Abstract
Activation of Toll-like receptor (TLR) signaling rapidly induces the expression of inflammatory genes, which is sustained for a defined period of time. However, uncontrolled and excessive inflammation may lead to the development of diseases. 4-1BB ligand (4-1BBL) plays an essential role in sustaining the expression of inflammatory cytokines by interacting with TLRs during macrophage activation. Here, we show that inhibition of 4-1BBL signaling reduced the inflammatory responses in macrophages and ameliorated endotoxin-induced sepsis in mice. A 4-1BB-Fc fusion protein significantly reduced TNF production in macrophages by blocking the oligomerization of TLR4 and 4-1BBL. Administration of 4-1BB-Fc suppressed LPS-induced sepsis by reducing TNF production, and the co-administration of anti-TNF and 4-1BB-Fc provided more protection against LPS-induced sepsis. Therefore, these observations suggest that inhibition of the TLR/4-1BBL complex formation may be highly efficacious in protecting against sustained inflammation, and that 4-1BB-Fc treatment may be a potential therapeutic option for inflammatory diseases.
Keywords: Toll-like receptor, inflammation, innate immunity, TNF, 4-1BBL
INTRODUCTION
Inflammation is a beneficial process that limits cellular and tissue damage in order to protect a host from microbial infection or tissue injury. Cytokines and chemokines are released by immune cells during these responses, over a prolonged but regulated period of time, whereas uncontrolled and excessive inflammation is believed to play a critical role in driving pathology associated with inflammatory diseases [1;2]. Therefore, understanding the mechanism underlying sustained cytokine responses may offer a new treatment option for inflammatory diseases.
TNF is the most significant and potent pro-inflammatory cytokine, and plays a critical role in the pathology of many inflammatory diseases [3;4]. The role of TNF in sepsis has been well documented in animal models, which has led to the development of several therapeutic approaches such as anti-TNF and TNFR-IgG-Fc fusion proteins to inhibit the binding of TNF to its receptors [3;5]. TNF blockers including etanercept, adalimumab, and infliximab have been approved for, and are widely used for, the treatment of inflammatory diseases such as Crohn’s disease, rheumatoid arthritis, and psoriasis [6–8]. However, about 40% of patients do not show any response to current TNF therapies in clinical trials, and it is not clear why some patients do not respond [3;9;10]. In addition to the limited efficiency, anti-TNF therapies have also revealed side effects including an increased chance of Tuberculosis infection and the unforeseen phenomenon of inducing autoimmune and inflammatory diseases in some patients [11;12]. Therefore, further studies are necessary to determine the mechanism of this paradoxical inflammation and to develop a safer strategy for treating inflammatory diseases that will avoid these unpredictable side effects [13;14].
Our recent data show that a member of the TNF superfamily, 4-1BB ligand (4-1BBL) can play a crucial role in regulating the maintenance of inflammatory responses in innate immune cells. LPS-induced TNF production is sustained for 24 hours in vitro in wildtype (WT) macrophages. However, we found that genetic ablation of 4-1BBL curtails this prolonged TNF production in macrophages while TNF levels are unaffected during the first few hours. Explaining this phenomenon, we showed that the TLR response induces the expression of 4-1BBL during the early phase of macrophage activation, and subsequently the newly expressed 4-1BBL interacts with the TLRs to initiate a secondary late-phase signaling response to sustain TNF expression [15]. The TLR-4-1BBL complex interacts with an adaptor protein TIRAP and a kinase IRAK2, but not with MyD88 and TRIF, to activate a downstream signaling cascade that includes TRAF6-TAK1-TAB1 [16]. Unlike the early phase of TLR responses, this later phase controlled by 4-1BBL does not induce the activation of NF-κB pathway, while MAPKs, protein kinases, and transcription factors, such as CREB and C/EBP that play an essential role in sustaining expression of TNF. Specifically, we found that inhibition of the 4-1BBL-TIRAP interaction significantly reduced TNF production in macrophages in vitro, and improved survival of mice in a model of endotoxin-induced sepsis [16], suggesting that the 4-1BBL-mediated signaling may be a target of anti-inflammation treatments.
Here, we demonstrate that blockade of TLR4-4-1BBL oligomerization by treatment with anti-4-1BBL or 4-1BB-Fc fusion protein significantly reduces sustained TNF production in LPS-stimulated macrophages, and that administration of 4-1BB-Fc ameliorates endotoxin-induced septic shock in mice. Additionally, co-administration of anti-TNF and 4-1BB-Fc alleviated LPS-induced sepsis. Therefore, we conclude that late phase 4-1BBL-mediated TLR signaling plays an essential role in sustaining inflammation and that targeting of this pathway can provide a potential strategy for the treatment of some inflammatory diseases driven by TLRs.
