Abstract
Objectives:
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related deaths worldwide. CA19-9 is a glycoprotein that predicts poor prognosis in pancreatic and biliary malignancies. We evaluated it as a prognostic biomarker for patients with HCC.
Methods:
We prospectively enrolled 145 patients with HCC, diagnosed using American Association for Study of Liver Diseases criteria, between October 2008 and November 2012. We examined whether baseline serum CA19-9 levels predicted overall survival. We also examined immunostains of hepatic resections and explants of patients with elevated and normal serum CA19-9.
Results:
In a cohort of predominantly hepatitis C and B patients, CA19-9 ≥100 U/ml was associated with a 2.7-fold increased mortality (hazard ratio (HR): 2.72; 95% confidence interval (CI): 1.52–4.88, P<0.001). It remained a significant predictor (HR: 2.58; 95% CI: 1.41–4.72, P=0.002) in a multivariable model adjusted for Child–Pugh score, alpha-fetoprotein, Barcelona Clinic Liver Cancer stage, and Model for End-Stage Liver Disease. CA19-9 immunohistochemistry performed on a subset of liver resection and explant specimens showed increased CA19-9 immunostaining of non-tumor liver parenchyma in patients with elevated serum CA19-9. It also showed staining of native and reactive bile ducts, and of progenitor-like cells at the periphery of cirrhotic nodules.
Conclusions:
Elevated serum CA19-9 ≥100 U/ml is an independent predictor of poor overall survival in this hypothesis-generating study. The unfavorable prognosis seen with elevated serum levels may be related to progenitor-like cells in the non-tumor liver.
INTRODUCTION
Hepatocellular carcinoma (HCC) incidence rates have tripled in the United States over the last 30 years.1 Alpha-fetoprotein (AFP) level has been the most commonly used prognostic marker in HCC. Decreasing AFP levels have been used to predict successful HCC downstaging and response to anti-angiogenic therapy, whereas high AFP levels have been associated with poor liver transplant outcomes.2, 3, 4 However, it lacks both sensitivity and specificity as a prognostic biomarker. Various studies have also examined explanted livers for genetic signatures to identify favorable outcomes.5, 6, 7 However, this can be technically challenging, and tumor tissue is often unavailable when deciding upon the optimal treatment strategy.
CA19-9, or sialyl Lewis A antigen, is expressed on cancer cells as a glycolipid and an O-linked glycoprotein.8 Serum levels may be elevated in benign conditions and in pancreatic, biliary, gastric, esophageal, and colonic cancers.9,10 Elevated CA19-9 may also predict prognosis and response to therapy in patients with pancreatic malignancies, cholangiocarcinoma (CC), or mixed HCC-cholangiocarcinoma (HCC-CC).11, 12 It has not been extensively studied in HCC patients. More recently, elevated preoperative CA19-9 levels have been associated with worse survival in HCC patients who were resected or underwent liver transplant.13, 14 In this study, we examined whether elevated CA19-9 at the time of HCC diagnosis is predictive of increased mortality in a heterogeneous cohort of patients who underwent different treatments. If further validated, this biomarker may be used to help assess the appropriateness of various treatment approaches.
METHODS
Subjects
We prospectively enrolled 145 patients aged 18 years or older with HCC, diagnosed using American Association for Study of Liver Diseases criteria, between October 2008 and November 2012. Subjects were followed until December 2012. Data were prospectively collected as part of another study where body mass index measurements were needed. Thus, patients with ascites or Child–Pugh C cirrhosis were excluded to avoid confounding with ascites. Patients with cancer within the last 5 years, prior or current systemic chemotherapy, or previous liver transplant were also excluded. Patients with prior local therapy or resection were eligible as long as they had recurred and had measurable disease by Response Evaluation Criteria in Solid Tumors criteria. The protocol was approved by the Institutional Review Board at Columbia University Medical Center, and written informed consent was obtained from all subjects prior to enrollment.
