Abstract
Reflexes initiated by the carotid body, the principal O2-sensing organ, are critical for maintaining cardio-respiratory homeostasis during hypoxia. O2 sensing by the carotid body requires carbon monoxide (CO) generation by heme oxygenase-2 (HO-2) and hydrogen sulfide (H2S) synthesis by cystathionine-γ-lyase (CSE). We report that O2 stimulated the generation of CO, but not that of H2S, and required two cysteine residues in the heme regulatory motif (Cys265 and Cys282) of HO-2. CO stimulated protein kinase G (PKG)–dependent phosphorylation of Ser377 of CSE, inhibiting the production of H2S. Hypoxia decreased the inhibition of CSE by reducing CO generation resulting in increased H2S, which stimulated carotid body neural activity. In carotid bodies from mice lacking HO-2, compensatory increased abundance of nNOS (neuronal nitric oxide synthase) mediated O2 sensing through PKG-dependent regulation of H2S by nitric oxide. These results provide a mechanism for how three gases work in concert in the carotid body to regulate breathing.
INTRODUCTION
Cellular communication is mediated through diverse signaling molecules, including proteins, lipids, amino acids, and gases. Gaseous messengers are distinctive among signaling molecules in that they cannot be stored in vesicles. This suggests that gaseous messenger signaling depends on tightly regulated in situ enzymatic synthesis instead of exocytotic machinery. Despite a growing recognition of the widespread importance of gaseous messengers in various biological processes (1), little is known about their regulation under physiological conditions.
The gaseous messenger carbon monoxide (CO) is important for oxygen (O2) sensing (2–4). In the carotid body, changes in blood O2 concentrations are transduced into changes in CO production. CO signaling during normoxia inhibits the carotid body sensory nerve activity, whereas decreased CO generation during hypoxia increases the carotid body neural output (2, 3). CO acts similarly in the brain to alter cerebral blood flow (4). High CO concentrations during normoxia elicit vasoconstriction, whereas decreased CO signaling during hypoxia results in vasodilation. CO synthesis in the carotid body (2, 3) and in the brain (4) is catalyzed by the constitutively expressed enzyme heme oxygenase-2 (HO-2) (5). It is unclear how changes in O2 concentrations affect CO synthesis by HO-2.
Whereas CO generation is sensitive to changes in O2, CO by itself does not trigger responses to hypoxia. Instead, CO affects the generation of another gaseous messenger, hydrogen sulfide (H2S) (3, 4, 6, 7). H2S mediates increased carotid body sensory nerve activity (3, 7) and cerebral vasodilation (4) during hypoxia. Emerging evidence suggests that CO suppresses H2S signaling by inhibiting the H2S-synthesizing enzymes cystathionine-γ-lyase (CSE) (7) and cystathionine β-synthase (CBS) (4, 6, 8, 9). CSE is the predominant source of H2S in peripheral tissues such as the carotid body (7, 10), whereas CBS is responsible for most of the H2S production in the brain (11, 12). It is believed that CO directly inhibits CBS by binding to its heme moiety (6, 8, 9). Unlike CBS, however, CSE is not a heme-containing protein, indicating that CO inhibits CSE by an alternative and unknown mechanism.
O2 sensing by the carotid body is critical for regulation of vital functions including breathing, heart rate, and blood pressure under hypoxic conditions (13). Given the critical roles of CO and H2S signaling for sensory function of the carotid body (3, 7), we sought to determine how changes in O2 affect CO and H2S generation by carotid body chemoreceptor cells. The limited availability of carotid body tissue (wet weight of the mouse carotid body is ~25 µg) necessitated first studying the effects of O2 on CO and H2S generation in human embryonic kidney (HEK) 293 cells heterologously expressing HO-2 and CSE. The mechanisms identified in vitro were then validated in mouse carotid bodies.
RESULTS
Cys265 and Cys282 are critical for O2-sensitive CO generation by HO-2
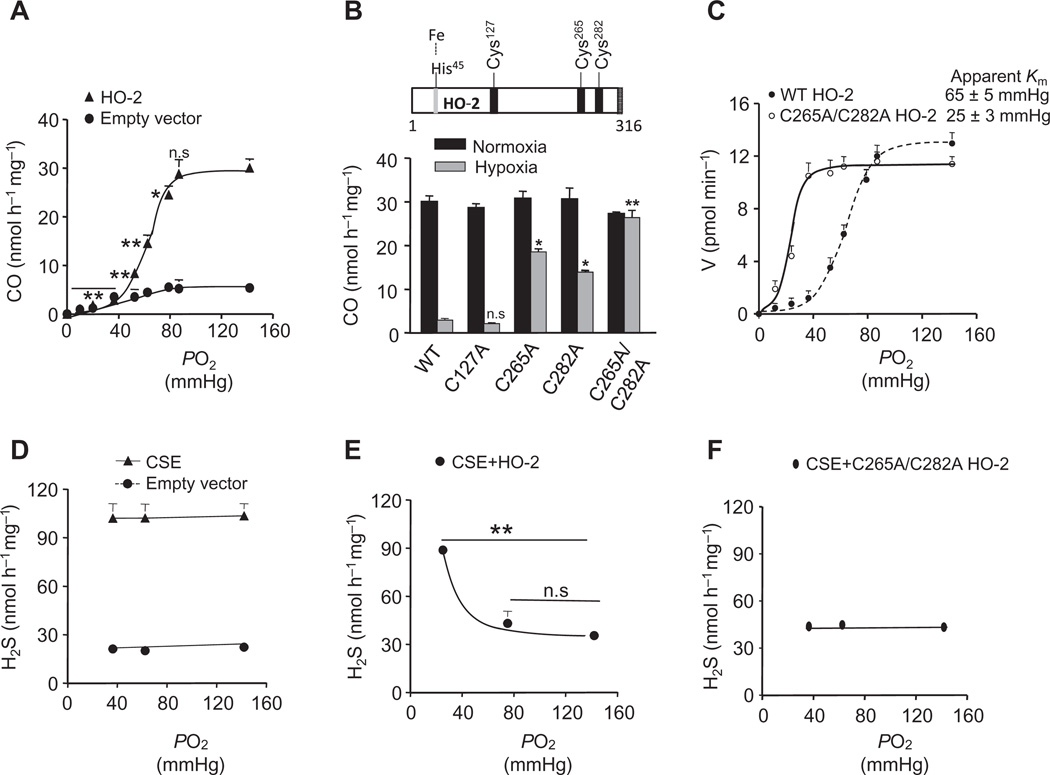
We first examined the effect of changes in the partial pressure of O2 (PO2) on CO generation in HEK-293 cells heterologously expressing HO-2. Cells expressing HO-2 generated more CO under normoxia (PO2 = 142 ± 3 mmHg) than did empty vector–transfected cells. Reducing the PO2 from ~142 to ~90 mmHg had no significant effect, whereas further reductions in PO2 resulted in a progressive decrease in CO generation in a stimulus-dependent manner as compared with normoxia (Fig. 1A and fig. S1A). These findings demonstrated that CO generation by HO-2 was inherently responsive to changes in PO2.
Fig. 1. Effect of O2 on CO and H2S generation in HEK-293 cells expressing HO-2 and CSE alone or together.
(A) CO generation as a function of PO2 in the medium in cells transfected with the empty vector and HO-2 vector. (B) Effect of mutating cysteine residues in HO-2 on CO generation. Top: Schematic representation of cysteine residues in the N-terminal and heme regulatory motif of HO-2. Bottom: CO production in cells expressing either wild-type (WT) or mutant (C127A, C265A, C282A, or C265A/C282A) HO-2 in response to normoxia and hypoxia. (C) Rate of CO generation as a function of PO2 in WT and mutant HO-2 (C265A/C282A)–expressing cells. Apparent Km values are derived by iterative curve fitting. (D to F) H2S generation as a function of PO2 in cells transfected with the empty vector or CSE vector (D) or vectors encoding CSE and HO-2 (E) or CSE and mutant HO-2 (C265A/C282A) (F). The graphs in (A) to (F) represent means ± SEM (n = 3 to 5 independent experiments). *P < 0.05; **P < 0.01; n.s., not significant (P > 0.05). See fig. S1 for data on Western blot analysis of HO-2– and CSE-expressing HEK cells.
