Abstract
Background
The Weight Loss Maintenance Trial tested strategies for maintenance of weight loss. Personal contact was superior to interactive technology and self-directed conditions.
Purpose
We aimed to identify behavioral mediators of the superior effect of personal contact vs. interactive technology and of personal contact vs. self-directed arms.
Methods
Overweight/obese adults at risk for cardiovascular disease (n=1,032) who lost at least 4 kg were randomized to personal contact, interactive technology, or self-directed. After 30 months, 880 participants had data on weight and behavioral strategies.
Results
Reported increase of intake of fruits and vegetables and physical activity and more frequent self-weighing met criteria as mediators of the better outcome of personal contact vs. interactive technology. Increased intake of fruits and vegetables, more frequent self-weighing, and decreased dessert consumption were mediators of the difference between personal contact vs. self-directed.
Conclusion
Inducing changes in the identified behaviors might yield better outcomes in future weight loss maintenance trials. (ClinicalTrials.gov number NCT00054925)
Keywords: Obesity, Weight maintenance, Weight regain, Behavioral strategies, Mediators
Introduction
Obesity is a chronic, relapsing condition requiring long-term treatment, and development and dissemination of effective and affordable weight loss maintenance interventions remains an urgent public health priority [1, 2]. Lifestyle behavioral intervention is a promising approach to promoting long-term weight loss maintenance. A review of randomized clinical trials of weight loss maintenance identified 14 English-language studies that enrolled adults into behaviorally based weight maintenance treatment following an initial weight loss [3]. Ten of these trials provided a personal contact intervention, i.e., ongoing therapist and/or social support, and four studied an internet-based intervention. The studies had varying periods of follow-up, many shorter than 18 months, and often had one or more methodological limitations, such as small sample size, high attrition rate, or poor generalizability to men and ethnic minorities. Despite these limitations, trials of personal contact interventions have generally found that they are efficacious in reducing weight regain [3]. Studies of Internet interventions have had mixed results; some have found that in-person behavioral weight loss maintenance intervention is superior to internet intervention, whereas others have not [3].
The Weight Loss Maintenance Trial is a completed randomized clinical trial that was not included in the above review. The design of both phases and primary outcomes has been published elsewhere [4–6]. Briefly, the Weight Loss Maintenance Trial compared two behavioral maintenance interventions to a self-directed control in 1,032 randomized participants who had lost at least 4 kg (overall mean, 8.5 kg; SD, 4.2) during a 6-month nonrandomized behavioral group intervention (phase 1) [6]. The two active weight maintenance interventions in phase 2 were personal contact, involving brief, monthly personal contact with an interventionist, and an Internet-based intervention, named interactive technology, delivering the same content [7]. At 30-month follow-up, participants in the personal contact group had regained significantly less weight than those in either the interactive technology or self-directed group (4.0 vs. 5.2 vs. 5.5 kg, respectively), but the interactive technology and self-directed groups did not differ [5].
Once a significant difference is found between interventions with multiple behavioral elements, questions remain about why the better one was successful (what elements were the mediators of treatment effect) and for whom it was successful (moderators). Identification of possible mediators of treatment effect can be heuristic in generating changes in the intervention or measures to be tested in future, confirmatory intervention studies.
Baron and Kenny [8] defined terms and outlined analytic strategy for identifying a probable mediator. Their approach emphasized that a plausible assumption of causality must underlie the hypothesized direct and indirect influences in a mediated association between two variables, and there continues to be consensus on this point. However, as Kraemer et al. [9] posited, without strong evidence for a causal pathway, the results can be ambiguous, with even “reverse” causality apparently supported by analytic results. The MacArthur approach, developed by Kraemer and colleagues in the MacArthur Foundation Research Network on Psychopathology and Development [10, 11], focuses on the association between an intervention in a randomized clinical trial and variables representing trait status either clearly before (moderators) or after (mediators) the intervention begins. Thus, the first condition of causality (result follows event chronologically) is met a priori.
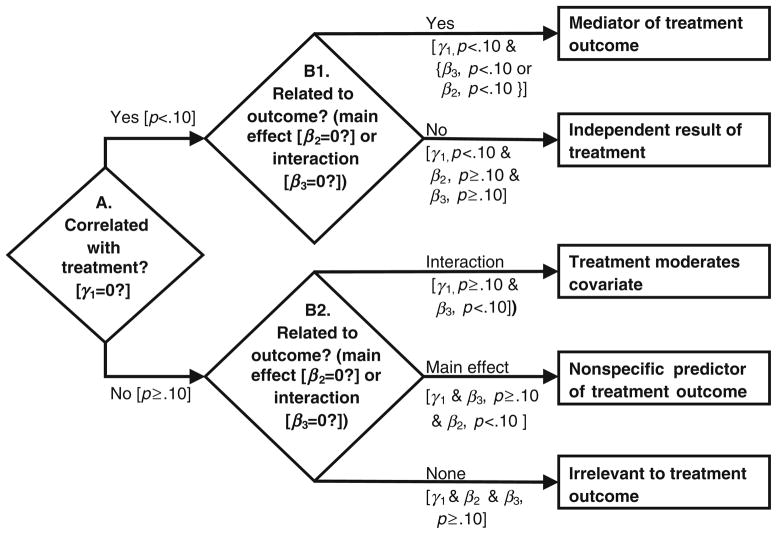
Once a significant treatment effect is shown, mediator analyses under the MacArthur approach “identify possible mechanisms through which a treatment might achieve its effects” ([10], p. 878). An attractive feature of the MacArthur approach is the decision tree for classifying the role of various postintervention measures in a randomized clinical trial (Fig. 1, adapted from Kraemer et al. [10], Table, p. 880). To be designated as a mediator, a variable must: (1) be correlated with treatment (e.g., means differ between treatment groups that differ in outcome: ‘Yes’ on step A) and (2) have an effect on the outcome of interest while adjusting for treatment (‘Yes’ on step B1). This may be either a main effect or an interaction with treatment. A ‘No’ on step A indicates a measure does not meet the definition of a mediator. A variable can be further classified based on the result of step B2 into one of three categories. More details about Fig. 1 are presented in the “Methods.”
Fig. 1.
Decision flow chart for classifying the role of postintervention measures in an experimental study
We are not aware of any weight loss maintenance trials that have formally examined mediators of treatment effects by applying the MacArthur approach [10]. Daniels et al. [12] demonstrated use of a Bayesian approach to estimate the direct and indirect effects of a continuous mediator on a binary outcome. They found that the effect of a 12-month face-to-face weight management program, delivered after 6 months of lifestyle modification, was not mediated by number of days of self-monitoring food intake. In a randomized clinical trial of a 12-month weight loss intervention, baseline to 12-month changes in psychosocial traits (e.g., self-efficacy and self-perception) were evaluated as mediators of treatment effect [13]. That study differs from the present one on two counts—randomized intervention for initial weight loss (vs. weight loss maintenance) and the nature of the putative mediators (psychological constructs vs. behavioral strategies). They also do not consider the possibility that a mediator operated through an interaction, one of the differences between the MacArthur and Baron–Kenny approaches [11].
