are 98% identical. Importantly, the high similarity between the rat and the human nucleotide sequences leads to 95% amino-acid identity between the active neuropeptides4.
The promoter region of the gene contains a number of predicted transcription factor binding sites that are conserved across rat, mouse and human4,11,12. These predicted protein–DNA interaction sequences regulate basal and stimulus-induced CART mRNA expression — possibly through interactions with nuclear transcription factors4,11-13,171. FIGURE 1 shows the structure of rat CART 55–102, one of the active CART peptides, including the three conserved disulphide bridges. The HuGo gene nomenclature committee has approved “CARTPT” for the gene symbol and “CART prepropeptide” as the gene name.
CART peptides as neurotransmitters
CART peptides satisfy the general requirements for being peptide neurotransmitters. Although the descriptions of these requirements vary, they generally include demonstrations that the peptides are present in tissues, are biologically active and are released by Ca2+-dependent mechanisms. CART mRNAs and peptides are present in the brain (see, for example, REFS 1,2,14,15), and they produce many effects when they are injected into the brain or are applied to cells in culture; injections with antibodies against CART peptides can oppose these effects, as discussed below. CART peptides in the brain are found only in neurons — for example, in the nucleus accumbens16,17 — and coexist with other neurotransmitters such as GAbA (γ-aminobutyric acid)17 and substance P18,19. They are found in dense core vesicles16,17, and proCART has a leader sequence1 that facilitates its entry into vesicles and its subsequent processing1. Moreover, K+-induced release of CART peptides from hypothalamic explants is Ca2+-dependent20.
It has been known for many years that various peptides that are found in the brain are also found in the gut — they are referred to as ‘brain–gut’ peptides. Indeed, CART peptides are also found in the gut21, and there is evidence that they are biologically active there22. evidence suggests that CART peptides also have a hormonal role: they are found in peripheral blood and in pituitary portal blood23 as well as in tissues that have a hormonal role, such as the anterior and posterior pituitary and the adrenal medulla24. CART peptide levels in the blood and in some brain regions have a diurnal rhythm25.
CART receptors
Shortly after Douglass et al.’s landmark paper1 describing CART mRNAs and peptides, many laboratories attempted to find a receptor for the peptides using receptor-binding techniques. Receptor-binding studies usually require demonstrations of high-affinity ligand–receptor interactions, saturability and specificity. However, these early attempts met with failure: no specific receptor binding could be detected in brain homogenates or slices. The reasons that were proposed to explain this lack of specific binding included a supposition of a low-affinity binding site that could not be detected using this approach, and high levels of non-specific binding. Although there was no progress in the search for a CART receptor for several years, the fact that CART peptides were active in various experiments was good evidence that CART receptors exist. For example, it was found that central administration of CART peptides increased c-Fos mRNA levels in various areas of the brain26. In addition, applying CART 55–102 to hippocampal primary cells in culture resulted in an inhibition of voltage-dependent intracellular Ca2+ signalling27. This effect was blocked by treating the cells with pertussis toxin, suggesting that the observed signalling occurred through activation of the inhibitory G proteins Gi/o. Furthermore, central administration of CART peptides resulted in phosphorylation of cyclic AMP-response-element-binding protein (CREB) in some hypothalamic neurons28. Application of the peptide to AtT20 cells, a mouse pituitary tumour cell line, activated extracellular signal-regulated kinase (ERK) signalling by increasing the levels of phosphorylated ERK, and this was reduced by pertussis toxin29, again indicating that CART peptides activate Gi/o proteins. CART signalling in cultures of bovine granulosa cells also seemed to involve a Gi/o mechanism30. The combined evidence of several laboratories thus suggests that CART peptides can activate G-protein signalling pathways (FIG. 2), which strongly supports the existence of a G-protein-coupled receptor (GPCR) for CART peptides. Although some skepticism and caution is warranted, it is difficult to explain how the peptides could exert the effects they do without the existence of relevant receptors.
Figure 2. Proposed CART receptor signalling.
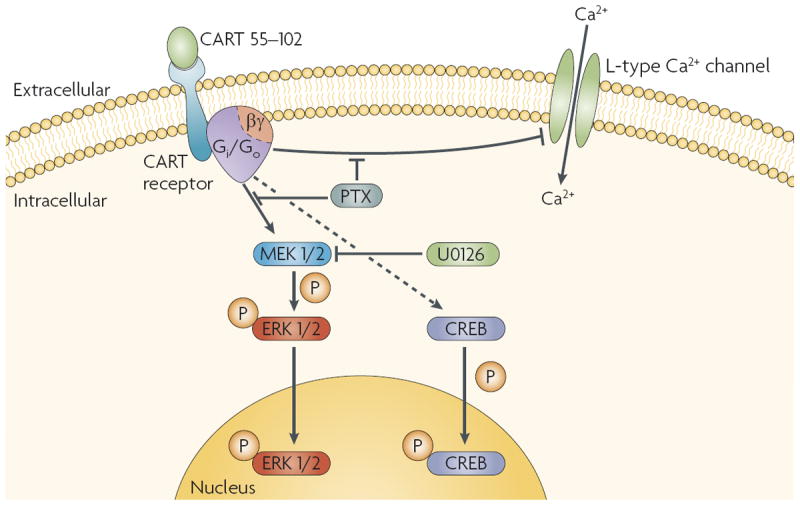
Several studies on CART (cocaine- and amphetamine-regulated transcript)-peptide-induced cell signalling have demonstrated that CART peptides activate at least three signalling mechanisms. First, CART 55–102 inhibited voltage-gated L-type Ca2+ channels through a pertussis toxin (PTX; an inhibitor of inhibitory-G-protein (Gi/Go)-dependent signalling)-sensitive mechanism in hippocampal neurons27. Second, CART 55–102 increased the phosphorylation of cyclic AMP-response-element-binding protein (CREB) in the nuclei of corticotropin-releasing hormone (CRH) neurons in the hypothalamic paraventricular nucleus in fasted and fed rats28. Third, CART 55–102 increased extracellular signal-regulated kinase (ERK) phosphorylation in AtT20 and GH3 cells, an effect that was blocked by U0126, an inhibitor of MEK kinases, and by PTX29. The dashed arrow indicates that it is not yet known whether this effect of CART 55–102 is mediated by inhibitory G proteins.
Because CART-peptide signalling was established in AtT20 cells, binding studies were subsequently conducted in these cells31. They identified a high-affinity, saturable binding site that was specific for the active peptides (CART 55–102 and CART 62–102). Another binding study, which used a complex of CART 55–102 and green fluorescent protein (GFP), also revealed binding in HepG2 cells and in dissociated hypothalamic cells32. However, the binding was not obviously saturable at high concentrations, suggesting that the ligand might have been binding to a low-affinity binding site or receptor32. A subsequent study33 found that CART-peptide binding in both differentiated and non-differentiated PC12 cells had characteristics of receptor binding. Specific CART-peptide binding was identified in primary cultures of rat nucleus accumbens34. The binding had receptor-like characteristics, and the ligand-binding affinity was reduced in the presence of GTP analogues but not ATP analogues, indicating that the binding was to a GPCR. overall, although the binding approach has been generally successful, some results have shown the binding to be low and variable, and more consistent assays are needed. It is of note that either of the two biologically active CART peptides can be active while the other is without effect in the same preparation — the possibility of multiple receptors cannot be ignored10.
To summarize, the CART receptor seems to be a GPCR that is coupled to Gi/o. The evidence for the existence of a CART receptor supports the hypothesis that CART peptides are neurotransmitter and/or hormonal substances. Moreover, this sets the stage for drug screening, which hopefully will result in the identification of small-molecule agonists and antagonists. As discussed below, CART peptides are involved in many processes, and there are many possible uses for CART-system-related drugs as therapeutic agents35.
CART in the regulation of body weight
Early findings
Soon after the discovery of CART peptides1-3, it was suggested that they were involved in food intake. This suggestion was based on the distribution of CART peptides in the brain24, namely in regions that included the arcuate nucleus, the lateral hypothalamus, the paraventricular nucleus (PvN) and the nucleus accumbens14,24, all of which have a role in the regulation of food intake. In addition, CART mRNA and peptides have been demonstrated to be present in peripheral feeding-relevant regions36-40.
Lambert et al.41,42 showed that, in rats, intracerebro-ventricular (ICv) administration of CART peptide fragments decreased food intake, and ICv administration of antibodies directed against CART peptide increased feeding. Moreover, CART-peptide-containing cell bodies in the hypothalamic arcuate nucleus were found to be surrounded by neuropeptide Y (NPY)-immunoreactive nerve terminals, suggesting that there is an interaction between CART peptides and NPY, a peptide that stimulates feeding. Importantly, it was demonstrated by Kristensen and co-workers43 that, in addition to the anorectic effects of CART peptides, CART mRNA in the arcuate nucleus was decreased in food-deprived animals, and intraperitoneal administration of leptin increased CART mRNA levels in the arcuate nucleus. Moreover, in animals with disrupted leptin signalling, CART mRNA was nearly absent in the arcuate nucleus43. These and other core observations44 stimulated many additional studies on the involvement of the CART system in the regulation of feeding and body weight.
Effects of CART peptides on feeding and energy regulation in animal studies
CART peptides and their effects have been found in feeding-relevant parts of the brain, as noted above. CART peptides were also demonstrated to be present in the gut20-22,36-40 and in vagal nerves45,46, which have a role in feeding. Moreover, ICv and intra-cisternal injection of CART 55–102 inhibited gastric-acid secretion and gastric emptying47-50.
Injection of CART peptide into the nucleus accumbens resulted in inhibition of feeding51, indicating that this region, which has high levels of endogenous CART peptides, might be involved in the CART system’s anorexigenic effects. Activating serotonin-4 receptors in the nucleus accumbens increased CART mRNA levels and reduced the drive to eat, whereas reducing CART peptide levels in food-deprived mice by administering short interfering RNAs against CART mRNA abolished the anorectic effects of a serotonin-4 receptor agonist52. Knock down of CART mRNA also reduced the anorectic effect of MDMA (3,4-methylenedioxy-N-methyl-amphetamine), suggesting that CART peptides in the nucleus accumbens mediate the appetite-suppressing effect of MDMA52. It was also shown that injection of CART 55–102 into the lateral ventricles resulted in increased c-Fos levels in feeding areas of the brain26, suggesting a physiological action of CART peptides in these areas. Moreover, chronic (10-day) ICv administration of CART peptide inhibited food intake53. Mice that lacked the CART gene (CARTPT−/− mice) gained body weight54-56, and viral delivery of CART mRNA through the ICv cannulae suppressed weight gain in diet-induced obese rats57. However, not all studies of CARTPT−/− mice are in agreement58. This lack of agreement could be due to varying genetic backgrounds in the various groups of CARTPT−/− mice, or to other differences. Aside from the contradictions in studies with CARTPT−/− mice, there is abundant evidence in animal studies that CART peptides can directly regulate body weight and feeding.