We next sought to delineate the mechanism underlying the inherent O2 sensitivity of HO-2. HO-2 has three cysteine residues (Cys127, Cys265, and Cys282; numbering of amino acid residues corresponds to the human protein unless otherwise stated) in the heme regulatory motif (Fig. 1B) (14). We investigated whether these cysteine residues are required for the sensitivity of HO-2 to O2. Substituting alanine for either Cys265 or Cys282 reduced the O2 sensitivity of HO-2, whereas mutation of both Cys265 and Cys282 eliminated modulation of CO generation by hypoxia (PO2 = 36 ± 4 mmHg; Fig. 1B). In contrast, substitution of alanine for Cys127 had no effect on CO production during hypoxia (Fig. 1B).
We then investigated whether changes in O2 affinity accounted for the loss of O2-sensitive CO generation in cells expressing double mutant (C265A/C282A) HO-2. We found that the apparent Michaelis constant (Km) of wild-type HO-2 for O2 was 65 ± 5 mmHg (~88 µM), a value close to that reported previously (15). In contrast, the Km of HO-2C265A/C282A was 25 ± 3 mmHg (~34 µM; Fig. 1C and fig. S1B). These findings suggest that Cys265 and Cys282 confer low O2 affinity for HO-2, thereby rendering CO generation sensitive to changes in PO2.
O2-dependent HO-2 activity inhibits H2S generation by CSE
In contrast to the intrinsic O2 sensitivity of CO generation by HO-2, H2S generation in CSE-expressing HEK-293 cells was unaffected by changes in PO2 (Fig. 1D and fig. S1C). To reconcile the observations that CSE is not directly sensitive to O2, but H2S generation from CSE increases during hypoxia in the carotid body (7), we hypothesized that CSE was regulated by O2-sensitive CO generation. Whereas cells expressing only CSE displayed steady H2S generation regardless of changes in PO2, cells coexpressing HO-2 and CSE exhibited decreased H2S concentrations during normoxia and increased concentrations during hypoxia (Fig. 1E and fig. S1D), recapitulating the O2-dependent H2S generation in the carotid body (7). However, H2S concentrations remained unchanged during hypoxia when CSE was coexpressed with the O2-insensitive mutant HO-2C265A/C282A (Fig. 1F and fig. S1E). These results demonstrate that CO is an inhibitor of CSE and that O2-dependent CO generation contributes to increased H2S generation by CSE in hypoxic cells.
CO inhibits CSE through cyclic guanosine monophosphate
To delineate the mechanism by which CO inhibits CSE, we first considered whether CO inhibits calmodulin, a calcium-dependent activator of CSE (12). CO might inhibit CSE activity by reducing the pool of intracellular calcium available to stimulate calmodulin. However, the intracellular Ca2+ concentrations ([Ca2+]i) were similar in cells that coexpressed HO-2 and CSE and in cells that only expressed CSE (fig. S2, A and B). The calmodulin inhibitor W7 [N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride] did not affect H2S generation (fig. S2C), arguing against a mechanism in which CO inhibits H2S by regulating calmodulin activity.
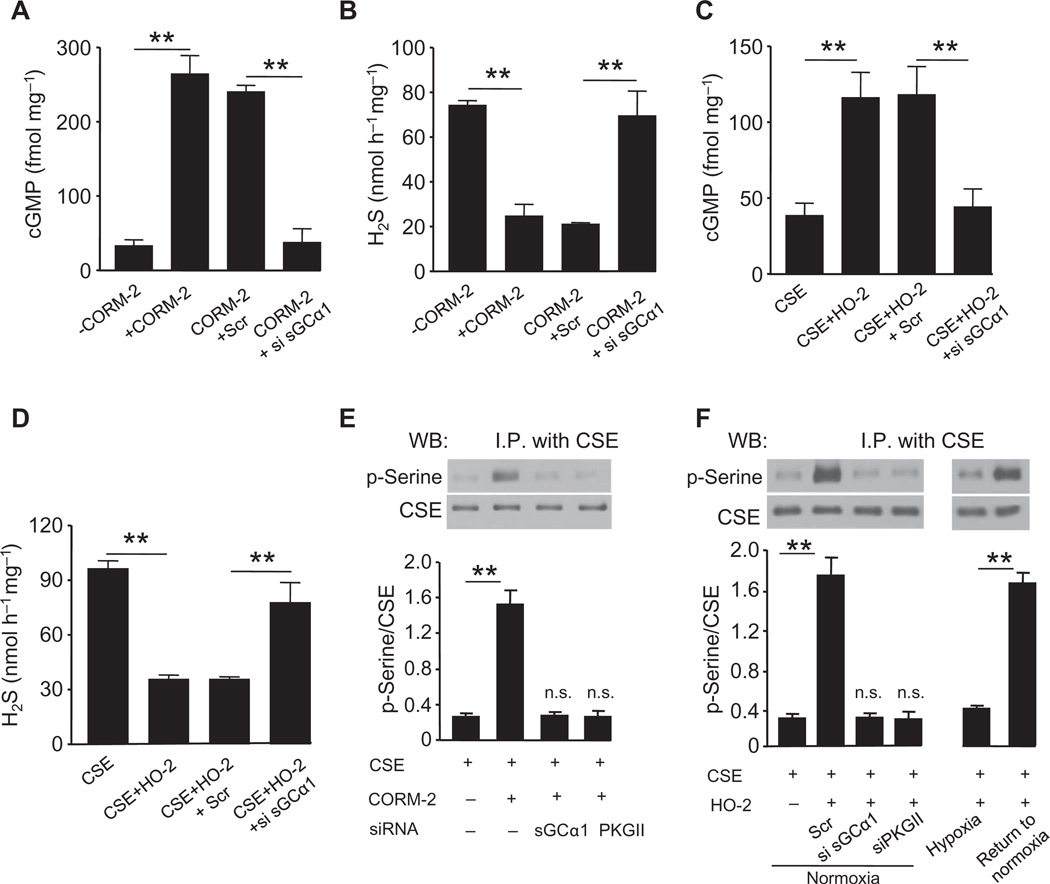
CO binds to and activates soluble guanylyl cyclase (sGC) (16), which catalyzes the synthesis of the second messenger cyclic guanosine monophosphate (cGMP). To determine whether CO regulates H2S generation through sGC/cGMP signaling, we challenged CSE-expressing cells with the CO donor [Ru(CO)3Cl2]2 [tricarbonyldichlororuthenium (II) dimer] (CORM-2) (17). CORM-2 increased cGMP concentrations and reduced H2S generation (Fig. 2, A and B). Transfection of a small interfering RNA (siRNA) targeting the α1 subunit of sGC (fig. S3A) prevented cGMP accumulation and preserved H2S generation in CORM-2–treated cells (Fig. 2, A and B).
Fig. 2. CO inhibits H2S generation through sGC-dependent cGMP production.
(A to D) Effects of siRNA silencing of sGCα1 on the generation of cGMP and H2S in response to either the CO donor CORM-2 (A and B) or coexpression of HO-2 (C and D) in HEK-293 cells expressing CSE. Scr, scrambled RNA. (E and F) Analysis of serine phosphorylation of CSE. Top: representative immunoblot; bottom: densitometric analysis. Effects of siRNA silencing of either sGCα1 or cGMP-dependent PKG II on serine phosphorylation in cells expressing CSE in response to CORM-2 (E) or in CSE/HO-2–coexpressing cells under normoxia or hypoxia or hypoxic cells returned to normoxia. (F) The graphs in (A) to (F) represent means ± SEM (n = 4 to 5 independent experiments). **P < 0.01. See fig. S3 for data on silencing sGC and PKG II by siRNA. I.P., immunoprecipitation.
To confirm that HO-2–derived CO stimulates sGC/cGMP signaling as does exogenous CO, we measured cGMP and H2S concentrations in cells coexpressing HO-2 and CSE. Cells expressing HO-2 as well as CSE produced more cGMP under normoxia than did cells expressing only CSE (Fig. 2C). The increase in cGMP was associated with suppressed H2S production (Fig. 2D). siRNA-mediated silencing of sGC reduced cGMP concentrations and prevented HO-2 from inhibiting H2S generation under normoxia (Fig. 2, C and D). Hypoxia, which inhibits HO-2 activity, reduced cGMP concentrations and increased H2S generation. Hypoxia had no further effect on cGMP or H2S concentrations in cells with sGC knockdown (fig. S3, B and C). Together, these results demonstrate that CO, through sGC/cGMP signaling, inhibited CSE-dependent H2S production.