Data collection
Patients were recruited in outpatient oncology and liver clinics. Upon enrollment, subjects completed an epidemiologic questionnaire and underwent a physical exam. Clinical data were obtained from the questionnaire and corroborated by the electronic medical record. Baseline fasting blood samples were drawn, including basic metabolic and hepatic panels, complete blood count, viral and autoimmune serologies, iron studies, ceruloplasmin, and alpha-1 antitrypsin to determine the etiology of liver disease. Other underlying causes of liver diseases were confirmed by clinical history and/or liver biopsies. Alcohol intake was considered significant for patients who were reported consuming two or more standard drinks per day (28 gm) for a year at any point in their lifetime. Diabetes mellitus was based on clinical history or enrollment fasting glucose level ≥126 mg/dl. Survival data were obtained by using information from office visits, hospitalizations, or from the national social security death index. Tumor markers AFP and CA19-9 levels were obtained using the Associated Regional and University Pathologists, a national reference laboratory where Roche CA19-9 electrochemiluminescent immunoassay and Beckman Coulter Access DxI AFP assays were used. Clinical data such as tumor number, largest tumor diameter, and evidence of vascular invasion were obtained by evaluating pre-treatment imaging by one radiologist (SV).
Pathological examination
CA19-9 immunohistochemistry was performed on paraffin-embedded liver specimens from 10 and 12 randomly selected patients with elevated serum CA19-9 (≥38 U/ml or median) and low serum CA19-9 (<38 U/ml), respectively. Primary antibodies against CA19-9 antigen were used (Ventana, Roche, Rocklin, CA). We stained sections that included both tumor and adjacent non-tumor liver parenchyma (Supplementary Figure 1). The positive CA19-9 stain of native bile ducts as well as of reactive ductules represents an internal positive control for the staining. A positive CA19-9 stain of native larger portal bile ducts in non-tumoral liver parenchyma for both patients with high and low serum CA19-9 serves as an additional internal control. At last, separate staining of the non-tumor liver away from the tumor showed similar staining to peritumoral liver. The slides were stained by the clinical pathology immunohistochemistry laboratory using an automated procedure validated for clinical pathology use. Because CA19-9 marked both bile ducts and cells with hepatobilliary features, cytokeratin 7 (CK7; a marker of bile ducts and progenitor cells) immunostain was examined on serial sections to assess for similarity in staining pattern and cell types.
Radiological examination
A radiologist blinded to patient serum CA19-9 levels reviewed the closest pre-treatment imaging study (magnetic resonance imaging or computed tomography scan) within 1–2 months of enrollment date. Tumors were characterized as central, intrahepatic, or peripheral to determine whether CA19-9 levels correlated with tumor location. Central tumors were defined subjectively as tumors near the hilum, segment 1, or near the origins of main right and left portal vein. Peripheral tumors were defined as <3 cm from the margin of the liver if within the liver, or were on the liver surface. Intrahepatic tumors were defined as any tumors between central and peripheral tumors.
CA19-9 elevation has also been associated with benign and malignant biliary obstruction,9, 10 so we examined whether CA19-9 levels correlated with biliary dilatation seen on imaging or elevations in biochemical indices related to biliary obstruction. The radiologist measured intrahepatic bile duct size within each liver segment, with dilatation defined as >4 mm.15 Extrahepatic biliary dilatation was defined as >4 mm in patients under 50-year old and defined as greater than the calculated age-appropriate diameter in those over 50-year old (common bile duct size increases approximately 1 mm per decade).16, 17
Statistical analysis
Associations between CA19-9 levels (≥100 or <100 U/ml) and patient enrollment clinical and tumor characteristics (all as categorical variables) were analyzed using X2-tests. We used a cutoff of CA19-9 ≥100 U/ml in our main analysis because levels <100 U/ml are often seen in cirrhosis and hyperbilirubinemic patients.18 Pearson's correlation coefficients were used to examine associations between biomarker levels and other continuous variables. We analyzed the relationship between CA19-9 and time to death using Kaplan–Meier curves and Cox Proportional Hazards Models. In addition to using a CA19-9 cutoff of 100 U/ml, we defined other cutoffs for low and high levels of CA19-9 using quartiles of baseline serum levels (18, 38, and 88 U/ml).