There is also evidence that CART peptides interact with other factors that affect body weight, feeding and energy expenditure (see also Box 1). For example, as mentioned above, CART might mediate the effects of serotonin-4 receptor activation on food intake52. Second, CART is colocalized with melanocyte stimulating hormone (MSH)59-63, an inhibitor of food intake, in the rat arcuate nucleus. Third, CART mRNA levels in the rat arcuate nucleus were increased by administration of leptin64, and leptin receptors were found on CART-peptide-containing cells in the arcuate nucleus and other hypothalamic regions65, indicating that CART peptides might be mediators of leptin effects in these hypothalamic areas. Recent data suggest that glucocorticoids might modulate the interaction between CART and leptin66. Fourth, there is evidence for an interaction between CART and the endocannabinoid system. The endocannabinoid anandamide increases appetite by activating Cb1 receptors, whereas Cb1 receptor antagonists inhibit food intake. Treating wild-type mice with a Cb1 receptor agonist increased CART peptide levels in the nucleus accumbens, and the Cb1 antagonist rimonabant had no effect on feeding in CARTPT−/− mice67, suggesting that CART is involved in the orexigenic effects of anandamide67. Fifth, CART peptides are expressed in arcuate neurons that project to the PvN and regulate the release of thyrotropin-releasing hormone (TRH)68-70. TRH-containing neurons in the PvN in turn regulate the release of thyroid-stimulating hormone from the pituitary, which exerts an effect on energy homeostasis by increasing heat generation in brown adipose tissue and muscle. Thus, by regulating TRH release, CART peptides can influence the pituitary–thyroid axis, with a resultant effect on energy expenditure. Finally, injection of CART 55–102 into the rat PvN or arcuate nucleus increases the expression of the mRNA for uncoupling protein, again relating CART peptides to energy expenditure71,72. These examples show that, even though there are a number of seemingly disparate mechanisms involved in feeding, CART peptides are implicated in many of them.
Box 1. CART peptides regulate pancreatic islet cell function.
There is evidence that CART (cocaine- and amphetamine-regulated transcript) peptides regulate islet cell function in the pancreas, and that CART peptides play a part in type 2 diabetes. It was noted that CART peptides were expressed in pancreatic islet tumours and in the normal islets of Langerhans in the somatostatin-producing islet cells in the rat167. This paper suggested that a role for CART peptides in islet function needed to be identified. It was then found that, in rats, CART peptides were expressed in several islet cell types during development but mainly in somatostatin cells in the adult168, confirming and extending the previous report167. It has also been reported37,56,168 that CART peptides are expressed in both sensory and autonomic nerve fibres and ganglion cells in the pancreas, increasing the possible roles for CART peptides. The authors concluded that the CART system could play a part in islet development and maintenance. The same group56 showed that mice that lacked the CART gene (CARTPT) had impaired insulin secretion and glucose tolerance, along with altered β-cell morphology that was independent of the increased body weight. Glucose transporters declined with age in the CARTPT-knockout mice, indicating dysfunction of their β-cells; in addition, glucose-stimulated insulin secretion was impaired both in vivo and in vitro56. CART 55–102 has complex effects on islet hormone secretion that depend on whether cyclic AMP-elevating agents are present169. For example, in the presence of the incretin hormone glucagon-like peptide 1 (GLP1) or forskolin, insulin secretion from INS-1 (insulinoma) cells or isolated rat islets was markedly augmented by CART peptide. Furthermore, in two rat models of type 2 diabetes, CART peptides were expressed at much higher levels than normal in the islet β-cells. Taken together, these data suggest a role for the CART system in type 2 diabetes and in the regulation of islet cell function.
Effects of CART peptides on body weight and energy metabolism in humans
Animal studies are strongly indicative of a role for CART peptides in feeding behaviour, and the results from studies in humans strongly support this idea. For example, obese members of an Italian family had a missense mutation (leu34Phe) in their CART gene73. This missense mutation led to a CART peptide deficiency in the sera74. expression of the mutated cDNA in AtT20 cells resulted in decreased CART peptide levels compared with levels in cells that expressed the wild-type cDNA75. Moreover, expression of the mutated proCART in AtT20 cells showed that it was mis-sorted, poorly processed and secreted74 (FIG. 3). Thus, the missense mutation had cellular effects that reduced CART peptide levels and affected the cellular distribution of CART peptide. A different study of over 500 subjects76 showed that the 5′ region of the CART gene was highly polymorphic, and that a polymorphism at −156 kb (that is, at a site that is in linkage disequilibrium with the CART gene) might associate with obesity. Another study in several hundred French subjects, some of whom were morbidly obese, identified a single-nucleotide polymorphism in the CART gene (-3608T>C) that might contribute to the genetic risk for obesity77. It was recently suggested that the CART peptides might be involved in lipid metabolism and artherogenesis78, and that CART peptides might influence fat distribution79 and contribute to dyslipidaemia80. viewed collectively, these studies are quite supportive of a role for CART in regulating feeding and body weight in humans. However, not all polymorphisms in the CART gene are associated with obesity 81-83, perhaps because not all of them would be expected to alter the peptide structure and functional activity.
Figure 3. Effects of a missense mutation resulting in Leu34Phe in proCART.
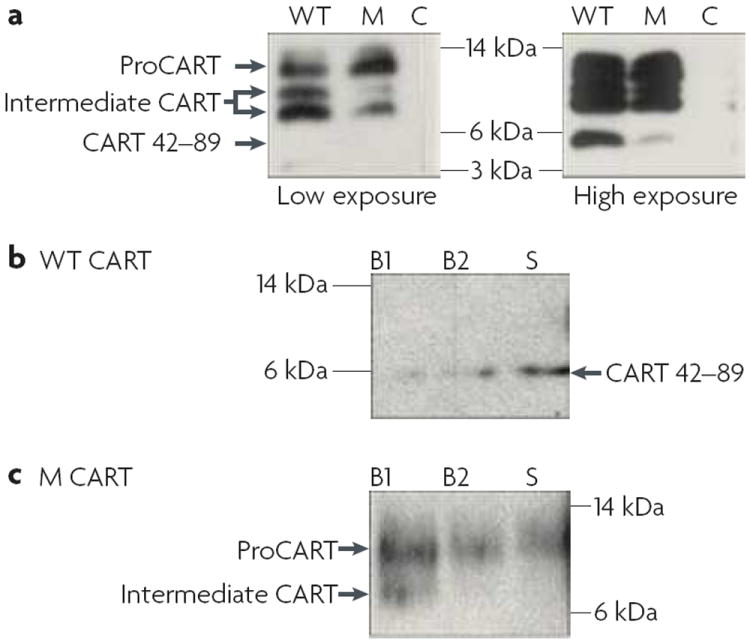
Western-blot analysis of CART (cocaine- and amphetamine-regulated transcript)-peptide production, processing and release in transfected AtT20 cells reveals the effects of a Leu34Phe mutation in the CARTPT gene. a ∣ Non-transfected AtT20 cells (C) showed no detectable CART peptide after either low or high exposure. Transfection of cells with a gene encoding wild-type (WT) proCART resulted in WT proCART largely being processed into intermediate CART peptides (~8 kDa) and active CART 42–89 (5.2 kDa) after high exposure. Transfection with a gene encoding mutated Leu34Phe proCART (M) resulted in M proCART being partially processed to intermediate CART peptides but only minimally processed to bioactive CART 42–89 after high exposure. b,c ∣ CART peptides secreted into the medium from cells transfected with either a gene encoding WT proCART (b) or a gene encoding M proCART (c) were analysed. Cells were incubated in basal medium (DMEM) for two 30-minute periods (B1 and B2) and then were incubated in stimulation medium for 30 minutes (S). Western blots of cell media show that in cells expressing WT CART peptides, basal secretion of an active form of CART peptide (5.2 kDa) is very small, but secretion is increased with stimulation. Cells expressing a gene encoding M proCART showed higher basal secretion of M proCART (10 kDa) and intermediate proCART (~8 kDa), but no increase was observed with stimulation. It is therefore thought that the Leu34Phe mutation73 results in a deficiency of bioactive CART peptides that are released by Ca2+-dependent mechanisms. The mutation seems to promote constitutive release of CART peptides, whereas WT CART peptides are mostly released by stimulation. The sera from the carriers of the mutation produced consistent results (not shown). Figure reproduced, with permission, from REF. 74 © (2006) Endocrine Society.
Some problematic results
Some experimental findings do not obviously support the idea that CART peptides affect body weight and are anorexigenic. For example, in rats, ICv injection of CART peptide reduced the intake of a liquid diet if the peptide reached the fourth ventricle84; however, the effects of the peptide (in this region) were not specific to nutrients but rather produced a conditioned taste aversion. Thus, CART peptide effects on food intake might be secondary to the production of such an aversive state. Also, ICv administration of CART peptide altered licking patterns, suggesting an effect on motor control of eating85,86, and high doses of ICv peptides can result in seizures87. It is possible that the motor and anxiogenic actions of CART peptides could produce some of these effects on feeding and body weight (see below). Moreover, it was reported that injection of CART peptide into discrete hypothalamic nuclei resulted in increased, rather than decreased, food intake72,88. Thus, although the evidence that CART peptides are anorexigenic is strong, some inconsistent observations need to be resolved or clarified.
In summary, CART peptides seem to have anorexi-genic effects, although the site(s) and mechanisms of these effects are not yet fully known. The literature indicates that there could be many sites and mechanisms. because most published experiments have involved increasing CART peptide levels by injection, studies with CART depletion or CART receptor blockers are needed. As CART peptides have a major impact on body weight in humans and animals, the CART system is important for understanding obesity and is a promising target for anti-obesity drug development35.
The CART system and addiction
Drug abuse is a significant problem, and much progress has been made in identifying the mechanisms and neuronal substrates of addiction. There is substantial evidence connecting the CART system and drug abuse. In post-mortem tissues from human victims of cocaine overdose, CART mRNA levels were increased in the nucleus accumbens and decreased in the ventral tegmen-tal area (VTA)89,90. Also, in a Korean population, an association was found between a polymorphism in intron 1 of the CART gene and alcoholism, but not between this polymorphism and bipolar disorder or schizophrenia91.
