Abstract
Amyloid beta (Aβ) peptides are the major components of senile plaques, one of the main pathological hallmarks of Alzheimer disease (AD). However, Aβ peptides’ functions are not fully understood and seem to be highly pleiotropic. We hypothesized that plasma Aβ peptides concentrations could be a suitable endophenotype for a genome-wide association study (GWAS) designed to (i) identify novel genetic factors involved in amyloid precursor protein metabolism and (ii) highlight relevant Aβ-related physiological and pathophysiological processes. Hence, we performed a genome-wide association meta-analysis of four studies totaling 3 528 healthy individuals of European descent and for whom plasma Aβ1–40 and Aβ1–42 peptides levels had been quantified. Although we did not observe any genome-wide significant locus, we identified 18 suggestive loci (P<1 × 10−5). Enrichment-pathway analyses revealed canonical pathways mainly involved in neuronal functions, for example, axonal guidance signaling. We also assessed the biological impact of the gene most strongly associated with plasma Aβ1–42 levels (cortexin 3, CTXN3) on APP metabolism in vitro and found that the gene protein was able to modulate Aβ1–42 secretion. In conclusion, our study results suggest that plasma Aβ peptides levels are valid endophenotypes in GWASs and can be used to characterize the metabolism and functions of APP and its metabolites.
Keywords: Aβ, peptides, alzheimer, ctxn3, elderly, gwas, plasma
INTRODUCTION
The amyloid beta (Aβ) peptides are the major components of senile plaques—one of the main pathological hallmarks of Alzheimer disease (AD). Abnormal Aβ levels and aggregation of oligomeric Aβ peptides in the brain are thought to trigger a cascade of events that leads to synapse and neuron loss, progressive cognitive impairment and ultimately dementia.
The two major Aβ species (Aβx–40 and Aβx–42) are produced by sequential endoproteolysis of amyloid precursor protein (APP) by β-secretase and γ-secretase complex. Amyloid precursor protein can also undergo non-amyloidogenic proteolysis by α-secretase, which cleaves APP within the Aβ sequence and thereby precludes Aβ generation.1 Although APP metabolism has been extensively studied, it is likely that some of the factors involved in this complex process have yet to be identified and characterized.
Beyond the role of Aβ peptides in the AD process, little is known about their physiological roles which seem to be highly pleiotropic. Several lines of evidence suggest that Aβ peptides have a broad spectrum of biological functions: (i) they are produced by many different cell types; (ii) they are present in peripheral tissues as well as in the brain; (iii) they may act as ligands for various receptors and other molecules;2,3 (iv) they variously display neurotrophic, antioxidant, antimicrobial, vaso-constrictive and platelet aggregation-modulating properties at physiological concentrations.4–8
With a view to identifying novel factors involved in APP metabolism and characterizing Aβ-related physiological and pathophysiological processes, we hypothesized that Aβ peptides concentrations in biological fluids may constitute a suitable endophenotype for a genome-wide association study (GWAS). We decided to focus on plasma Aβ peptides concentrations for several reasons: (i) plasma samples are easy to collect and thus facilitate meta-analyses of individuals with both Aβ peptides concentrations and GWAS data; (ii) plasma Aβ peptides are associated with risk of incident dementia and hypertension;9–12 (iii) plasma Aβ1–40 and Aβ1–42 levels are heritable traits.13
Within this context, we developed a genome-wide association meta-analysis of four studies totaling 3 528 healthy individuals of European descent and for whom plasma Aβ1–40 and Aβ1–42 peptides levels had been assayed.
MATERIALS AND METHODS
Populations
The three-city (3C) study
The 3C study is a prospective cohort study of vascular risk factors for dementia. The methodology of the study has been described in detail elsewhere.14 The 3C study’s protocol was approved by the independent ethics committee at Kremlin-Bicêtre University Hospital (Paris). In 1999–2000, a sample of 9 294 community dwellers aged 65 and over was selected from the electoral rolls of three French cities: Bordeaux (n = 2 104), Dijon (n = 4 931) and Montpellier (n = 2 259). Of these, 880 participants were excluded due to lack of a blood sample or lack of participation in any of the follow-up examinations. This left a sample of 8 414 participants. A case-cohort study (3C1) was performed after 4 years of follow-up, in order to investigate non-standard risk markers for dementia, stroke and coronary heart disease.10 This sub-cohort was composed of 1 254 participants randomly selected in strata according to center, age (in 5-year age groups) and gender. Participants diagnosed with prevalent dementia at baseline or incident dementia during follow-up were excluded from the present analysis. Participants for whom at least one plasma Aβ concentration or covariate was missing and participants with an aberrant plasma Aβ concentration (more than four standard deviations above or below the mean) were also excluded. Finally, we excluded individuals of non-European descent or with missing genetic information. These selection steps allowed us to define a sample of 909 individuals. Another subset of 1 169 participants from the 3C Dijon Center (3C2) in whom plasma Aβ levels had been recently assayed was also available. A sample of 911 individuals was analyzed after application of the selection steps mentioned above.
The Rotterdam study
The Rotterdam study is an ongoing, prospective, population-based cohort study investigating risk factors and incidence of cardiovascular, neurodegenerative, locomotor and ophthalmological diseases in elderly people.15,16 From 1990–1993, all 10 275 residents of Ommoord (a district of Rotterdam) aged 55 years or older were invited to participate in an extensive home interview and two visits to the research center; 7 983 (78%) agreed. At the baseline clinical examination, blood samples were drawn from 7 050 individuals, of whom 7 047 underwent screening for dementia. Prevalent dementia was diagnosed in 334 of the latter. Hence, the cohort at risk of dementia comprised 6 713 participants. A random sub-cohort of 1 756 people was drawn from this source population for plasma Aβ concentration assessment.9 Individuals for whom at least one plasma Aβ concentration or co-variable measurement was missing were excluded, leading to final analysis set of 1 490 individuals.
The Pittsburgh cardiovascular health study cognition study (CHS-CS)
This study began in 1992–1994, at the time when the participants underwent an initial brain MRI scan.17 In 2002–2003, the incidence of dementia and mild cognitive impairment (MCI) diagnosed in 1998–1999 in the CHS-CS population was determined. Of the 924 participants examined in 1992–1994, a total of 532 normal and MCI participants were available for study in 1998–1999. These participants had undergone annual cognitive tests from 1989–1990 to 1998–1999 and complete neurologic and neuropsychological examinations in 1998–1999 and 2002–2003. In addition to the brain MRI data set obtained in 1992–1994, a second MRI session was performed in 1998–1999 and 157 participants also underwent MRI in 2002–2003. The brain MRI in 2002–2003 was performed when a participant’s status changed from normal to MCI, from MCI to dementia or from normal to dementia. Participants were included in this analysis if they were alive at both time points, had available blood samples from both 1998–1999 and 2002–2003 and had been classified according to the CHS’ cognitive criteria. Application of the exclusion criteria used in the 3C and Rotterdam study lead to an analysis set of 73 participants.
The Alzheimer’s disease neuroimaging initiative study (ADNI)
The ADNI study is a prospective, multicentre, longitudinal neuroimaging study that was launched in the USA in 2004 by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, the Food and Drug Administration, private pharmaceutical companies and non-profit organizations.18 It includes 819 adult participants aged 55–90 who fulfilled the entry criteria (a clinical diagnosis of amnestic MCI, probable AD or cognitively normal). The collected data encompasses clinical information, neuroimaging data and biological samples for molecular biomarker measurement (for more details, see http://www.adni-info.org). We further classified participants as ‘stable’ if they did not change their diagnostic status from cognitively normal to MCI/AD or from MCI to AD during a follow-up period of at least 36 months. Participants other than stable cognitively normals were excluded. After applying the same criteria as in the 3C study, 145 participants were included in the analyses.
Genotyping and imputation
All the above-mentioned studies used different Illumina platforms for genome-wide genotyping; these are described in detail elsewhere19–21 and summarized in Supplementary Material Table 1. In order to have a common set of single-nucleotide polymorphisms (SNPs) available for meta-analysis and after selecting individuals and genotyped SNPs on the basis of call rates, minor allele frequency and Hardy–Weinberg equilibrium test thresholds, SNPs were imputed in each study using the Hapmap 2 release 22 CEU population as a reference panel (see Supplementary Material Table 1 for precise filters used). All data sets were recoded to match the ‘+’ strand.