To determine whether CA19-9 independently predicted prognosis, clinically relevant variables that achieved statistical significance in the univariable analysis were included in the multivariable Cox proportional hazards models. We included Barcelona Clinic Liver Cancer Staging (BCLC Stage B+C vs. 0+A) and Milan (outside vs. within) in two separate multivariable models because elements of Milan criteria (one lesion≤5 cm or three lesions≤3 cm) are part of the BCLC staging classification. Because macrovascular invasion was more prevalent in patients with elevated CA19-9 (≥100 U/ml) and different treatment interventions might confound the outcome of the analysis, these variables were included in a third multivariable model. Subjects contributed person-time for survival analyses from the date of enrollment to the date of death or date of censoring. Subjects were censored if they were lost to follow-up or at the end of study period (December 2012).
To further evaluate the predictive value of CA19-9 in this hypothesis-generating study, we assessed hazard ratios for CA19-9 levels in patient subgroups with different clinical characteristics and for different cut points of AFP. An AFP >1000 ng/ml has been associated with worse outcomes after liver transplantation.19, 20 AFP >100 ng/ml has also been associated with increased mortality, and levels <100 ng/ml have been associated with successful downstaging with loco-regional therapy.2, 21 We used median AFP (45 ng/ml), normal AFP (≤8 ng/ml), AFP >100 ng/ml, and AFP>1000 ng/ml as various cutoffs for low and high AFP levels. All analyses were performed with SAS Version 9.3.
RESULTS
Patient clinical characteristics
We prospectively enrolled 145 patients from 2008 to 2012. No patients had CC on explant pathology, and one patient had mixed HCC-CC. Four patients had macroregenerative nodules and complete tumor necrosis. These patients were included in the analysis.
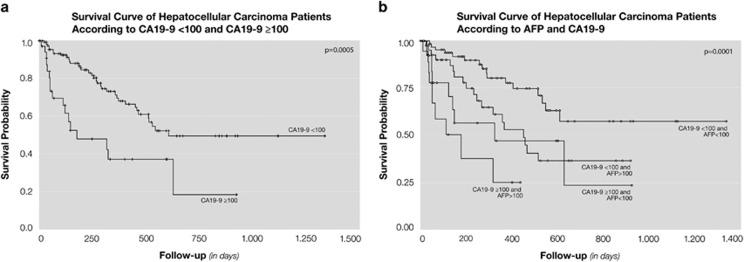
The median age of subjects was 62 years; 79% were male, 59% had underlying hepatitis C virus, and 16% had hepatitis B virus (Table 1). More than half (57%) were outside Milan criteria (one lesion≤5 cm or three lesions all <3 cm) and 61% had BCLC stage B and C at enrollment. Fifty percent of the patients were within University of California, San Francisco criteria (one lesion≤6.5 cm or three lesions with largest lesion ≤4.5 cm and total tumor diameter≤8 cm). Median survival of the cohort was 18.2 months (interquartile range could not be calculated because of censoring) and median follow-up was 8.3 months (interquartile range=385 days). Eighteen patients were lost to follow-up; baseline CA19-9 levels were not significantly different in these subjects. Twenty-two percent of the patients had an elevated CA19-9 ≥100 U/ml. Median survival was 20.3 months and 5.9 months for patients with CA19-9 <100 U/ml and CA19-9 ≥100 U/ml, respectively (Figure 1a). CA19 ≥100 U/ml was significantly associated with ≥3 tumors, peripheral tumors, and macrovascular invasion defined by imaging (Table 1).