CO induces protein kinase G–dependent phosphorylation of CSE
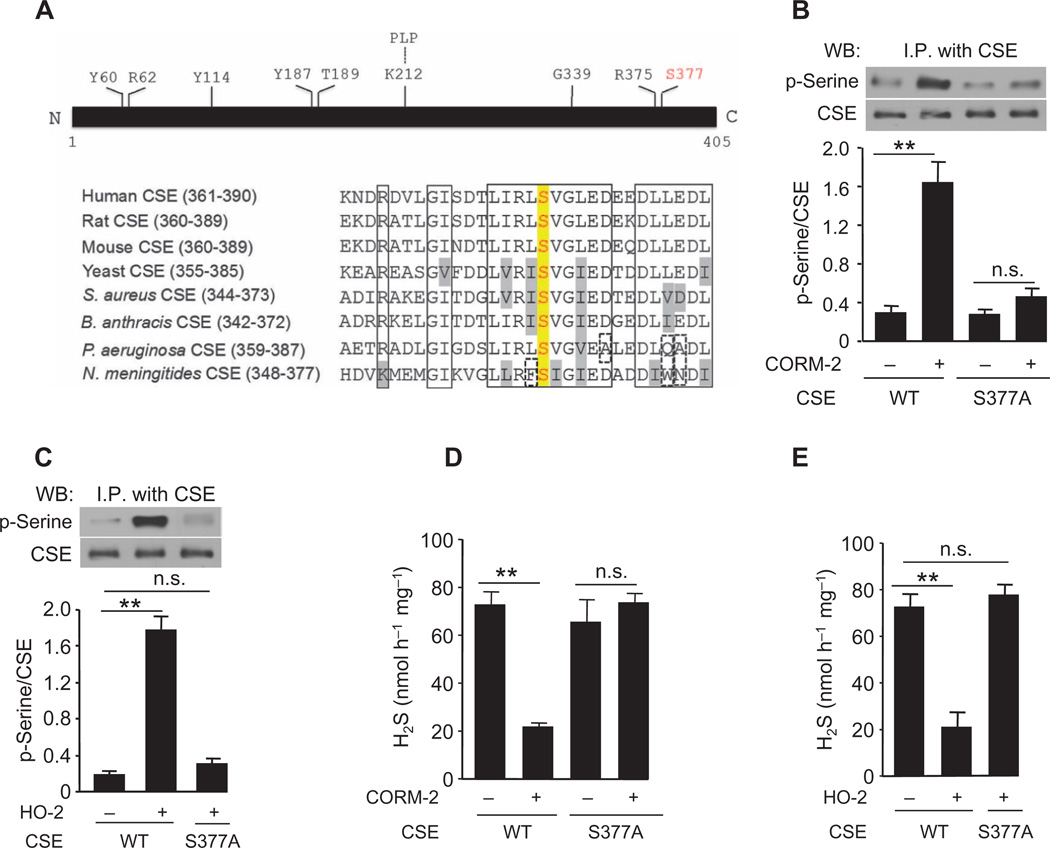
A major biological action of cGMP is the activation of cGMP-dependent protein kinase G (PKG) I and II, which phosphorylate serine and threonine residues in target proteins (18). We explored whether activation of cGMP signaling by CO stimulates PKG to phosphorylate CSE. CSE immunoprecipitates from CSE-expressing HEK-293 cells were immunoblotted with antibodies recognizing phosphoserine. Treatment of CSE-expressing cells with CORM-2 induced an increase in the serine phosphorylation of CSE (Fig. 2E). Likewise, cells coexpressing HO-2 and CSE also exhibited a robust increase in serine phosphorylation of CSE under normoxia (Fig. 2F). siRNA-mediated silencing of either sGC or PKG II, which is more abundant in the mouse carotid body than PKG I (fig. S3D), prevented phosphorylation of CSE evoked by CORM-2 in CSE/HO-2–coexpressing cells under normoxia (Fig. 2, E and F, and fig. S3E). Exposure of HO-2/CSE–coexpressing cells to hypoxia markedly reduced phosphorylation of CSE, which completely recovered after being returned to normoxic conditions (Fig. 2F), demonstrating the reversible nature of O2-dependent CSE phosphorylation.
We next sought to identify the serine residue in CSE that is phosphorylated by PKG. CSE belongs to a family of pyridoxal-5-phosphate–dependent enzymes (19). Sequence alignment of CSE across species revealed that Ser377 is located within a highly conserved putative PKG recognition sequence (Fig. 3A) (18). Mutating Ser377 to alanine (S377A) abolished CORM-2– or HO-2–induced serine phosphorylation of CSE (Fig. 3, B and C). Neither CORM-2 nor HO-2 coexpression inhibited H2S generation from cells expressing CSES377A (Fig. 3, D and E). Cells expressing CSE with the phosphomimetic substitution S377E exhibited a >90% reduction in basal H2S concentrations (fig. S3F), suggesting that phosphorylation of Ser377 attenuated H2S synthesis. Together, these results point to the reversible phosphorylation of Ser377 by PKG as a switch by which CO regulates H2S generation by CSE.
Fig. 3. Inhibition of H2S generation by CO requires phosphorylation of CSE at Ser377.
(A) Top: Amino acid residues in CSE required for H2S generation. Bottom: Sequence alignment of CSE across species reveals that Ser377 is evolutionarily conserved (red). Conserved residues are outlined by a solid box. Conservative substitutions are shown in gray, and nonconservative substitutions are outlined by a dashed box. (B and C) Comparison of the serine phosphorylation of exogenously expressed WT and mutant (S377A) CSE in cells treated with the CO donor CORM-2 (B) and in cells coexpressing HO-2 (C). Top: representative immunoblots; bottom: densitometric analysis. (D and E) Effects of CORM-2 (D) and HO-2 expression (E) on H2S generation in cells expressing WT or mutant CSE (S377A). The graphs in (B) to (E) represent means ± SEM (n = 3 to 4 independent experiments). **P < 0.01. See fig. S3 for data on the effect of the phosphomimetic mutation S377E on H2S generation.
PKG signaling inhibits H2S generation and sensory nerve activity of the carotid body
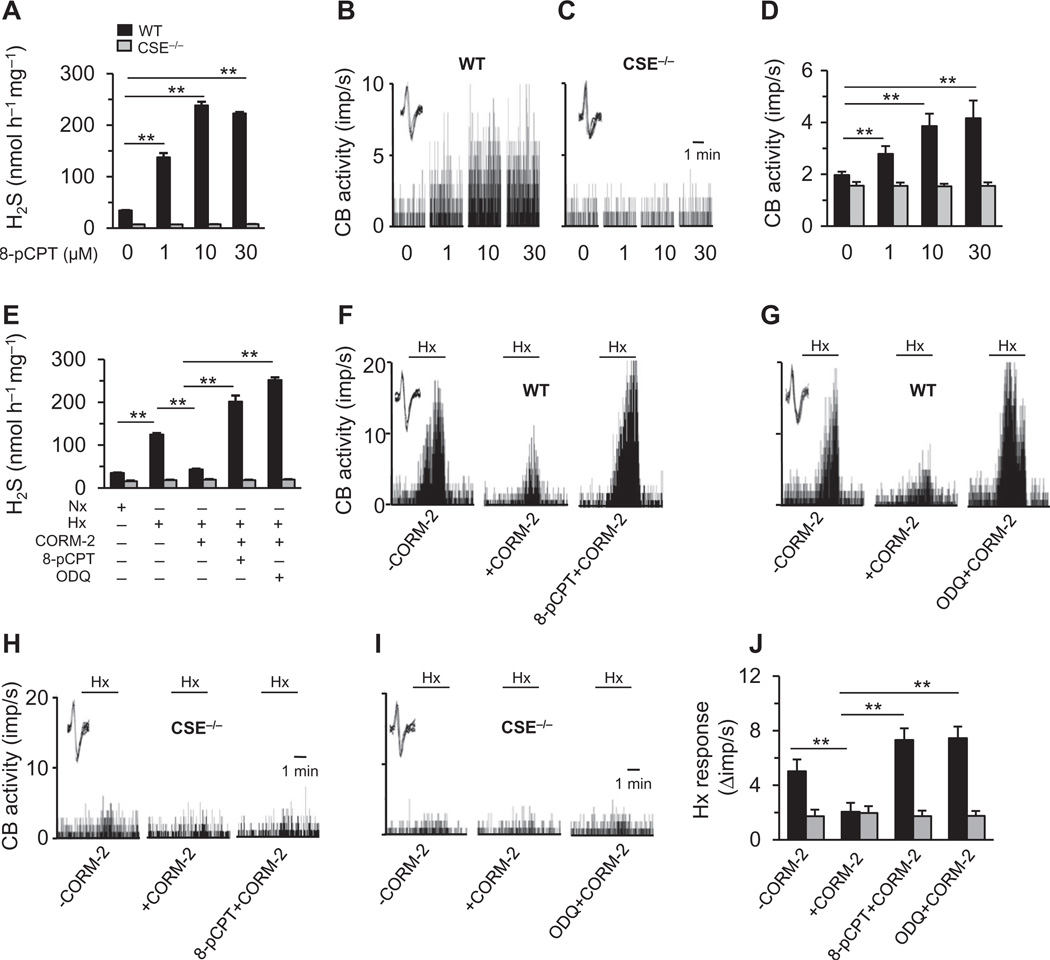
We assessed the relevance of PKG regulation of CSE on carotid body function. Because carotid bodies produce high amounts of CO during normoxia (3), we hypothesized that activation of PKG by CO inhibits H2S generation from CSE and maintains low sensory activity, whereas inhibiting PKG, similar to exposure to hypoxia, should increase H2S concentrations and augment carotid body sensory nerve activity. Indeed, application of a PKG inhibitor, (Rp)-8-pCPT-cGMPS (8-pCPT), increased H2S generation in a dose-dependent manner in wild-type carotid bodies with a concomitant increase in sensory nerve activity under normoxia (PO2 = 142 ± 3 mmHg). However, the PKG inhibitor did not affect H2S concentrations or sensory nerve activity in CSE-null carotid bodies (Fig. 4, A to D).