Amyloid beta peptide assays
The assays used in the different studies have been described in detail elsewhere.9,10,17,22 Briefly, in the 3C study, non-fasting plasma samples were collected in tubes containing sodium EDTA as an anticoagulant. After centrifugation, plasma samples were divided into aliquots in polypropylene tubes, stored at −80 °C and only thawed immediately before Aβ quantification. The plasma Aβ peptide assay was performed using an INNO-BIA kit (Innogenetics NV, Ghent, Belgium) based on a multiplex xMAP (Luminex, Austin, TX, USA) technique.
In the Rotterdam study, non-fasting blood samples obtained at baseline were placed in Vacutainer tubes (Becton Dickenson, Franklin Lakes, NJ, USA) containing sodium citrate. These samples were put on ice immediately and centrifuged within 60 min. Aliquots of plasma were stored at −80 °C. Plasma Aβ concentrations were determined using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) method (Pfizer, New York, NY, USA).
In the CHS-CS, the samples used for the assays (collected in 1998–1999 and 2002–2003) were defrosted in 2006 and measured at the same time using the same method. Plasma Aβ1–40 and Aβ1–42 levels were measured with a double-antibody sandwich ELISA based on a combination of the mouse monoclonal antibody 6E10 (which is specific for an epitope present within amino acid residues 3–16 of Aβ) and two different, specific anti-Aβ1–40 and -Aβ1–42 antibodies.
In the ADNI study, plasma samples were obtained from the ADNI biofluid repository at the University of Pennsylvania. The plasma samples were collected at the participating centers. After overnight fasting, plasma was collected in the morning by venipuncture and placed in Vacutainer tubes containing potassium K3 ethylene tetraacetate as an anticoagulant. After centrifugation, samples were placed in polypropylene transfer tubes (13 ml, Sarstedt Inc., Newton, NC, USA catalog number 60.541), frozen and shipped on dry ice to the UPenn Biomarker Core Laboratory, where they were stored temporarily at −80 °C. Within several weeks of receipt, the samples were thawed, aliquoted by 500 μl into polypropylene tubes (1.5 ml, Thermo Fisher Scientific, Waltham, MA, USA catalog number 05-408-129) and stored at −80 °C pending biochemical analyses. The plasma levels of Aβ1–40 and Aβ1–42 were quantified with the same INNO- BIA kit (Innogenetics NV, Ghent, Belgium) as in the 3C study.
Of note, all the characteristics of the populations are described in the Supplementary Table 3.
Cell culture and western blot analyses
The HEK293-APP695wt cell line was maintained in DMEM supplemented with 10% FBS, penicillin and streptomycin at 37 °C in a humidified atmosphere with 5% CO2. Transient transfection was performed using X-tremeGENE HP DNA Transfection Reagent (Roche Applied Science, Rotkreuz, Switzerland) according to the manufacturer’s recommendations. The pCNV6entry-mycCTXN3 vector (NM_001048252) containing RC215242 (a Myc-DDK-tagged ORF clone of the Homosapiens cortexin 3 gene) was purchased from Origene (Rockville, MD, USA). After 48 h of transfection, supernatants were replaced. After 4 h, cells were finally lysed and supernatants were recovered. Cell extracts (10–30 μg) were analyzed by SDS–PAGE. The antibody against the c-myc epitope (Life Technologies, catalog number 46-0603, Grand Island, NY, USA) was diluted 1/5000; The primary antibody used to measuring holo-APP and APP-CTF is the APPCter-C17, a well-characterized rabbit antibody raised against the last 17 amino acids of the human APP sequence23,24 and was diluted 1/10 000. A monoclonal mouse antibody (Sigma-Aldrich, St Louis, MO, USA) was used to detect β-actin (dilution: 1/10 000). Immunoreactive complexes were revealed using the ECL western blotting kit (Amersham, Piscatway, NJ, USA). Membranes were digitized using the ChemiDoc MP System (Bio-Rad, Marnes-la-Coquette, France).
Elisa
Aβ1–40 and Aβ1–42 peptides concentrations were measured using sandwich ELISAs (the Human Amyloid β (1–40) Assay Kit (IBL-Hamburg, Germany) and the INNOTEST β-Amyloid (1–42) (Innogenetics NV)), according to the manufacturer’s recommendations. The results were read at 450 nm with a spectrophotometer (Labsystems Multiskom MS, Waltham, MA, USA). At least three independent triplicate experiments in were performed. All ELISAs were performed twice on a same sample.
Immunofluorescence
Cells were washed with PBS and fixed in PBS containing 4% paraformaldehyde for 30 min at room temperature. Cells were permeabilized with 0.25% Triton X-100 in PBS for 10 min. After blocking in 1% bovine serum albumin (BSA), cells were incubated for 2 h at room temperature with primary antibodies listed above (diluted 1/100 in 1% BSA in PBS). Cells were then washed three times with PBS. Appropriate secondary antibodies (Alexa Fluor, Invitrogen, Grand Island, NY, USA) diluted at 1/400 were used. After washing, slides were mounted with Fluoromount (Sigma-Aldrich).
Proximity ligation assay (PLA)
All reagents used in the PLA were purchased from Olink Bioscience (Uppsala, Sweden). The PLAs were performed according to the manufacturer’s instructions by using anti-myc (diluted 1/100) and anti-holoAPP (diluted 1/100) as primary antibodies.
Genome-wide association analyses
Linear regression models were used to assess associations between genetic markers and plasma Aβ levels in each study. In each data set, plasma Aβ levels were transformed as z-scores by subtracting the mean and dividing by the standard deviation, in order to account for differences in assay methods. SNPs data were analyzed either as allele dosages or imputation probabilities, depending on the imputation software used (see Supplementary Material Table 1). All models were systematically adjusted for age at blood collection and gender. An additional adjustment for study center was done in the 3C1 sample. Population substructures were taken into account by adjustment for principal components when they were significantly associated with plasma Aβ levels. Of note, all these adjustments did not notably modify the association observed for the best signals (see Table 1 and Supplementary Table 5 for comparison).
Table 1.
Results of a genome-wide meta-analysis of plasma Aβ levels
| SNP | Chr. | Position | Intragenic | Closest gene (50 kb) | Minor/major alleles | MAF (%) | Aβ1–42
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direction | Effect size | s.e. | I2 (%) | P for Cochran’s Q | P in GWAS | |||||||
| Aβ1–42 | ||||||||||||
| rs11241936 | 5 | 127006007 | No | CTXN3 | C/T | 31.2 | +++ − + | 0.08 | 0.02 | 38 | 0.17 | 2.32e - 07 |
| rs12761450 | 10 | 61555898 | Yes | ANK3 | T/G | 41.5 | −−−−− | − 0.07 | 0.02 | 0 | 0.83 | 1.24e - 06 |