Table 1. Clinical characteristics of hepatocellular carcinoma patients associated with CA19-9 Level.
| Variable | CA19-9 <100 | CA19-9 ≥100 | P value |
|---|---|---|---|
| Age | 0.10 | ||
| ≥62.0 | 61 (54%) | 12 (38%) | |
| <62.0 | 52 (46%) | 20 (63%) | |
| Gender | 0.24 | ||
| Male | 92 (81%) | 23 (72%) | |
| Female | 21 (19%) | 9 (28%) | |
| Chronic hepatitis B | 21 (19%) | 2 (6%) | 0.09 |
| Chronic hepatitis C | 63 (56%) | 22 (69%) | 0.19 |
| Diabetes mellitus | 37 (33%) | 11 (34%) | 0.86 |
| Alcohol history | 35 (31%) | 5 (16%) | 0.09 |
| Child–Pugh class | 0.12 | ||
| A | 76 (67%) | 16 (50%) | |
| B | 37 (33%)* | 16 (50%) | |
| MELD | 0.13 | ||
| <10 | 60 (53%) | 12 (37%) | |
| ≥10 | 53 (47%) | 20 (63%) | |
| Within Milan criteria | 0.06 | ||
| Within | 53 (47%) | 9 (28%) | |
| Outside | 60 (53%) | 23 (72%) | |
| BCLC stage | 0.31 | ||
| 0 + A | 46 (41%) | 10 (31%) | |
| B + C | 67 (59%) | 22 (69%) | |
| AFP | 0.45 | ||
| ≥47.9 ng/ml | 55 (49%) | 18 (56%) | |
| <47.9 ng/ml | 58 (51%) | 14 (44%) | |
| Liver transplant | 30 (27%) | 5 (16%) | 0.19 |
| Resection | 22 (19%) | 5 (16%) | 0.56 |
| Chemotherapy | 14 (12%) | 5 (16%) | 0.60 |
| Loco-regional therapy | 91 (81%) | 18 (56%) | 0.002 |
| Tumor numbers | 0.002 | ||
| Single | 40 (35%) | 9 (28%) | |
| 2–3 | 42 (37%) | 3 (9%) | |
| >3 or multiple | 22 (19%) | 12 (38%) | |
| Infiltrative | 9 (8%) | 8 (25%) | |
| Tumor size | 0.42 | ||
| <3cm | 46 (41%) | 10 (31%) | |
| 3–5cm | 31 (27%) | 8 (25%) | |
| >5cm | 36 (32%) | 14 (44%) | |
| Common hepatic biliary dilatation | 43 (38%) | 16 (50%) | 0.24 |
| Intrahepatic biliary dilatation (>4mm) | 27 (24%) | 10 (31%) | 0.29 |
| Metastases | 16 (14%) | 6 (19%) | 0.58 |
| Vascular invasion | 17 (15%) | 10 (31%) | 0.03 |
| Peripheral tumor | 86 (76%) | 29 (91%) | 0.02 |
| Central tumor | 88 (78%) | 26 (81%) | 0.26 |
AFP, Alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; MELD, Model for End-Stage Liver Disease. *Two patients were Child–Pugh B on enrollment, and decompensated to Child–Pugh C.
*Bold type signifies P≤0.05.
Figure 1.
(a) Survival curve of hepatocellular carcinoma patients according to CA19-9 ≥100. (b) Survival curve of hepatocellular carcinoma patients according to AFP and CA19-9.
Thirty-six percent of subjects died during follow-up (46% when excluding patients who underwent liver transplant). Twenty-four percent underwent liver transplant over the course of follow-up and 18% were resected. One of the 35 transplanted patients died from recurrent HCC. 16% of the patients had biopsy proven metastatic HCC; 19 patients were treated with sorafenib while one patient was treated with erlotinib. Five patients were treated with an insulin growth factor receptor inhibitor study drug in addition to sorafenib as part of a clinical trial. Most (75%) patients underwent loco-regional therapy such as transcatheter arterial chemoembolization or radiofrequency ablation in their treatment course.