The primary aim of the current analysis is to apply the MacArthur approach to investigate which behavioral strategies on a Weight Management Strategies questionnaire meet formal criteria for status as likely mediators of the greater efficacy of personal contact vs. self-directed and personal contact vs. interactive technology in the Weight Loss Maintenance Trial. This is an exploratory, hypothesis-generating analysis.
Methods
The details of the two-phased Weight Loss Maintenance Trial design have been published [4–6]. In brief, phase 1 was a group-based behavioral intervention led by a trained interventionist over 20 sessions [6]. Intervention goals included 180 min/week of moderate-to-vigorous physical activity, reduced caloric intake, and adoption of the dietary approaches to stop hypertension (DASH) dietary program, which emphasizes eating fruits, vegetables, and low-fat dairy products while reducing total and saturated fat [14, 15]. Participants were taught to keep food and physical activity self-monitoring records and to calculate caloric intake. Participants who lost at least 4 kg in phase 1 were randomly assigned to one of three arms (self-directed, interactive technology, and personal contact) in the 30-month maintenance portion of the trial (phase 2).
The personal contact arm of phase 2 included monthly individual contact with an interventionist, during which key components of phase 1 were reinforced. Most personal contact sessions were by phone and lasted from 5 to 15 min; face-to-face individual sessions occurred approximately every 4th month and ranged from 45 to 60 min in duration. Personal contact sessions consisted of an update of progress, support from the interventionist, accountability for previous goals, and a discussion of barriers and successes. Participants in the interactive technology arm had unlimited access to a project-specific website designed to support weight loss maintenance. Participants were encouraged to login at least weekly to enter their current weight, caloric intake, and physical activity minutes. Features of the Interactive technology intervention included goal setting and action planning exercises, graphing of personal data over time, a bulletin board offering social support among participants, modules teaching problem solving and motivation, and automated e-mail and phone calls prompting login behavior after periods of no contact [7]. Unlike the personal contact intervention, the interactive technology intervention did not include personal feedback from or interaction with an interventionist. At randomization, those in the self-directed arm received a printed lifestyle guideline with the phase 1 diet and physical activity goals. They met briefly with a study interventionist at the 12-month data collection visit.
Participants
Participants in the present study (N=880) were randomized to phase 2 of the Weight Loss Maintenance Trial and had both a weight measurement at 30-month follow-up and complete responses to the Weight Management Strategies questionnaire. Although the primary outcome analysis for phase 2 was based on a dataset containing multiply imputed data [5], we did not use imputed data in the present mediator analysis, because the Weight Management Strategies questionnaire, which is the primary focus of this report, was not included in the phase 2 multiple imputation.
Inclusion criteria for entry to phase 1 of the Weight Loss Maintenance Trial 1 included: BMI between 25 and 45 kg/m2, taking medication for hypertension, dyslipidemia, or both; aged 25 or greater, and willingness to abstain from weight loss medications and bariatric surgery during the study. Exclusion criteria included active or recent cardiovascular disease, medication-treated diabetes mellitus, recent weight loss of >9 kg, weight loss surgery, and other medical or psychiatric conditions that were contraindications to study participation. Randomization into phase 2 required a weight loss of at least 4 kg during phase 1 and was stratified on clinic, race (African-American or not), and amount of phase 1 weight loss. Of those randomized, 964 (93 %) provided a 30-month weight and 880 of these (91 %) provided complete behavioral strategies data on the Weight Management Strategies questionnaire.
Measures
All measures were either participant self-report or collected by staff who were masked to participant treatment assignment.
Outcome
The outcome of interest in this report is weight change (kg) from randomization to 30-month follow-up. A positive value indicates weight gain. On both occasions, participants were weighed in light indoor clothes without shoes on a high-quality, calibrated digital scale. Weight was measured on two separate days, and an average of these weights was used to calculate the outcome measure.
Mediators
As potential mediators of weight control in personal contact (vs. interactive technology or self-directed), we used responses to the Weight Management Strategies questionnaire developed by the weight loss maintenance measurement committee and administered at the last follow-up visit, 30 months after randomization. This Weight Management Strategies questionnaire queried participants about 19 behavioral weight loss maintenance strategies they may have used during phase 2 of the Weight Loss Maintenance Trial: (“since you completed your weekly weight loss groups in weight loss maintenance phase 1, that is, since you were randomized into phase 2 of the study, have you done any of the following in order to control your weight?”). Responses on 18 items were “yes” or “no”. Eight of these were strategies not encouraged in phase 1 or phase 2 of the Weight Loss Maintenance Trial (e.g., used over-the-counter meal replacements), whereas ten items listed behaviors encouraged during phase 1, either cutting back a behavior (e.g., dessert consumption) or increasing it (e.g., physical activity). Finally, we asked “in the last 30 days, how often have you weighed yourself?” Responses were on a 5-point scale (from 1=every day to 5=not at all).
Concurrent Validation
In order to calculate concurrent validity of the Weight Management Strategies questionnaire items, we chose prospectively measured dietary and physical activity variables from the primary outcomes dataset. Physical activity was objectively measured using a triaxial accelerometer (RT3, Stayhealthy Inc, Monrovia, California). Participants were asked to wear the accelerometer above the left hip during waking hours for seven consecutive days just before randomization to phase 2 and 30-month follow-up. Moderate-to-vigorous physical activity was defined as ≥3 metabolic equivalents and previously published RT3 cut points (>1,316.5 counts/min) were used [16]. Minutes of moderate-to-vigorous (MVPA) were computed taking a weighted average of daily weekday and weekend activity (weekly MVPA=(5× average daily weekday MVPA)+(2×average daily weekend MVPA). Further details have been described elsewhere in detail [17]. Two measures of dietary intake were abstracted from the Block Food Frequency questionnaire: total kilocalories per day and servings of fruits and vegetables per day [18]. This is an extensively validated questionnaire for reporting customary daily intake of food group servings, scored to obtain kilocalories and nutrients.
Data Analysis
Preliminary analyses evaluated whether intervention arm, baseline weight, or race-sex distribution differed between the participants with 30-month weight who completed the Weight Management Strategies questionnaire (n=880) and those who did not (n=84).
In our main analyses, we followed the MacArthur approach for identifying mediators of treatment in a randomized clinical trial (Fig. 1) [10]. In view of the exploratory and hypothesis-generating nature of this approach ([10], p. 882) and to reduce the probability of missing a meaningful association (a type II error), the critical p value for significance was set at 0.10. In view of the challenges of achieving successful weight loss maintenance, missing a potentially useful mediator seems worse than tentatively proposing one that is not later confirmed. For the same reason, we did not adjust for multiple tests.