| rs12611088 | 19 | 48764642 | Yes | XRCC1 | A/G | 37.1 | +++++ | 0.07 | 0.02 | 0 | 0.54 | 1.30e - 06 |
| rs17655565 | 12 | 50978225 | Yes | KRT86 | C/T | 10.9 | +++++ | 0.12 | 0.02 | 0 | 0.61 | 1.76e - 06 |
| rs12656502 | 5 | 108009646 | No | None | A/G | 22.8 | ++++ − | 0.08 | 0.02 | 22 | 0.28 | 3.27e - 06 |
| rs2176862 | 3 | 46625997 | No | LOC100132146 | T/C | 5.4 | ++++ − | 0.15 | 0.03 | 34 | 0.19 | 4.49e - 06 |
| rs7138951 | 12 | 79999764 | Yes | ACSS3 | G/A | 9.4 | ++++ − | 0.12 | 0.03 | 0 | 0.56 | 5.03e - 06 |
| rs10819795 | 9 | 102408184 | No | MURC | A/T | 2.5 | − ++++ | 0.23 | 0.05 | 34 | 0.19 | 7.36e -06 |
| Aβ1–40 | ||||||||||||
| rs3015469 | 14 | 50226910 | No | SAV1 | G/A | 31.4 | +++ −− | 0.04 | 0.02 | 34 | 0.19 | 2.39e - 02 |
| rs1335688 | 13 | 62292794 | No | None | C/T | 15.3 | +++++ | 0.07 | 0.02 | 44 | 0.13 | 1.14e - 03 |
| rs1995809 | 4 | 148063874 | Yes | TTC29 | G/A | 5.4 | +++++ | 0.04 | 0.03 | 15 | 0.32 | 2.71e - 01 |
| rs7151302 | 14 | 32753868 | Yes | NPAS3 | C/T | 13.6 | −−− + | − 0.08 | 0.02 | 65 | 0.02 | 3.08e - 04 |
| rs12422267 | 12 | 131167549 | Yes | EP400NL | G/A | 9.5 | +++++ | 0.04 | 0.02 | 23 | 0.27 | 1.04e - 01 |
| rs2403083 | 8 | 86295401 | Yes | E2F5 | C/A | 26.1 | −−−−− | − 0.04 | 0.02 | 0 | 0.72 | 3.44e - 02 |
| rs1341320 | 1 | 57739116 | Yes | DAB1 | G/A | 6.4 | −−−−− | − 0.09 | 0.03 | 0 | 0.88 | 2.33e - 03 |
| rs4263408 | 4 | 39461671 | No | UBE2K | T/C | 43.0 | − + − ++ | − 0.03 | 0.02 | 62 | 0.03 | 5.76e - 02 |
| rs8001893 | 13 | 33120261 | Yes | STARD13 | A/C | 2.5 | ++++? | 0.14 | 0.05 | 0 | 0.87 | 8.52e - 03 |
| rs108961 | 11 | 2714561 | Yes | KCNQ1 | T/C | 47.4 | +++++ | 0.03 | 0.02 | 72 | 0.007 | 2.85e - 02 |
| SNP | Chr. | Position | Intragenic | Closest gene (50 kb) | Minor/Major alleles | MAF (%) | Aβ1–40
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direction | Effect size | s.e. | I2 (%) | P for Cochran’s Q | P in GWAS | |||||||
| Aβ1–42 | ||||||||||||
| rs11241936 | 5 | 127006007 | No | CTXN3 | C/T | 31.2 | +++ − + | 0.03 | 0.02 | 27 | 0.24 | 1.22e - 01 |
| rs12761450 | 10 | 61555898 | Yes | ANK3 | T/G | 41.5 | ++ −− + | −0.01 | 0.02 | 0 | 0.52 | 7.51e - 01 |
| rs12611088 | 19 | 48764642 | Yes | XRCC1 | A/G | 37.1 | ++++ − | 0.07 | 0.02 | 22 | 0.28 | 7.58e - 04 |
| rs17655565 | 12 | 50978225 | Yes | KRT86 | C/T | 10.9 | +++++ | 0.07 | 0.03 | 0 | 0.79 | 3.74e - 02 |
| rs12656502 | 5 | 108009646 | No | None | A/G | 22.8 | −− ++ − | 0.04 | 0.02 | 53 | 0.07 | 1.45e -01 |
| rs2176862 | 3 | 46625997 | No | LOC100132146 | T/C | 5.4 | − +++ − | 0.05 | 0.04 | 6 | 0.37 | 2.74e -01 |
| rs7138951 | 12 | 79999764 | Yes | ACSS3 | G/A | 9.4 | ++++ − | 0.10 | 0.04 | 15 | 0.32 | 1.06e - 02 |
| rs10819795 | 9 | 102408184 | No | MURC | A/T | 2.5 | − ++ − + | 0.09 | 0.07 | 37 | 0.17 | 2.18e -01 |
| Aβ1–40 | ||||||||||||
| rs3015469 | 14 | 50226910 | No | SAV1 | G/A | 31.4 | +++++ | 0.11 | 0.02 | 54 | 0.07 | 8.35e - 07 |
| rs1335688 | 13 | 62292794 | No | None | C/T | 15.3 | +++++ | 0.14 | 0.03 | 0 | 0.69 | 1.04e - 06 |
| rs1995809 | 4 | 148063874 | Yes | TTC29 | G/A | 5.4 | +++ − + | 0.22 | 0.05 | 51 | 0.08 | 1.60e - 06 |
| rs7151302 | 14 | 32753868 | Yes | NPAS3 | C/T | 13.6 | −−−−− | −0.14 | 0.03 | 0 | 0.57 | 1.71e - 06 |
| rs12422267 | 12 | 131167549 | Yes | EP400NL | G/A | 9.5 | +++++ | 0.17 | 0.03 | 29 | 0.23 | 1.87e - 06 |
| rs2403083 | 8 | 86295401 | Yes | E2F5 | C/A | 26.1 | −−−−− | −0.11 | 0.02 | 0 | 0.96 | 3.27e - 06 |
| rs1341320 | 1 | 57739116 | Yes | DAB1 | G/A | 6.4 | −− ++ | −0.18 | 0.04 | 0 | 0.56 | 5.40e - 06 |
| rs4263408 | 4 | 39461671 | No | UBE2K | T/C | 43.0 | −− ++ | −0.09 | 0.02 | 47 | 0.11 | 6.98e - 06 |
| rs8001893 | 13 | 33120261 | Yes | STARD13 | A/C | 2.5 | ++++? | 0.30 | 0.07 | 0 | 0.61 | 7.11e - 06 |
| rs108961 | 11 | 2714561 | Yes | KCNQ1 | T/C | 47.4 | +++++ | 0.09 | 0.02 | 34 | 0.20 | 9.34e - 06 |
SNP: SNPs with the lowest P-value in a given locus for a particular plasma Aβ concentration. Chr.: chromosome. Position: positions of the SNPs (based on dbSNP build 130, Hg18 coordinates). MAF: minor allele frequency. Direction: directions of effect. The order is: 3C1, 3C2, Rotterdam, ADNI and CHS. s.e.: standard error.
Genome-wide meta-analysis
Single-nucleotide polymorphisms with a minor allele frequency below 1% or bad imputation quality (info score<0.8 or r-squared<0.3, depending on the imputation software used) were excluded (see Supplementary Table 2 for number of SNPs removed during filtering). A fixed-effect, inverse-variance weighted meta-analysis was then performed. Quantile-quantile (QQ) plots and the genomic inflation factor (lambda) were used to detect potential population stratification in each data set and in the meta-analyses (see Supplementary Material Figure 1). Additional correction on genomic inflation factor was performed and did not change the results. Genome-wide significance was defined for P-values below 5 × 10 −8 and suggestive association was defined for P-values comprised between 1 × 10 −5 and 5 × 10−8. SNPs showing suggestive associations with plasma Aβ levels were examined manually and forest plots were used to detect extreme heterogeneity or aberrant values. Heterogeneity was also assessed by performing random-effect meta-analyses and the Cochran’s Q-test.
Of note, the METAL script used for the GWAS analysis is described in the Supplementary method.
Pathway analyses
We selected a list of 1 762 genes containing at least one SNP associated with both plasma Aβ1–40 and Aβ1–42 (P <0.05) concentrations with the same direction of effect for pathway analyses using two softwares: Ingenuity Pathway Analysis and WebGestalt.
Ingenuity Pathway Analysis (IPA, November 2012) was used to detect enriched canonical pathways. Canonical pathways were determined by analyzing a ratio of the number of genes that map to the pathway divided by the total number of genes in the pathway using the Ingenuity knowledge base. This base contains expertly curated biological interactions and functional annotations from literature. We considered only direct and indirect experimentally observed relationships for this analysis. A Fisher exact test was used and to assess the significance of a pathway, we applied a Benjamini–Hochberg multiple testing correction.
WebGestalt (WEB-based Gene SeT AnaLysis Toolkit) incorporates different public resources and provides a gene-set enrichment analysis. We used the web version updated on 30th of January, 2013 and conducted a gene-set enrichment analysis on our gene list using Gene Ontology (version 1.2, 11/11/2012) as a reference set. We selected only gene ontology categories with a minimum of two genes. The enrichment analysis is based on an hypergeometric test and we corrected the P-value by a Benjamini–Hochberg multiple testing correction.