Univariable analysis
In the univariable analysis, CA19-9 ≥100 U/ml was associated with an almost threefold increase in mortality (HR: 2.72; 95% CI: 1.52–4.88, P<0.001). Other cut-points for CA19-9 yielded similar results, although not all were statistically significant (Table 2). Other factors associated with increased mortality were BCLC stage B and C, presence of metastases, elevated AFP, HCC outside Milan criteria, having a higher Model for End-Stage Liver Disease (MELD) score, and having Child–Pugh B liver disease (Table 2). Etiology of liver disease, such as hepatitis B or C, was not a statistically significant predictor and thus was not included in the multivariate analysis.
Table 2. Univariable analysis of variables associated with worse survival in patients with hepatocellular carcinoma.
| Factor | (n) | Reference (n) | HR | 95% CI | P value |
|---|---|---|---|---|---|
| Age (years) | ≥62 | <62 | 0.92 | 0.53–1.58 | 0.75 |
| Gender | Male | Female | 0.96 | 0.50–1.82 | 0.89 |
| Hepatitis B virus | Positive | Negative | 0.70 | 0.38–1.27 | 0.24 |
| Hepatitis C virus | Positive | Negative | 1.12 | 0.64–1.97 | 0.68 |
| Alcohol history | Positive | Negative | 0.42 | 0.19–0.92 | 0.03 |
| Metastasis | Positive | Negative | 2.42 | 1.31–4.48 | 0.004 |
| Macrovascular invasion | Positive | Negative | 3.10 | 1.73–5.54 | 0.001 |
| Milan criteria | Outside | Within | 6.39 | 2.88–14.18 | <0.001 |
| Child–Pugh class | B | A | 1.91 | 1.10–3.35 | 0.02 |
| BCLC Stage | B + C | 0 + A | 3.11 | 1.60–6.06 | 0.0009 |
| AFP median (ng/ml) | ≥47 | <47 | 2.44 | 1.37–4.35 | 0.002 |
| aMELD | 1.07 | 1.01–1.14 | 0.02 | ||
| CA19-9 (U/ml) | ≥18 | <18 | 1.66 | 0.85–3.22 | 0.14 |
| ≥38 | <38 | 1.73 | 1.00–3.00 | 0.04 | |
| ≥88 | <88 | 2.48 | 1.38–4.45 | 0.002 | |
| ≥100 | <100 | 2.72 | 1.52–4.88 | <0.001 |
AFP, Alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; HR, hazard ratio; MELD, Model for End-Stage Liver Disease; n, number of patients. Median survival was 609 days and 178 days for patients with CA19-9<100 U/ml and CA19-9≥100 U/ml, respectively.
Continuous variable.
Bold type signifies *P≤0.05.
Multivariable analysis
A CA19-9 ≥100 U/ml independently predicted a 2.6-fold increase in mortality (HR: 2.58; 95% CI: 1.41–4.72, P=0.002) (Table 3) in a multivariable model that adjusted for Child–Pugh class, BCLC stage, AFP levels, and MELD score. In a second model that substituted BCLC stage with Milan (outside vs. within), results were similar (HR: 2.73; 95% CI: 1.49–5.02, P=0.001). CA19-9 remained an independent predictor (HR: 2.16; 95% CI: 1.07–4.34, P=0.03) in the third multivariable model that adjusted for treatment interventions (chemotherapy, loco-regional therapy, transplant, and resection), macrovascular invasion, Child–Pugh class, BCLC stage, median AFP, and MELD score (Table 4).
Table 3. Multivariable analysis for overall survival in hepatocellular carcinoma patients: Cox proportional hazards model.
| Factor | (n) | Reference (n) | HR | 95% CI | P value |
|---|---|---|---|---|---|
| CA19-9 (U/ml) | ≥100 | <100 | 2.58 | 1.41–4.72 | 0.002 |
| Child–Pugh class | B | A | 2.72 | 1.33–5.55 | 0.006 |
| BCLC stage | B+C | 0+A | 3.57 | 1.74–7.32 | 0.0005 |
| AFP median (ng/ml) | ≥47 | <47 | 2.27 | 1.24–4.15 | 0.008 |
| aMELD | 1.00 | 0.93–1.07 | 0.92 |
AFP, Alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; HR, hazard ratio; MELD, Model for End-Stage Liver Disease; n, number of patients. All variables in the above multivariable model are adjusted for each other.