Psychostimulants activate CART-peptide-containing neurons in the nucleus accumbens
When initial studies showed an upregulation of CART gene transcription in the striatum after acute injections of either cocaine or amphetamine1, the assumption was that the CART system was involved in the action of psychostimulants. More recently it was shown that administration of methamphetamine, MDMA or ethanol also increases CART mRNA levels in the nucleus accumbens52,92,93. Some studies had difficulty reproducing the original findings with cocaine or amphetamine94-96, but elevations in CART mRNA were again found when higher dosages were given or repeated dosing was used97-99. It was subsequently shown that acute cocaine administration after injection of forskolin, which activates adenylyl cyclase, did increase CART levels96, suggesting that a preliminary activation of CART gene expression makes it more responsive to upregulation by psychostimulants. Recently it was shown that acute administration of cocaine increased the number of CART-peptide-expressing cells in the nucleus accumbens that stained for c-Fos100; this finding supports the notion that cocaine activates CART-peptide-expressing neurons in the nucleus accumbens, even though cocaine does not reliably alter CART peptide levels. The finding also suggests that changes in CART peptide levels per se might not be the best indicator of a role for the CART system in some process. Drugs can affect many neurons without necessarily causing a change in the levels of neurotransmitters, because the neurotransmitters can be replaced, at least in the short term, by synthesis (see REF. 100 for discussion). Taken together, these data indicate that CART-peptide-containing neurons in the nucleus accumbens are activated by acute administration of psychostimulants.
The CART system and mesolimbic dopamine
Additional data support the idea that CART-peptide-containing neurons in the ventral striatum are activated by psycho-stimulants. Psychostimulants increase dopamine levels in the synaptic cleft and in extra-neuronal spaces, and it has been shown that there are dopamine receptors on CART-peptide-containing neurons101-103. Also, CART neurons in the nucleus accumbens receive nerve terminals that stain positive for tyrosine hydroxylase, which implies that there is a direct dopaminergic input to CART-peptide-containing cells16-17. Interestingly, there is also evidence for a reciprocal CART peptide input to dopamine neurons in the ventral midbrain104-106. Some CART peptide is also found in the medial prefrontal cortex, and cocaine self-administration transiently increases CART gene transcription in this region, indicating that CART peptides might be involved in tolerance and dependence in this region as well107.
Given that CART neurons in the nucleus accumbens are indirectly activated by psychostimulants through dopamine, the functional effects of CART peptides become the next issue. For this discussion, we will restrict our attention to the role of CART peptides in the nucleus accumbens, for which significant data exist. The first observation was that injections of CART 55–102 alone into the nucleus accumbens had no observable effect on the animals’ locomotor activity. Although intra-accumbal injections of dopamine or amphetamine increased locomotor activity as expected, co-injection of these compounds with CART peptide resulted in a relative reduction in the locomotor activity108,109. Thus, CART peptide blunted the locomotor-activating effects of the stimulants. Another paper showed that intra-accumbal injection of CART peptide inhibited the expression of behavioural sensitization that is normally induced by amphetamine administration110. Furthermore, a recent paper indicates that CART peptide reduces the rewarding effects of cocaine in drug self-administration studies111. FIGURE 4 shows that the break point, a reflection of how hard animals are willing to work for a cocaine injection — and sometimes considered a measure of reward — is dose-responsively reduced when CART peptide is injected into the nucleus accumbens111. Cocaine administration elevates dopamine levels and therefore increases the activity that is associated with elevated dopamine levels; CART peptides in the nucleus accumbens might act to blunt the effects of dopamine.
Figure 4. CART peptides as potential regulators of dopamine activity in the nucleus accumbens.
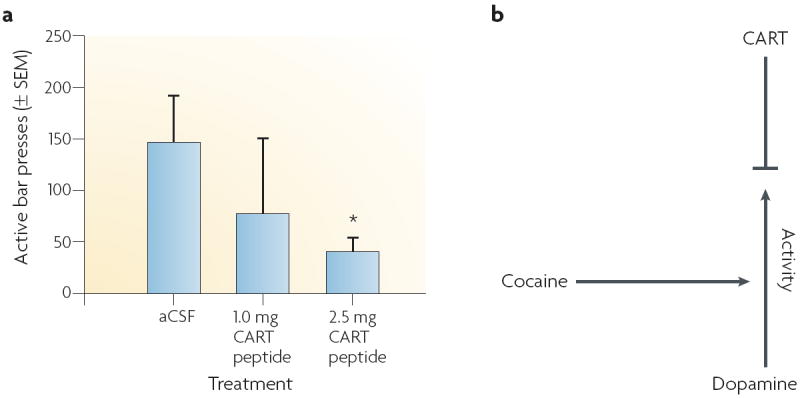
a ∣ The effect of bilateral CART (cocaine- and amphetamine-regulated transcript)-peptide infusions (0.0, 1.0 or 2.5 μg per side) into the nucleus accumbens on cocaine self-administration. Bilateral CART peptide infusions reduced the break point of cocaine self-administration in a dose-dependent manner. The break point is a reflection of how hard the animals are willing to work for a cocaine injection, and can be considered to be a measure of reward. b ∣ Cocaine administration elevates dopamine levels and therefore increases the locomotor activity that is associated with elevated dopamine levels. According to one hypothesis, CART peptides in the nucleus accumbens act to blunt this increased activity — perhaps through normal regulatory responses in this region, although the precise mechanism of the blunting is not yet clear. aCSF, artificial cerebrospinal fluid; SEM, standard error of the mean. Data in part a from REF. 108.
These findings suggest that CART peptides have a homeostatic function in the nucleus accumbens. It is postulated that as dopamine levels rise after the administration of psychostimulants, CART systems are activated to reduce or control the functional effects of the rise in dopamine (although the mechanisms and specificity of the CART effect are not yet clear). This hypothesis is interesting from a functional point of view, but also from the point of view of using the CART system as a target for developing medications for psychostimulant abuse.
Endocrine regulation
CART peptides are present in each of the three components of the hypothalamic–pituitary–adrenal (HPA) axis24,112 and in portal blood23. Application of CART 55–102 to hypothalamic explants stimulated the release of corticotropin-releasing hormone, TRH and NPY, and reduced the release of alpha-MSH113. These early findings suggested that CART peptide can regulate hormone release, and much work has investigated its effects on prolactin secretion. In dispersed anterior pituitary cells, prolactin release was suppressed by administration of CART peptide114. In another study, CART peptide had no effect by itself on prolactin release from anterior pituitary cell cultures, but it did inhibit TRH-induced prolactin release115. Moreover, ICV injection of CART peptide increased prolactin and growth hormone levels in the blood, as did intravenous administration of CART peptide, although at later time points116. Another report found that CART peptides in the anterior pituitary were localized mainly in lactotrophs, and in the posterior pituitary with oxytocin-containing cells; CART mRNA was significantly increased in the anterior pituitary and supraoptic nuclei of lactating rats, suggesting a feedback regulation of lactation on CART mRNA levels117. Thus, the basic findings suggest a role for CART peptide in regulating the release of prolactin and other hormones from the pituitary, although the exact mechanisms are currently unknown and might prove to be controversial118.
The CART system and stress
CART peptide and mRNA are present at many levels of the HPA axis and in other ‘stress-related’ areas, including the arcuate nucleus, the PvN, the pituitary, the medullary C1 adrenaline-containing cells, the intermediolateral cell column of the spinal cord, and the adrenal medulla1,14,18,23,24,63,112,119-124 (FIG. 5). It is somewhat remarkable that the CART system is present at all of these levels. As expected from its localization, the CART system has been functionally implicated in stress. CART peptides are released into the pituitary portal blood after hypotensive stress23; this important finding in rats indicates that CART peptides could function as releasing factors, and there is other evidence for this (see the section on endocrine regulation). ICv injection of CART 55–102 resulted in c-Fos elevations in rat PvN neurons, particularly in the corticotrophin-releasing factor (CRF)-containing neurons, suggesting that CART peptides in the hypothalamus have a modulating role on CRF release125,126. Furthermore, ICv administration of CART peptide elevated plasma levels of adrenocorticotropic hormone and corticosterone113.
Figure 5. The involvement of the CART system in the stress response.
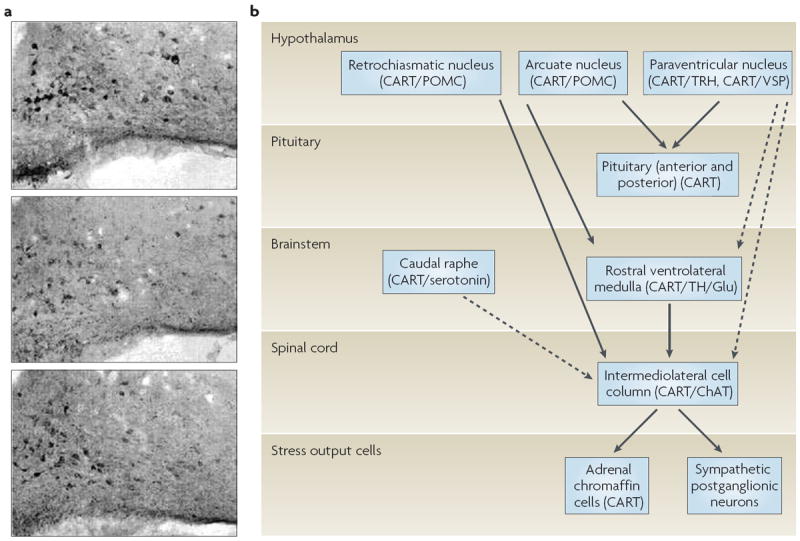
CART (cocaine- and amphetamine-regulated transcript) peptides are, strikingly, present in many parts of the stress axis, and CART-peptide-containing neurons respond to stress presumably by releasing CART peptides. a ∣ Staining cells in the arcuate nucleus for the presence of CART peptide. The top image shows normal (untreated) tissue. The middle image shows tissue from an adrenalectomized animal: the number of CART-peptide-containing cells was reduced by ~40%. The bottom image shows tissue from an adrenalectomized animal that was given hormone replacement. The number of CART-peptide-containing cells in the replacement condition was not significantly different from that in the control condition shown in the top image. b ∣ CART peptides are strikingly found in most of the key regions and tissues that are involved in the stress response119-121. ChAT, choline acetyltransferase; POMC, pro-opiomelanocortin; TH, tyrosine hydroxylase; TRH, thyrotropin-releasing hormone; VSP, vasopressin. Part a reproduced, with permission, from REF. 127 © (2003) Elsevier/North-Holland Biomedical Press. Part b modified, with permission, from REF. 124 © (2006) Elsevier Science Inc.