All analyses were carried out using SAS, Release.9.2. We estimated the total treatment effect (τ) in the analysis sample, in the unadjusted model Y=τX+ε1, where X is the binary treatment indicator (personal contact vs. self-directed, or personal contact vs. interactive technology), and Y is the weight change.
Correlation of strategy measure (Z) with treatment (step A in Fig. 1) was evaluated in a logistic regression of each treatment contrast on each strategy: logit(Z)=γ0+γ1X+ε2, where cumulative logit was used in scales with three or more levels. The assumption of proportional odds was tested, and the generalized logit was used if the assumption was rejected. The strength of the association between treatment arm and behavioral strategy is measured by the odds ratio, defined as exp(γ1).
To measure relationship of the mediator to change in weight (Y) given treatment (step B in Fig. 1), analysis of covariance was utilized, where the model was: Y=β0+β1X+β2Z+β3(X*Z)+ε3. If the interaction (β3) was significant, we carried out post hoc contrasts. If the interaction was not significant, the model was rerun without it to estimate the main effect (β2) of the potential mediator. When the main effect test was significant, we then estimated the mediated association of treatment with outcome (ζ) using SAS “type 1” sums of squares, in which the parameter estimates on the second or later term entered in the model is adjusted for variables already entered. The model is Y=ζ0+ζ1Z+ζ2X+ε4. Finally, we estimated the indirect effect. For a continuous variable this is the product of the X-to-Z and Z-to-Y effects [19]; however, where the X-to-Z path is estimated in a logistic model, the mediated or indirect effect is estimated with the difference τ−ζ2 [20, 21]. We ran the models in each strategy separately.
In a post hoc analysis to evaluate concurrent validity, the magnitude of change represented by endorsing physical activity or dietary intake items on the Weight Management Strategies questionnaire was estimated using regression of each of these on change during phase 2 in corresponding measures of moderate-to-vigorous physical activity and nutrient intake, adjusting for treatment within each of the two paired treatment comparisons (personal contact vs. interactive technology or personal contact vs. self-directed). We selected these data from the existing primary outcome dataset, which includes previously imputed values [5], to match our analysis sample. Only this part of the analysis used imputed data, and the sample remained the same; 80 % had no imputed values. We verified that the imputation methods, as reported in Svetkey et al. [5] were appropriate for our purposes. The analyses for this step were repeated identically over the five copies of imputed data then combined according to Rubin’s rules [22], using SAS PROC MIANALYZE which adjusts the standard errors for the uncertainty inherent in imputed data.
Results
Of the 1,032 participants randomized to phase 2 of the Weight Loss Maintenance Trial, 152 were not analyzable for the following reasons: death (n=3), failed to attend final data collection visit (n=65), or failed to complete the Weight Management Strategies questionnaire (n=84), leaving 880 analyzable participants. Table 1 presents participant characteristics and weight outcomes. The sample was 38 % male and 36 % African-American. Mean age and BMI were 55.9 years (SD=8.7) and 30.9 kg/m2 (SD=4.7), respectively. For personal contact vs. interactive technology analyses, n=593 participants, whereas for personal contact vs. self-directed, n=579.
Table 1.
Patient characteristics and weight outcomes (N=880), mean (SD), or percentage
| SD (n=287) | IT (n=301) | PC (n=292) | All (n=880) | |
|---|---|---|---|---|
| % of randomized | 84.2 | 86.7 | 85.6 | 85.5 |
| Race and sex distribution (%) | ||||
| AA men | 9.8 | 12.0 | 13.4 | 11.7 |
| AA women | 25.4 | 24.3 | 22.3 | 24.0 |
| Non-AA men | 28.2 | 25.9 | 25.7 | 26.6 |
| Non-AA women | 36.6 | 37.9 | 38.7 | 37.7 |
| Age | 56.0 (8.6) | 56.0 (8.5) | 55.8 (9.1) | 55.9 (8.7) |
| BMI at randomization | 30.7 (4.5) | 31.0 (4.8) | 31.0 (4.7) | 30.9 (4.7) |
| Weight at randomization (kg) | 86.9 (15.0) | 88.1 (15.2) | 88.1 (17.1) | 87.7 (15.8) |
| Change in weight (kg) from randomization to 30 month follow-up | 5.9 (6.3) | 5.2 (5.8) | 4.0 (5.3) | 5.0 (5.9) |
AA African American, BMI body mass index, kg kilogram, SD self directed, IT interactive technology, PC personal contact
The analysis sample did not differ from those who failed to complete the questionnaire (n=84) for treatment arm or weight change; however, the relative frequency of missing data differed across race-sex groups (p=0.0108). African-American women were most likely to have missing data (14 % of those with 30-month weight) vs. African-American men (9 %), Non African-American women (8 %), and non-African-American men (5 %).
For personal contact vs. interactive technology, the treatment effect (1.2 kg (95 % confidence limits (CL), 0.3, 2.1), p=0.0079) was very similar to that reported on the intent-to-treat sample, 1.2 kg [5]. In the personal contact vs. self-directed contrast, the estimate was somewhat larger (1.9 kg (95 % CL, 1.0), 2.9, p<0.0001), compared with 1.5 kg.
Table 2 lists the frequency and percentage of endorsement (Yes) in each trial arm for binary items, and the distribution of responses for those with three or more responses. Three strategies (use of laxatives, diuretics, and nicotine) were dropped from the analysis because these behaviors were reported by ≤1 % of participants. Three strategies (tried another weight loss program, used over-the-counter or diet meals, and cut back on sugared beverages) were created by summing responses to similar items to yield short ordinal scales. Results for tests of difference between treatments are indicated in Table 2 with symbols (*) and (**).
Table 2.