Software
Individual study analyses were performed with either R version 2.15.1 (2012-06-22),25 Plink,26 ProbABEL27 or SNPTEST version 2.2 (https://mathgen.stats.ox.ac.uk/genetics_software/snptest/snptest.html). Genome-wide meta-analysis was performed with METAL.28 Manhattan plots and quantiles-quantiles plots were generated for each study and for meta-analyses with R functions taken from the Getting Genetics Done blog (http://gettinggeneticsdone.blogspot.com/2011/04/annotated-manhattan-plots-and-qq-plots.html). For the top SNPs, random-effect meta-analyses were performed with the metafor R package.29 Forest plots were generated using the rmeta R package (http://CRAN.R-project.org/package=rmeta). Regional association plots were generated using LocusZoom version 1.1 (http://genome.sph.umich.edu/wiki/LocusZoom_Standalone). Pathway analyses were performed using Ingenuity Pathway Analysis (http://www.ingenuity.com/products/ipa) and WebGestalt (http://bioinfo.vanderbilt.edu/webgestalt/).30
RESULTS
The total sample was composed of 3 528 non-demented participants from European descent from the three-city study from France (n = 1 820), the Rotterdam study from the Netherlands (n = 1 490), the Cardiovascular Health Study–Cognition Study from the US (CHS-CS; n = 73) and the Alzheimer’s Disease Neuroimaging Initiative from the US (ADNI; n = 145). As expected, plasma Aβ1–40 and Aβ1–42 concentrations were strongly correlated in each study and significantly differed across studies (one-way analysis of variance (ANOVA) P-values<10 −16 for plasma Aβ1–42 and Aβ1–40 concentrations, see Supplementary Material Table 3). This latter observation led us to transform plasma Aβ levels into z-scores for each study. In each population, associations between Aβ1–42 or Aβ1–40 levels and SNPs were then evaluated in linear regression models adjusted for age, gender, center and principal components. After removing SNPs with low minor allele frequencies or bad imputation quality scores from each study (see Supplementary Material Table 2), we selected 2 316 178 SNPs present at least in the three-city and Rotterdam studies for meta-analysis. We filtered SNPs showing extreme heterogeneity (P-value in the Cochran’s Q-test <0.0001), excluding 1 725 SNPs for Aβ1–42 and 1 744 SNPs for Aβ1–40. QQ plots of the P-values were then generated (see Supplementary Material Figure 1). For both Aβ1–42 and Aβ1–40, no inflation of P-values was observed; the overall genomic inflation factors (lambda) were 1.02 for Aβ1–42 and 0.99 for Aβ1–40.
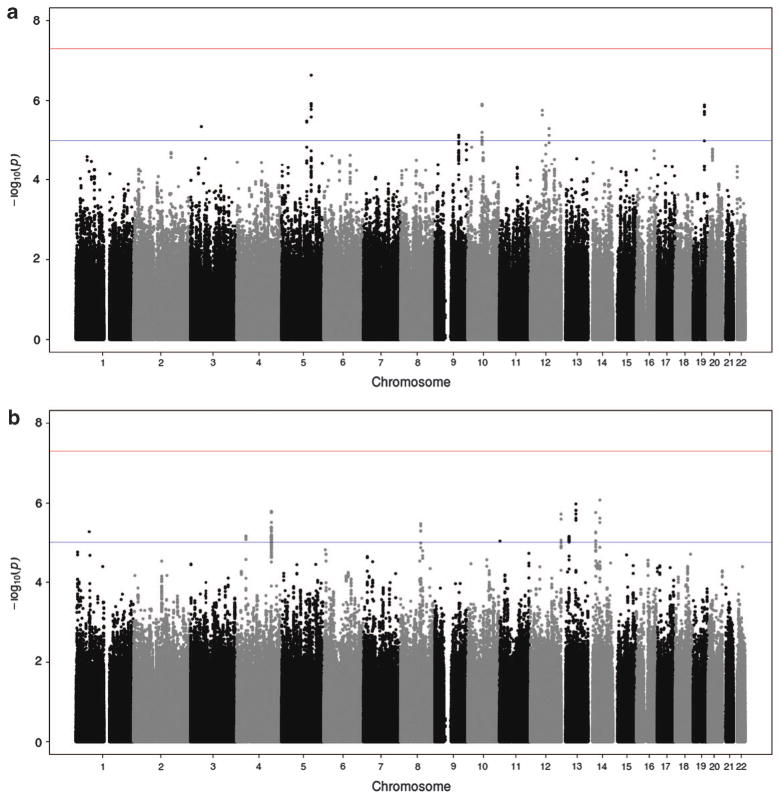
Manhattan plots of plasma Aβ1–40 and Aβ1–42 P-values are presented in Figure 1. Although none of the meta-analyzed SNPs attained genome-wide significance (P-values<5 × 10 −8), several suggestive signals (with P-values between 5 × 10 −8 and 1 × 10 −5) were identified. The SNPs with the lowest P-value in these loci are presented in Table 2 (see also detailed results in Supplementary Material Table 4 and forest plots and regional association plots in Supplementary Material Figures 2 to 19). We were unable to set up a replication stage due to the low number of available cohorts with both GWAS and plasma Aβ concentration data. We therefore decided to develop a number of complementary statistical, bioinformatics and biological approaches to assess the relevance of our results.
Figure 1.
Results of a genome-wide meta-analysis of plasma Aβ levels. The plots represent the - log10(P-values) of the SNPs on the y axis and chromosome positions on the x axis for (a) Aβ1-42 and (b) Aβ1 - 40 plasma levels. The red line indicates the genome-wide significance threshold (5 × 10−8) and the blue line indicates the suggestive significance threshold (1 × 10−5). SNP, single-nucleotide polymorphism.
Table 2.
Results of the ingenuity pathway or Webgestalt analysis for genes which presented at least one SNP associated with both plasma Aβ1–40 and Aβ1–42 (P <0.05) concentrations and with the same direction of effect
| Ingenuity canonical pathways | Corrected P-valuea | Webgestalt gene ontology categories | GO ID | Corrected P-valuea |
|---|---|---|---|---|
| Protein kinase A signaling | 3.72E - 06 | Neuron development | GO:0048666 | 9.48E - 10 |
| Axonal guidance signaling | 2.00E - 05 | Neuron projection development | GO:0031175 | 9.48E - 10 |
| Synaptic long term potentiation | 3.47E - 04 | Nervous system development | GO:0007399 | 1.28E - 09 |
| Melatonin signaling | 1.07E - 03 | Axon guidance | GO:0007411 | 1.60E - 09 |
| Role of NFAT in cardiac hypertrophy | 2.34E - 03 | Neurogenesis | GO:0022008 | 2.04E - 09 |
| Corticotropin releasing hormone signaling | 2.34E - 03 | Generation of neurons | GO:0048699 | 2.04E - 09 |
| Dopamine-DARPP32 feedback in cAMP signaling | 2.88E - 03 | Cell projection organization | GO:0030030 | 2.09E - 09 |
| ErbB signaling | 2.88E - 03 | Axonogenesis | GO:0007409 | 2.29E - 09 |
| Fcγ receptor-mediated phagocytosis in macrophages and monocytes | 3.02E - 03 | Cell projection | GO:0042995 | 2.55E - 09 |
| Synaptic long term depression | 3.09E - 03 | Cell junction | GO:0030054 | 2.55E - 09 |
| CREB signaling in neurons | 3.16E - 03 | Neuron projection morphogenesis | GO:0048812 | 2.61E - 09 |
| GNRH signaling | 3.16E - 03 | Cell morphogenesis involved in neuron differentiation | GO:0048667 | 2.61E - 09 |
| Cellular effects of sildenafil (Viagra) | 3.80E - 03 | Neuron differentiation | GO:0030182 | 2.61E - 09 |
| Netrin signaling | 7.24E - 03 | Multicellular organismal signaling | GO:0035637 | 3.43E - 09 |
| Neuregulin signaling | 8.13E - 03 | Transmission of nerve impulse | GO:0019226 | 1.40E - 08 |
| Hepatic cholestasis | 1.15E - 02 | Cell development | GO:0048468 | 3.13E - 08 |
| Renin-angiotensin signaling | 1.38E - 02 | Neuron projection | GO:0043005 | 5.17E - 08 |
| α-Adrenergic signaling | 1.51E - 02 | Cell projection morphogenesis | GO:0048858 | 5.33E - 08 |
| Endothelin-1 signaling | 1.74E - 02 | Cell morphogenesis involved in differentiation | GO:0000904 | 5.43E - 08 |
| Calcium signaling | 1.74E - 02 | Plasma membrane | GO:0005886 | 5.51E - 08 |
| G beta gamma signaling | 1.74E - 02 | Cell part morphogenesis | GO:0032990 | 6.74E - 08 |
| Gap junction signaling | 2.04E - 02 | Cell periphery | GO:0071944 | 7.59E - 08 |
| GABA receptor signaling | 3.02E - 02 | Cell projection part | GO:0044463 | 8.12E - 08 |
| P2Y purigenic receptor signaling pathway | 3.24E - 02 | Synapse | GO:0045202 | 1.03E - 07 |
| PTEN signaling | 3.24E - 02 | Cell adhesion | GO:0007155 | 1.11E - 07 |
| Sonic hedgehog signaling | 3.24E - 02 | Biological adhesion | GO:0022610 | 1.23E - 07 |
| PI3K signaling in B lymphocytes | 3.24E - 02 | Membrane | GO:0016020 | 2.25E - 07 |
Benjamini–Hochberg correction.