RESULTS AND DISCUSSION
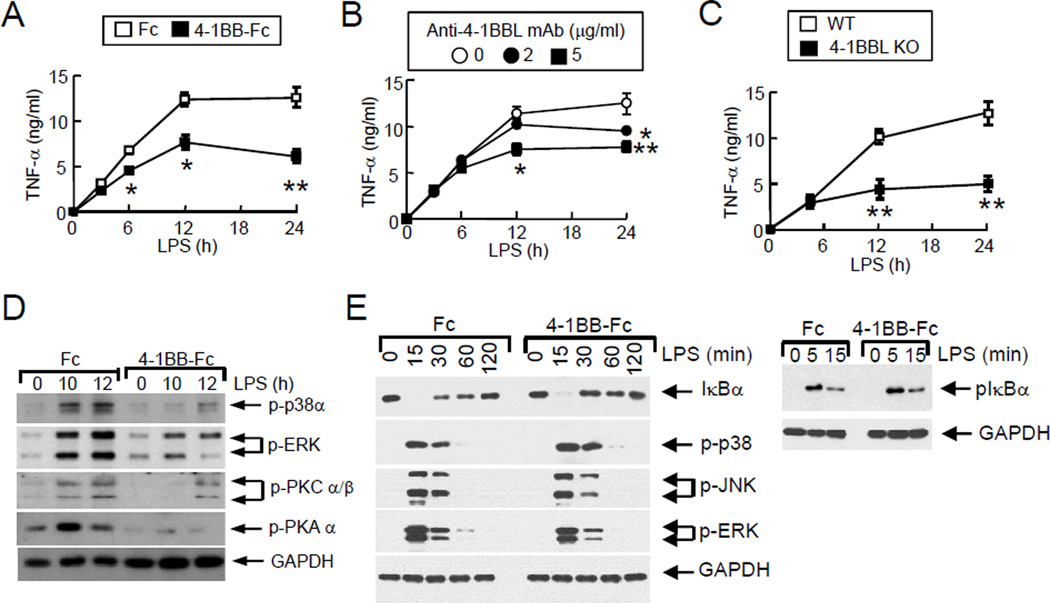
Inhibition of TLR4 and 4-1BBL oligomerization reduces inflammatory responses in macrophages
We previously showed that 4-1BBL-mediated sustained TNF production in macrophages is initiated by the cross-linking and subsequent oligomerization of 4-1BBL and TLRs, which is mediated by an adaptor protein TIRAP, but not by MyD88 and TRIF [15;16]. LPS-induced TNF production was suppressed by inhibiting the aggregation of 4-1BBL, suggesting that aggregation of 4-1BBL is essential for the activation of downstream signaling pathways similar to other TNF superfamily members that form trimeric or higher order oligomeric structures [4;17;18]. A recombinant 4-1BB-Fc protein is composed of a disulfide-linked homodimer of the 4-1BB extracellular domain with the Fc domain of human IgG, and can bind 4-1BBL (Supplemental Figure 1A). Because anti-4-1BBL and 4-1BB-Fc only theoretically bind two 4-1BBL molecules, we hypothesized that they may inhibit aggregation of 4-1BBL with TLRs and reduce sustained TNF production in macrophages. WT macrophages were then treated with LPS in the absence or presence of anti-4-1BBL or 4-1BB-Fc, and TNF production was measured at different times. LPS-induced TNF production was not affected for the first 4 hours in cultures of 4-1BB-Fc-treated macrophages, and TNF levels between isotype or anti-4-1BBL Ab-treated cells were comparable at 6 hours. However, production of TNF was not sustained in anti-4-1BBL- or 4-1BB-Fc-treated cells, compared to the controls (Figure 1A & B), which is similar to the lack of sustained TNF production observed in LPS-treated 4-1BBL-deficient macrophages (Figure 1C) [15]. These results indicate that inhibition of TLR-4-1BBL oligomerization results in a marked reduction of TNF production in macrophages. Interestingly, inhibition by 4-1BB-Fc was more pronounced than that mediated by anti-4-1BBL (Figure 1A & B), suggesting that inhibition by 4-1BB-Fc was more efficient at blocking the oligomerization of TLR4 with 4-1BBL. It has been shown that expression of 4-1BBL is induced at the early phase of TLR signaling. The newly formed 4-1BBL translocates to the surface of macrophages, and interacts with TLR to form a complex that recruit an adaptor protein TIRAP for the activation of downstream signaling pathways [15;16]. Thus, it is suggested that treatment of anti-4-1BBL or 4-1BB-Fc inhibits the formation of TLR-4-1BBL-TIRAP complex at the late phase of macrophage activation, which results in the reduced TNF production.
Figure 1. Inhibition of LPS-induced macrophage activation by blocking 4-1BBL-mediated signaling.