Experiments have suggested that there might be a mutual interaction between elements of the HPA axis and CART peptides. Adrenalectomy reduced the number of CART-peptide-expressing neurons in the PVN and mediobasal hypothalamus, and glucocorticoid replacement partially reversed the reduction125,127-129 (FIG. 5). Moreover, CRF administration increased CART mRNA levels in CATH.a cells (a locus coeruleus-like cell line) in culture and increased the release of CART peptide from rat anterior pituitary segments12,130. Nitric oxide, a regulator of stress responses, colocalizes with CART peptides in some neurons in the rat hypothalamus131. Intraperitoneal injection of corticosterone enhanced blood CART peptide levels, and this was prevented by injection of metyrapone (an inhibitor of corticosterone synthesis)132, suggesting a role for corticosterone in CART-peptide expression and/or release. However, chronic (14-day) administration of corticosterone in drinking water decreased CART peptide levels in the blood and pituitary of rats130. Together, these findings suggest that there is an interaction between corticosterone and CART peptides.
In light of these data, it is important to establish the effects of whole-animal stress on CART peptides. It has already been noted that hypotensive stress resulted in increases in CART peptide levels in portal blood23. Inflammation induced by administration of bacterial lipopolysaccharides, which are known activators of the HPA axis, increased CART mRNA levels in the hypothalamus of rats133. Further, chronic (20-day) cold stress in rats increased CART mRNA levels in the arcuate nucleus, again suggesting a role for CART peptides in energy expenditure and thermogenesis134. exposing male rats to forced swimming also increased CART peptide levels in the PvN135. In addition, acute and chronic restraint stress increased CART mRNA levels in the hippocampus and amygdala136 and, conversely, adrenalectomy reduced CART levels in the hippocampal dentate gyrus — an effect that was reversed by corticosterone replacement. It is clear that CART peptides are associated with stress and HPA axis activity; the current challenge is to elucidate the precise involvement and effects of CART peptides in this axis.
The CART system in anxiety and depression
There is evidence for an involvement of CART peptides in anxiety-like behaviour. CART peptides are expressed in several parts of the limbic system, which is thought to have a role in regulating emotion. These regions include the central and basomedial nuclei of the amygdala, the bed nucleus of the stria terminalis, and the hippocampus1,14. It was reported that, in rats and mice, ICv injection of small CART peptide fragments increased anxiety-like behaviour in the elevated plus maze137. The authors suggested that CART might be an anxiety/arousal peptide and that some of its other physiological effects might be secondary to this role. In another study, ICv administration of CART 55–102, but not CART 62–102, increased anxiety-like behaviour as measured in the elevated plus maze and in a social-interaction test138, and the effects were reduced by anxiolytic drugs such as diazepam. The different results for the two active CART peptides are compatible with the idea that there might be more than one CART receptor. Taken together, the evidence suggests that CART peptides have a role in the anxiety-like behaviours that are measured in these animal models, and possible mechanisms of these effects have been discussed139. Another potentially relevant study140 found that CART peptides were elevated in the cerebrospinal fluid of patients with Huntington’s disease; anxiety is a major feature of this disease.
CART peptide and mRNA are present in brain regions that are associated with depression, including the hippocampus, the locus coeruleus, parts of the midbrain raphe nuclei, the amygdala and the hypothalamus1,14. Recently it was shown that CART mRNA is downregulated in the frontal cortex of rats that have been subjected to a chronic mild-stress paradigm, an animal model of depression141. The CART system’s suggested connection to depression in humans derives from a study of an Italian family with early-onset obesity and a missense mutation in the CART gene142. Just as obesity co-segregated with the mutation among the family members, high levels of both anxiety and depression were found in family members with the mutation73. Although the number of subjects in this study was low, the results are intriguing. It is as yet unclear whether the anxiety and depression are consequences of other problems arising from the mutation, or whether they are a more direct consequence of the lack of CART peptide.
Additional functions of the CART system
CART peptide immunoreactivity has been observed in areas that are associated with cardiovascular control in rats, including the intermediolateral cell column of the spinal cord, portions of the rostral ventrolateral medulla and the intracardiac ganglia1,14,121,143-145. Moreover, after drug-induced hypotension approximately half of the CART-peptide-containing neurons stained positive for c-Fos in spinal cord segments T5–T13, suggesting the possibility of a role for CART in cardiovascular regulation. ICv injections of CART 55–102 in conscious rabbits increased mean arterial pressure and renal-nerve activity, presumably by altering sympatho-adrenal outflow146, and similar effects of CART peptides have been found in studies of anaesthetized rats147. because the cardiovascular effects occurred in experimental conditions that also resulted in inhibition of food intake, it has been speculated that there might be a relationship between the regulation of cardiovascular function and energy expenditure. More recently, CART peptide has been shown to evoke a long-lasting and dose-dependent constriction of isolated brain cerebral arterioles; these effects were dependent on endothelin A receptors148. Additional studies will be needed to investigate these possible mechanisms and to determine whether and how they apply to humans.
CART peptides are involved in a number of additional physiological processes. A series of studies have implicated CART peptides in pain14,149-153. CART peptides are antinociceptive in the formalin test149 and can reverse the hyperalgesia and allodynia in a model of chronic neuropathic pain153. bone remodelling also involves hypothalamic CART peptides154,155, and the CART system is now considered to be part of the new field of neuroskeletal biology156. CART peptides also have neurotrophic and neuroprotective properties157-159, and a few papers suggest that CART peptides play a part in CNS development55,160-162.
Conclusions and future perspectives
Although CART peptides were named after the experimental condition in which they were discovered, it is now fully appreciated that CART is not only involved in the actions of cocaine and amphetamines. Indeed, CART peptides are widely (but discretely) localized throughout the CNS and periphery, and it is therefore not surprising that they seem to be involved in a number of physiological processes. Many active substances like acetylcholine or NPY have multiple functions, again at least partly because they are widely distributed throughout the body. It is therefore difficult to espouse a single role for CART and premature to attempt to list all of the roles of CART peptides. At the moment our plates are full unravelling the CART system’s many physiological roles and mechanisms of action, and understanding the constituents of the system, such as the CART-peptide receptor(s).
The CART system might well function in conjunction with other active substances. CART peptides colocalize with many other active substances within cells, and it seems unlikely that they regulate many of the processes discussed above by themselves. For example, food intake is known to be regulated by tens of different neurotransmitters and hormones163-166. Nevertheless, it seems clear that the CART system is an important component of the regulation of feeding, body weight and energy expenditure.
Although there has been much research on the localization, processing and expression of CART peptides, the current frontiers are to further elucidate their physiological roles and to identify, clone and study the receptor(s) for CART peptides. Identifying the receptor(s) might facilitate screening for small-molecule agonists and antagonists, which in turn will be useful tools for additional studies of CART-peptide functions and mechanisms.
Acknowledgments
The authors acknowledge the helpful suggestions of Drs Nestler, Hunter, Sundler, Wierup and Vrang, and the support of the US National Institutes of Health.
Glossary
- Intracisternal injection
The injection of a substance into the space between the cerebellum and the medulla, which is filled with cerebrospinal fluid.
- Tyrosine hydroxylase
The enzyme that catalyzes the conversion of Ltyrosine to dihydroxyphenylalanine (DoPA), which is a precursor of dopamine.
- Elevated plus maze
A test that is used to assess anxietylike behaviour in animals, usually rats or mice. The basic measure is the animal’s preference — measured as duration of inhabitation — for dark, enclosed places over bright, more open places. More time spent in the bright and open areas suggests a lower level of anxiety.
- Formalin test
A model of chronic pain that is usually carried out in rats or mice as a test for substances that reduce pain. It involves a subcutaneous injection of formalin into the hind paw, which causes severe inflammation. The pain measurement can be the time the animal spends with weight on the injected paw or the time the animal spends biting and licking the injected paw.
Footnotes
Competing interests statement
The authors declare competing financial interests: see web version for details.