We first hypothesized that the genetic determinants of plasma Aβ concentrations were clustered within one or more specific biological pathways, rather than being randomly distributed. This type of analysis can indicate relevant pools of genes likely to genetically modulate APP metabolism and thus can provide clues about the function of Aβ peptides and APP. Hence, we generated a list of 1 762 genes containing at least one SNP associated with both plasma Aβ1–40 and Aβ1–42 (P <0.05) concentrations with the same direction of effect. Using Ingenuity Pathway Analysis (IPA), we observed 27 significantly enriched canonical pathways (out of 287) after multiple testing correction. These results are presented in Table 2. Interestingly, some of the pathways have been already described as being involved in APP metabolism (e.g., protein kinase A signaling and netrin signaling).31–34 Other pathways appear to be related to potential physiological functions of APP in the brain (e.g., axonal guidance signaling; see Supplementary Material Table 6)35,36 or directly associated with Aβ peptides’ properties, for example, blood pressure modulation (renin-angiotensin signaling).11 As pathway analyses depend on the tool, algorithm and pathway database used, we attempted to confirm our findings using the Webgestalt software. As a result, we observed 120 gene ontology categories significantly enriched (P<0.05 after correction) (See Table 2 for the most significant pathways). In line with the IPA findings, most of the significantly enriched pathways were related to potential physiological functions of APP in the brain and particularly, axonal guidance (see Supplementary Material Table 7 for the list of genes associated with Aβ plasma level and involved in this pathway).
To account for the eventuality of an artificial enrichment due to linkage disequilibrium between SNPs, we repeated IPA and Webgestalt analyses after filtering SNPs with r2 value ≥0.5 when comparing different SNPs. This resulted in the exclusion of three and four SNPs, respectively, and we were still able to identify axon guidance pathways displaying the overrepresentation of genes associated with Aβ plasma levels in our GWA data set (data not shown). We finally assessed the degree of consistency among data sets. We did not independently test CHS and ADNI because of their restricted sample size, and focused on results obtained from the Rotterdam study and in the combined 3C study. We observed consistent results among these data sets. For instance, using IPA, the Axonal guidance pathway was enriched in both Rotterdam and 3C studies (respectively, P = 7.1 × 10 −3 and P = 4.4 × 10 −3 after multiple testing correction). A similar observation was obtained using Webgestalt (P = 1.2 × 10 −6 in 3C and 8.0 × 10 −4 in Rotterdam after multiple testing correction).
We next took the gene most strongly associated with the plasma Aβ1–42 level (cortexin 3, CTXN3) in the meta-analysis and studied its influence on APP metabolism at the biological level. The results are presented in Figure 2. We tested the effect of CTXN3 overexpression on Aβ peptide secretion in a HEK cell line that stably expressed the APP695wt isoform. Following CTXN3 overexpression, Aβ1–42 secretion was significantly lower than in control experiments (37% lower; P = 0.02). A trend in the same direction was observed for Aβ1–40 secretion (27% lower, P = 0.10). Furthermore, we observed colocalization of APP and CTXN3 in HEK293 cells overexpressing both proteins. In order to control partially for potential biases due to proteins overexpression, we repeated the immunofluorescence experiments from endogenous APP and overexpressed CTXN3 in HEK293 cells (HEK293 cells do not express CTXN3). We still observed colocalization of APP and CTXN3 in this model. In conclusion, these data suggest that CTXN3 may lead to a decrease in Aβ peptide production by directly modulating APP metabolism.
Figure 2.
Overexpression of CTXN3-myc in a HEK293 cell line. (a) Representative western blots of extracts from HEK293-APP695wt—stably transfected with APP695wt—and transiently transfected with CTXN3-myc cDNA or a control empty plasmid (vector). Anti-APP (APPCter-C17), anti-myc (Invitrogen 46-0603) and anti-actin antibodies have been used for western blots. This experiment was repeated three times. (b) Measurement of secreted Aβ1–40 and Aβ1–42 by ELISA following transfection of the CTXN3-myc expression vector in APP-HEK293 cells. Variations in Aβ1–42 (left) and Aβ1–40 (right) secretion from three independent experiments (performed in duplicate) are shown. (c) Representative confocal images from immunofluorence staining of HEK293-APP695wt cells (APP695) or HEK293 (endogenous APP) transfected with CTXN3-myc using anti-myc (Invitrogen 46-0603, green) and anti-APP (APPCter-C17, red) antibodies. (d) An in situ proximity ligation assay measuring the interaction between overexpressed CTXN3 and holoAPP in HEK293 cells transfected as previously described above (in panel c). *P<0.05 in a Mann–Whitney non-parametric test); AU: arbitrary units.
Finally, we tried to replicate previously reported associations between SNPs and plasma Aβ1–42 levels and explored whether SNPs associated with cerebrospinal fluid concentrations of Aβ1–42 or AD risk would also be associated with plasma Aβ levels (see Table 3 and Supplementary Material Table 8). Only rs2075650, located in the APOE locus presented nominal level of association with plasma Aβ1–42 concentrations (P-value = 2.81 × 10 −2; see Table 3).
Table 3.
Results of a meta-analysis of plasma Aβ levels for SNPs known to be associated with the Alzheimer’s disease (AD) risk
| SNP | Chr. | Position | Gene | Minor/major alleles | MAF (%) | Aβ1–42
|
Aβ1–40
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direction | Effect size | s.e. | P in GWAS | I2 (%) | Direction | Effect size | s.e. | P in GWAS | I2 (%) | ||||||
| rs3818361 | 1 | 205851591 | CR1 | A/G | 19.4 | −−−− + | −0.0341 | 0.0184 | 6.41e - 02 | 0 | + − + − − | −0.0189 | 0.0255 | 4.61e - 01 | 60 |
| rs744373 | 2 | 127611085 | BIN1 | G/A | 28.9 | + −− ++ | −0.0046 | 0.0163 | 7.78e - 01 | 0 | + − − + − | −0.009 | 0.0225 | 6.91e - 01 | 41 |
| rs9296559 | 6 | 47560229 | CD2AP | C/T | 30.8 | ++ −− + | 0.005 | 0.0166 | 7.64e - 01 | 0 | ++ − ++ | 0.0136 | 0.0228 | 5.49e - 01 | 18 |
| rs11767557 | 7 | 142819261 | EPHA1 | C/T | 19.9 | −−− + − | −0.0205 | 0.0181 | 2.56e - 01 | 0 | −−− ++ | −0.0193 | 0.0249 | 4.39e - 01 | 54 |
| rs11136000 | 8 | 27520436 | CLU | T/C | 39.2 | − + −− + | −0.0032 | 0.0152 | 8.35e - 01 | 11 | −−−− + | −0.0151 | 0.0209 | 4.68e - 01 | 0 |
| rs610932 | 11 | 59695883 | MS4A6A | T/G | 43.8 | ++ −−− | 0.0035 | 0.0148 | 8.16e - 01 | 26 | + + − + − | 0.0185 | 0.0203 | 3.62e - 01 | 36 |
| rs3851179 | 11 | 85546288 | PICALM | T/C | 36.7 | −−− ++ | −0.0076 | 0.0151 | 6.16e - 01 | 63 | −−− ++ | −0.0366 | 0.0208 | 7.90e - 02 | 58 |
| rs3764650 | 19 | 997520 | ABCA7 | G/T | 8.7 | + −− ++ | −0.0136 | 0.0267 | 6.11e - 01 | 0 | + −−− + | −0.0305 | 0.0355 | 3.91e - 01 | 0 |
| rs2075650 | 19 | 50087459 | APOE | G/A | 14.0 | −−−− + | −0.0466 | 0.0212 | 2.81e - 02 | 77 | −− + − + | 0.0167 | 0.0307 | 5.87e -01 | 23 |
| rs3865444 | 19 | 56419774 | CD33 | A/C | 32.4 | −− + −− | −0.0016 | 0.0159 | 9.21e - 01 | 0 | −− + −− | −0.0156 | 0.022 | 4.78e - 01 | 0 |
Chr.: chromosome. Position: positions of the SNPs (based on dbSNP build 130, Hg18 coordinates). MAF: minor allele frequency. Direction: directions of effect. The order is: 3C1, 3C2, Rotterdam, ADNI and CHS. s.e.: standard error.