WT macrophages were treated with LPS plus 5 µg/ml of human Fc fragment (Fc, □) or 4-1BB-Fc (■) (A), or LPS plus anti-4-1BBL (clone TKS-1) with rat IgG to adjust the amount of antibodies (B). WT (□) or 4-1BBL−/− (■) peritoneal macrophages were treated with LPS (C). Culture supernatants were collected at the indicated times to measure TNF levels. (D & E) Inhibition of the activation of late phase (D) or early phase (E) signaling by 4-1BB-Fc. Macrophages were stimulated with LPS, and Fc or 4-1BB-Fc. Cell lysates were prepared at the indicated times, and phosphorylation of kinases was analyzed by immunoblotting. GAPDH level was examined as an internal control. Data are shown as the mean ± SD. *; p<0.05, **; p<0.01.
Association of TLR with 4-1BBL activates several signaling pathways including MAPKs, protein kinase A (PKA), PKC, and phosphoinositide 3-kinase (PI3K) [16]. Thus, we further tested whether activation of these molecules was inhibited by treatment with 4-1BB-Fc in LPS-treated macrophages. Activation of p38α, ERK, PKCα/β, and PKAα at late times following LPS stimulation was observed in macrophages, and addition of 4-1BB-Fc reduced the phosphorylation of these enzymes (Figure 1D). To rule out the possibility that the early phase activation for autocrine TNF signaling affects the late phase, we further analyzed the activation of LPS-induced early phase NF-κB and MAPK signaling in Fc- or 4-1BB-Fc-treated macrophages. Macrophages were treated with LPS plus Fc or 4-1BB-Fc, and cell lysates were prepared to analyze the activation of NF-κB and MAPK signaling pathways. As shown in Figure 1E, phosphorylation and degradation of IκBα were comparable between Fc- and 4-1BB-Fc-treated macrophages. Additionally, LPS-induced phosphorylation of p38α, JNK, and ERK was the same in Fc- or 4-1BB-Fc-treated cells. Furthermore, our previous studies have shown that the expression level of 4-1BBL is low in naive macrophages while expression of 4-1BBL is detected after 4–6 hours of macrophages activation [15;16], which implies that 4-1BB-Fc does not affect the early phase of macrophage activation. Therefore, this result indicates that activation of the early phase signaling for autocrine TNF activity is not inhibited by blocking the 4-1BBL signaling by 4-1BB-Fc.
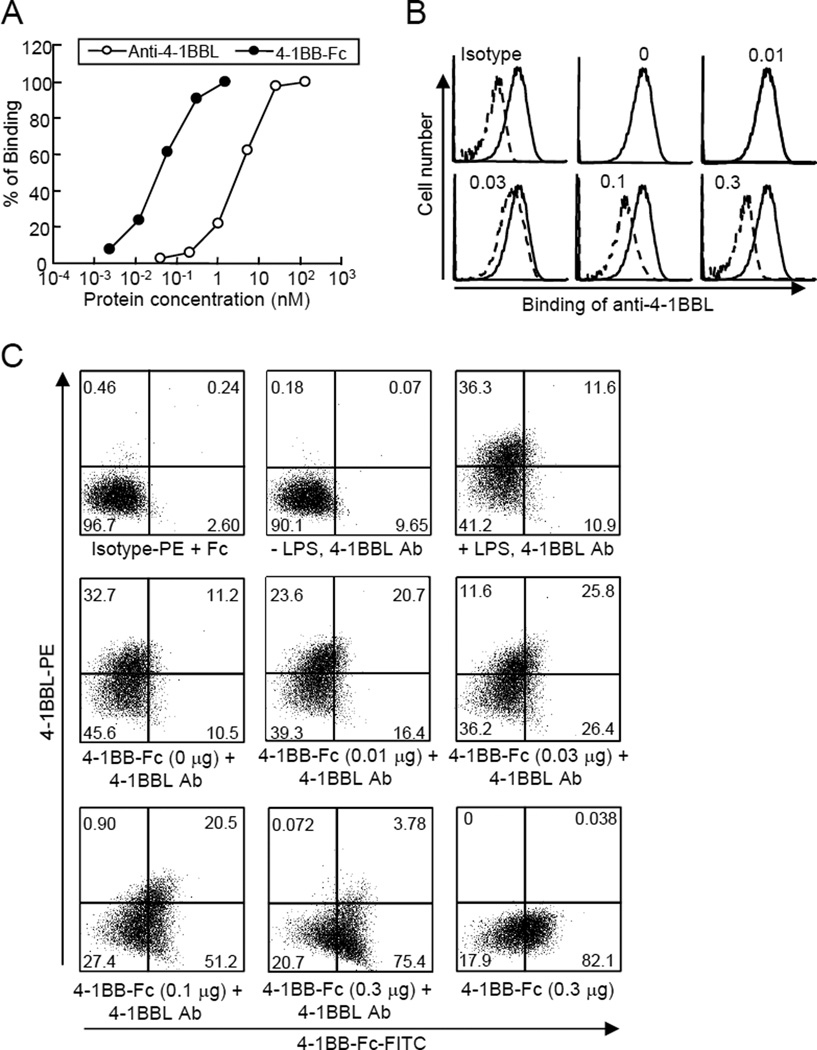
Analysis of binding activity of 4-1BB-Fc to 4-1BBL
Next, we tested the binding activity of anti-4-1BBL and 4-1BB-Fc to 4-1BBL by ELISA. We found that binding of 4-1BB-Fc to recombinant His-tagged 4-1BBL was saturated at a lower concentration of the protein than that of anti-4-1BBL, indicating that the binding affinity of 4-1BB-Fc may be much higher than that of anti-4-1BBL (Figure 2A). TNF production was reduced from the earlier time point in the cells treated with 4-1BB-Fc than anti-4-1BBL (Figure 1A & B), and the affinity of 4-1BB-Fc is higher than that of anti-4-1BBL. Thus, we suggest that 4-1BB-Fc interacts with 4-1BBL more strongly than anti-4-1BBL to inhibit TNF production efficiently.