References
- 1.Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. This basic paper established the field of CART peptide research. It also demonstrated the psychostimulant effect which, although sometimes controversial, has been confirmed several times, as summarized in reference 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiess J, Villarreal J, Vale W. Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry. 1981;20:1982–1988. doi: 10.1021/bi00510a038. [DOI] [PubMed] [Google Scholar]
- 3.Thim L, Kristensen P, Nielsen PF, Wulff BS, Clausen JT. Tissue-specific processing of cocaine- and amphetamine-regulated transcript peptides in the rat. Proc Natl Acad Sci USA. 1999;96:2722–2727. doi: 10.1073/pnas.96.6.2722. This study elucidated the tissue-specific processing and sequence of specific CART peptides. Reference 8 was a strong follow-up to this work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez G. The CART gene: structure and regulation. Peptides. 2006;27:1913–1918. doi: 10.1016/j.peptides.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 5.Thim L, et al. Purification and characterisation of a new hypothalamic satiety peptide, cocaine and amphetamine regulated transcript (CART), produced in yeast. FEBS Lett. 1998;428:263–268. doi: 10.1016/s0014-5793(98)00543-2. [DOI] [PubMed] [Google Scholar]
- 6.Ludvigsen S, Thim L, Blom AM, Wulff BS. Solution structure of the satiety factor, CART, reveals new functionality of a well-known fold. Biochemistry. 2001;40:9082–9088. doi: 10.1021/bi010433u. [DOI] [PubMed] [Google Scholar]
- 7.Dey A, et al. Biological processing of the cocaine and amphetamine-regulated transcript precursors by prohormone convertases, PC2 and PC1/3. J Biol Chem. 2003;278:15007–15014. doi: 10.1074/jbc.M212128200. [DOI] [PubMed] [Google Scholar]
- 8.Stein J, Shah R, Steiner DF, Dey A. RNAi-mediated silencing of prohormone convertase (PC) 5/6 expression leads to impairment in processing of cocaine- and amphetamine-regulated transcript (CART) precursor. Biochem J. 2006;400:209–215. doi: 10.1042/BJ20060506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein J, Steiner DF, Dey A. Processing of cocaine- and amphetamine-regulated transcript (CART) precursor proteins by prohormone convertases (PCs) and its implications. Peptides. 2006;27:1919–1925. doi: 10.1016/j.peptides.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Dylag T, Kotlinksa J, Rafalski P, Pachuta A, Silberring J. The activity of CART peptide fragments. Peptides. 2006;27:1926–1933. doi: 10.1016/j.peptides.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez G, Lakatos A, Kuhar MJ. Characterization of the cocaine- and amphetamine-regulated transcript (CART) peptide gene promoter and its activation by a cyclic AMP-dependent signaling pathway in GH3 cells. J Neurochem. 2002;80:885–893. doi: 10.1046/j.0022-3042.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- 12.Dominguez G, Kuhar MJ. Transcriptional regulation of the CART promoter in CATH.a cells. Brain Res Mol Brain Res. 2004;126:22–29. doi: 10.1016/j.molbrainres.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 13.Barrett P, Davidson J, Morgan P. CART gene promoter transcription is regulated by a cyclic adenosine monophosphate response element. Obes Res. 2002;10:1291–1298. doi: 10.1038/oby.2002.175. This and other work by Barrett was among the first to demonstrate regulation of CART gene expression by transcription factors. [DOI] [PubMed] [Google Scholar]
- 14.Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ. Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol. 1998;391:115–132. [PubMed] [Google Scholar]
- 15.Gautvik KM, et al. Overview of the most prevalent hypothalamus-specific mRNAs, as identified by directional tag PCR subtraction. Proc Natl Acad Sci USA. 1996;93:8733–8738. doi: 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith Y, Koylu EO, Couceyro P, Kuhar MJ. Ultrastructural localization of CART (cocaine- and amphetamine- regulated transcript) peptides in the nucleus accumbens of monkeys. Synapse. 1997;27:90–94. doi: 10.1002/(SICI)1098-2396(199709)27:1<90::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 17.Smith Y, Kieval J, Couceyro PR, Kuhar MJ. CART peptide-immunoreactive neurones in the nucleus accumbens in monkeys: ultrastructural analysis, colocalization studies, and synaptic interactions with dopaminergic afferents. J Comp Neurol. 1999;407:491–511. doi: 10.1002/(sici)1096-9861(19990517)407:4<491::aid-cne3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Vrang N. Anatomy of hypothalamic CART neurons. Peptides. 2006;27:1970–1980. doi: 10.1016/j.peptides.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Hubert GW, Kuhar MJ. Colocalization of CART with substance P but not enkephalin in the rat nucleus accumbens. Brain Res. 2005;1050:8–14. doi: 10.1016/j.brainres.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Murphy KG, et al. Quantification and synthesis of cocaine- and amphetamine-regulated transcript peptide (79–102)-like immunoreactivity and mRNA in rat tissues. J Endocrinol. 2000;166:659–668. doi: 10.1677/joe.0.1660659. [DOI] [PubMed] [Google Scholar]
- 21.Kuhar MJ, Yoho LL. CART peptide analysis by Western blotting. Synapse. 1999;33:163–171. doi: 10.1002/(SICI)1098-2396(19990901)33:3<163::AID-SYN1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Ekblad E. CART in the enteric nervous system. Peptides. 2006;27:2024–2030. doi: 10.1016/j.peptides.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Larsen PJ, et al. Cocaine- and amphetamine-regulated transcript is present in hypothalamic neuroendocrine neurones and is released to the hypothalamic-pituitary portal circuit. J Neuroendocrinol. 2003;15:219–226. doi: 10.1046/j.1365-2826.2003.00960.x. This study demonstrated the presence of CART peptides in the portal blood, which is fundamental to a role for them in endocrine regulation. [DOI] [PubMed] [Google Scholar]
- 24.Koylu EO, et al. Immunohistochemical localization of novel CART peptides in rat hypothalamus, pituitary and adrenal gland. J Neuroendocrinol. 1997;9:823–833. doi: 10.1046/j.1365-2826.1997.00651.x. This paper and reference 14 presented a detailed description of CART peptide distribution. The distribution suggested many roles for the CART system in endocrine function, stress and feeding. [DOI] [PubMed] [Google Scholar]
- 25.Vicentic A. CART peptide diurnal variations in blood and brain. Peptides. 2006;27:1942–1948. doi: 10.1016/j.peptides.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 26.Vrang N, Tang-Christensen M, Larsen PJ, Kristensen P. Recombinant CART peptide induces c-Fos expression in central areas involved in control of feeding behaviour. Brain Res. 1999;818:499–509. doi: 10.1016/s0006-8993(98)01349-3. [DOI] [PubMed] [Google Scholar]
- 27.Yermolaieva O, Chen J, Couceyro PR, Hoshi T. Cocaine- and amphetamine-regulated transcript peptide modulation of voltage-gated Ca2+ signaling in hippocampal neurons. J Neurosci. 2001;21:7474–7480. doi: 10.1523/JNEUROSCI.21-19-07474.2001. This was one of the first papers to demonstrate intracellular signalling by CART peptides, and it also gave evidence of a G-protein coupled CART receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkar S, Wittmann G, Fekete C, Lechan RM. Central administration of cocaine- and amphetamine-regulated transcript increases phosphorylation of cAMP response element binding protein in corticotrophin-releasing hormone-producing neurons but not in prothyrotropin-releasing hormone-producing neurons in the hypothalamic paraventricular nucleus. Brain Res. 2004;999:181–192. doi: 10.1016/j.brainres.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 29.Lakatos A, Prinster S, Vicentic A, Hall RA, Kuhar MJ. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci Lett. 2005;384:198–202. doi: 10.1016/j.neulet.2005.04.072. [DOI] [PubMed] [Google Scholar]
- 30.Sen A, Bettegowda A, Jimenez-Krassel F, Ireland JJ, Smith GW. Cocaine- and amphetamine-regulated transcript regulation of follicle-stimulating hormone signal transduction in bovine granulosa cells. Endocrinology. 2007;148:4400–4410. doi: 10.1210/en.2007-0332. [DOI] [PubMed] [Google Scholar]
- 31.Vicentic A, Lakatos A, Kuhar MJ. CART (cocaine- and amphetamine-regulated transcript) peptide receptors: specific binding in AtT20 cells. Eur J Pharmacol. 2005;528:188–189. doi: 10.1016/j.ejphar.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 32.Keller PA, et al. Characterization and localization of cocaine- and amphetamine-regulated transcript (CART) binding sites. Peptides. 2006;27:1328–1334. doi: 10.1016/j.peptides.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Maletinska L, et al. Cocaine- and amphetamine-regulated transcript (CART) peptide specific binding in pheochromocytoma cells PC12. Eur J Pharmacol. 2007;559:109–114. doi: 10.1016/j.ejphar.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Jones DC, Kuhar MJ. CART receptor binding in primary cell cultures of the rat nucleus accumbens. Synapse. 2008;62:122–127. doi: 10.1002/syn.20476. [DOI] [PubMed] [Google Scholar]
- 35.Hunter RG, Kuhar MJ. CART peptides as targets for CNS drug development. Curr Drug Targets CNS Neurol Disord. 2003;2:201–205. doi: 10.2174/1568007033482896. [DOI] [PubMed] [Google Scholar]
- 36.Couceyro P, Paquet M, Koylu E, Kuhar MJ, Smith Y. Cocaine- and amphetamine-regulated transcript (CART) peptide immunoreactivity in myenteric plexus neurons of the rat ileum and co-localization with choline acetyltransferase. Synapse. 1998;30:1–8. doi: 10.1002/(SICI)1098-2396(199809)30:1<1::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Wierup N, Gunnarsdottir A, Ekblad E, Sundler F. Characterization of CART-containing neurons and cells in the porcine pancreas, gastro-intestinal tract, adrenal and thyroid glands. BMC Neurosci. 2007;8:51. doi: 10.1186/1471-2202-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekblad E, Kuhar M, Wierup N, Sundler F. Cocaine- and amphetamine-regulated transcript: distribution and function in rat gastrointestinal tract. Neurogastroenterol Motil. 2003;15:545–557. doi: 10.1046/j.1365-2982.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 39.Ellis LM, Mawe GM. Distribution and chemical coding of cocaine- and amphetamine-regulated transcript peptide (CART)-immunoreactive neurons in the guinea pig bowel. Cell Tissue Res. 2003;312:265–274. doi: 10.1007/s00441-002-0678-9. [DOI] [PubMed] [Google Scholar]