Weight management strategies, with response distributions over treatment groups
| Since being randomized into phase 2 | SD (n=287) n (%) |
IT (n=301) n (%) |
PC (n=292) n (%) |
|---|---|---|---|
| Have you | |||
| Tried another weight loss program (sum of 3 items: weight loss group, Internet-based program, video/televised program)*, + | |||
| None (0) | 214 (75 %) | 224 (74 %) | 236 (81 %) |
| One type of program (1) | 63 (22 %) | 62 (21 %) | 44 (15 %) |
| Two or three types of programs (2) | 10 (3 %) | 15 (5 %) | 12 (4 %) |
| Routinely used OTC meal replacements and/or diet/low calorie meals (sum of 2 items) + | |||
| Neither (0) | 227 (79 %) | 244 (81 %) | 248 (85 %) |
| One or both (1) | 60 (21 %) | 57 (19 %) | 44 (15 %) |
| Increased physical activity*, + | 207 (72 %) | 201 (67 %) | 228 (78 %) |
| In order to control your weight, have you cut back on | |||
| Number of times eating fast food | 230 (80 %) | 240 (80 %) | 239 (82 %) |
| Number of times eating out | 150 (52 %) | 157 (52 %) | 171 (59 %) |
| Portion sizes | 232 (81 %) | 259 (86 %) | 247 (85 %) |
| Amount of desserts + | 224 (78 %) | 249 (83 %) | 246 (84 %) |
| Sugared beverages (sum of 2 items: full-calorie soda, other sugared beverages) | |||
| Neither (0) | 36 (12 %) | 43 (14 %) | 38 (13 %) |
| One but not both (1) | 34 (12 %) | 44 (15 %) | 35 (12 %) |
| Both soda and other (2) | 217 (76 %) | 214 (71 %) | 219 (75 %) |
| Amount of alcohol | 169 (59 %) | 174 (58 %) | 163 (56 %) |
| In order to control your weight have you increased | |||
| Intake of fruits and vegetables*, + | 230 (80 %) | 245 (81 %) | 257 (88 %) |
| Intake of water or reduced-calorie beverages | 236 (82 %) | 259 (86 %) | 247 (85 %) |
| Frequency of weighing in the last 30 days*, + | |||
| Every day (1, reference level) | 43 (15 %) | 77 (26 %) | 65 (22 %) |
| Several times a week (2) | 57 (20 %) | 74 (25 %) | 103 (35 %) |
| Once per week (3) | 69 (24 %) | 81 (27 %) | 71 (24 %) |
| 1–3 times per month (4) | 76 (26 %) | 55 (18 %) | 42 (15 %) |
| Not at all (5) | 42 (15 %) | 14 (5 %) | 11 (4 %) |
SD self-directed, IT interactive technology, PC personal contact, OTC over the counter
PC vs. IT, p < 0.10,
PC vs. SD, p < 0.10
Table 3 summarizes the classification of candidate mediators, for personal contact vs. interactive technology and for personal contact vs. self-directed, based on the results of testing the association of reported use of behavioral strategies with treatment and with weight change (steps A and B in Fig. 1). For each step, the p value, slope, and standard error (SE) for the association of each mediator with treatment (γ̂1) and the effect on weight change of each mediator (β̂2 or β̂3) are given. Frequency of weighing did not meet the assumption of proportional hazards; in this case, the difference in slope for each response is given, with “every day” as the reference value. Finally, Table 3 also lists the estimated slope parameter, with standard error, of treatment controlling for mediator (ζ̂2), with the corresponding estimate of the indirect effect (τ̂−ζ̂2) of treatment via mediator (columns under indirect effect).
Table 3.
Classification of putative mediators, by treatment contrast (slopes not different from 0 (p>0.10) are not tabled)
| Classification | df | Association with treatment (γ1) | Impact on weight change (β̂2 or β̂3) | Indirect effect
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment effect, given mediator (ζ̂2) | |||||||||||
|
|
|
|
|||||||||
| p | Slope * | SE | p | Slope (kg) | SE | p | Slope (kg) | SE | (τ̂−ζ̂2) (kg)† | ||
| PC vs. IT groups (N=593) | |||||||||||
| Mediators of treatment effect on weight change | |||||||||||
| Increased physical activity | 1 | 0.0023 | −0.57 | 0.19 | 0.0155 | −1.25 | 0.51 | 0.0192 | 1.08 | 0.46 | 0.14 |
| Increased intake of fruits and vegetables | 1 | 0.0265 | −0.52 | 0.23 | 0.0002 | −2.39 | 0.63 | 0.0199 | 1.06 | 0.45 | 0.16 |
| Frequency of weighing last month** (overall) | 4 | 0.0805 | <0.0001 | 0.0101 | 1.15 | 0.45 | 0.07 | ||||
| >Once a week (2)–every day (1) | −0.50 | 0.23 | 1.35 | 0.61 | |||||||
| Once per week (3)–every day (1) | −0.04 | 0.23 | 0.87 | 0.63 | |||||||
| 1–3 times (4)–every day (1) | 0.10 | 0.27 | 3.56 | 0.71 | |||||||
| Not at all (5)–every day (1) | 0.07 | 0.44 | 5.51 | 1.17 | |||||||
| Independent outcome of treatment | |||||||||||
| Tried another weight loss program | 2 | 0.0678 | 0.4169 | ||||||||
| Treatment moderates effect of behavioral strategy on weight change | |||||||||||
| None | |||||||||||
| Nonspecific predictor of weight change | |||||||||||
| Cut back No. of times eating fast food | 1 | 0.5137 | 0.0452 | ||||||||
| Cut back portion sizes | 1 | 0.6162 | 0.0028 | ||||||||
| Cut back amount of desserts | 1 | 0.6179 | <0.0001 | ||||||||
| Cut back sugared beverages | 2 | 0.3146 | 0.0899 | ||||||||
| Increased intake of water/reduced-calorie beverages | 1 | 0.6162 | 0.0655 | ||||||||
| Irrelevant to treatment effect or weight change | |||||||||||
| Used OTC meal replacements/low calorie meals | 1 | 0.2112 | 0.6665 | ||||||||
| Cut back No. of times eating out | 1 | 0.1173 | 0.2640 | ||||||||
| Cut back amount of alcohol | 1 | 0.6256 | 0.4656 | ||||||||
| PC vs. SD groups (N=579) | |||||||||||
| Mediator of treatment effect on weight change | |||||||||||
| Cut back amount of desserts | 1 | 0.0575 | −0.41 | 0.21 | <0.0001 | −2.69 | 0.61 | 0.0003 | 1.75 | 0.48 | 0.16 |
| Increased intake of fruits and vegetables | 1 | 0.0103 | −0.60 | 0.23 | 0.0007 | −2.25 | 0.66 | 0.0004 | 1.74 | 0.48 | 0.17 |
| Frequency of weighing last month** (overall) | 4 | <0.0001 | 0.0002 | 0.0058 | 1.38 | 0.50 | 0.53 | ||||
| >Once a week (2)–every day (1) | −0.18 | 0.26 | 1.37 | 0.72 | |||||||
| Once per week (3)–every day (1) | 0.38 | 0.26 | 2.16 | 0.74 | |||||||
| 1–3 times (4)–every day (1) | 1.01 | 0.28 | 3.39 | 0.77 | |||||||
| Not at all (5)–every day (1) | 1.75 | 0.39 | 3.18 | 0.98 | |||||||
| Independent outcome of treatment | |||||||||||
| Tried another weight loss program | 2 | 0.0914 | 0.7254 | ||||||||
| Used OTC meal replacements/low calorie meals | 1 | 0.0684 | 0.9815 | ||||||||
| Increased physical activity | 1 | 0.0980 | 0.2889 | ||||||||
| Treatment moderates effect of behavioral strategy on weight change | |||||||||||
| Cut back portion sizes×treatment interaction | 1 | 0.2331 | 0.0688 | ||||||||
| Yes–No|PC | 0.0790 | −1.63 | 0.93 | ||||||||
| Yes–No|SD | <0.0001 | −2.31 | 1.26 | ||||||||
| Nonspecific predictor of weight change | |||||||||||
| None | |||||||||||
| Irrelevant to treatment effect or weight change | |||||||||||
| Cut back No. of times eating fast food | 1 | 0.6001 | 0.1782 | ||||||||
| Cut back No. of times eating out | 1 | 0.1278 | 0.3272 | ||||||||
| Cut back sugared beverages | 2 | 0.8580 | 0.9945 | ||||||||
| Cut back amount of alcohol | 1 | 0.4563 | 0.7975 | ||||||||
| Increased intake of water/reduced-calorie beverages | 1 | 0.4457 | 0.3463 | ||||||||
PC=Personal Contact, IT=Interactive Technology, SD=Self-Directed, OTC=Over the counter.