Fig. 4. PKG-dependent cGMP signaling in the carotid body.
(A to D) Effect of the PKG inhibitor 8-pCPT on (A) H2S generation and (B to D) baseline sensory nerve activity of WT and CSE−/− mouse carotid bodies. (E to J) Effect of CORM-2 on hypoxia-evoked (E) H2S generation and (F to J) sensory nerve response of WT and CSE−/− mouse carotid bodies with or without 8-pCPT or the sGC inhibitor ODQ. The insets in the tracings of (B), (C), and (F) to (I) show superimposed action potentials of the sensory nerve fiber from which the integrated carotid body sensory nerve activity [CB activity; impulses per second (imp/s)] was derived. Black horizontal bars marked with “Hx” represent the duration of the hypoxic challenge (n = 3 independent experiments for H2S measurements and n = 6 carotid bodies for each genotype and treatment for sensory nerve activity measurements). **P < 0.01. See fig. S4 for data on YC-1.
To further assess the role of CO-induced PKG signaling, we treated wild-type carotid bodies with the CO donor CORM-2 or the sGC activator YC-1 [3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole] (20). Both CORM-2 and YC-1 blocked the increase in H2S concentrations in response to hypoxia, and pretreating the carotid bodies with a PKG inhibitor or the sGC inhibitor ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) prevented CORM-2 or YC-1 from inhibiting H2S generation (Fig. 4E; YC-1 data in fig. S4), demonstrating that CO signals through the sGC/PKG pathway to inhibit H2S generation. Neither activating nor inhibiting the sGC/PKG signaling pathway altered H2S concentrations in CSE-null carotid bodies (Fig. 4E; YC-1 data in fig. S4), providing further evidence that CSE is the target of CO-induced sGC/PKG signaling. Likewise, both CORM-2 and YC-1 reduced carotid body sensory nerve excitation by hypoxia in wild-type but not in CSE-null carotid bodies, and these effects were reversed by either a PKG inhibitor or the sGC inhibitor ODQ (Fig. 4, F to J; YC-1 data in fig. S4).
HO-2 governs O2 sensing of the carotid body
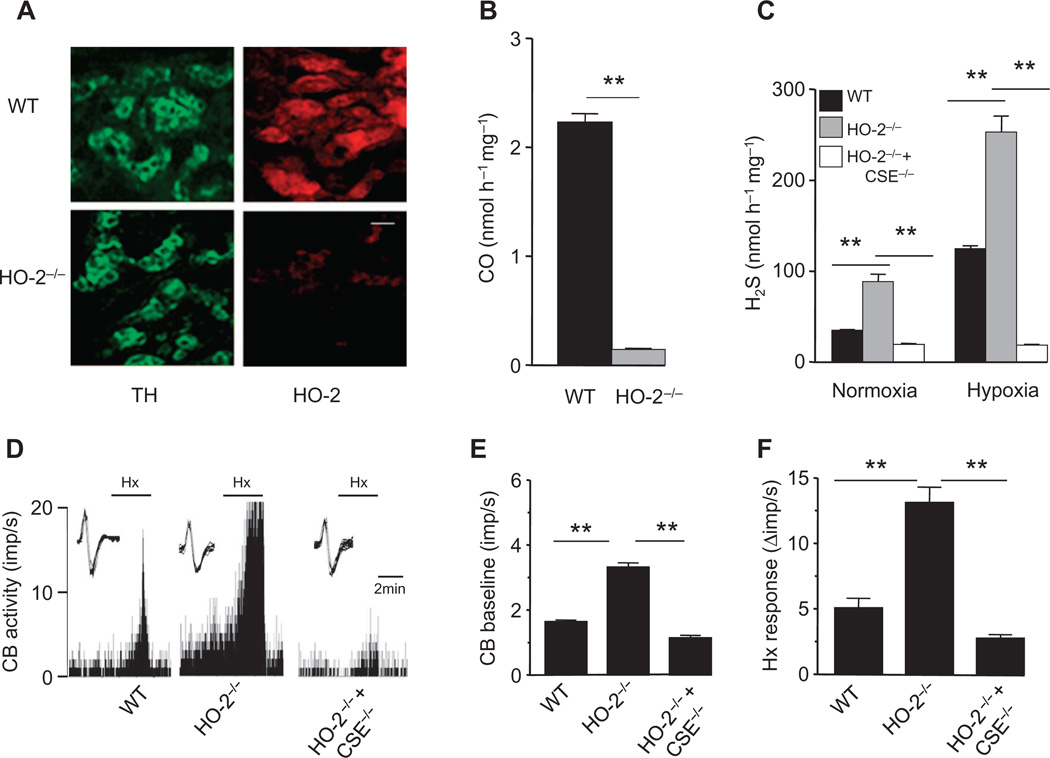
The inherent O2 sensitivity of HO-2 and its ability to regulate the generation of the effector molecule H2S led us to investigate whether carotid body O2 sensing is fundamentally governed by HO-2. In carotid bodies from wild-type mice, HO-2 was present in glomus cells, which are the primary O2-sensing cells of the carotid body (13), whereas HO-2 was absent in carotid bodies from HO-2–null mice (Fig. 5A), which exhibited a >90% reduction in CO production compared to wild-type carotid bodies (Fig. 5B). If HO-2–dependent CO generation is obligatory for carotid body O2 sensing, HO-2–null mice should exhibit augmented basal sensory nerve activity and H2S concentrations in the carotid body. Indeed, H2S generation and baseline sensory nerve activity were greater in HO-2–null carotid bodies than in wild-type (Fig. 5, C to E). However, H2S concentrations and sensory nerve activity still increased in hypoxia (Fig. 5, C, D, and F), suggesting the existence of a residual regulatory mechanism that does not involve HO-2. HO-2/CSE double-null carotid bodies did not exhibit a sensory nerve response to hypoxia, demonstrating that this residual O2-sensing mechanism exploited H2S generation by CSE to trigger sensory nerve excitation (Fig. 5, C to F).
Fig. 5. Gaseous messenger generation and sensory nerve activity in HO-2–null carotid bodies.
(A) Adjacent carotid body sections immunostained for HO-2 and tyrosine hydroxylase (TH), a marker of glomus cells, in WT and HO-2−/− mice. Scale bar, 20 µm. (B) CO generation in carotid bodies of WT and HO-2−/− mice. (C to F) H2S generation (C) and sensory nerve activity (D to F) of the carotid bodies of WT, HO-2−/−, and HO-2−/− + CSE−/− mice. In tracings of (D), the insets present superimposed action potentials of the sensory nerve fiber from which the integrated carotid body sensory nerve activity (CB activity; imp/s) was derived, and black bars marked with “Hx” represent the duration of the hypoxic challenge. Images in (A) are representative of three mice per genotype. The graphs in (B), (C), (E), and (F) represent means ± SEM (n = 3 independent experiments for CO and H2S measurements and n = 6 carotid bodies for each genotype for sensory nerve activity measurements). **P < 0.01.