- 40.Gunnarsdottir A, Wierup N, Larsson LT, Kuhar MJ, Ekblad E. CART-peptide immunoreactivity in enteric nerves in patients with Hirschsprung’s disease. Eur J Pediatr Surg. 2007;17:184–189. doi: 10.1055/s-2007-965164. [DOI] [PubMed] [Google Scholar]
- 41.Lambert PD, et al. A role for novel CART peptide fragments in the central control of food intake. Neuropeptides. 1997;31:620–621. [Google Scholar]
- 42.Lambert PD, et al. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–298. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 43.Kristensen P, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. This landmark paper linked hypothalamic CART peptides to feeding and regulation by leptin. [DOI] [PubMed] [Google Scholar]
- 44.Larsen PJ, et al. Ups and downs for neuropeptides in body weight homeostasis: pharmacological potential of cocaine amphetamine regulated transcript and pre-proglucagon-derived peptides. Eur J Pharmacol. 2002;440:159–172. doi: 10.1016/s0014-2999(02)01426-7. [DOI] [PubMed] [Google Scholar]
- 45.de Lartigue G, Dimaline R, Varro A, Dockray GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci. 2007;27:2876–2882. doi: 10.1523/JNEUROSCI.5508-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Broberger C, Holmberg K, Kuhar MJ, Hokfelt T. Cocaine- and amphetamine-regulated transcript in the rat vagus nerve: a putative mediator of cholecystokinin-induced satiety. Proc Natl Acad Sci USA. 1999;96:13506–13511. doi: 10.1073/pnas.96.23.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okumura T, Yamada H, Motomura W, Kohgo Y. Cocaine-amphetamine-regulated transcript (CART) acts in the central nervous system to inhibit gastric acid secretion via brain corticotrophin-releasing factor system. Endocrinology. 2000;141:2854–2860. doi: 10.1210/endo.141.8.7588. [DOI] [PubMed] [Google Scholar]
- 48.Asakawa A, et al. Cocaine-amphetamine-regulated transcript influences energy metabolism, anxiety and gastric emptying in mice. Horm Metab Res. 2001;33:554–558. doi: 10.1055/s-2001-17205. [DOI] [PubMed] [Google Scholar]
- 49.Smedh U, Moran TH. Peptides that regulate food intake: separable mechanisms for dorsal hindbrain CART peptide to inhibit gastric emptying and food intake. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1418–R1426. doi: 10.1152/ajpregu.00665.2002. [DOI] [PubMed] [Google Scholar]
- 50.Smedh U, Moran TH. The dorsal vagal complex as a site for cocaine- and amphetamine-regulated transcript peptide to suppress gastric emptying. Am J Physiol Regul Integr Comp Physiol. 2006;291:R124–R130. doi: 10.1152/ajpregu.00234.2004. [DOI] [PubMed] [Google Scholar]
- 51.Yang SC, Shieh KR, Li HY. Cocaine- and amphetamine-regulated transcript in the nucleus accumbens participates in the regulation of feeding behavior in rats. Neuroscience. 2005;133:841–851. doi: 10.1016/j.neuroscience.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 52.Jean A, et al. Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci USA. 2007;104:16335–16340. doi: 10.1073/pnas.0701471104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larsen PJ, Vrang N, Petersen PC, Kristensen P. Chronic intracerebroventricular administration of recombinant CART(42–89) peptide inhibits and causes weight loss in lean and obese Zucker (fa/fa) rats. Obes Res. 2000;8:590–596. doi: 10.1038/oby.2000.76. [DOI] [PubMed] [Google Scholar]
- 54.Asnicar MA, et al. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology. 2001;142:4394–4400. doi: 10.1210/endo.142.10.8416. [DOI] [PubMed] [Google Scholar]
- 55.Moffett M, et al. Studies of cocaine- and amphetamine-regulated transcript (CART) knockout mice. Peptides. 2006;27:2037–2045. doi: 10.1016/j.peptides.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 56.Wierup N, et al. CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology and increased body weight. Regul Pept. 2005;129:203–211. doi: 10.1016/j.regpep.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 57.Qing K, Chen Y. Central CART gene delivery by recombinant AAV vector attenuates body weight gain in diet-induced-obese rats. Regul Pept. 2007;140:21–26. doi: 10.1016/j.regpep.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 58.Steiner RC, Hsiung HM, Picciotto MR. Cocaine self-administration and locomotor sensitization are not altered in CART knockout mice. Behav Brain Res. 2006;171:56–62. doi: 10.1016/j.bbr.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 59.Tian DR, et al. Changes of hypothalamic alpha-MSH and CART peptide expression in diet-induced obese rats. Peptides. 2004;25:2147–2153. doi: 10.1016/j.peptides.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 60.Vrang N, Kristensen P, Tang-Christensen M, Larsen PJ. Effects of leptin on arcuate pro-opiomelanocortin and cocaine-amphetamine-regulated transcript expression are independent of circulating levels of corticosterone. J Neuroendocrinol. 2002;14:880–886. doi: 10.1046/j.1365-2826.2002.00855.x. [DOI] [PubMed] [Google Scholar]
- 61.Dhillo WS, et al. Hypothalamic interactions between neuropeptide Y, agouti-related protein, cocaine- and amphetamine-regulated transcript and alpha-melanocyte-stimulating hormone in vitro in male rats. J Neuroendocrinol. 2002;14:725–730. doi: 10.1046/j.1365-2826.2002.00832.x. [DOI] [PubMed] [Google Scholar]
- 62.Adam CL, Archer ZA, Findlay PA, Thomas L, Marie M. Hypothalamic gene expression in sheep for cocaine- and amphetamine-regulated transcript, pro-opiomelanocortin, neuropeptide Y, agouti-related peptide and leptin receptor and responses to negative energy balance. Neuroendocrinology. 2002;75:250–256. doi: 10.1159/000054716. [DOI] [PubMed] [Google Scholar]
- 63.Elias CF, et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron. 1998;21:1375–1385. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 64.Wang ZW, et al. Comparing the hypothalamic and extrahypothalamic actions of endogenous hyperleptinemia. Proc Natl Acad Sci USA. 1999;96:10373–10378. doi: 10.1073/pnas.96.18.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elias CF, et al. Characterization of CART neurons in rat and human hypothalamus. J Comp Neurol. 2001;26:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- 66.Germano CM, et al. Time course effects of adrenalectomy and food intake on CART expression in the hypothalamus. Brain Res. 2007;1166:55–64. doi: 10.1016/j.brainres.2007.05.077. [DOI] [PubMed] [Google Scholar]
- 67.Osei-Hyiaman D, et al. Cocaine- and amphetamine-related transcript is involved in the orexigenic effect of endogenous anandamide. Neuroendocrinology. 2005;81:273–282. doi: 10.1159/000087925. [DOI] [PubMed] [Google Scholar]
- 68.Fekete C, et al. Association of cocaine- and amphetamine-regulated transcript- immunoreactive elements with thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and its role in the regulation of the hypothalamic-pituitary-thyroid axis during fasting. J Neurosci. 2000;20:9224–9234. doi: 10.1523/JNEUROSCI.20-24-09224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fekete C, Lechan RM. Neuroendocrine implications for the association between cocaine- and amphetamine regulated transcript (CART) and hypophysiotropic thyrotropin-releasing hormone (TRH) Peptides. 2006;27:2012–2018. doi: 10.1016/j.peptides.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 70.Wittmann G, Liposits Z, Lechan RM, Fekete C. Medullary adrenergic neurons contribute to the cocaine- and amphetamine-regulated transcript-immunoreactive innervation of thyrotropin-releasing hormone synthesizing neurons in the hypothalamic paraventricular nucleus. Brain Res. 2004;1006:1–7. doi: 10.1016/j.brainres.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 71.Wang C, Billington CJ, Levine AS, Kotz CM. Effect of CART in the hypothalamic paraventricular nucleus on feeding and uncoupling protein gene expression. Neuroreport. 2000;11:3251–3255. doi: 10.1097/00001756-200009280-00040. [DOI] [PubMed] [Google Scholar]
- 72.Kong WM, et al. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. FASEB J. 2003;17:1688–1690. doi: 10.1096/fj.02-0805fje. [DOI] [PubMed] [Google Scholar]
- 73.del Giudice EM, et al. Mutational screening of the CART gene in obese children: identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes. 2001;50:2157–2160. doi: 10.2337/diabetes.50.9.2157. This paper demonstrated a relationship between a mutation in the CART gene and obesity among family members. The mutation disrupts CART peptide processing and levels (see reference 74) [DOI] [PubMed] [Google Scholar]
- 74.Yanik T, Dominguez G, Kuhar MJ, Del Giudice EM, Loh YP. The Leu34Phe ProCART mutation leads to cocaine- and amphetamine-regulated transcript (CART) deficiency: a possible cause for obesity in humans. Endocrinology. 2006;147:39–43. doi: 10.1210/en.2005-0812. [DOI] [PubMed] [Google Scholar]
- 75.Dominguez G, del Giudice EM, Kuhar MJ. CART peptide levels are altered by a mutation associated with obesity at codon 34. Mol Psychiatry. 2004;9:1065–1066. doi: 10.1038/sj.mp.4001578. [DOI] [PubMed] [Google Scholar]
- 76.Yamada K, et al. Sequencing of the putative promoter region of the cocaine- and amphetamine-regulated-transcript gene and identification of polymorphic sites associated with obesity. Int J Obes Relat Metab Disord. 2002;26:132–136. doi: 10.1038/sj.ijo.0801848. [DOI] [PubMed] [Google Scholar]
- 77.Guerardel A, et al. Analysis of sequence variability in the CART gene in relation to obesity in a Caucasian population. BMC Genet. 2005;6:19. doi: 10.1186/1471-2156-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vasseur F, et al. Impact of a CART promoter genetic variation on plasma lipid profile in a general population. Mol Genet Metab. 2007;90:199–204. doi: 10.1016/j.ymgme.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 79.Challis BG, et al. The CART gene and human obesity: mutational analysis and population genetics. Diabetes. 2000;49:872–875. doi: 10.2337/diabetes.49.5.872. [DOI] [PubMed] [Google Scholar]
- 80.Fu M, et al. Association of the cocaine and amphetamine-regulated transcript gene with type 2 diabetes mellitus. Zhonghua Nei Ke Za Zhi. 2002;41:805–808. [PubMed] [Google Scholar]
- 81.Walder K, Morris C, Ravussin E. A polymorphism in the gene encoding CART is not associated with obesity in Pima Indians. Int J Obes Relat Metab Disord. 2000;24:520–521. doi: 10.1038/sj.ijo.0801196. [DOI] [PubMed] [Google Scholar]
- 82.Echwald SM, et al. Sequence variants in the human cocaine and amphetamine-regulated transcript (CART) gene in subjects with early onset obesity. Obes Res. 1999;7:532–536. doi: 10.1002/j.1550-8528.1999.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 83.Kenealy SJ, Kim KS, Hu Z, Rothschild MF. Genetic linkage mapping of the porcine cocaine- and amphetamine- regulated transcript (CART) gene. J Anim Sci. 1999;77:791–792. doi: 10.2527/1999.773791x. [DOI] [PubMed] [Google Scholar]