the odds ratio is exp[γ1];
4 df Wald Chi-sq test, assumption of proportional odds rejected p<0.03, OR for level on scale relative to weighing ever day;
τ̂ = 1.2 for PC vs. IT, =1.9 for PC vs. SD
Mediator Analysis: Personal Contact Versus Interactive Technology
Correlations with Treatment
The relative frequency of using four behavioral strategies was significantly different (H0, γ1=0, p<0.10) between personal contact vs. interactive technology (Table 3 (PC vs. IT groups (N=593), columns under association with treatment (γ1))), meeting the criterion of a potential mediator of weight loss maintenance (step A in Fig. 1). Participants in the interactive technology arm were less likely to report increase in physical activity or consumption of fruits and vegetables and more likely to report trying another weight management program than those in the personal contact arm (67 vs. 78 % (OR, 0.56), 81 vs. 88 % (OR, 0.60), and 26 vs. 19 % (OR, 1.44), respectively). Participants in the personal contact arm were also more likely to report weighing themselves more than once a week; whereas, those in the interactive technology arm were less likely to weigh that frequently (OR for frequency relative to “every day” ranges from 0.6 to 1.1).
Relationship to Outcome
None of the behavioral weight loss strategies had a significant interaction (β3) with treatment arm, indicating that the effect of the behavioral strategy was not conditional on treatment arm. Three of the above potential mediators (increased physical activity, increased intake of fruits and vegetables, and more frequent weighing) had a significant main effect on change in weight from randomization to 30-month follow-up (β2; Table 3 (PC vs. IT groups (N=593), columns under impact on weight change (β2 or β3))), thus meeting criteria as a mediator (B1 in Fig. 1). Increase in each of these behaviors was associated with less weight regain (negative slope). For instance, the difference in weight regain between positive and negative responders to “increased intake of fruits and vegetables” was −2.39 kg (SE=0.63). Figure 2 illustrates the nonlinear association between frequency of weighing with weight loss maintenance, with the greatest weight regain (≥6 kg) occurring in individuals who reported weighing less than once per week. The fourth potential mediator (tried another weight loss program) was correlated with treatment (p=0.0678, OR=1.40) but not with weight change, so it was classified as an independent outcome of treatment.
Fig. 2.
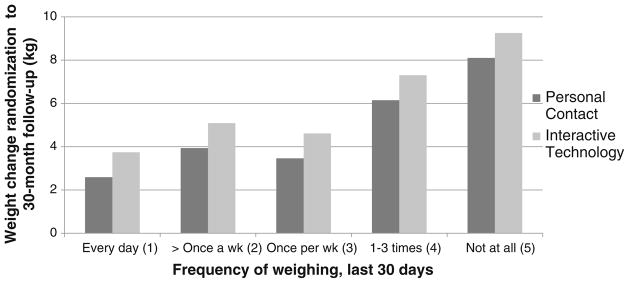
Relationship between frequency of weighing and weight change over 30 months in the personal contact vs. interactive technology comparison
Five behavioral strategies are classified as nonspecific predictors of weight change (B2 in Fig. 1, main effect only) since they were not correlated with treatment but did significantly impact outcome. Decrease in number of times eating fast food, portion sizes, amount of desserts, and consumption of sugared beverages, as well as increase in intake of water or diet beverages predicted less weigh regain in both treatment arms. For the remaining three behavioral strategies, both β2 and β3 were not significant, so they were classified as irrelevant to treatment effect or weight change.
Mediator Analysis: Personal Contact Versus Self-directed
Correlations with Treatment
The relative frequency of using six behavioral strategies was significantly different (H0, γ1=0, p<0.10) between personal contact and self-directed and thus were potential mediators of weight loss maintenance (Table 3 (PC vs. SD groups (N= 579), under association with treatment (γ1)); step A in Fig. 1). Individuals in the personal contact arm were less likely to report participating in another weight loss program, and to use meal replacements or low-calorie, prepared meals than those in self-directed (19 vs. 25 % (OR, 1.40) and 15 vs. 21 % (OR, 1.49), respectively) and were more likely to report increase in physical activity, cutting back on desserts, and increased consumption of fruits and vegetables (78 vs. 72 % (OR, 0.73), 84 vs. 78 % (OR, 0.66), and 88 vs. 80 % (OR, 0.55), respectively.) The contrast between personal contact and self-directed treatments on frequency of weighing is particularly marked (p<0.0001), with self-directed participants almost six times more likely than those in personal contact to report not weighing at all in the last 30 days (OR, 5.8 (95 % CL, 2.7, 12.4)). As shown in Table 2, 41 % of participants in the self-directed arm reported weighing themselves only a few times a month or not at all, whereas 19 % of those in the personal contact arm reported this.
Relationship to Outcome
As shown in Table 3 (PC vs. SD groups (N=579), columns under impact on weight change (β2 or β3)), three behavioral strategies were significantly associated with weight change, after controlling for treatment, thus meeting criteria as a mediator of weight loss maintenance (β2; step B1 in Fig. 1). Increased fruit and vegetable intake and frequency of weighing and decrease in amount of desserts were associated with less weight regain. The other three potential mediators had a non-significant association with the outcome and are classified as independent outcome of treatment.
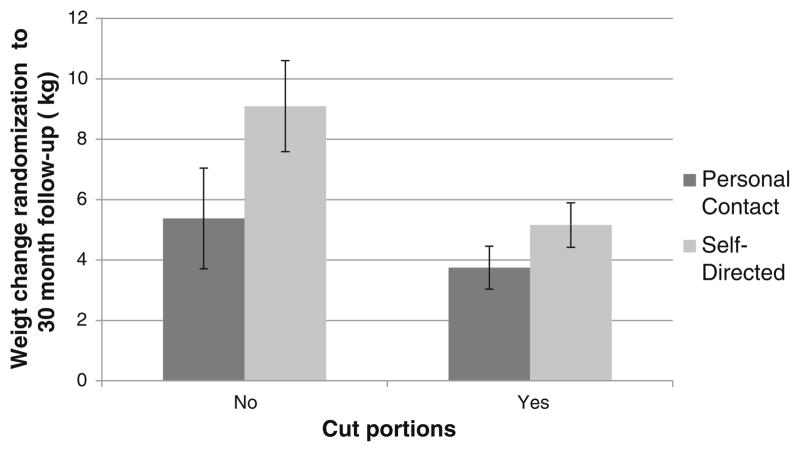
One strategy was not associated with treatment (Table 2; step A in Fig. 1) but had a significant interaction (β3; step B2 in Fig. 1) with treatment arm, indicating that the effect on the outcome was conditional on treatment arm, and is therefore classified as moderated by treatment. As depicted in Fig. 3, failure to cut back on portion size was associated with significantly greater weight regain at 30 months among those in the self-directed arm (9.1 kg (95 % CL=7.6, 10.6)) than in personal contact (5.4 kg (95 % CL=3.7), 7.1; p=0.0013). Finally, there was no nonspecific predictor of weight change, and five behavioral strategies were found to be irrelevant to treatment effect or weight change.