Figure 2. Binding properties of anti-4-1BBL and 4-1BB-Fc.
(A) Binding of anti-4-1BBL and 4-1BB-Fc to recombinant 4-1BBL was measured by ELISA. Anti-His Abs (0.2 µg/ml) were coated, and recombinant his-tagged mouse 4-1BBL (0.2 µg/ml) was added. Diluted 4-1BB-Fc or biotinylated anti-4-1BBL was added, followed by incubation with anti-human Fc-HRP or streptavidin-HRP. (B & C) Competition analysis for binding of anti-4-1BBL and 4-1BB-Fc to macrophages. Macrophages were stimulated with LPS for 8 hours. (B) Cells were incubated with different amounts of 4-1BB-Fc (solid line) for 2 hours followed by staining with anti-4-1BBL-PE (dashed line). Binding of anti-4-1BBL was analyzed by FACS. Numbers in the histograms indicate the amount of 4-1BB-Fc (µg) added to the cells. (C) LPS-treated macrophages were incubated with 4-1BB-Fc for 2 hours, and further stained with anti-4-1BBL-PE (0.5 µg) and FITC-conjugated anti-Fc antibodies. Binding of anti-4-1BBL and 4-1BB-Fc was analyzed by FACS. Result is shown as dot plots. Rat IgG-PE was used as an isotype control. The numbers indicate the percentage of each cell population.
We further tested whether 4-1BB-Fc and anti-4-1BBL bound shared epitopes of 4-1BBL. LPS-treated macrophages were incubated with different concentrations of 4-1BB-Fc, and then further incubated with PE-conjugated anti-4-1BBL (Figure 2B) or with PE-conjugated anti-4-1BBL and FITC-conjugated anti-Fc (Figure 2C). We found that the binding of anti-4-1BBL to macrophages was inhibited by pre-incubation with 4-1BB-Fc (Figure 2B). In addition, the number of anti-4-1BBL-interacting macrophages decreased while that of 4-1BB-Fc-bound cells increased by addition of 4-1BB-Fc (Figure 2C), suggesting that 4-1BB-Fc and anti-4-1BBL may share some epitopes of 4-1BBL. Consistent with the finding that 4-1BB-Fc might possess a higher binding affinity to 4-1BBL, smaller amount of 4-1BB-Fc was sufficient to inhibit the binding of anti-4-1BBL. These results indicate that we cannot combine anti-4-1BBL and 4-1BB-Fc further increase inhibition of LPS-induced TNF production in macrophages.
The extracellular domain of 4-1BB consists of 4 repeated cysteine rich domains (CRDs) (also known as TNFR homologous motifs). To determine the CRD in 4-1BB-Fc that is essential for the interaction with 4-1BBL and inhibition of LPS-induced inflammatory activity, we generated 4-1BB-Fc molecules with a deletion of each CRD (Supplemental Figure 1A). First, we tested the binding activity of the CRD deletion mutants of 4-1BB-Fc to the recombinant 4-1BBL by ELISA. Compared to the full-length (FL) 4-1BB-Fc, binding to 4-1BBL of mutants with deletion of the third or fourth CRD was not significantly, or only partially, affected. However, deletion of the first or second CRD diminished binding of 4-1BB-Fc to 4-1BBL (Supplemental Figure 1B), corresponding to a previous study also showed that both the first and second CRD are essential for the binding of 4-1BB-Fc to 4-1BBL [19]. We further evaluated the inhibitory activities of 4-1BB-Fc mutant molecules on sustained TNF production in vitro. Peritoneal macrophages were treated with LPS, and FL or mutant 4-1BB-Fc proteins were added after 4 hours. Culture supernatants were collected after 4, 12, and 24 hours of LPS stimulation to measure TNF levels by ELISA. Production of TNF by LPS was not affected in Fc-treated macrophages compared to PBS-treated cells (Supplemental Figure 1C) while FL 4-1BB-Fc significantly reduced TNF levels. LPS-induced TNF production was reduced by the addition of 4-1BB-Fc FL or mutants with a deletion of the third or fourth CRD, but was not affected by Fc or 4-1BB-Fc mutants with a deletion of the first or second CRD (Supplemental Figure 1C). Collectively, our results indicate that the first and second CRDs of 4-1BB play an essential role in interacting with 4-1BBL to inhibit the sustained TNF production driven by LPS.