- 84.Aja S, Robinson BM, Mills KJ, Ladenheim EE, Moran TH. Fourth ventricular CART reduces food and water intake and produces a conditioned taste aversion in rats. Behav Neurosci. 2002;116:918–921. [PubMed] [Google Scholar]
- 85.Aja S, Sahandy S, Ladenheim EE, Schwartz GJ, Moran TH. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1862–R1867. doi: 10.1152/ajpregu.2001.281.6.R1862. [DOI] [PubMed] [Google Scholar]
- 86.Aja S, Schwartz GJ, Kuhar MJ, Moran TH. Intracerebroventricular CART peptide reduces rat ingestive behavior and alters licking microstructure. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1613–R1619. doi: 10.1152/ajpregu.2001.280.6.R1613. [DOI] [PubMed] [Google Scholar]
- 87.Keating GL, Kuhar MJ, Rye DB. High dose CART induces abnormal EEG activity and behavioral seizures. Neuropeptides. 2008;42:199–204. doi: 10.1016/j.npep.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 88.Abbott CR, et al. Evidence of an orexigenic role for cocaine- and amphetamine-regulated transcript after administration into discrete hypothalamic nuclei. Endocrinology. 2001;142:3457–3463. doi: 10.1210/endo.142.8.8304. [DOI] [PubMed] [Google Scholar]
- 89.Albertson DN, et al. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–924. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jung SK, et al. Association between polymorphism in intron 1 of cocaine- and amphetamine-regulated transcript gene with alcoholism, but not with bipolar disorder and schizophrenia in Korean population. Neurosci Lett. 2004;365:54–57. doi: 10.1016/j.neulet.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 92.Salinas A, Wilde JD, Maldve RE. Ethanol enhancement of cocaine- and amphetamine-regulated transcript mRNA and peptide expression in the nucleus accumbens. J Neurochem. 2006;97:408–415. doi: 10.1111/j.1471-4159.2006.03745.x. [DOI] [PubMed] [Google Scholar]
- 93.Ogden CA, et al. Candidate genes, pathways and mechanisms for bipolar (manic-depressive) and related disorders: an expanded convergent functional genomics approach. Mol Psychiatry. 2004;9:1007–1029. doi: 10.1038/sj.mp.4001547. [DOI] [PubMed] [Google Scholar]
- 94.Vrang N, Larsen PJ, Kristensen P. Cocaine-amphetamine regulated transcript (CART) expression is not regulated by amphetamine. Neuroreport. 2002;13:1215–1218. doi: 10.1097/00001756-200207020-00029. [DOI] [PubMed] [Google Scholar]
- 95.Marie-Claire C, et al. Fos but not Cart (cocaine and amphetamine regulated transcript) is overexpressed by several drugs of abuse: a comparative study using real-time quantitative polymerase chain reaction in rat brain. Neurosci Lett. 2003;345:77–80. doi: 10.1016/s0304-3940(03)00307-0. [DOI] [PubMed] [Google Scholar]
- 96.Jones DC, Kuhar MJ. Cocaine-amphetamine-regulated transcript expression in the rat nucleus accumbens is regulated by adenylyl cyclase and the cyclic adenosine 5′-monophosphate/protein kinase A second messenger system. J Pharmacol Exp Ther. 2006;317:454–461. doi: 10.1124/jpet.105.096123. [DOI] [PubMed] [Google Scholar]
- 97.Fagergren P, Hurd YL. Mesolimbic gender differences in peptide CART mRNA expression: effects of cocaine. Neuroreport. 1999;10:3449–3452. doi: 10.1097/00001756-199911080-00034. [DOI] [PubMed] [Google Scholar]
- 98.Brenz Verca MS, Widmer DA, Wagner GC, Dreyer J. Cocaine-induced expression of the tetraspanin CD81 and its relation to hypothalamic function. Mol Cell Neurosci. 2001;17:303–316. doi: 10.1006/mcne.2000.0942. [DOI] [PubMed] [Google Scholar]
- 99.Hunter RG, Vicentic A, Rogge G, Kuhar MJ. The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol. 2005;517:45–50. doi: 10.1016/j.ejphar.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 100.Hubert GW, Kuhar MJ. Cocaine administration increases the fraction of CART cells in the rat nucleus accumbens that co-immunostain for c-Fos. Neuropeptides. 2008;42:339–343. doi: 10.1016/j.npep.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beaudry G, Zekki H, Rouillard C, Levesque D. Clozapine and dopamine D3 receptor antisense reduce cocaine- and amphetamine-regulated transcript expression in the rat nucleus accumbens shell. Synapse. 2004;51:233–240. doi: 10.1002/syn.10302. [DOI] [PubMed] [Google Scholar]
- 102.Hunter RG, et al. Regulation of CART mRNA in the rat nucleus accumbens via D3 dopamine receptors. Neuropharmacology. 2006;50:858–864. doi: 10.1016/j.neuropharm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Hubert GW, Kuhar MJ. Colocalization of CART peptide with prodynorphin and dopamine D1 receptors in the rat nucleus accumbens. Neuropeptides. 2006;40:409–415. doi: 10.1016/j.npep.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine- regulated transcript peptide 55–102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–792. [PubMed] [Google Scholar]
- 105.Shieh KR. Effects of the cocaine- and amphetamine-regulated transcript peptide on the turnover of central dopaminergic neurons. Neuropharmacology. 2003;44:940–948. doi: 10.1016/s0028-3908(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 106.Dallvechia-Adams S, Kuhar MJ, Smith Y. Cocaine- and amphetamine-regulated transcript peptide projections in the ventral midbrain: colocalization with γ-aminobutyric acid, melanin-concentrating hormone, dynorphin, and synaptic interactions with dopamine neurons. J Comp Neurol. 2002;448:360–372. doi: 10.1002/cne.10268. [DOI] [PubMed] [Google Scholar]
- 107.Freeman WM, et al. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ. Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther. 2003;307:1038–1044. doi: 10.1124/jpet.103.052332. [DOI] [PubMed] [Google Scholar]
- 109.Kim JH, Creekmore E, Vezina P. Microinjection of CART peptide 55–102 into the nucleus accumbens blocks amphetamine-induced locomotion. Neuropeptides. 2003;37:369–373. doi: 10.1016/j.npep.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 110.Kim S, Yoon HS, Kim JH. CART peptide 55–102 microinjected into the nucleus accumbens inhibits the expression of behavioral sensitization by amphetamine. Regul Pept. 2007;144:6–9. doi: 10.1016/j.regpep.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 111.Jaworski JN, Hansen ST, Kuhar MJ, Mark GP. Injection of CART (cocaine- and amphetamine-regulated transcript) peptide into the nucleus accumbens reduces cocaine-self-administration in rats. Behav Brain Res. 2008;191:266–271. doi: 10.1016/j.bbr.2008.03.039. This drug self-administration paper demonstrated that CART peptides might suppress cocaine-induced reward. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Couceyro PR, Koylu EO, Kuhar MJ. Further studies on the anatomical distribution of CART by in situ hybridization. J Chem Neuroanat. 1997;12:229–241. doi: 10.1016/s0891-0618(97)00212-3. [DOI] [PubMed] [Google Scholar]
- 113.Stanley SA, et al. Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res. 2001;893:186–194. doi: 10.1016/s0006-8993(00)03312-6. This was one of the first papers to demonstrate a relationship between CART peptides and the stress-response system. [DOI] [PubMed] [Google Scholar]
- 114.Kuriyama G, et al. Cocaine- and amphetamine-regulated transcript peptide in the rat anterior pituitary gland is localized in gonadotrophs and suppresses prolactin secretion. Endocrinology. 2004;145:2542–2550. doi: 10.1210/en.2003-0845. [DOI] [PubMed] [Google Scholar]
- 115.Raptis S, et al. Cocaine- and amphetamine-regulated transcript co-contained in thyrotropin-releasing hormone (TRH) neurons of the hypothalamic paraventricular nucleus modulates TRH-induced prolactin secretion. Endocrinology. 2004;145:1695–1699. doi: 10.1210/en.2003-1576. [DOI] [PubMed] [Google Scholar]
- 116.Baranowska B, Wolinska-Witort E, Martynska L, Chmielowska M, Baranowska-Bik A. Effects of cocaine-amphetamine regulated transcript (CART) on hormone release. Regul Pept. 2004;122:55–59. doi: 10.1016/j.regpep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 117.Smith SM, et al. Cocaine- and amphetamine-regulated transcript is localized in pituitary lactotropes and is regulated during lactation. Endocrinology. 2006;147:1213–1223. doi: 10.1210/en.2005-1392. [DOI] [PubMed] [Google Scholar]
- 118.Baranowska B, et al. Controversial opinions on the role of CART in prolactin release. Neuro Endocrinol Lett. 2007;27:60–62. [PubMed] [Google Scholar]
- 119.Dun NJ, Dun SL, Kwok EH, Yang J, Chang J. Cocaine- and amphetamine-regulated transcript-immunoreactivity in the rat sympatho-adrenal axis. Neurosci Lett. 2000;283:97–100. doi: 10.1016/s0304-3940(00)00935-6. [DOI] [PubMed] [Google Scholar]
- 120.Dun SL, Chianca DA, Jr, Dun NJ, Yang J, Chang JK. Differential expression of cocaine- and amphetamine-regulated transcript-immunoreactivity in the rat spinal preganglionic nuclei. Neurosci Lett. 2000;294:143–146. doi: 10.1016/s0304-3940(00)01575-5. [DOI] [PubMed] [Google Scholar]
- 121.Dun SL, Ng YK, Brailoiu GC, Ling EA, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide-immunoreactivity in adrenergic C1 neurons projecting to the intermediolateral cell column of the rat. J Chem Neuroanat. 2002;23:123–132. doi: 10.1016/s0891-0618(01)00147-8. [DOI] [PubMed] [Google Scholar]
- 122.Dall Vechia S, Lambert PD, Couceyro PC, Kuhar MJ, Smith Y. CART peptide immunoreactivity in the hypothalamus and pituitary in monkeys: analysis of ultrastructural features and synaptic connections in the paraventricular nucleus. J Comp Neurol. 2000;416:291–308. doi: 10.1002/(sici)1096-9861(20000117)416:3<291::aid-cne2>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 123.Broberger C. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 1999;848:101–113. doi: 10.1016/s0006-8993(99)01977-0. [DOI] [PubMed] [Google Scholar]
- 124.Dun SL, Brailoiu GC, Yang J, Chang JK, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide and sympatho-adrenal axis. Peptides. 2006;27:1949–1955. doi: 10.1016/j.peptides.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 125.Vrang N, Larsen PJ, Tang-Christensen M, Larsen LK, Kristensen P. Hypothalamic cocaine-amphetamine regulated transcript (CART) is regulated by glucocorticoids. Brain Res. 2003;965:45–50. doi: 10.1016/s0006-8993(02)04064-7. [DOI] [PubMed] [Google Scholar]
- 126.Smith SM, et al. Cocaine- and amphetamine-regulated transcript activates the hypothalamic-pituitary-adrenal axis through a corticotropin-releasing factor receptor-dependent mechanism. Endocrinology. 2004;145:5202–5209. doi: 10.1210/en.2004-0708. [DOI] [PubMed] [Google Scholar]