Continuous variable.
Bold type signifies *P≤0.05.
Table 4. Multivariable analysis for overall survival in hepatocellular carcinoma patients including treatment variables and macrovascular invasion: Cox proportional hazards model.
| Factor | (n) | Reference (n) | HR | 95% CI | P value |
|---|---|---|---|---|---|
| CA19-9 (U/ml) | ≥100 | <100 | 2.16 | 1.07–4.34 | 0.03 |
| Child–Pugh class | B | A | 1.81 | 0.77–4.23 | 0.18 |
| BCLC Stage | B+C | 0+A | 2.40 | 1.11–5.16 | 0.03 |
| AFP median (ng/ml) | ≥47 | <47 | 2.19 | 1.08–4.43 | 0.03 |
| aMELD | 1.05 | 0.96–1.15 | 0.28 | ||
| Loco-regional therapy | Positive | Negative | 0.54 | 0.25–1.16 | 0.11 |
| Resection | Positive | Negative | 0.59 | 0.23–1.50 | 0.27 |
| Transplant | Positive | Negative | 0.15 | 0.04–0.55 | 0.004 |
| Chemotherapy | Positive | Negative | 0.82 | 0.35–1.91 | 0.64 |
| Macrovascular invasion | Positive | Negative | 1.76 | 0.84–3.71 | 0.14 |
AFP, Alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; HR, hazard ratio; MELD, Model for End-Stage Liver Disease; n, number of patients. All variables in the above multivariable model are adjusted for each other.
Continuous variable.
Bold type signifies *P≤0.05.
We next evaluated the prognostic value of CA19-9 in patient subgroups with different clinical characteristics, including those with high or low AFP levels. Among patients with AFP >100 ng/ml, median survival was longer when comparing those with low CA19-9 (<100 U/ml) to those with high CA19-9 (≥100 U/ml) levels (15 vs. 6 months; P=0.0001, log-rank) (Figure 1b). This was even more pronounced with higher AFP levels. In the group of patients with AFP >1000 ng/ml, median survival was 12 months compared with 2 months when comparing those with low CA19-9 to those with high CA19-9 (P=0.01, log-rank). CA19-9 ≥100 U/ml remained predictive of mortality after stratifying results by other clinical characteristics. CA19-9 ≥100 U/ml was associated with a two- to threefold increase in mortality in patients with BCLC stage B or C HCC, and HCC outside of Milan criteria (HR 2.84; 95% CI: 1.50–5.41, P=0.001 and HR 2.04; 95% CI: 1.09–3.81, P=0.02, respectively) (Table 5).
Table 5. Stratified analysis comparing hepatocellular carcinoma patients with CA19-9 ≥100 vs. <100 U/ml.
| Factor | HR | 95% CI | P value |
|---|---|---|---|
| Child–Pugh class: A | 1.37 | 0.56–3.37 | 0.48 |
| Child–Pugh class: B | 5.67 | 2.24–14.34 | <0.001 |
| Within Milan criteria | 2.49 | 0.48–12.97 | 0.27 |
| Outside Milan criteria | 2.04 | 1.09–3.81 | 0.02 |
| BCLC Stage: 0 + A | 1.57 | 0.33–7.39 | 0.32 |
| BCLC Stage: B + C | 2.84 | 1.50–5.41 | 0.001 |
| AFP (ng/ml): <47 (median) | 3.65 | 1.42–9.34 | 0.007 |
| AFP (ng/ml): ≥47 (median) | 2.15 | 1.02–4.56 | 0.04 |
| AFP (ng/ml): <20 | 6.15 | 2.10–18.03 | 0.0009 |
| AFP (ng/ml): ≥20 | 1.79 | 0.88–3.61 | 0.11 |
| AFP (ng/ml): <1000 | 2.18 | 1.01–4.68 | 0.04 |
| AFP (ng/ml): ≥1000 | 3.23 | 1.23–8.48 | 0.02 |
AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; HR, hazard ratio.