DISCUSSION
To our knowledge, the present study constitutes the first genome-wide association meta-analysis of plasma Aβ concentrations in non-demented participants. Although we did not observe any genome-wide significant locus, we identified 18 suggestive loci. Pathway analyses showed enrichment of canonical pathways involved in neuronal functions, for example, axonal guidance signaling. We also assessed the biological impact of the gene most strongly associated with plasma Aβ1–42 levels (cortexin 3, CTXN3) on APP metabolism in vitro and found that the gene protein was able to modulate Aβ1–42 secretion.
Set-up and performance of the study was subject to several methodological and technological limitations. First, environmental factors modulating Aβ plasma concentrations are poorly known. Thus, it is difficult to assess whether differences in alimentary habits or in medications across samples could influence our results. Second, plasma Aβ concentrations were assayed using different approaches and thus were not fully comparable. Therefore, we transformed the data into z-scores prior to the statistical analyses to minimize between-center variability. Third, few previous studies had both plasma Aβ concentrations and GWAS data available at the time of this study. This limited the power of our meta-analysis and we failed to generate results that were statistically significant on the genome-wide scale. Given the high number of association tests usually performed in GWASs, the likelihood of false-positive results is high and is mitigated by applying highly conservative alpha-risk corrections (e.g., the Bonferroni correction in many cases). Furthermore, our results may be influenced by the simultaneous involvement of many biological processes modulating Aβ peptide concentrations in plasma (e.g., peptide production, secretion, degradation and/or clearance); this would have weakened our ability to detect significant genome-wide signals. Finally, the main limitation of our study relates to the difficulty in obtaining relevant replication samples; this made it impossible for us to discriminate between false and true positives. One option would have been to split our data sets between discovery and replication stages. However, we considered that an excessively underpowered discovery stage would have led to a large number of false negatives.
Given these caveats, we used complementary approaches. The first was based on pathway enrichment analyses using the IPA software. The most enriched canonical pathway was protein kinase A signaling, which had already been described as modulator of APP metabolism.31,32 Remarkably, we also observed that a large proportion of the enriched canonical pathways were involved in neuronal functions and development. This is in line with the reported involvement of APP and its metabolites in neurogenesis and synapse formation/function.35–37 Furthermore, we observed that canonical pathway renin-angiotensin signaling was enriched in genes associated with plasma Aβ concentrations. This is of particular interest, as blood pressure modulation is one of the suspected physiological functions of Aβ peptides.5,11 With importance, we confirmed the enrichment of pathway involved in the development of neurons and in neuronal functions using the Webgestalt software.
The existence of equilibrium between plasma and brain Aβ peptides is debated even if some evidences seems to indicate that plasma Aβ peptides do not reflect a dynamic equilibrium between brain, CSF and plasma compartments.38–43 One can argue that actors of the APP metabolism driving Aβ peptide production and/or degradation are not the same between organs/compartments. However, we detected enrichment of pathways in axon guidance using two different tools (IPA and Webgestalt) and a list of 1 762 genes containing at least one SNP associated with both plasma Aβ1–40 and Aβ1–42 (P<0.05) concentrations with the same direction of effect. We indeed postulate that correlation between plasma Aβ1–40 and Aβ1–42 are representative of physiological processes leading to Aβ peptide productions (i.e., amyloid precursor protein metabolism). Of note, we observed, as expected, strong correlation between plasma Aβ1–40 and Aβ1–42 (Supplementary Table 3). Taken together, these observations indicate that we were able to detect processes involved in neuronal development although working on plasma phenotype. This suggests that mechanisms controlling Aβ peptide production and/or degradation could be in part constant across organs/compartments. As a consequence, analyzing such a plasma phenotype appears to be relevant to search for cerebral actors of the APP metabolism.
Our second approach was based on biological relevance and determination of whether genes corresponding to our best signals might be directly involved in APP metabolism. We noticed that the disabled homolog 1 (DAB1) gene was associated with plasma Aβ concentrations in our GWAS. The corresponding protein is a partner of APP and has been described as a modulator of metabolism.44,45 In addition to characterizing genes known to be involved in Aβ peptides production, we also focused on the best signal obtained for plasma Aβ1–42 concentration. This locus contained the CTXN3 gene, the product of which had not previously been described as a modulator of APP metabolism. We showed that CTXN3 overexpression was associated with significantly lower Aβ1–42 secretion and that CTXN3 may co-localize with APP. Nevertheless, little is known about the CTXN3 protein (described for the first time in 2007): it has a single membrane-spanning domain and appears to be expressed only in the kidney and in the brain.46 Another interesting gene of this locus, MEGF10, encodes a phagocytic receptor that could have a role in Aβ1–42 uptake in the brain47 and a suggestive association with cognitive decline in AD patients has been recently reported within this gene.48
Previous genetic studies of plasma Aβ levels have reported a linkage peak on chromosome 10q49 and subsequently, several associations with variants in candidate genes of this region, including CTNNA3,50,51 PLAU52,53 and IDE.54–57 Another group reported an haplotypic association spanning the MMP3 gene.58 Using the same SNPs, we did not replicate theses findings in our study (Supplementary Table 8). Of note, our second best signal for plasma Aβ1–42 levels was located within the 10q linkage peak, in an intron of the ANK3 gene. Further studies will be needed to assess whether this gene is responsible for our signal and the 10q linkage peak previously reported.49 Variants in this gene are associated with risk of bipolar disorder,59 schizophrenia60 and austism spectrum disorders.61 Links between these conditions and APP metabolism have been suggested,62–64 which warrant further studies of ANK3.
Several GWAS have also analyzed genetic associations with Aβ1–42 concentrations in CSF.65–67 Only one SNP associated with CSF concentrations of Aβ1–42 and tau was also associated with plasma concentrations of Aβ1–42 at nominal significance level in our study (Supplementary Table 8). This SNP, rs2075650, is located in the APOE locus, which is suspected to influence AD pathophysiology through impaired Aβ peptides clearance from the brain.68
In addition, apart from this APOE SNP, we did not also find any significant association between known genetic risk factors for AD18,69,70 and plasma Aβ concentrations (Table 3). As our analyses were performed in non-demented samples, the genes within our suggestive loci may not be involved in the AD process. Of note, we did not observe any significant associations between CTXN3 and AD risk in the European Alzheimer’s Disease Initiative GWAS of 2 032 AD cases and 5 328 controls.19
In conclusion, our study results suggest that the use of plasma Aβ peptides are valid endophenotypes in GWASs and can be used to characterize the metabolism and functions of APP and its metabolites. Our identification of CTXN3’s involvement in APP metabolism in vitro indicates that our genome-wide association meta-analysis was able to pick up new factors in this metabolic pathway. Consequently, systematic screening of the other suggestive loci might be relevant.
