Abstract
Genetic testing is poised to play a greater role in the diagnosis and management of pulmonary arterial hypertension (PAH). Physicians who manage PAH should know the heritable PAH phenotypes, inheritance patterns, and responsible genes. They also should know indications, potential risks and benefits, and the issues surrounding genetic counselling and testing for patients with PAH.
The clinical classification of pulmonary hypertension includes 5 diagnostic groups, with diagnostic group 1 encompassing pulmonary arterial hypertension (PAH), a rare disorder that elevates pulmonary arterial pressure and ultimately causes right ventricular failure. The diagnostic subgroups of PAH include idiopathic PAH (IPAH), heritable PAH (HPAH), and PAH associated (APAH) with other disorders. The 4th World Symposium on Pulmonary Hypertension added HPAH to include patients with PAH and more than 1 family member diagnosed with PAH or with an identified mutation known to cause PAH. The classification committee of the 5th World Symposium on Pulmonary Hypertension designated pulmonary capillary hemangiomatosis (PCH) and pulmonary veno-occlusive disease (PVOD) as group 1, recognizing similarities to diagnostic group 1 PAH and unique clinicopathologic features of these poorly understood disorders.
In the past 15 years, there have been discoveries of genetic mutations that can cause HPAH, PCH, or PVOD. Identification of mutation carriers who are at increased risk for the development of PAH, PCH, or PVOD may facilitate earlier diagnosis and treatment of these disorders.
The aims of this review were to (1) identify HPAH phenotypes, (2) describe HPAH inheritance patterns, (3) identify genes that cause HPAH, and (4) describe elements of genetic counselling and testing for families with HPAH.
PAH Phenotypes, Inheritance Patterns, and Gene Mutations
Patients with IPAH represent the most common HPAH phenotype. A family history of PAH is often lacking. As a result, physicians often diagnose patients with IPAH before the heritable nature of the disease becomes apparent. For this reason, genetic counselling and testing must be considered when a physician diagnoses IPAH, as well as when a physician recognizes more than 1 family member affected by PAH.
PAH accompanying hereditary hemorrhagic telangiectasia (HHT), PCH, or PVOD represent rare phenotypes of HPAH. The physician should also consider genetic counselling and testing for patients affected by these uncommon disorders.
Idiopathic PAH and Heritable PAH Without HHT
In 1984, Loyd et al.1 described 14 families affected by PAH. These family pedigrees suggested autosomal dominant inheritance with incomplete penetrance. This pattern of inheritance is common to most forms of HPAH and amplifies the challenge of identifying heritable disease. To date, investigators have identified 4 principal genetic causes of HPAH occurring without HHT.
BMPR2
The major gene associated with HPAH is bone morphogenetic protein receptor type 2 (BMPR2). BMPR2 mutations cause approximately 75% of familial cases of PAH and 5% to 20% of cases of IPAH. More than 350 BMPR2 mutations have been described, and most families have a unique mutation.
BMPR2 encodes a protein that is a cell surface receptor (BMPRII). BMPRII is a member of the transforming growth factor β (TGFβ) superfamily of receptors, which signal intracellularly after binding ligand. BMPR2 mutations appear to trigger inappropriate cell growth and proliferation.
SMAD9
The discovery of BMPR2 suggested additional candidate genes to screen for mutations that may contribute to PAH. Exploration of the TGFβ pathway identified 3 somatic mutations in SMAD9 in patients with PAH.
CAV1
The availability of whole exome sequencing provided an opportunity to search for novel gene mutations associated with familial PAH. This approach resulted in the discovery that mutations in caveolin-1 (CAV1), a gene that encodes a protein involved in plasma membrane integrity and nitric oxide production, is associated with a minority of PAH cases.
KCNK3
In 2013, investigators again used whole exome sequencing to investigate a family affected by PAH without mutations in any of the known PAH genes. This approach resulted in the discovery that mutations in KCNK3, a gene that codes for 2 pore domain potassium channels expressed in pulmonary artery smooth muscle cells, are associated with a minority of PAH cases. Mutations in KCNK3 account for approximately 3% of familial PAH cases and 1% of IPAH cases.
PAH With Hereditary Hemorrhagic Telangiectasia
HHT is a vascular disease that has many clinical manifestations, including bleeding from and shunt physiology through arteriovenous malformations in the nasal mucosa, lung, liver, brain, and gastrointestinal tract. PAH is present in some patients with HHT and often presents without the stigmata of HHT in younger individuals. PAH with HHT is inherited in an autosomal dominant manner.
ACVRL1 and ENG
Scientists also demonstrated that PAH accompanying HHT can involve mutations in activin-receptor-like kinase 1 (ACVRL1) or endoglin (ENG). Like BMPR2, ACVRL1 and ENG affect vascular proliferation, and their pleiotropic nature may explain the spectrum of vascular pathologic conditions observed in patients with these mutations.