Neuronal nitric oxide synthase abundance is increased in HO-2–null glomus cells
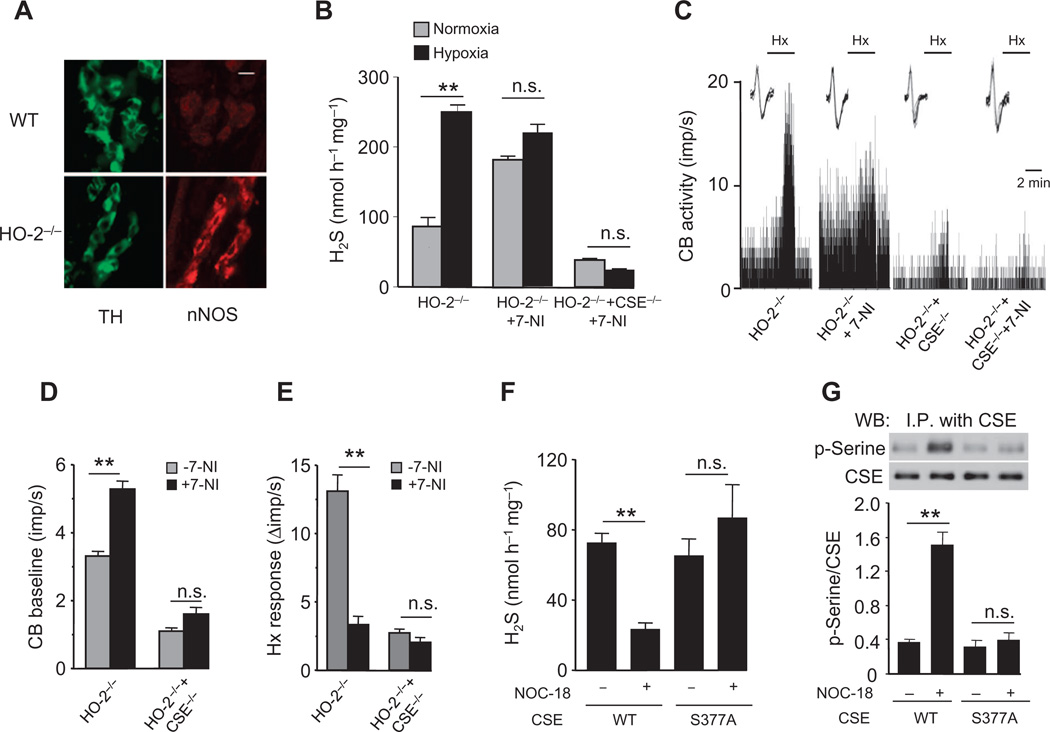
Immunocytochemistry of HO-2–null carotid bodies revealed neuronal nitric oxide synthase (nNOS) staining in glomus cells (Fig. 6A). In contrast, nNOS staining was not detectable either in glomus cells or in nerve fibers of wild-type carotid bodies (Fig. 6A). nNOS generates nitric oxide (NO) in an O2-dependent manner (21), and similar to CO, NO inhibits carotid body sensory nerve activity (22). Treatment of HO-2–null carotid bodies with the nNOS inhibitor 7-nitroindazole (7-NI) (23, 24) increased baseline H2S concentrations and sensory nerve activity, suggesting that NO could also be a physiological inhibitor of H2S generation. 7-NI also eliminated the O2 sensitivity of HO-2–null carotid bodies (Fig. 6, B to E). 7-NI did not affect H2S concentrations or sensory nerve activity in HO-2/CSE double-null carotid bodies (Fig. 6, B to E).
Fig. 6. NO signaling in HO-2–null carotid body.
(A) Adjacent carotid body sections immunostained for nNOS and tyrosine hydroxylase (TH), amarker of glomus cells, inWT and HO-2−/− mice. Scale bar, 20 µm. (B to E) Effect of the nNOS inhibitor 7-NI on H2S generation (B) and sensory nerve activity (C to E) of carotid bodies from HO-2−/− and HO-2−/− + CSE−/− mice. In tracings in (C), the insets present superimposed action potentials of the sensory nerve fiber from which the integrated carotid body sensory nerve activity (CB activity; imp/s) was derived, and black bars marked with “Hx” represent the duration of the hypoxic challenge. (F and G) Effect of the NO donor NOC-18 on H2S generation (F) and CSE serine phosphorylation (G) in HEK-293 cells expressing WT or mutant CSE (S377A). Images in (A) are representative of three mice per genotype. The graphs in (B) and (D) to (G) represent means ± SEM (n = 3 for each genotype and treatment for H2S measurements, n = 6 to 8 carotid bodies for each genotype and treatment for sensory nerve activity measurements, and n = 3 independent experiments for H2S measurements and CSE phosphorylation in HEK-293 cells). **P < 0.01.
NO activates sGC (25) and can thus substitute for CO in the same sGC/PKG signaling pathway to inhibit H2S generation by CSE. To determine whether NO also inhibits H2S generation by inducing the phosphorylation of CSE at Ser377, we treated cells expressing CSE with the NO donor NOC-18 [3,3-bis(aminoethyl)-1-hydroxy-2-oxo-1-triazene] (26). Similar to the CO donor, the NO donor decreased H2S generation and increased serine phosphorylation of CSE in cells expressing wild-type CSE, but not in those expressing CSES377A (Fig. 6, F and G). These results demonstrate that NO and CO regulate H2S generation in a similar manner, through phosphorylation of CSE at Ser377.
DISCUSSION
Emerging evidence suggests that CO and H2S are critical for O2 sensing by the carotid body (3, 7, 10); however, the molecular mechanisms by which O2 affect the generation of these gaseous messengers have been elusive. Our results establish that HO-2 is sensitive to changes in O2 availability, even in a heterologous system, such that CO generation is high under normoxia and low during hypoxia. This unique O2 sensitivity requires Cys265 and Cys282, which lower the O2 affinity of HO-2 and enable the enzyme to transduce changes in O2 into changes in CO generation. The carotid body sensory nerve activity begins to increase only when the arterial blood PO2 drops to anywhere between 60 and 80 mmHg [see (13) for references], which coincides with the apparent Km of HO-2 for O2 of ~65 mmHg. Thus, the low affinity of HO-2 for O2 is physiologically critical to O2 sensing by the carotid body.
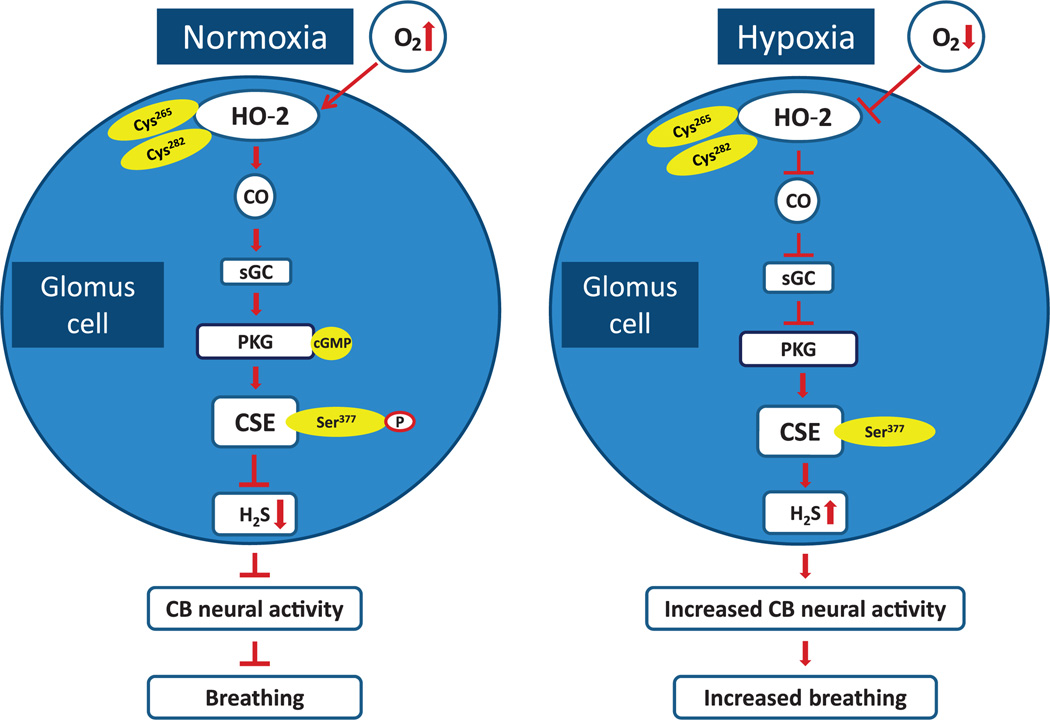
In contrast, H2S generation by CSE is not directly sensitive to changes in PO2. CO is a physiological inhibitor of H2S generation by CSE (7). Increased H2S generation during hypoxia is due to decreased CO generation, resulting in reduced inhibition of CSE. CO inhibits CSE by stimulating sGC/PKG signaling, in contrast to the heme-dependent inhibition of CBS by CO (4, 6, 8, 9). PKG phosphorylates CSE at Ser377, rendering it inactive. CSE phosphorylation is O2-dependent and reversible, further suggesting that this phosphorylation event functions in a signaling capacity. The complex interplay between three gases—O2, CO, and H2S—and its impact on carotid body sensory nerve activity and breathing is summarized in Fig. 7. The tight posttranslational regulation of CSE activity by PKG-dependent phosphorylation suggests a possible role for protein phosphatases and/or cGMP-dependent phosphodiesterases, which remain to be studied.