Fig. 3.
Treatment moderates the relationship between cutting back portions and weight outcomes in the personal contact vs. self-directed comparison
Indirect Effect
The right-most columns (indirect effect) of Table 3 show the adjusted effect of treatment after accounting for a mediator, plus the estimated indirect effect for each. The treatment contrast continues to be significant (p<0.02) even after adjusting for the mediator. The rightmost column (τ̂−ζ̂2) shows the reduction in treatment effect attributable to the mediator (in estimated weight regain, in kilograms). For instance, increased physical activity accounts for 0.14/1.2 of treatment effect for personal contact vs. interactive technology contrast, about 12 %.
Concurrent Validation
In the personal contact vs. interactive technology group, those who claimed increased fruit and vegetable intake on the Weight Management Strategies questionnaire reported smaller mean decline in servings of fruits and vegetables on the Block Food Frequency questionnaire (0.8 serving/day (95 % CL=−0.1), 1.7, p=0.0820). Those who responded “Yes” had a decline of 1.8, but those who said ‘No’ were down 2.6 servings/day. We failed to observe an association of frequency of weighing or increased physical activity with any of the three prospective change measures.
For personal contact vs. self-directed, there was a significant difference in the mean change in kilocalories per day over phase 2 (−170.1 (95 % CL=−299.2), −41.0, p=0.0104) between those who reported cutting back on desserts (mean increase of 9.3 kcal/day) and those who did not (up 179.4 kcal/day). As in the personal contact vs. interactive technology comparisons, those who claimed increased fruit and vegetable intake reported significantly smaller mean decline in servings of fruits and vegetables on the Block Food Frequency questionnaire (1 serving/day (CL=0.1), 1.8, p=0.0343). Those who responded “Yes” had a decline of 1.8, but those who said “No” were down 2.8 servings/day. We failed to detect an association of frequency of weighing with any of the three prospective change measures.
Discussion
The Weight Loss Maintenance Trial provides unique information on potential behavioral mediators of weight loss maintenance. We found that increased fruit and vegetable consumption and more frequent self-weighing mediated the superior effect of the personal contact arm on weight loss maintenance in comparison to both the interactive technology and self-directed arms. In addition, increase in physical activity mediated the difference between personal contact and interactive technology effects on weight loss maintenance, and decreased dessert consumption mediated the difference between personal contact and self-directed effects.
The relationship between increased fruit and vegetable consumption and improved weight management outcome has been observed in previous intervention studies (for a comprehensive review, see Rolls et al. [23]). In the Women’s Health Initiative Dietary Modification Trial, a study of a low-fat diet intervention designed to prevent breast and colorectal cancer in postmenopausal women, increase in fruit and vegetable servings was associated with greater weight loss over 7.5 years [24]. Similarly, among moderately overweight middle-aged men participating in the Multiple Risk Factor Intervention Trial, a multifactor intervention program for modifying coronary heart disease risk factors, increased consumption of foods of lower energy density (e.g., fruits and vegetables) was associated with more sizeable, sustained weight loss over 6 years [25].
Although previous studies have demonstrated an association between fruit and vegetable intake and longer-term weight outcomes, the current study is the first to specifically identify increased consumption of fruits and vegetables as a mediator of the effect of a behavioral lifestyle intervention on weight loss maintenance. Based on our study, it appears that the more personal nature and/or the specific content covered in interactions with personal contact interventionists, in contrast to the less personal or less targeted information delivered in the interactive technology intervention (or in the case of the self-directed condition, no intervention), was more likely to promote fruit and vegetable consumption, a core focus of the DASH dietary approach [14, 15]. Although the two interventions were intended to cover the same content, the interactive technology intervention does not appear to have emphasized fruit and vegetable consumption to the same degree as the personal contact intervention. These findings should be interpreted carefully, however, given that our concurrent validity analyses found that those who reported increasing fruits and vegetables during the maintenance phase of the study did not demonstrate greater increases in fruits and vegetables as assessed by Block Food Frequency questionnaire in comparison to those who did not endorse increasing fruit and vegetable consumption on the Weight Management Strategies questionnaire. We instead found that those who reported increasing consumption of fruits and vegetables on the Weight Management Strategies questionnaire decreased intake of fruits and vegetables as assessed by the Block Food Frequency questionnaire to a lesser extent than those who denied increasing fruits and vegetables on the Weight Management Strategies questionnaire. Although further research should distinguish whether it is increasing fruits and vegetables or avoiding decreasing fruit and vegetable consumption that is most important in avoiding weight regain following a weight loss, this dietary component appears to be a significant strategy in weight loss maintenance.
The association between self-monitoring of weight and weight management outcomes has also been observed in a number of clinical trials [26–29] and in the National Weight Control Registry [30], an annual evaluation of adults who have lost at least 30 lb (13.6 kg) and kept it off for at least 1 year [31]. In the Pound of Prevention Trial [29], the Weigh to Be Trial [26], and the Study to Prevent Regain Trial (STOP Regain) [27], more frequent self-weighing was associated with less weight gain, greater weight loss, and better weight loss maintenance, respectively; however, none of these trials have reported formal mediation analyses. The current study suggests that the Weight Loss Maintenance Trial’s personal contact intervention was superior at encouraging participants to weigh themselves regularly, both in relation to the interactive technology and self-directed arms. Moreover, as nicely depicted in Fig. 2, weighing at least once a week, perhaps even daily, appears to be protective of weight regain.
Although it is somewhat intuitive that being in no intervention (self-directed) would be associated with less frequent monitoring of weight, which in our study was markedly lower when comparing those in self-directed to personal contact, it is less clear why the interactive technology intervention, which had several features to encourage weighing and provide weight-related feedback, was not as successful at promoting this behavior. Based on our findings, it appears that personal contact with an interventionist trained in motivational interviewing and behavioral weight loss/maintenance strategies has a superior impact on this self-regulation strategy. It is not known, however, whether it was interventionists’ encouragement of the behavior of self-weighing itself, or the provision of accountability and impactful feedback about weight outcomes, that reinforced this important behavior. Interactive technology features today may be more sophisticated than they were at the time the Weight Loss Maintenance Trial was conducted and, based on our findings, those engaged in delivering technology-based interventions for weight loss maintenance should continue placing significant efforts on enhancing weight-related feedback and support (e.g., linking automated feedback about weight to a personal goal; setting up a system where weight data can be released to a real person, such as a primary care doctor or primary support person).