Pulmonary Veno-occlusive Disease/PCH
PCH and PVOD are rare disorders defined by histopathologic examination of lung tissue. PCH was originally described as a malignant proliferation of endothelial cells lining capillary-like channels, often accompanied by obstruction of pulmonary veins, alveolar hemosiderosis, and interstitial fibrosis. PVOD was first described as a condition that mimicked PAH clinically, but fibrous involvement of the pulmonary veins with capillary engorgement and alveolar hemosiderosis, rather than pulmonary arteriopathy, characterized PVOD.
Both PVOD and PCH present with nonspecific symptoms of progressive dyspnea, cough, fatigue, and occasionally hemoptysis. Hypoxemia and severe reductions of diffusion capacity of carbon monoxide are often identified. Chest computed tomography characteristically shows centrilobular ground glass opacities, septal lines, lymphadenopathy, and pleural effusion. An autosomal recessive pattern of inheritance characterizes both familial PCH and PVOD.2
EIF2AK4
Recently, researchers identified mutations in the gene eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4) as being causative for PCH/PVOD. EIF2AK4 is a protein member of a family of kinases that respond to cellular stress. Additionally, EIF2AK4 regulates angiogenesis.
Genetic Counselling and Genetic Testing for PAH
Overview
Health professionals must first recognize which patients with PAH are candidates for genetic counselling and testing. Patients diagnosed with familial PAH, HHT with PAH, and familial PCH/PVOD are candidates for genetic counselling and testing. Furthermore, consensus guideline committees recommend that patients with IPAH (and by extension sporadic PCH/PVOD) be advised about the availability of genetic counselling and testing because of the possibility that they carry a disease causing mutations.3,4
Genetic counselling
Genetic counseling is often performed by a genetic counsellor and should precede genetic testing and review the inheritance of PAH, HHT with PAH, or PCH/PVOD and the possible psychosocial effects of genetic test results.
Genetic counselling educates symptomatic patients before they decide whether or not to undergo genetic testing. Patients need to understand that genetic test results will allow them to know whether they carry a PAH gene mutation and whether other members of their family may have inherited an increased risk for the development of PAH. Professional societies recommend that health providers encourage patients to disclose genetic test results to their families. Subsequent genetic testing of asymptomatic family members also should include counselling by a genetic counsellor or geneticist, or both.
In families with an identified PH-associated mutation, family members without the mutation are unlikely to develop PAH (their risk of PAH is approximately equal to the risk for the general population). The identification of the family’s mutation in a family member substantially increases the risk that the person will develop PAH in their lifetime. However, for those with BMPR2 gene mutations, the risk differs according to sex. The risk of PAH among those with the family mutation is approximately 40% for females, and 15% for males.5
Patients may have concerns about possible discrimination based on genetic test results. In the United States, the Genetic Information Nondiscrimination Act (GINA) protects asymptomatic HPAH mutation carriers from having their health insurance access or rates changed based on genetic test results. However, GINA does not provide protection against discrimination in life, disability, or long-term care insurance.
Genetic testing for asymptomatic children presents special counselling challenges. Professionals often must balance the desire of parents to test their children against the rights of the children to maintain their autonomy and future choice about genetic testing. Test results may have harmful psychosocial effects on children and their parents. Identification of a familial mutation is often associated with feelings of guilt in the parent who has passed the mutation on to the child. Professional societies stress the importance of assessing whether or not a child and family will receive a timely substantial benefit as a result of the test.5 If the benefits are uncertain or will be deferred to later in life, predictive testing in asymptomatic children may not be justified.
Genetic testing for PAH
Indications, benefits, and risks
The most commonly cited reason for genetic testing for PAH is to provide information for current and future children. However, genetic testing can also provide information for adult family members about their risk for the development of PAH. For families affected by PAH, genetic tests can estimate the risk that future children will acquire PAH. Preimplantation genetic diagnosis has been used to avoid transmission of PAH gene mutations to offspring once a familial mutation was identified. Prenatal genetic testing (eg, amniocentesis or chorionic villus sampling) is used rarely, because most couples would not terminate a pregnancy for a disorder with such low penetrance.