Fig. 7. O2 sensing in the carotid body.
Schematic presentation of the signaling pathways associated with the interplay between three gases—O2, CO, and H2S—in glomus cells of the carotid body (CB) and their impact on CB neural activity and breathing. Cys265 and Cys282 are located in the heme regulatory motif of HO-2. Ser377 is the target residue in the putative PKG recognition sequence in CSE.
Alternative mechanisms for H2S generation during hypoxia have been proposed in trout gill chemoreceptors (27), in cyclostome blood vessels (28), and in Caenorhabditis elegans neurons (29). Increased H2S concentrations during hypoxia in lower vertebrates have been attributed to decreased oxidative degradation of H2S (30). While such a mechanism may operate in lower vertebrates, our results indicate that in the mammalian carotid body, H2S generation by CSE is not directly sensitive to changes in O2; instead, it is regulated through O2-dependent CO generation by HO-2.
A role for HO-2 in O2 sensing by the carotid body has been previously proposed (2, 31). Consistent with this possibility, genetic knockout of HO-2, like hypoxia, increased the baseline sensory nerve activity and H2S generation of the carotid body. However, we did find that HO-2–null carotid bodies still responded to hypoxia as previously reported (32). Further investigation revealed that loss of HO-2 in glomus cells prompts an unanticipated compensatory increase of another O2-sensitive gaseous messenger–generating enzyme, nNOS, which catalyzes the generation of NO. Although the mechanisms underlying the compensatory increase of nNOS in glomus cells of HO-2–null mice remain to be investigated, inhibition of nNOS eliminated sensory nerve excitation and H2S generation in response to hypoxia in HO-2–null carotid bodies. The O2 affinity of nNOS is much lower (apparent Km = 350 µM or ~250 mmHg) (21, 22) than that of HO-2 (apparent Km = 88 µM or ~65 mmHg), suggesting that even a modest reduction in PO2 will lead to decreased NO production by nNOS. Similar to CO, NO also inhibits carotid body sensory nerve activity (21, 22) and inhibits CSE by inducing the phosphorylation of Ser377. Collectively, these findings suggest that HO-2 governs carotid body O2 sensing by affecting H2S generation through PKG-dependent phosphorylation of CSE. In the absence of HO-2 activity, nNOS provides an alternative mechanism by which H2S generation can be regulated according to O2 availability, thereby providing an important fail-safe redundancy for a vital homeostatic process.
How might regulation of H2S by either CO or NO contribute to O2 sensing by the carotid body? The prevailing model of O2 sensing by the carotid body suggests that hypoxia increases the sensory nerve activity by inhibiting O2-sensitive K+ channels in glomus cells, leading to depolarization-induced voltage-gated Ca2+ influx and release of neurotransmitter(s) to excite sensory nerve endings [see (13) for references]. Similar to hypoxia, H2S increases carotid body sensory nerve activity by inhibiting O2-sensitive K+ channels in glomus cells, resulting in voltage-gated Ca2+ influx [see (33) for references]. Furthermore, hypoxia is ineffective in causing voltage-gated Ca2+ influx and neurotransmitter secretion in CSE-null glomus cells (10). Together, these findings suggest that H2S transduces the hypoxic stimulus to changes in ion channel function and neurotransmitter release from glomus cells to elicit sensory nerve excitation.
Dysregulation of CO and H2S production in the carotid body can give rise to an abnormal chemosensory reflex, resulting in pathological consequences such as hypertension, pulmonary edema, and poor ventilatory adaptation to hypoxia (3, 34–37). Solely by correcting abnormal CO or H2S concentrations, it has been possible to successfully control hypertension in spontaneous hypertensive rats and prevent pulmonary edema in rats exposed to hypobaric hypoxia (3). Given the explosion of interest in surgically transecting carotid bodies as a therapeutic strategy in patients with essential hypertension and heart failure (38, 39), we believe that pharmacologically perturbing components of the sGC/PKG signaling pathway identified in this study is a more viable and safer approach for treating carotid body–related morbidities. Moreover, whereas gaseous messengers are important for O2 sensing, their biological functions are by no means confined to it. It is becoming increasingly recognized that dysregulated gaseous signaling contributes to diverse diseases, including hypertension (3, 11) and Parkinson’s disease (40). Given that HO-2, NOS, and CSE are all present in the vasculature as well as in neurons (41–43), dysregulated crosstalk between CO, NO, and H2S may contribute to the pathophysiology of these disorders. Accordingly, this newfound understanding of gaseous messenger signaling likely has substantial implications in a diverse set of physiological and pathophysiological conditions.
MATERIALS AND METHODS
Plasmids, cell culture, and transfection
HO-2 (rat) was cloned into pcDNA3.1 with N-terminal myc tag. Mouse CSE was cloned into pCMV-myc. Mutations of HO-2 and CSE were performed using the QuikChange II XL mutagenesis kit (Agilent Technologies, #200521) according to the manufacturer’s instructions. Mutations were verified by Sanger sequencing at the University of Chicago Core sequencing facility. HEK-293 cells were cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, #11995-065), 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 U/ml) in 5% CO2 atmosphere at 37°C. Cells were plated on 60-mm plates and transfected with 0.5 µg of plasmid pcDNA encoding either wild-type or mutant HO-2 and/or 2 µg of plasmid DNA encoding either wild-type or mutant CSE using Lipofectamine (Invitrogen, #18324-012) according to the manufacturer’s protocol.
Immunoblot and immunoprecipitation assays
Immunoblot assays (HO-2, CSE, sGCα1, PKG II, and phosphoserine) and immunoprecipitation of CSE in HEK-293 cell lysates were performed as described (44). The following primary antibodies were used: anti–HO-2 (1:1000; Abcam, #ab90492), anti-CSE (1:1000; Novus Biologicals, #NBP1-52849), anti-sGCα1 (1:1000; Abcam, #ab50358), anti-CGK2 (PKG II; 1:1000; Abcam, #ab110124), anti-phosphoserine (1:1000; Chemicon International, #AB1603), and anti–α-tubulin (1:1000; Sigma-Aldrich, #T6199).
Measurements of H2S and CO
H2S and CO generation in HEK-293 cells and carotid bodies was determined as previously described (7). In the experiments involving carotid bodies, six tissues from three mice were pooled in a given experiment. In experiments assessing the effect of PO2 on H2S and CO generation, sealed tubes containing the reaction medium along with the center well were flushed with either 21% O2 or varying proportions of O2-N2 gas mixtures for 15 min. PO2 in the reaction medium was determined by a blood gas analyzer (ABL5, Radiometer). H2S concentrations were calculated from a standard curve relating Na2S concentration to absorption at 620-nm light. CO concentrations were calculated from a standard curve relating CORM-2 ([Ru(CO)3Cl2]2; 1 mol of CORM-2 is equivalent to 0.7 mol of CO) to absorption at 590-nm light. The generation of H2S and CO was expressed as nanomoles per hour per milligram of protein.