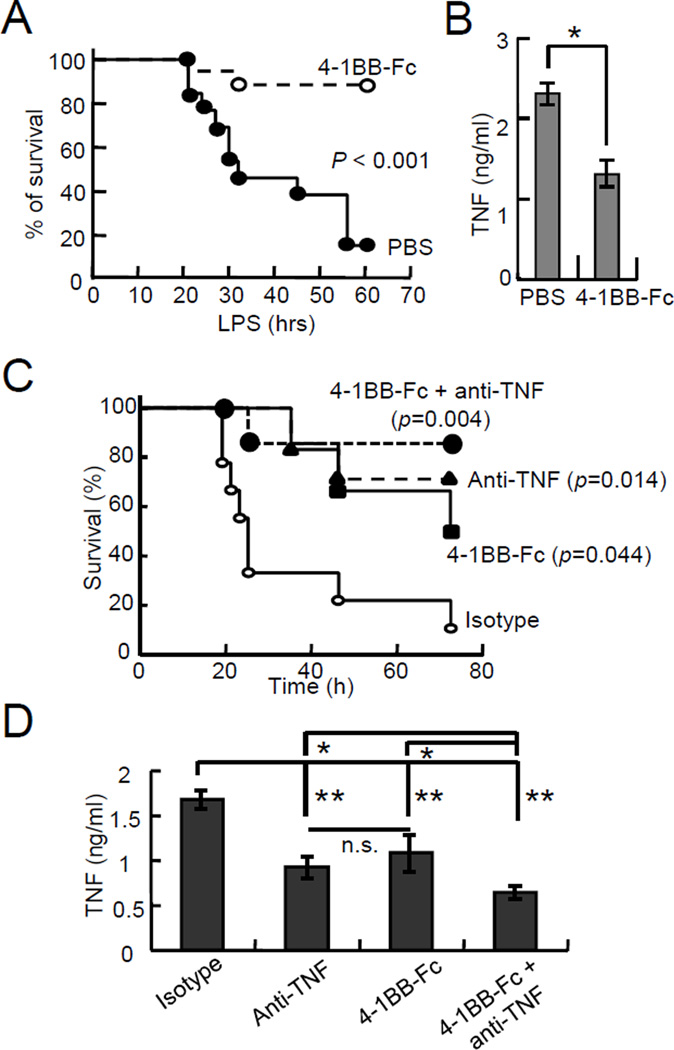
Inhibition of TLR4-4-1BBL-mediated signaling by 4-1BB-Fc protects mice from endotoxin-induced sepsis
We next wished to test whether inhibition of 4-1BBL-mediated TLR signaling could ameliorate endotoxin-induced septic shock in mice. Administration of 4-1BB-Fc significantly improved survival of LPS-injected mice compared to PBS-injected animals (Figure 3A), and TNF levels in sera were also substantially reduced in 4-1BB-Fc-administered mice (Figure 3B). We also tested whether Fc fragment of 4-1BB-Fc affected TNF production and survival of mice in LPS-induced sepsis. TNF levels in sera of LPS-injected mice were comparable between PBS- or Fc-injected mice while 4-1BB-Fc administration substantially reduced the TNF concentrations in the sera (Supplemental Figure 2A). Additionally, survival of mice did not differ between PBS and Fc administration (Supplemental Figure 2B), indicating that Fc does not block the endotoxin-induced sepsis. These results are similar to our finding that 4-1BBL-deficient mice are protected from the lethality of endotoxin-induced sepsis, and that administration of anti-4-1BBL improved the survival of LPS-injected mice compared with isotype control [15]. Therefore, these results suggest that inhibition of 4-1BBL-mediated signaling by 4-1BB-Fc may be used for the treatment of inflammatory disorders.
Figure 3. Amelioration of LPS-induced sepsis by blocking 4-1BBL-mediated signaling.
(A & B) Mice were administered with PBS (n=12, ●) or 4-1BB-Fc (n=15, 50 µg per mouse, ○) i.p., followed by LPS (500 µg per mouse) i.p. injection after 1 hour. Survival of mice was monitored (A), and blood samples were collected after 2 hours of LPS injection to measure TNF levels (B).