Tests and costs
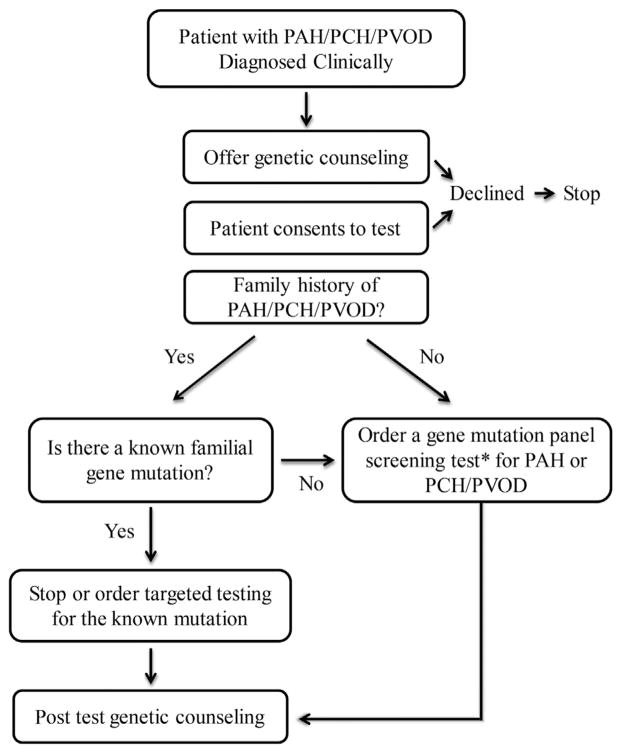
Genetic testing ideally starts with a symptomatic PAH (not APAH), PCH, or PVOD patient (Fig. 1). Identification of a mutation then allows for inexpensive targeted testing of consenting asymptomatic family members. At this time, identification of a mutation in symptomatic patients with PAH generally does not change management, and genetic testing primarily allows for improved risk stratification of other family members. Genetic testing is most helpful when it is able to identify members of the family who are not genetically at risk for PAH and who can forgo serial evaluations to screen for PAH. For at-risk asymptomatic family members, regular screening by echocardiography and awareness of disease symptoms should enable timely diagnosis and intervention to avoid disease progression without treatment.
Figure 1.
An approach to genetic testing and counselling for symptomatic patients with pulmonary capillary hemochromatosis/pulmonary veno-occlusive disease or with idiopathic or familial pulmonary arterial hypertension (PAH) but not associated PAH. The clinician offers genetic counselling to patients, making them aware of the possibility that they carry a disease-causing mutation and the importance of that knowledge for other family members. The patient may consent to genetic testing, in which case the clinician chooses a test based on the clinical circumstances.
Clinical genetic testing is available in North America and Europe, with results generally available in 8 weeks or less. Clinicians may order a variety of tests for BMPR2, ACVRL1, CAV1, ENG, SMAD9, KCNK3, and EIF2AK4, depending on the clinical circumstance (Table 1).
Table 1.
Genetic Tests for PAH, PCH, or PVOD
| Indication | Test | Comments |
|---|---|---|
| Identify mutation in a patient with PAH, not APAH | Sequencing of BMPR2 | May miss large deletions or duplications without MLPA or array comparative genomic hybridization |
| Multigene panel including BMPR2 | ||
| Targeted array comparative genomic hybridization | Often combined with sequencing-based tests for maximal sensitivity | |
| Identify mutation in a patient with PVOD/ PCH | Direct sequencing of EIF2AK4 | |
| Risk stratify a family member when the mutation is known | Targeted test | Lowest cost |
APAH, associated pulmonary arterial hypertension; MLPA, multiplex-dependent probe amplification; PAH, pulmonary arterial hypertension; PCH, pulmonary capillary hemangiomatosis; PVOD, pulmonary veno-occlusive disease.
The cost of genetic testing for PAH varies widely. Targeted DNA sequencing for a known familial gene is inexpensive (typically a few hundred dollars). However, next- generation sequencing-based gene panels that test all genes known to cause PAH for sequence variants and deletions/duplications are more expensive (typically a few thousand dollars). Although insurers generally cover genetic testing for PAH, preauthorization is often necessary to ensure reimbursement.
Whole exome sequencing
Rarely, in families with HPAH and multiple affected family members, a mutation will not be identified after exhaustive testing of the known genes for HPAH. In these cases, whole exome sequencing can be used to identify novel genes for PAH. This strategy may also be useful to identify the cause of sporadic PAH in children that may be caused by de novo or spontaneous new mutations. At this time, because of a variety of complexities, including high cost and professional interpretation, exome sequencing should be undertaken in concert with a clinical geneticist only.
Conclusions
Genetic testing is available for PAH and should be considered to identify asymptomatic individuals who are at increased risk of the development of PAH. Genetic testing is a personal choice because there is currently insufficient information to alter care in symptomatic patients based on genotype. Genotype-positive asymptomatic individuals should undergo close longitudinal clinical follow-up to enable early diagnosis and treatment.
Acknowledgments
Funding Sources
Funding was received from NIH K23 HL 098743 (E.D.A.) and Intermountain Research & Medical Foundation (C.G.E.).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Loyd JE, Primm RK, Newman JH. Familial primary pulmonary hypertension: clinical patterns. Am Rev Respir Dis. 1984;129:194–7. doi: 10.1164/arrd.1984.129.1.194. [DOI] [PubMed] [Google Scholar]
- 2.Langleben D. Pulmonary capillary hemangiomatosis: the puzzle takes shape. Chest. 2014;145:197–9. doi: 10.1378/chest.13-2513. [DOI] [PubMed] [Google Scholar]
- 3.Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007;131:1917–28. doi: 10.1378/chest.06-2674. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–94. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 5.Committee on Ethics, American College of Obstetricians and Gynecologists, Gynecologists; Committee on Genetics, American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 410: Ethical issues in genetic testing. Obstet Gynecol. 2008;111:1495–502. doi: 10.1097/AOG.0b013e31817d252f. [DOI] [PubMed] [Google Scholar]