Supplementary Material
Acknowledgments
The work was made possible by the generous participation of the control participants and their families. This work was supported by the National Foundation for Alzheimer’s disease and related disorders, the Institut Pasteur de Lille, the Centre National de Génotypage, Inserm, FRC (fondation pour la recherche sur le cerveau) and Rotary. This work has been developed and supported by the LABEX (laboratory of excellence program investment for the future) DISTALZ grant (Development of Innovative Strategies for a Transdisciplinary approach to ALZheimer’s disease). JC was funded by the MEDIALZ Project (Grant 11001003) financed by ERDF (European Regional Development Fund) and Conseil Régional Nord Pas de Calais. The three-city study was performed as a part of collaboration between the Institut National de la Santé et de la Recherche Médicale (Inserm), the Victor Segalen Bordeaux II University and Sanofi-Synthélabo. The Fondation pour la Recherche Médicale funded the preparation and initiation of the study. The 3C study was also funded by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, MGEN, Institut de la Longévité, Agence Française de Sécurité Sanitaire des Produits de Santé, the Aquitaine and Bourgogne Regional Councils, Fondation de France and the joint French Ministry of Research/INSERM “Cohortes et collections de données biologiques” programme. Lille Génopôle received an unconditional grant from Eisai. The Rotterdam study is sponsored by the Erasmus Medical Center and Erasmus University Rotterdam, The Netherlands Organization for Scientific Research (I), The Netherlands Organization for Health Research and Development (ZonMW), the Research Institute for Diseases in the Elderly (RIDE), The Netherlands Genomics Initiative, the Ministry of Education, Culture and Science, the Ministry of Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. Further support was obtained from the Netherlands Consortium for Healthy Ageing. Dr Ikram was supported by a ZonMW Veni grant: 916.13.054. The cardiovascular health study (CHS) research was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086; and NHLBI grants HL080295, HL087652, HL105756 with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG023629 from the National Institute on Aging (NIA). A full list of CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. The provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR000124, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center. ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; GE Healthcare; Innogenetics, N.V.; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Synarc Inc.; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of California, Los Angeles. This research was also supported by NIH grants P30 AG010129 and K01 AG030514.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
References
- 1.De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J Cell Sci. 2000;113(Pt 11):1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- 2.Yazawa H, Yu ZX, Takeda, Le Y, Gong W, Ferrans VJ, et al. Beta amyloid peptide (Abeta42) is internalized via the G-protein-coupled receptor FPRL1 and forms fibrillar aggregates in macrophages. FASEB J. 2001;15:2454–2462. doi: 10.1096/fj.01-0251com. [DOI] [PubMed] [Google Scholar]
- 3.Maezawa I, Jin L-W, Woltjer RL, Maeda N, Martin GM, Montine TJ, Montine KS. Apolipoprotein E isoforms and apolipoprotein AI protect from amyloid precursor protein carboxy terminal fragment-associated cytotoxicity. J Neurochem. 2004;91:1312–1321. doi: 10.1111/j.1471-4159.2004.02818.x. [DOI] [PubMed] [Google Scholar]
- 4.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 5.Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. Beta-amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 6.Li QX, Whyte S, Tanner JE, Evin G, Beyreuther K, Masters CL. Secretion of Alzheimer’s disease Abeta amyloid peptide by activated human platelets. Lab Invest. 1998;78:461–469. [PubMed] [Google Scholar]
- 7.Kontush A. Alzheimer’s amyloid-beta as a preventive antioxidant for brain lipo-proteins. Cell Mol Neurobiol. 2001;21:299–315. doi: 10.1023/A:1012629603390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Oijen M, Hofman A, Soares HD, Koudstaal PJ, Breteler MMB. Plasma Abeta(1–40) and Abeta(1–42) and the risk of dementia: a prospective case-cohort study. Lancet Neurol. 2006;5:655–660. doi: 10.1016/S1474-4422(06)70501-4. [DOI] [PubMed] [Google Scholar]
- 10.Lambert J-C, Schraen-Maschke S, Richard F, Fievet N, Rouaud O, Berr C, et al. Association of plasma amyloid beta with risk of dementia: the prospective three-city study. Neurology. 2009;73:847–853. doi: 10.1212/WNL.0b013e3181b78448. [DOI] [PubMed] [Google Scholar]
- 11.Lambert J-C, Dallongeville J, Ellis KA, Schraen-Maschke S, Lui J, Laws S, et al. Association of plasma aβ peptides with blood pressure in the elderly. PLoS One. 2011;6:e18536. doi: 10.1371/journal.pone.0018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ibrahim-Verbaas CA, Zorkoltseva IV, Amin N, Schuur M, Coppus AMW, Isaacs A, et al. Linkage analysis for plasma amyloid beta levels in persons with hypertension implicates Aβ-40 levels to presenilin 2. Hum Genet. 2012;131:1869–1876. doi: 10.1007/s00439-012-1210-2. [DOI] [PubMed] [Google Scholar]
- 13.Ertekin-Taner N, Graff-Radford N, Younkin LH, Eckman C, Adamson J, Schaid DJ, et al. Heritability of plasma amyloid beta in typical late-onset Alzheimer’s disease pedigrees. Genet Epidemiol. 2001;21:19–30. doi: 10.1002/gepi.1015. [DOI] [PubMed] [Google Scholar]
- 14.3C Study Group. Vascular factors and risk of dementia: design of the three-city study and baseline characteristics of the study population. Neuroepidemiology. 2003;22:316–325. doi: 10.1159/000072920. [DOI] [PubMed] [Google Scholar]
- 15.Hofman A, van Duijn CM, Franco OH, Ikram MA, Janssen HLA, Klaver CCW, et al. The Rotterdam study: 2012 objectives and design update. Eur J Epidemiol. 2011;26 :657–686. doi: 10.1007/s10654-011-9610-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikram MA, van der Lugt A, Niessen WJ, Krestin GP, Koudstaal PJ, Hofman A. The Rotterdam scan study: design and update up to 2012. Eur J Epidemiol. 2011;26:811–824. doi: 10.1007/s10654-011-9624-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez OL, Kuller LH, Mehta PD, Becker JT, Gach HM, Sweet RA. Plasma amyloid levels and the risk of AD in normal subjects in the cardiovascular health study. Neurology. 2008;70:1664–1671. doi: 10.1212/01.wnl.0000306696.82017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toledo JB, Vanderstichele H, Figurski M, Aisen PS, Petersen RC, Weiner MW, et al. Factors affecting Aβ plasma levels and their utility as biomarkers in ADNI. Acta Neuropathol. 2011;122:401–413. doi: 10.1007/s00401-011-0861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert J-C, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 20.Psaty BM, O’Donnell CJ, Gudnason V, Lunetta KL, Folsom AR, Rotter JI, et al. Cohorts for heart and aging research in genomic epidemiology (CHARGE) consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet. 2009;2:73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saykin AJ, Shen L, Foroud TM, Potkin SG, Swaminathan S, Kim S, et al. Alzheimer’s disease neuroimaging initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement. 2010;6:265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figurski MJ, Waligórska T, Toledo J, Vanderstichele H, Korecka M, Lee VMY Alzheimer’s Disease Neuroimaging Initiative. Improved protocol for measurement of plasma β-amyloid in longitudinal evaluation of Alzheimer’s Disease Neuroimaging Initiative study patients. Alzheimers Dement. 2012;8:250–260. doi: 10.1016/j.jalz.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sergeant N, David J-P, Champain D, Ghestem A, Wattez A, Delacourte A. Progressive decrease of amyloid precursor protein carboxy terminal fragments (APP-CTFs), associated with tau pathology stages, in Alzheimer’s disease. J Neurochem. 2002;81:663–672. doi: 10.1046/j.1471-4159.2002.00901.x. [DOI] [PubMed] [Google Scholar]
- 24.Chapuis J, Hot D, Hansmannel F, Kerdraon O, Ferreira S, Hubans C, et al. Transcriptomic and genetic studies identify IL-33 as a candidate gene for Alzheimer’s disease. Mol Psychiatry. 2009;14:1004–1016. doi: 10.1038/mp.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- 26.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aulchenko YS, Struchalin MV, Duijn CM. ProbABEL package for genome-wide association analysis of imputed data. BMC Bioinformatics. 2010;11:134. doi: 10.1186/1471-2105-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genome-wide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 30.Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013;41:W77–W83. doi: 10.1093/nar/gkt439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marambaud P, Chevallier N, Ancolio K, Checler F. Post-transcriptional contribution of a cAMP-dependent pathway to the formation of alpha- and beta/gamma-secretases-derived products of beta APP maturation in human cells expressing wild-type and Swedish mutated beta APP. Mol Med. 1998;4:715–723. [PMC free article] [PubMed] [Google Scholar]