Measurements of cGMP
cGMP concentrations were determined by a chemiluminescent enzyme-linked immunosorbent assay (Cell Biolabs, # STA-506) according to the manufacturer’s instructions. Before the experiment, cells were treated with 1 mM IBMX (3-isobutyl-1-methylxanthine) to inhibit degradation of cGMP by phosphodiesterases. cGMP concentrations were normalized to protein concentration as determined by bicinchoninic acid assay.
Measurements of intracellular calcium concentrations
Intracellular calcium concentrations ([Ca2+]i) in HEK-293 cells were measured using Fura-2AM as previously described (10). Background fluorescence was subtracted from signals. Image intensity at 340 nm was divided by 380-nm image intensity to obtain the ratiometric image. Ratios were converted to free [Ca2+]i using calibration curves constructed in vitro by adding Fura-2 (50 µM, free acid) to solutions containing known concentrations of Ca2+ (0 to 2000 nM). The recording chamber was continually superfused with solution from gravity-fed reservoirs.
Preparation of mice
Experiments were approved by the Institutional Animal Care and Use Committee of the University of Chicago and were performed on age-matched, male Bl6, HO-2−/− and CSE−/− mice (from S. H. Snyder and R. Wang) unless otherwise noted. Double HO-2 and CSE knockout mice were created by crossing HO-2−/− females with CSE−/− males. Animals were euthanized by intracardiac injection (0.1 ml) of euthanasia solution (Beuthanasia-D Special, Schering-Plough).
Immunocytochemistry
Carotid bodies were harvested from anesthetized mice and perfused with heparinized saline followed by 4% paraformaldehyde for 30 min. Adjacent carotid body sections (8 µm thick) were immunostained with either rabbit anti–tyrosine hydroxylase antibody (1:4000; Pel-Freez, #P40101), rabbit anti–HO-2 antibody (1:200 dilution; Abcam, #ab90492), or rabbit anti-nNOS antibody (1:200; Cell Signaling, #4231) as previously described (3, 7).
Carotid body sensory nerve activity
Sensory nerve activity was recorded from ex vivo carotid bodies harvested from anesthetized mice as previously described (3, 7). Briefly, the carotid sinus nerve was treated with collagenase, and a few nerve bundles were isolated. Action potentials from one of the nerve bundles were recorded using a suction electrode (~20-µm-diameter tip). In general, two to three action potentials of varying size and amplitude were seen in a given nerve bundle. Action potentials of similar height, duration, and shape (“single” unit) were selected using Spike histogram software (LabChart 7 Pro) for analysis of the sensory nerve activity. To obtain a stable baseline sensory nerve activity, carotid bodies were first superfused for 1 hour with normoxia-equilibrated medium. Subsequently, baseline sensory nerve activity under normoxia was recorded for 5 min. Sensory nerve responses to hypoxia were monitored for 3 min. The PO2 in the medium was determined by a blood gas analyzer (ABL 5). Hypoxic response was measured as the difference between the sensory nerve activity under baseline and during hypoxia (Δimp/s).
Quantitation of PKG isoform mRNA
The mRNA expression of PKG I and PKG II was analyzed in the carotid bodies by quantitative real-time polymerase chain reaction (Bio-Rad), and the data were normalized with 18S mRNA as previously described (45). Two carotid bodies were pooled in a given experiment. The primers used for PKG I (Prkg1), PKG II (Prkg2), and 18S ribosomal RNA (rRNA) were as follows: Prkg1, CCTTGCAGGGGGAGGATGTAA (forward), TTGGCGAAGAAGGCAGCTTC (reverse); Prkg2, CTGCGGAGCAAAGTGGCAGA (forward), CCTGCAGCTTGTTCAGCTGGAT (reverse); 18S rRNA, GTAACCCGTTGAACCCCATT (forward), CCATCCAATCGGTAGTAGCG (reverse).
Drugs
The following drugs were used: CORM-2 (Sigma-Aldrich, #288144),ODQ (Tocris Bioscience, #0880), YC-1 (Sigma-Aldrich, #Y102), 8-pCPT (Enzo Life Sciences, #BML-CN206-001), 7-NI (Tocris Bioscience, #0602), W7 (Tocris Bioscience, #0369), NOC-18 (Sigma-Aldrich, #A5581), and IBMX (Sigma-Aldrich, #I5879).
All drugs were freshly prepared during the experiment. The following concentrations of drugs were used: CORM-2, 10 µM; 8-pCPT, 10 µM; ODQ, 15 µM; YC-1, 30 µM; W7, 100 µM; and NOC-18, 250 µM. In experiments involving HEK-293 cells, cells were treated with desired concentration of drugs 30 min before the experiment. In the studies with ex vivo carotid bodies, the chemoreceptor tissue was continuously superfused with desired concentration of drugs added to the reservoirs. In the experiments with 7-NI, mice were treated with 7-NI (10 mg/kg intraperitoneally) 1 hour before harvesting carotid bodies for recording sensory nerve activity.
Data analysis and statistics
All data were reported as means ± SEM derived from three independent biological experiments, unless otherwise stated in the figure legends. Statistical analysis was performed with either one-way or two-way analysis of variance (ANOVA) with repeated measures followed by post hoc Tukey’s test. For the analysis of normalized data, the Mann-Whitney test was used. P values <0.05 were considered significant.
Acknowledgments
We thank N. Wang for help with immunocytochemistry and R. Wang for providing CSE knockout mice.
Funding: This work was supported by NIH grants P01 HL-90554 and UH2-HL-123610 (to N.R.P.) and U.S. Public Health Service grant DA-000226 and Research Scientist Award DA-00074 (to S.H.S.). M.M.G. is supported by the NIH Medical Scientist Training Program Award T32 GM-007309.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencesignaling.org/cgi/content/full/8/373/ra37/DC1
Fig. S1. Western blot analysis of HEK-293 cells exogenously expressing HO-2 and CSE.
Fig. S2. Calcium-dependent calmodulin activity is not required for inhibition of CSE by CO.
Fig. S3. Effect of siRNA targeting sGCα1 on hypoxia-induced changes in cGMP concentrations and H2S generation in HEK-293 cells.
Fig. S4. Effects of YC-1 with or without either PKG or sGC inhibitor on H2S generation and the sensory nerve activity of wild-type and CSE−/− mouse carotid bodies.