(C & D) Effect of 4-1BB-Fc and anti-TNF on the amelioration of LPS-induced sepsis. Mice were injected i.p. with isotype (n=10, ○), 4-1BB-Fc (n=6, 30 µg per mouse, ■), anti-TNF (n=5, 500 µg per mouse, ▲), or 4-1BB-Fc plus anti-TNF (n=6, ●), followed by injection of LPS (500 µg per mouse, i.p.) after 1 hour. Survival of the mice was monitored (C), and TNF levels in sera were measured after 2 hours of LPS injection (D). Data are shown as the mean ± SD. P values are shown. *, p<0.01, and **, p<0.005. n.s., not significant.
Co-administration of anti-TNF and 4-1BB-Fc protects mice from sepsis-induced lethality
The role of TNF in sepsis has led to the development of therapeutic strategies that target TNF activity, such as anti-TNF and TNFR-IgG-Fc fusion proteins [3;5;20;20;21]. Unlike the TNF blockers, our strategy for targeting 4-1BBL-mediated TLR signaling blocks sustained production of TNF. Thus, we hypothesized that co-administration of anti-TNF and 4-1BB-Fc would ameliorate endotoxin-induced sepsis to a greater extent than either by reducing the circulating levels of TNF as well as the activity of TNF. To test this, smaller amounts of 4-1BB-Fc and anti-TNF were administered into LPS-injected mice. The survival of the mice was significantly improved by administration of anti-TNF or 4-1BB-Fc, and co-administration further protected mice from sepsis (Figure 3C). TNF levels were significantly reduced in mice treated with anti-TNF or 4-1BB-Fc compared to the isotype Abs-injected mice, while TNF concentrations were comparable between anti-TNF- or 4-1BB-Fc-injected mice. Furthermore, co-administration of anti-TNF and 4-1BB-Fc additively reduced TNF in LPS-injected mice to the levels lower than those in mice treated with anti-TNF or 4-1BB-Fc alone (Figure 3D). These data suggest that inhibition of TNF activity and sustained TNF production ameliorates LPS-induced septic shock.
Since TNF is the most potent and important pro-inflammatory cytokine in the pathology of many inflammatory diseases, strategies for targeting TNF activity have been developed and successfully used for the treatment of inflammatory diseases [3;5]. Despite the fact that TNF blockers are widely used for the treatment of inflammatory diseases, not all patients respond to current TNF therapies [3;9;10]. Thus, there is a strong demand to develop strategies that are more effective for the treatment of inflammatory diseases. Since 4-1BBL plays an essential role in the development of inflammatory diseases [22–26], targeting the 4-1BBL-mediated TLR pathway may be useful as a treatment of inflammatory diseases. In addition to a strategy of inhibiting the late phase 4-1BBL-mediated TNF production by targeting TIRAP activity with an inhibitory peptide, 4-1BB-Fc blocks the oligomerization of TLR with 4-1BBL and also reduces sustained TNF production. Additionally, the finding that administration of 4-1BB-Fc with anti-TNF further improves the survival of mice in LPS-induced sepsis strongly suggests that our strategy of inhibiting TNF activity and blocking TNF production could be an effective method for the treatment of inflammatory diseases.
CONCLUDING REMARKS
Our data have demonstrate that blockade of TLR4-4-1BBL oligomerization by 4-1BB-Fc treatment significantly reduces sustained TNF production in LPS-stimulated macrophages. Additionally, administration of 4-1BB-Fc alleviates endotoxin-induced septic shock in mice, and co-administration of anti-TNF and 4-1BB-Fc highly efficiently ameliorates LPS-induced sepsis. Therefore, targeting of late phase 4-1BBL-mediated TLR signaling can provide a potential strategy for the treatment of some inflammatory diseases driven by TLRs.
MATERIALS AND METHODS
Mice
C57BL/6 background wildtype and 4-1BBL-deficient mice were previously described [15]. Protocols for the use of animals were approved by the Institutional Animal Care and Use Committee.
Reagents
Antibodies against ant-IκBα, anti-phospho-IκBα, anti-phospho-PKCα/β, anti-phospho-p38α, anti-phospho-ERK, and anti-phospho-JNK antibodies (Cell Signaling); anti-His and anti-phospho-PKAα (Santa Cruz Biotechnology); anti-GAPDH antibody (MAB374, Chemicon); LPS from E. coli O111:B4 for in vivo experiment (Sigma-Aldrich); LPS from E. coli O111:B4 for in vitro experiment (List Biological Laboratories). Low endotoxin anti-mouse TNF antibodies (clone MP6-XT22) and isotype rat IgG1 were purchased from Biolegend (San Diego, CA). Anti-4-1BBL antibodies were prepared [27].