- 32.Su Y, Ryder J, Ni B. Inhibition of Abeta production and APP maturation by a specific PKA inhibitor. FEBS Lett. 2003;546:407–410. doi: 10.1016/s0014-5793(03)00645-8. [DOI] [PubMed] [Google Scholar]
- 33.Lourenço FC, Galvan V, Fombonne J, Corset V, Llambi F, Müller U, Bredesen DE, Mehlen P. Netrin-1 interacts with amyloid precursor protein and regulates amyloid-beta production. Cell Death Differ. 2009;16:655–663. doi: 10.1038/cdd.2008.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rama N, Goldschneider D, Corset V, Lambert J, Pays L, Mehlen P. Amyloid precursor protein regulates netrin-1-mediated commissural axon outgrowth. J Biol Chem. 2012;287:30014–30023. doi: 10.1074/jbc.M111.324780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Tang BL. The amyloid precursor protein and postnatal neurogenesis/neuroregeneration. Biochem Biophys Res Commun. 2006;341:1–5. doi: 10.1016/j.bbrc.2005.12.150. [DOI] [PubMed] [Google Scholar]
- 36.Chasseigneaux S, Allinquant B. Functions of Aβ, sAPPα and sAPPβ: similarities and differences. J Neurochem. 2012;120(Suppl 1):99–108. doi: 10.1111/j.1471-4159.2011.07584.x. [DOI] [PubMed] [Google Scholar]
- 37.Lazarov O, Demars MP. All in the family: how the APPs regulate neurogenesis. Front Neurosci. 2012;6:81. doi: 10.3389/fnins.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghersi-Egea JF, Gorevic PD, Ghiso J, Frangione B, Patlak CS, Fenstermacher JD. Fate of cerebrospinal fluid-borne amyloid beta-peptide: rapid clearance into blood and appreciable accumulation by cerebral arteries. J Neurochem. 1996;67:880–883. doi: 10.1046/j.1471-4159.1996.67020880.x. [DOI] [PubMed] [Google Scholar]
- 39.Vanderstichele H, Van Kerschaver E, Hesse C, Davidsson P, Buyse MA, Andreasen N, et al. Standardization of measurement of beta-amyloid(1–42) in cerebrospinal fluid and plasma. Amyloid. 2000;7:245–258. doi: 10.3109/13506120009146438. [DOI] [PubMed] [Google Scholar]
- 40.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehta PD, Pirttila T, Patrick BA, Barshatzky M, Mehta SP. Amyloid beta protein 1–40 and 1–42 levels in matched cerebrospinal fluid and plasma from patients with Alzheimer disease. Neurosci Lett. 2001;304:102–106. doi: 10.1016/s0304-3940(01)01754-2. [DOI] [PubMed] [Google Scholar]
- 42.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 43.Giedraitis V, Sundelöf J, Irizarry MC, Gårevik N, Hyman BT, Wahlund L-O, et al. The normal equilibrium between CSF and plasma amyloid beta levels is disrupted in Alzheimer’s disease. Neurosci Lett. 2007;427:127–131. doi: 10.1016/j.neulet.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 44.Gavín R, Ferrer I, del Río JA. Involvement of Dab1 in APP processing and beta-amyloid deposition in sporadic Creutzfeldt-Jakob patients. Neurobiol Dis. 2010;37:324–329. doi: 10.1016/j.nbd.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Kwon OY, Hwang K, Kim J-A, Kim K, Kwon IC, Song HK, et al. Dab1 binds to Fe65 and diminishes the effect of Fe65 or LRP1 on APP processing. J Cell Biochem. 2010;111:508–519. doi: 10.1002/jcb.22738. [DOI] [PubMed] [Google Scholar]
- 46.Wang HT, Chang JW, Guo Z, Li BG. In silico-initiated cloning and molecular characterization of cortexin 3, a novel human gene specifically expressed in the kidney and brain, and well conserved in vertebrates. Int J Mol Med. 2007;20:501–510. [PubMed] [Google Scholar]
- 47.Singh TD, Park S-Y, Bae J-S, Yun Y, Bae Y-C, Park R-W, et al. MEGF10 functions as a receptor for the uptake of amyloid-β. FEBS Lett. 2010;584:3936–3942. doi: 10.1016/j.febslet.2010.08.050. [DOI] [PubMed] [Google Scholar]
- 48.Sherva R, Tripodis Y, Bennett DA, Chibnik LB, Crane PK, de Jager PL, et al. Genome-wide association study of the rate of cognitive decline in Alzheimer’s disease. Alzheimers Dement. 2013;10:45–52. doi: 10.1016/j.jalz.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ertekin-Taner N, Graff-Radford N, Younkin LH, Eckman C, Baker M, Adamson J, et al. Linkage of plasma Abeta42 to a quantitative locus on chromosome 10 in late-onset Alzheimer’s disease pedigrees. Science. 2000;290:2303–2304. doi: 10.1126/science.290.5500.2303. [DOI] [PubMed] [Google Scholar]
- 50.Ertekin-Taner N, Ronald J, Asahara H, Younkin L, Hella M, Jain S. Fine mapping of the alpha-T catenin gene to a quantitative trait locus on chromosome 10 in late-onset Alzheimer’s disease pedigrees. Hum Mol Genet. 2003;12:3133–3143. doi: 10.1093/hmg/ddg343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyashita A, Arai H, Asada T, Imagawa M, Matsubara E, Shoji M, et al. Genetic association of CTNNA3 with late-onset Alzheimer’s disease in females. Hum Mol Genet. 2007;16:2854–2869. doi: 10.1093/hmg/ddm244. [DOI] [PubMed] [Google Scholar]
- 52.Ertekin-Taner N, Ronald J, Feuk L, Prince J, Tucker M, Younkin L, et al. Elevated amyloid beta protein (Abeta42) and late onset Alzheimer’s disease are associated with single nucleotide polymorphisms in the urokinase-type plasminogen activator gene. Hum Mol Genet. 2005;14:447–460. doi: 10.1093/hmg/ddi041. [DOI] [PubMed] [Google Scholar]
- 53.Papassotiropoulos A, Tsolaki M, Wollmer MA, Molyva D, Thal DR, Huynh K-D, et al. No association of a non-synonymous PLAU polymorphism with Alzheimer’s disease and disease-related traits. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:21–23. doi: 10.1002/ajmg.b.30103. [DOI] [PubMed] [Google Scholar]
- 54.Ertekin-Taner N, Allen M, Fadale D, Scanlin L, Younkin L, Petersen RC, et al. Genetic variants in a haplotype block spanning IDE are significantly associated with plasma Abeta42 levels and risk for Alzheimer disease. Hum Mutat. 2004;23:334–342. doi: 10.1002/humu.20016. [DOI] [PubMed] [Google Scholar]
- 55.Carrasquillo MM, Belbin O, Zou F, Allen M, Ertekin-Taner N, Ansari M, et al. Concordant association of insulin degrading enzyme gene (IDE) variants with IDE mRNA, Abeta, and Alzheimer’s disease. PLoS One. 2010;5:e8764. doi: 10.1371/journal.pone.0008764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bartl J, Scholz C-J, Hinterberger M, Jungwirth S, Wichart I, Rainer MK, et al. Disorder-specific effects of polymorphisms at opposing ends of the insulin degrading enzyme gene. BMC Med Genet. 2011;12:151. doi: 10.1186/1471-2350-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reitz C, Cheng R, Schupf N, Lee JH, Mehta PD, Rogaeva E, et al. Association between variants in IDE-KIF11-HHEX and plasma amyloid beta levels. Neurobiol Aging. 2012;33:199.e13–199.e17. doi: 10.1016/j.neurobiolaging.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reitz C, van Rooij FJA, Soares HD, de Maat MPM, Hofman A, Witteman JCM, et al. Matrix metalloproteinase 3 haplotypes and plasma amyloid beta levels: the Rotterdam study. Neurobiol Aging. 2010;31:715–718. doi: 10.1016/j.neurobiolaging.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira MAR, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bi C, Wu J, Jiang T, Liu Q, Cai W, Yu P, et al. Mutations of ANK3 identified by exome sequencing are associated with autism susceptibility. Hum Mutat. 2012;33:1635–1638. doi: 10.1002/humu.22174. [DOI] [PubMed] [Google Scholar]
- 62.Su Y, Ryder J, Li B, Wu X, Fox N, Solenberg P, et al. Lithium, a common drug for bipolar disorder treatment, regulates amyloid-beta precursor protein processing. Biochemistry. 2004;43:6899–6908. doi: 10.1021/bi035627j. [DOI] [PubMed] [Google Scholar]
- 63.Qing H, He G, Ly PTT, Fox CJ, Staufenbiel M, Cai F, et al. Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J Exp Med. 2008;205:2781–2789. doi: 10.1084/jem.20081588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lahiri DK, Sokol DK, Erickson C, Ray B, Ho CY, Maloney B. Autism as early neurodevelopmental disorder: evidence for an sAPPα-mediated anabolic pathway. Front Cell Neurosci. 2013;7:94. doi: 10.3389/fncel.2013.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han M-R, Schellenberg GD, Wang L-S Alzheimer’s Disease Neuroimaging Initiative. Genome-wide association reveals genetic effects on human Abeta42 and Tau protein levels in cerebrospinal fluids: a case control study. BMC Neurol. 2010;10:90. doi: 10.1186/1471-2377-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim S, Swaminathan S, Shen L, Risacher SL, Nho K, Foroud T, et al. Genome-wide association study of CSF biomarkers Abeta1–42, t-tau, and p-tau181p in the ADNI cohort. Neurology. 2011;76:69–79. doi: 10.1212/WNL.0b013e318204a397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cruchaga C, Kauwe JSK, Harari O, Jin SC, Cai Y, Karch CM, et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer’s disease. Neuron. 2013;78:256–268. doi: 10.1016/j.neuron.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lambert JC, Amouyel P. Genetics of Alzheimer’s disease: new evidences for an old hypothesis? Curr Opin Genet Dev. 2011;21:295–301. doi: 10.1016/j.gde.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hollingworth P, Harold D, Sims R, Gerrish A, Lambert J-C, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.