The association between physical activity and weight loss maintenance is well-documented in observational studies such as the National Weight Control Registry [31], but less so in randomized clinical trials of weight loss maintenance. National Weight Control Registry participants who have successfully maintained a weight loss of ≥30 lb (13.6 kg) for ≥1 year report an average of ~60 min of daily moderate intensity activity [32]; however, there is considerable variability in the amount of activity reported [33]. In a population-based survey by Kruger et al. [34], adults who reported 60–90 min of physical activity per day had the greatest odds for successful weight loss maintenance. In our study, similar to the face-to-face intervention of STOP Regain [35], the personal contact arm of the Weight Loss Maintenance Trial was more effective at encouraging increased activity than interactive technology or self-directed; however, the impact on weight regain was significant only in the personal contact vs. interactive technology contrast. These results need to be interpreted thoughtfully since we did not observe an association between retrospectively reported increases in physical activity and prospectively measured changes in physical activity. One explanation is that participants’ self-reported increased light activity did not meet the accelerometer-based threshold for moderate intensity. It is plausible that this increase in light activity, and perhaps a decrease in sedentary behavior, was associated with weight management. There is evidence that both higher levels of sedentary time and lower levels of light activity are associated with higher BMI [36, 37].
Cutting back on desserts, decreasing portions, eating less fast food, decreasing sugared beverages, and increasing water and diet beverages emerged as nonspecific predictors of weight loss maintenance in the personal contact vs. interactive technology comparison. These behaviors seem to be important in preventing weight regain among those participating in a behaviorally based weight maintenance intervention, regardless of the type of delivery channel. Cutting back desserts also predicted better weight control in the personal contact vs. self-directed comparison, and being in the personal contact intervention was associated with a greater reduction in dessert consumption compared with no intervention (self-directed); therefore, reduction of desserts is a potential mediator of the difference between personal contact and self-directed effects. Cutting back portions was moderated by treatment in the personal contact vs. self-directed comparison, indicating that the effect of failing to adopt this behavior depended on the presence or absence of treatment. More specifically, those in the self-directed group who denied decreasing portions gained significantly more weight (~9 kg) than those in the personal contact group (~5 kg) who also denied decreasing portion sizes. Those in the personal contact group who denied decreasing portions may have developed other healthy eating strategies (e.g., decreasing fat, increasing fiber, etc.) without necessarily altering portion size.
A unique feature of the current study is its ability to report on the proportion of participants in the various interventions who practiced various weight control strategies or enrolled in weight loss programs that are not typically encouraged in randomized clinical behavioral weight management trials. For example, we were uncertain before analyzing these data whether those in the control group would be more likely to participate in other commercial weight loss programs or to use over-the-counter diet products in the absence of an active intervention. Furthermore, we wondered whether any of these strategies would be significantly associated with weight loss maintenance outcomes or could help explain why we did not find larger effect sizes in our initial comparison of intervention and control conditions [5]. Interestingly, both interactive technology and self-directed participants were more likely to try another weight loss program than personal contact participants, and those in self-directed were also more likely to use over-the-counter meal replacement or diet meals; however, none of these weight management strategies was associated with greater weight loss maintenance. This area clearly needs further study, particularly since commercial weight loss programs are the most commonly reported approach to weight loss among successful dieters [27].
The current study is limited by measure-related factors; the questionnaire asked participants to report on behavior change that occurred during the maintenance phase of the Weight Loss Maintenance Trial at only one point in time, with a long retrospective period (30 months). Therefore, we cannot be completely confident that participants were reporting on their behaviors from the beginning of phase 2 to the end of phase 2, and not from the beginning of phase 1 to the end of phase 2. In short, there is always an unknown when time frame is specified in self-report instruments. Furthermore, we are unable to describe when the behaviors assessed were employed during the long intervention period and how engagement or changes in the behaviors may have related to weight trajectory. The current findings, particularly those that have been identified as “irrelevant to treatment outcome” and are inconsistent with past literature, need to be interpreted cautiously since recall issues are a factor. Additionally, future studies assessing the identified mediators should utilize prospective, rather than retrospective, measures. Another potential limitation of the current study is the inclusion of completers only in the analysis sample. We did find a difference between race-sex groups in the likelihood of being a completer. Completers may differ from non-completers in ways that could bias the results of this study. For instance, they may have dropped out because of less success maintaining weight loss.
Despite these limitations, we believe the current data offers rich information on the topic of weight loss maintenance, particularly since the Weight Loss Maintenance Trial is one of the largest and longest randomized clinical trials on weight loss maintenance to date and included such a diverse sample of participants. Results of the current study, however, may not apply to initial weight loss or to other populations.
To confirm that the behavioral mediators identified in this exploratory, hypothesis-generating study are indeed causal links between treatment and outcome (i.e., mechanisms of treatment effects), future studies should enhance the focus on these strategies and test them in well-designed trials (e.g., factorial designs or trials that isolate each of the mediators). Once more potent treatment interventions are developed and determined to be effective, they could be refined into innovative and cost effective treatments, such as internet and virtual coaching programs.
Acknowledgments
This study was supported by grants 5-U01 HL68734, 5-U01 HL68676, 5-U01 HL68790, 5-U01 HL68920, and 5-HL68955 from the National Heart, Lung, Blood Institute.
Footnotes
Conflict of interest statements: Four of the study authors (JWC, LJA, GJJ, and ATD) have a financial relationship with Healthways, Inc., a company with whom we partnered for the POWER Trial (Appel et al., 2011), which occurred after the Weight Loss Maintenance Trial was complete. This relationship presents no conflicts of interest with the organization that sponsored the research (NIH). Appel L.J., Clark J.M., Yeh H.C., Wang N.Y., Coughlin J.W., Daumit G., Miller E.R., Dalcin A., Jerome G.J., Geller S., Noronha G., Pozefsky T., Charleston J., Reynolds J.B., Durkin N., Rubin R.R., Louis T.A., and Brancati F.L. (2011). Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med. 365 [20]. doi: 10.1056/NEJMoa1108660, PMID:22085317.