Cells
HEK 293T cells were cultured in DMEM supplemented with 10% FBS. Peritoneal macrophages were obtained from thioglychollate-elicited mice. Briefly, mice were intraperitineally injected with 2 ml of 3% thioglychollate. After 3–4 days, the exudate cells were obtained by washing the mouse peritoneum with ice-cold PBS. Cells were further washed in culture medium.
Plasmid construction and expression of 4-1BB-Fc
Mammalian expression vectors for 4-1BB-Fc expression were constructed as follows. Full-length or truncated mutants of the extracellular domain of mouse 4-1BB cDNAs were generated by PCR and cloned into pFUSE-hIgG1-Fc (Invivogen, San Diego). Plasmids were transfected into HEK293 cells. Culture supernatants were collected after 3–4 days, and 4-1BB-Fc was purified using Protein G-agarose. Endotoxin was removed using a ProteoSpin Endotoxin Removal Kit (Norgen Biotek, Ontario, Canada), and the endotoxin levels were found to be less than 0.01 EU/µg of protein.
Immunoblotting
Macrophages were treated with LPS, and cell lysates were analyzed to detect the phosphorylation of kinases by immunoblotting.
Flow cytometry
Peritoneal macrophages were treated with LPS to induce the expression of 4-1BBL at the cell surface. After 8 hours, cells were incubated with different amounts of 4-1BB-Fc for 2 hour at 4 or 37°C. Cells were harvested using PBS-EDTA (2.5 mM), and further stained with anti-4-1BBL-PE and FITC-conjugated anti-human Fc-specific Abs. Rat IgG-PE was used as an isotype control.
Binding assay
Each well of ELISA plates were coated with anti-His Abs (0.2 µg/ml) followed by blocking with 1% BSA. His-tagged mouse 4-1BBL (0.2 µg/ml) was added and incubated. After washing with PBST, diluted 4-1BB-Fc or biotinylated anti-4-1BBL was added, followed by incubation with anti-human Fc-HRP or streptavidin-HRP. TMB was used as a substrate and absorbance at 450 nm was measured.
Endotoxin-induced sepsis in mice
Wildtype mice were intraperitoneally injected with isotype, anti-TNF, or 4-1BB-Fc. After an hour, LPS (500 µg per 20g body weight) was injected i.p. to induce sepsis. Survival of mice was monitored, and ~20 µl of blood samples were collected after 2 hours of LPS challenge.
Measurement of cytokine production
The concentrations of TNF in culture supernatants or blood sera were measured by ELISA (eBioscience).
Statistical analysis
Statistical significance was determined by the Student's t-test. Kaplan-Meier plotting, and a log-rank test were performed to determine significant differences in the survival of mice.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health grant AI088229 to Y. J. K., AI089624 to M.C., and by Grant-in-Aid (S1201013) from MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2012–2017 to H.Y.
Abbreviations
- 4-1BBL
4-1BB ligand
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no financial or commercial conflict of interest.
REFERENCES
- 1.Alessandri AL, Sousa LP, Lucas CD, Rossi AG, Pinho V, Teixeira MM. Resolution of inflammation: mechanisms and opportunity for drug development. Pharmacol. Ther. 2013;139:189–212. doi: 10.1016/j.pharmthera.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2002;2:364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 4.Vassalli P. The pathophysiology of tumor necrosis factors. Annual Reviews of Immunology. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. Ref Type: Journal (Full) [DOI] [PubMed] [Google Scholar]
- 5.Peppel K, Crawford D, Beutler B. A tumor necrosis factor (TNF) receptor-IgG heavy chain chimeric protein as a bivalent antagonist of TNF activity. J. Exp. Med. 1991;174:1483–1489. doi: 10.1084/jem.174.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pache I, Rogler G, Felley C. TNF-alpha blockers in inflammatory bowel diseases: practical consensus recommendations and a user's guide. Swiss. Med. Wkly. 2009;139:278–287. doi: 10.4414/smw.2009.12549. [DOI] [PubMed] [Google Scholar]
- 7.Upchurch KS, Kay J. Evolution of treatment for rheumatoid arthritis. Rheumatology.(Oxford) 2012;51(Suppl 6):vi28–vi36. doi: 10.1093/rheumatology/kes278. [DOI] [PubMed] [Google Scholar]
- 8.Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin. Arthritis Rheum. 2010;40:233–240. doi: 10.1016/j.semarthrit.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van AG, Hoffman I, Van SK, Vermeire S, Rutgeerts P. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. 2009;58:492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