- 127.Balkan B, Koylu EO, Kuhar MJ, Pogun S. The effect of adrenalectomy on cocaine and amphetamine-regulated transcript (CART) expression in the hypothalamic nuclei of the rat. Brain Res. 2001;917:15–20. doi: 10.1016/s0006-8993(01)02899-2. [DOI] [PubMed] [Google Scholar]
- 128.Balkan B, Koylu E, Pogun S, Kuhar MJ. Effects of adrenalectomy on CART expression in the rat arcuate nucleus. Synapse. 2003;50:14–19. doi: 10.1002/syn.10213. [DOI] [PubMed] [Google Scholar]
- 129.Savontaus E, Conwell IM, Wardlaw SL. Effects of adrenalectomy on AGRP, POMC, NPY and CART gene expression in the basal hypothalamus of fed and fasted rats. Brain Res. 2002;958:130–138. doi: 10.1016/s0006-8993(02)03674-0. [DOI] [PubMed] [Google Scholar]
- 130.Stanley SA, et al. Regulation of rat pituitary cocaine- and amphetamine-regulated transcript (CART) by CRH and glucocorticoids. Am J Physiol Endocrinol Metab. 2004;287:E583–E590. doi: 10.1152/ajpendo.00576.2003. [DOI] [PubMed] [Google Scholar]
- 131.Koylu EO, et al. Co-localization of cart peptide immunoreactivity and nitric oxide synthase activity in rat hypothalamus. Brain Res. 2000;868:352–357. doi: 10.1016/s0006-8993(00)02290-3. [DOI] [PubMed] [Google Scholar]
- 132.Vicentic A, Hunter RG, Kuhar MJ. Effect of corticosterone on CART peptide levels in rat blood. Peptides. 2005;26:531–533. doi: 10.1016/j.peptides.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 133.Sergeyev V, Broberger C, Hokfelt T. Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus. Brain Res Mol Brain Res. 2001;90:93–100. doi: 10.1016/s0169-328x(01)00088-2. [DOI] [PubMed] [Google Scholar]
- 134.Kong WM, et al. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. FASEB J. 2003;17:1688–1690. doi: 10.1096/fj.02-0805fje. [DOI] [PubMed] [Google Scholar]
- 135.Gozen O, et al. Sex differences in the regulation of cocaine and amphetamine-regulated transcript expression in hypothalamic nuclei of rats by forced swim stress. Synapse. 2007;61:561–568. doi: 10.1002/syn.20395. [DOI] [PubMed] [Google Scholar]
- 136.Hunter RG, et al. Regulation of CART mRNA by stress and corticosteroids in the hippocampus and amygdala. Brain Res. 2007;1152:234–240. doi: 10.1016/j.brainres.2007.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kask A, Schioth HB, Mutulis F, Wikberg JE, Rago L. Anorexigenic cocaine- and amphetamine-regulated transcript peptide intensifies fear reactions in rats. Brain Res. 2000;857:283–285. doi: 10.1016/s0006-8993(99)02383-5. [DOI] [PubMed] [Google Scholar]
- 138.Chaki S, Kawashima N, Suzuki Y, Shimazaki T, Okuyama S. Cocaine- and amphetamine-regulated transcript peptide produces anxiety-like behavior in rodents. Eur J Pharmacol. 2003;464:49–54. doi: 10.1016/s0014-2999(03)01368-2. [DOI] [PubMed] [Google Scholar]
- 139.Stanek LM. Cocaine- and amphetamine related transcript (CART) and anxiety. Peptides. 2006;27:2005–2011. doi: 10.1016/j.peptides.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 140.Bjorkqvist M, et al. Cocaine- and amphetamine-regulated transcript is increased in Huntington disease. Mov Disord. 2007;22:1952–1954. doi: 10.1002/mds.21447. [DOI] [PubMed] [Google Scholar]
- 141.Orsetti M, Di Briscoe F, Canonico PL, Genazzani AA, Ghi P. Gene regulation in the frontal cortex of rats exposed to the chronic mild stress paradigm, an animal model of human depression. Eur J Neurosci. 2008;27:2156–2164. doi: 10.1111/j.1460-9568.2008.06155.x. [DOI] [PubMed] [Google Scholar]
- 142.del Giudice EM, et al. Adolescents carrying a missense mutation in the CART gene exhibit increased anxiety and depression. Depress Anxiety. 2006;23:90–92. doi: 10.1002/da.20156. [DOI] [PubMed] [Google Scholar]
- 143.Fenwick NM, Martin CL, Llewellyn-Smith IJ. Immunoreactivity for cocaine- and amphetamine-regulated transcript in rat sympathetic preganglionic neurons projecting to sympathetic ganglia and the adrenal medulla. J Comp Neurol. 2006;495:422–433. doi: 10.1002/cne.20870. [DOI] [PubMed] [Google Scholar]
- 144.Burman KJ, Sartor DM, Verberne AJ, Llewellyn-Smith IJ. Cocaine- and amphetamine-regulated transcript in catecholamine and noncatecholamine presympathetic vasomotor neurons of rat rostral ventrolateral medulla. J Comp Neurol. 2004;476:19–31. doi: 10.1002/cne.20198. [DOI] [PubMed] [Google Scholar]
- 145.Richardson RJ, Grkovic I, Anderson CR. Cocaine- and amphetamine-related transcript peptide and somatostatin in rat intracardiac ganglia. Cell Tissue Res. 2006;324:17–24. doi: 10.1007/s00441-005-0087-y. [DOI] [PubMed] [Google Scholar]
- 146.Matsumura K, Tsuchihashi T, Abe I. Central human cocaine- and amphetamine-regulated transcript peptide 55–102 increases arterial pressure in conscious rabbits. Hypertension. 2001;38:1096–1100. doi: 10.1161/hy1101.092968. [DOI] [PubMed] [Google Scholar]
- 147.Hwang LL, Chen CT, Li TL, Chiu CZ, Chi SF. Central pressor effects of CART peptides in anesthetized rats. Neuropeptides. 2004;38:69–76. doi: 10.1016/j.npep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 148.Iliff JJ, Alkayed NJ, Gloshani KJ, Traystman RJ, West GA. Cocaine- and amphetamine-regulated transcript (CART) peptide: a vasoactive role in the cerebral circulation. J Cereb Blood Flow Metab. 2005;25:1376–1385. doi: 10.1038/sj.jcbfm.9600136. [DOI] [PubMed] [Google Scholar]
- 149.Kozsurek M, et al. Cocaine- and amphetamine-regulated transcript peptide (CART) is present in peptidergic C primary afferents and axons of excitatory interneurons with a possible role in nociception in the superficial laminae of the rat spinal cord. Eur J Neurosci. 2007;26:1624–1631. doi: 10.1111/j.1460-9568.2007.05789.x. [DOI] [PubMed] [Google Scholar]
- 150.Damaj MI, Martin BR, Kuhar MJ. Antinociceptive effects of supraspinal rat cart (55–102) peptide in mice. Brain Res. 2003;983:233–236. doi: 10.1016/s0006-8993(03)03094-4. [DOI] [PubMed] [Google Scholar]
- 151.Bannon AW, et al. Multiple behavioral effects of cocaine- and amphetamine-regulated transcript (CART) peptides in mice: CART 42–89 and CART 49–89 differ in potency and activity. J Pharmacol Exp Ther. 2001;299:1021–1026. [PubMed] [Google Scholar]
- 152.Damaj MI, Hunter RG, Martin BR, Kuhar MJ. Intrathecal CART (55–102) enhances the spinal analgesic actions of morphine in mice. Brain Res. 2004;1024:146–149. doi: 10.1016/j.brainres.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 153.Damaj MI, Zheng J, Martin BR, Kuhar MJ. Intrathecal CART (55–102) attenuates hyperalgesia and allodynia in a mouse model of neuropathic but not inflammatory pain. Peptides. 2006;27:2019–2023. doi: 10.1016/j.peptides.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 154.Elefteriou F, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 155.Ahn JD, Dubern B, Lubrano-Berthelier C, Clement K, Karsenty G. Cart overexpression is the only identifiable cause of high bone mass in melanocortin 4 receptor deficiency. Endocrinology. 2006;147:3196–3202. doi: 10.1210/en.2006-0281. [DOI] [PubMed] [Google Scholar]
- 156.Patel MS, Elefteriou F. The new field of neuroskeletal biology. Calcif Tissue Int. 2007;80:337–347. doi: 10.1007/s00223-007-9015-3. [DOI] [PubMed] [Google Scholar]
- 157.Louis JCM. Methods of preventing neuron degeneration and promoting neuron regeneration. #WO96/34619. Amgen Int Patent Appl Publication. 1996
- 158.Xu Y, et al. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci USA. 2006;103:14489–14494. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu B, Hu S, Yang M, Pan H, Zhu S. CART peptide promotes the survival of hippocampal neurons by upregulating brain-derived neurotrophic factor. Biochem Biophys Res Commun. 2006;347:656–661. doi: 10.1016/j.bbrc.2006.06.117. [DOI] [PubMed] [Google Scholar]
- 160.Brischoux F, Fellmann D, Risold PY. Ontogenetic development of the diencephalic MCH neurons: a hypothalamic ‘MCH area’ hypothesis. Eur J Neurosci. 2001;13:1733–1744. doi: 10.1046/j.0953-816x.2001.01552.x. [DOI] [PubMed] [Google Scholar]
- 161.Brischoux F, Griffond B, Fellmann D, Risold PY. Early and transient ontogenetic expression of the cocaine- and amphetamine-regulated transcript peptide in the rat mesencephalon: correlation with tyrosine hydroxylase expression. J Neurobiol. 2002;52:221–229. doi: 10.1002/neu.10077. [DOI] [PubMed] [Google Scholar]
- 162.Dun SL, Castellino SJ, Yang J, Chang JK, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide-immunoreactivity in dorsal motor nucleus of the vagus neurons of immature rats. Brain Res Dev Brain Res. 2001;131:93–102. doi: 10.1016/s0165-3806(01)00267-x. [DOI] [PubMed] [Google Scholar]
- 163.Adan RA, Vanderschuren LJ, la Fleur ES. Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol Sci. 2008;29:208–217. doi: 10.1016/j.tips.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 164.Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol Behav. 2007;92:263–271. doi: 10.1016/j.physbeh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 165.Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal satiety signals. Annu Rev Physiol. 2008;70:239–255. doi: 10.1146/annurev.physiol.70.113006.100506. [DOI] [PubMed] [Google Scholar]
- 166.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 167.Jensen PB, et al. The hypothalamic satiety peptide CART is expressed in anorectic and non-anorectic pancreatic islet tumors and in the normal islet of Langerhans. FEBS Lett. 1999;447:139–143. doi: 10.1016/s0014-5793(99)00291-4. [DOI] [PubMed] [Google Scholar]
- 168.Wierup N, et al. Cocaine- and amphetamine-regulated transcript (CART) is expressed in several islet cell types during rat development. J Histochem Cytochem. 2004;52:169–177. doi: 10.1177/002215540405200204. [DOI] [PubMed] [Google Scholar]
- 169.Wierup N, Bjorkqvist M, Kuhar MJ, Mulder H, Sundler F. CART regulates islet hormone secretion and is expressed in the beta-cells of type 2 diabetic rats. Diabetes. 2006;55:305–311. doi: 10.2337/diabetes.55.02.06.db04-1383. [DOI] [PubMed] [Google Scholar]
- 170.Hubert GW, Jones DC, Moffett MC, Rogge G, Kuhar MJ. CART peptides as modulators of dopamine and psychostimulants and interactions with the mesolimbic dopaminergic system. Biochem Pharmacol. 2008;75:57–62. doi: 10.1016/j.bcp.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Barrett P, et al. The differential regulation of CART gene expression in a pituitary cell line and primary cell cultures of ovine pars tuberalis cells. J Neuroendocrinol. 2001;13:347–352. doi: 10.1046/j.1365-2826.2001.00634.x. [DOI] [PubMed] [Google Scholar]