Contributor Information
J. W. Coughlin, Email: jwilder3@jhmi.edu, Johns Hopkins University School of Medicine, 600 North Wolfe Street, Meyer 101, Baltimore, MD 21287, USA
C. M. Gullion, Kaiser Permanente Center for Health Research, Oakland, CA, USA
P. J. Brantley, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, LA, USA
V. J. Stevens, Kaiser Permanente Center for Health Research, Oakland, CA, USA
A. Bauck, Kaiser Permanente Center for Health Research, Oakland, CA, USA
C. M. Champagne, Pennington Biomedical Research Center, Louisiana State University System, Baton Rouge, LA, USA
A. T. Dalcin, Johns Hopkins University School of Medicine, 600 North Wolfe Street, Meyer 101, Baltimore, MD 21287, USA
K. L. Funk, Kaiser Permanente Center for Health Research, Oakland, CA, USA
J. F. Hollis, Kaiser Permanente Center for Health Research, Oakland, CA, USA
G. J. Jerome, Towson University, Towson, MD, USA
L. F. Lien, Duke University Medical Center, Durham, NC, USA
C. M. Loria, National Heart, Lung, and Blood Institute, Bethesda, MD, USA
V. H. Myers, Klein Buendel, Inc., Golden, CO, USA
L. J. Appel, Johns Hopkins University School of Medicine, 600 North Wolfe Street, Meyer 101, Baltimore, MD 21287, USA
References
- 1.National Institutes of Health/National Heart LaBI. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: The evidence report. Obesity Research. 1998;6:51S–210S. [PubMed] [Google Scholar]
- 2.World Health Organization. Obesity: Preventing and managing the global epidemic. 2000. [PubMed] [Google Scholar]
- 3.Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: A review. J Cardiovasc Nurs. 2009;24:58–80. doi: 10.1097/01.JCN.0000317471.58048.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brantley PJ, Appel LJ, Hollis JF, et al. Design considerations and rationale of a multi-center trial to sustain weight loss: The weight loss maintenance trial. Clinical Trials. 2008;5:546–556. doi: 10.1177/1740774508096315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svetkey LP, Stevens VJ, Brantley PJ, et al. Comparison of strategies for sustaining weight loss: The weight loss maintenance randomized controlled trial. JAMA. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- 6.Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008;35:118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stevens VJ, Funk KL, Brantley PJ, et al. Design and implementation of an interactive website to support long-term maintenance of weight loss. J Med Internet Res. 2008;10:e1. doi: 10.2196/jmir.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 9.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 10.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 11.Kraemer HC, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychol. 2008;27:S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels MJ, Roy JA, Kim C, Hogan JW, Perri MG. Biometrics: Bayesian Inference for the causal effect of mediation. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teixeira PJ, Silva MN, Coutinho SR, et al. Mediators of weight loss and weight loss maintenance in middle-aged women. Obesity (Silver Spring) 2010;18:725–735. doi: 10.1038/oby.2009.281. [DOI] [PubMed] [Google Scholar]
- 14.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 15.Karanja NM, Obarzanek E, Lin PH, et al. Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension Trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99:S19–S27. doi: 10.1016/s0002-8223(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 16.Rowlands AV, Thomas PW, Eston RG, Topping R. Validation of the RT3 triaxial accelerometer for the assessment of physical activity. Med Sci Sports Exerc. 2004;36:518–524. doi: 10.1249/01.mss.0000117158.14542.e7. [DOI] [PubMed] [Google Scholar]
- 17.Jerome GJ, Young DR, Laferriere D, Chen C, Vollmer WM. Reliability of RT3 accelerometers among overweight and obese adults. Med Sci Sports Exerc. 2009;41:110–114. doi: 10.1249/MSS.0b013e3181846cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harlan LC, Block G. Use of adjustment factors with a brief food frequency questionnaire to obtain nutrient values. Epidemiology. 1990;1:224–231. doi: 10.1097/00001648-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 20.Li Y, Schneider JA, Bennett DA. Estimation of the mediation effect with a binary mediator. Stat Med. 2007;26:3398–3414. doi: 10.1002/sim.2730. [DOI] [PubMed] [Google Scholar]
- 21.Mackinnon DP, Warsi G, Dwyer JH. A simulation study of mediated effect measures. Multivariate Behav Res. 1995;30:41. doi: 10.1207/s15327906mbr3001_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Little RJA, Rubin DB. Statistical analysis with missing data. 2. New York: Wiley; 2002. [Google Scholar]
- 23.Rolls BJ, Ello-Martin JA, Tohill BC. What can intervention studies tell us about the relationship between fruit and vegetable consumption and weight management? Nutr Rev. 2004;62:1–17. doi: 10.1111/j.1753-4887.2004.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 24.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: The Women’s Health Initiative Dietary Modification Trial. JAMA. 2006;295:39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Stamler J, Dolecek TA. Relation of food and nutrient intakes to body mass in the special intervention and usual care groups in the Multiple Risk Factor Intervention Trial. Am J Clin Nutr. 1997;65:366S–373S. doi: 10.1093/ajcn/65.1.366S. [DOI] [PubMed] [Google Scholar]
- 26.Jeffery RW, Sherwood NE, Brelje K, et al. Mail and phone interventions for weight loss in a managed-care setting: Weigh-to-be one-year outcomes. Int J Obes Relat Metab Disord. 2003;27:1584–1592. doi: 10.1038/sj.ijo.0802473. [DOI] [PubMed] [Google Scholar]
- 27.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–1571. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 28.Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Ann Behav Med. 2005;30:210–216. doi: 10.1207/s15324796abm3003_5. [DOI] [PubMed] [Google Scholar]
- 29.Jeffery RW, French SA. Preventing weight gain in adults: The pound of prevention study. Am J Public Health. 1999;89:747–751. doi: 10.2105/ajph.89.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: A key component of successful weight loss maintenance. Obesity (Silver Spring) 2007;15:3091–3096. doi: 10.1038/oby.2007.368. [DOI] [PubMed] [Google Scholar]
- 31.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66:239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 32.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 33.Catenacci VA, Ogden LG, Stuht J, et al. Physical activity patterns in the National Weight Control Registry. Obesity (Silver Spring) 2008;16:153–161. doi: 10.1038/oby.2007.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruger J, Blanck HM, Gillespie C. Dietary practices, dining out behavior, and physical activity correlates of weight loss maintenance. Prev Chronic Dis. 2008;5:A11. [PMC free article] [PubMed] [Google Scholar]
- 35.Wing RR, Papandonatos G, Fava JL, et al. Maintaining large weight losses: The role of behavioral and psychological factors. J Consult Clin Psychol. 2008;76:1015–1021. doi: 10.1037/a0014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swartz AM, Tarima S, Miller NE, et al. Prediction of body fat in older adults by time spent in sedentary behavior. J Aging Phys Act. 2012;20:332–344. doi: 10.1123/japa.20.3.332. [DOI] [PubMed] [Google Scholar]
- 37.Dunton GF, Berrigan D, Ballard-Barbash R, Graubard B, Atienza AA. Joint associations of physical activity and sedentary behaviors with body mass index: Results from a time use survey of US adults. Int J Obes (Lond) 2009;33:1427–1436. doi: 10.1038/ijo.2009.174. [DOI] [PubMed] [Google Scholar]