- 10.Crombe V, Salleron J, Savoye G, Dupas JL, Vernier-Massouille G, Lerebours E, Cortot A, Merle V, Vasseur F, Turck D, Gower-Rousseau C, Lemann M, Colombel JF, Duhamel A. Long-term outcome of treatment with infliximab in pediatric-onset Crohn's disease: a population-based study. Inflamm. Bowel. Dis. 2011;17:2144–2152. doi: 10.1002/ibd.21615. [DOI] [PubMed] [Google Scholar]
- 11.Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A, Symmons DP. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Ann. Rheum. Dis. 2010;69:522–528. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallahi-Sichani M, Flynn JL, Linderman JJ, Kirschner DE. Differential risk of tuberculosis reactivation among anti-TNF therapies is due to drug binding kinetics and permeability. J. Immunol. 2012;188:3169–3178. doi: 10.4049/jimmunol.1103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat. Rev. Gastroenterol. Hepatol. 2012;9:496–503. doi: 10.1038/nrgastro.2012.125. [DOI] [PubMed] [Google Scholar]
- 14.Fiorino G, Danese S, Pariente B, Allez M. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-alpha agents. Autoimmun. Rev. 2014;13:15–19. doi: 10.1016/j.autrev.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Kang YJ, Kim SO, Shimada S, Otsuka M, Seit-Nebi A, Kwon BS, Watts TH, Han J. Cell surface 4-1BBL mediates sequential signaling pathways 'downstream' of TLR and is required for sustained TNF production in macrophages. Nat. Immunol. 2007;8:601–609. doi: 10.1038/ni1471. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Bang BR, Lu J, Eun SY, Otsuka M, Croft M, Tobias P, Han J, Takeuchi O, Akira S, Karin M, Yagita H, Kang YJ. The TNF family member 4-1BBL sustains inflammation by interacting with TLR signaling components during late-phase activation. Sci. Signal. 2013;6:ra87. doi: 10.1126/scisignal.2004431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eck MJ, Sprang SR. The structure of tumor necrosis factor-alpha at 2.6 A resolution. Implications for receptor binding. J. Biol. Chem. 1989;264:17595–17605. doi: 10.2210/pdb1tnf/pdb. [DOI] [PubMed] [Google Scholar]
- 18.Ameloot P, Declercq W, Fiers W, Vandenabeele P, Brouckaert P. Heterotrimers formed by tumor necrosis factors of different species or muteins. J. Biol. Chem. 2001;276:27098–27103. doi: 10.1074/jbc.M104486200. [DOI] [PubMed] [Google Scholar]
- 19.Yi L, Zhao Y, Wang X, Dai M, Hellstrom KE, Hellstrom I, Zhang H. Human and mouse CD137 have predominantly different binding CRDs to their respective ligands. PLoS. One. 2014;9:e86337. doi: 10.1371/journal.pone.0086337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spooner CE, Markowitz NP, Saravolatz LD. The role of tumor necrosis factor in sepsis. Clin. Immunol. Immunopathol. 1992;62:S11–S17. doi: 10.1016/0090-1229(92)90036-n. [DOI] [PubMed] [Google Scholar]
- 21.Fisher CJ, Jr, Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RM, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor: Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N. Engl. J. Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 22.Seo SK, Choi JH, Kim YH, Kang WJ, Park HY, Suh JH, Choi BK, Vinay DS, Kwon BS. 4-1BB-mediated immunotherapy of rheumatoid arthritis. Nat. Med. 2004;10:1088–1094. doi: 10.1038/nm1107. [DOI] [PubMed] [Google Scholar]
- 23.Jeon HJ, Choi JH, Jung IH, Park JG, Lee MR, Lee MN, Kim B, Yoo JY, Jeong SJ, Kim DY, Park JE, Park HY, Kwack K, Choi BK, Kwon BS, Oh GT. CD137 (4-1BB) deficiency reduces atherosclerosis in hyperlipidemic mice. Circulation. 2010;121:1124–1133. doi: 10.1161/CIRCULATIONAHA.109.882704. [DOI] [PubMed] [Google Scholar]
- 24.Seo SK, Park HY, Choi JH, Kim WY, Kim YH, Jung HW, Kwon B, Lee HW, Kwon BS. Blocking 4-1BB/4-1BB ligand interactions prevents herpetic stromal keratitis. J. Immunol. 2003;171:576–583. doi: 10.4049/jimmunol.171.2.576. [DOI] [PubMed] [Google Scholar]
- 25.Cheung CT, Deisher TA, Luo H, Yanagawa B, Bonigut S, Samra A, Zhao H, Walker EK, McManus BM. Neutralizing anti-4-1BBL treatment improves cardiac function in viral myocarditis. Lab Invest. 2007;87:651–661. doi: 10.1038/labinvest.3700563. [DOI] [PubMed] [Google Scholar]
- 26.Le NH, Kim CS, Tu TH, Choi HS, Kim BS, Kawada T, Goto T, Park T, Park JH, Yu R. Blockade of 4-1BB and 4-1BBL interaction reduces obesity-induced skeletal muscle inflammation. Mediators. Inflamm. 2013;2013:865159. doi: 10.1155/2013/865159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.