Author contributions: N.R.P. and G.K.K. designed the research; G.Y., C.V., Y.-J.P., V.V.M., G.R., J.N., and G.K.K. performed the experiments. G.Y., Y.-J.P., V.V.M., J.N., C.V., G.R., G.K.K., and N.R.P. analyzed the data; M.M.G. and S.H.S. contributed reagents; N.R.P., G.K.K., C.V., G.L.S., and S.H.S. wrote the paper.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Mustafa AK, Gadalla MM, Snyder SH. Signaling by gasotransmitters. Sci. Signal. 2009;2:re2. doi: 10.1126/scisignal.268re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prabhakar NR, Dinerman JL, Agani FH, Snyder SH. Carbon monoxide: A role in carotid body chemoreception. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1994–1997. doi: 10.1073/pnas.92.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peng YJ, Makarenko VV, Nanduri J, Vasavda C, Raghuraman G, Yuan G, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. Inherent variations in CO-H2S-mediated carotid body O2 sensing mediate hypertension and pulmonary edema. Proc. Natl. Acad. Sci. U.S.A. 2014;111:1174–1179. doi: 10.1073/pnas.1322172111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morikawa T, Kajimura M, Nakamura T, Hishiki T, Nakanishi T, Yukutake Y, Nagahata Y, Ishikawa M, Hattori K, Takenouchi T, Takahashi T, Ishii I, Matsubara K, Kabe Y, Uchiyama S, Nagata E, Gadalla MM, Snyder SH, Suematsu M. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc. Natl. Acad. Sci. U.S.A. 2012;109:1293–1298. doi: 10.1073/pnas.1119658109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maines MD. The heme oxygenase system: Past, present, and future. Antioxid. Redox Signal. 2004;6:797–801. doi: 10.1089/ars.2004.6.797. [DOI] [PubMed] [Google Scholar]
- 6.Shintani T, Iwabuchi T, Soga T, Kato Y, Yamamoto T, Takano N, Hishiki T, Ueno Y, Ikeda S, Sakuragawa T, Ishikawa K, Goda N, Kitagawa Y, Kajimura M, Matsumoto K, Suematsu M. Cystathionine β-synthase as a carbon monoxide–sensitive regulator of bile excretion. Hepatology. 2009;49:141–150. doi: 10.1002/hep.22604. [DOI] [PubMed] [Google Scholar]
- 7.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10719–10724. doi: 10.1073/pnas.1005866107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taoka S, West M, Banerjee R. Characterization of the heme and pyridoxal phosphate cofactors of human cystathionine β-synthase reveals nonequivalent active sites. Biochemistry. 1999;38:2738–2744. doi: 10.1021/bi9826052. [DOI] [PubMed] [Google Scholar]
- 9.Taoka S, Banerjee R. Characterization of NO binding to human cystathionine β-synthase: Possible implications of the effects of CO and NO binding to the human enzyme. J. Inorg. Biochem. 2001;87:245–251. doi: 10.1016/s0162-0134(01)00335-x. [DOI] [PubMed] [Google Scholar]
- 10.Makarenko VV, Nanduri J, Raghuraman G, Fox AP, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. Endogenous H2S is required for hypoxic sensing by carotid body glomus cells. Am. J. Physiol. Cell Physiol. 2012;303:C916–C923. doi: 10.1152/ajpcell.00100.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paul BD, Snyder SH. Modes of physiologic H2S signaling in the brain and peripheral tissues. Antioxid. Redox Signal. 2015;22:411–423. doi: 10.1089/ars.2014.5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar P, Prabhakar NR. Peripheral chemoreceptors: Function and plasticity of the carotid body. Compr. Physiol. 2012;2:141–219. doi: 10.1002/cphy.c100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoubrey WK, Huang TJ, Maines MD. Heme oxygenase-2 is a hemoprotein and binds heme through heme regulatory motifs that are not involved in heme catalysis. J. Biol. Chem. 1997;272:12568–12574. doi: 10.1074/jbc.272.19.12568. [DOI] [PubMed] [Google Scholar]
- 15.Migita CT, Matera KM, Ikeda-Saito M, Olson JS, Fujii H, Yoshimura T, Zhou H, Yoshida T. The oxygen and carbon monoxide reactions of heme oxygenase. J. Biol. Chem. 1998;273:945–949. doi: 10.1074/jbc.273.2.945. [DOI] [PubMed] [Google Scholar]
- 16.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH. Carbon monoxide: A putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 17.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: Characterization of biochemical and vascular activities. Circ. Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 18.Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J. Biol. Chem. 1991;266:15555–15558. [PubMed] [Google Scholar]
- 19.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem. J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friebe A, Schultz G, Koesling D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- 21.Prabhakar NR, Kumar GK, Chang CH, Agani FH, Haxhiu MA. Nitric oxide in the sensory function of the carotid body. Brain Res. 1993;625:16–22. doi: 10.1016/0006-8993(93)90132-7. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakar NR, Semenza GL. Gaseous messengers in oxygen sensing. J. Mol. Med. 2012;90:265–272. doi: 10.1007/s00109-012-0876-1. [DOI] [PubMed] [Google Scholar]
- 23.Babbedge RC, Bland-Ward PA, Hart SL, Moore PK. Inhibition of rat cerebellar nitric oxide synthase by 7-nitro indazole and related substituted indazoles. Br. J. Pharmacol. 1993;110:225–228. doi: 10.1111/j.1476-5381.1993.tb13796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore PK, Wallace P, Gaffen Z, Hart SL, Babbedge RC. Characterization of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles: Anti-nociceptive and cardiovascular effects. Br. J. Pharmacol. 1993;110:219–224. doi: 10.1111/j.1476-5381.1993.tb13795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. U.S.A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibuta S, Mashimo T, Zhang P, Ohara A, Yoshiya I. A new nitric oxide donor, NOC-18, exhibits a nociceptive effect in the rat formalin model. J. Neurol. Sci. 1996;141:1–5. doi: 10.1016/0022-510x(96)00102-5. [DOI] [PubMed] [Google Scholar]
- 27.Olson KR, Healy MJ, Qin Z, Skovgaard N, Vulesevic B, Duff DW, Whitfield NL, Yang GD, Wang R, Perry SF. Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R669–R680. doi: 10.1152/ajpregu.00807.2007. [DOI] [PubMed] [Google Scholar]
- 28.Olson KR, Forgan LG, Dombkowski RA, Forster ME. Oxygen dependency of hydrogen sulfide-mediated vasoconstriction in cyclostome aortas. J. Exp. Biol. 2008;211:2205–2213. doi: 10.1242/jeb.016766. [DOI] [PubMed] [Google Scholar]
- 29.Ma DK, Vozdek R, Bhatla N, Horvitz HR. CYSL-1 interacts with the O2-sensing hydroxylase EGL-9 to promote H2S-modulated hypoxia-induced behavioral plasticity in C. elegans. Neuron. 2012;73:925–940. doi: 10.1016/j.neuron.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olson KR, Donald JA, Dombkowski RA, Perry SF. Evolutionary and comparative aspects of nitric oxide, carbon monoxide and hydrogen sulfide. Respir. Physiol. Neurobiol. 2012;184:117–129. doi: 10.1016/j.resp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 32.Ortega-Sáenz P, Pascual A, Gómez-Díaz R, López-Barneo J. Acute oxygen sensing in heme oxygenase-2 null mice. J. Gen. Physiol. 2006;128:405–411. doi: 10.1085/jgp.200609591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhakar NR. Sensing hypoxia: Physiology, genetics and epigenetics. J. Physiol. 2013;591:2245–2257. doi: 10.1113/jphysiol.2012.247759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dempsey JA, Forster HV. Mediation of ventilatory adaptations. Physiol. Rev. 1982;62:262–346. doi: 10.1152/physrev.1982.62.1.262. [DOI] [PubMed] [Google Scholar]
- 35.Hackett PH, Roach RC, Schoene RB, Harrison GL, Mills WJ. Abnormal control of ventilation in high-altitude pulmonary edema. J. Appl. Physiol. 1988;64:1268–1272. doi: 10.1152/jappl.1988.64.3.1268. [DOI] [PubMed] [Google Scholar]
- 36.Trzebski A. Arterial chemoreceptor reflex and hypertension. Hypertension. 1992;19:562–566. doi: 10.1161/01.hyp.19.6.562. [DOI] [PubMed] [Google Scholar]
- 37.Tan ZY, Lu Y, Whiteis CA, Simms AE, Paton JF, Chapleau MW, Abboud FM. Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ. Res. 2010;106:536–545. doi: 10.1161/CIRCRESAHA.109.206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paton JF, Sobotka PA, Fudim M, Engelman ZJ, Engleman ZJ, Hart EC, McBryde FD, Abdala AP, Marina N, Gourine AV, Lobo M, Patel N, Burchell A, Ratcliffe L, Nightingale A. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61:5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 39.Marcus NJ, Del Rio R, Schultz EP, Xia XH, Schultz HD. Carotid body denervation improves autonomic and cardiac function and attenuates disordered breathing in congestive heart failure. J. Physiol. 2014;592:391–408. doi: 10.1113/jphysiol.2013.266221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vandiver MS, Paul BD, Xu R, Karuppagounder S, Rao F, Snowman AM, Ko HS, Lee YI, Dawson VL, Dawson TM, Sen N, Snyder SH. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M. Interactions of multiple gas-transducing systems: Hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid. Redox Signal. 2010;13:157–192. doi: 10.1089/ars.2009.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 43.Paul BD, Sbodio JI, Xu R, Vandiver MS, Cha JY, Snowman AM, Snyder SH. Cystathionine γ-lyase deficiency mediates neurodegeneration in Huntington’s disease. Nature. 2014;509:96–100. doi: 10.1038/nature13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan G, Peng YJ, Reddy VD, Makarenko VV, Nanduri J, Khan SA, Garcia JA, Kumar GK, Semenza GL, Prabhakar NR. Mutual antagonism between hypoxia-inducible factors 1α and 2α regulates oxygen sensing and cardio-respiratory homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2013;110:E1788–E1796. doi: 10.1073/pnas.1305961110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X, Kinsman B, Peng YJ, Kumar GK, Fox AP, Godley LA, Semenza GL, Prabhakar NR. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc. Natl. Acad. Sci. U.S.A. 2012;109:2515–2520. doi: 10.1073/pnas.1120600109. [DOI] [PMC free article] [PubMed] [Google Scholar]