CA19-9 immunohistochemistry
Pathology specimens from hepatic resections and liver transplant explants were available for 51 out of 145 (35%) patients. Liver biopsy specimens were available for 39 out of 145 (27%) patients. HCC was confirmed in all specimens except for one explant that was consistent with mixed HCC-cholangiocarcinoma. There were no pure cholangiocarcinomas based on available pathology review.
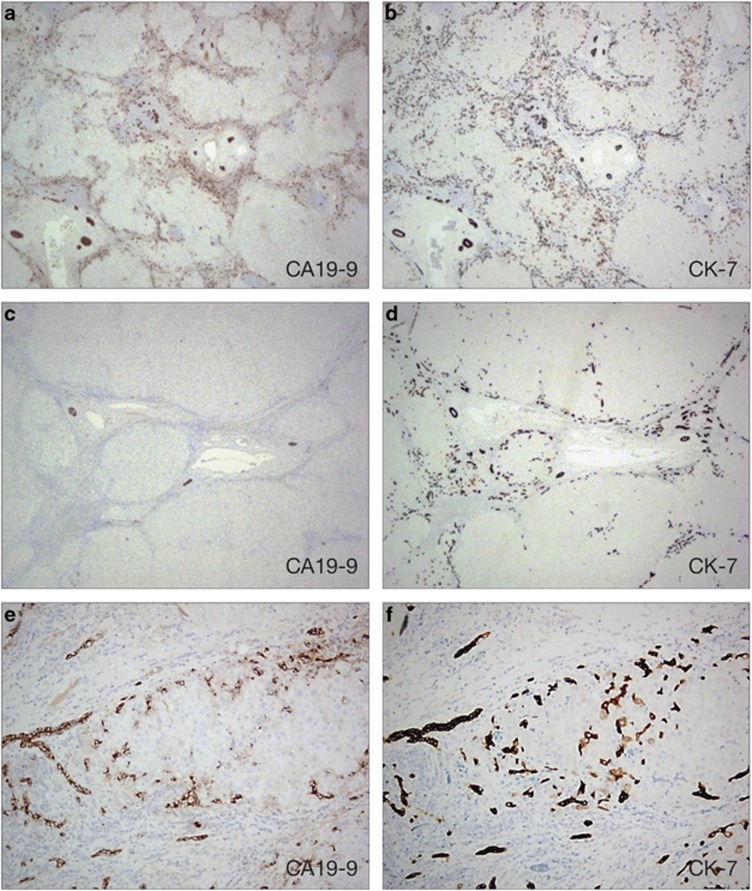
The tumor sections we stained were selected to include both tumor and adjacent non-tumor liver parenchyma. Interestingly, no tumors showed CA19-9 staining. In patients with elevated CA19-9 (≥100 IU/ml), we observed increased CA19-9 staining and a proliferative ductular reaction in the surrounding non-tumor liver parenchyma (Figure 2). We identified CA19-9 staining of native and reactive bile ducts, and also a population of small progenitor-like cells and cells with intermediate hepatobiliary phenotype. The CA19-9 staining was similar in the regions adjacent and away from the tumor. All patients (n=10) with elevated serum CA19-9 had positive staining, whereas only 5 out of 12 patients with low serum CA19-9 had this pattern. CK7, a marker of progenitor cells and bile ducts, showed a similar staining pattern as CA19-9 in non-tumoral liver in serial sections (Figure 2).22, 23 It highlighted native and reactive bile ducts as well as a population of more primitive cells with a cord-like arrangement and single-cell distribution at the periphery of the cirrhotic nodules. In contrast to CA19-9 stain, the CK7 stain was also positive in patients with low serum CA19-9, although with fewer positive cells and less ductular proliferation, when compared with patients with high serum CA19-9.
Figure 2.
Immunohistochemistry (IHC) of non-tumor parenchyma explanted liver paraffin sections. (a) CA19-9 Immunostain on patient with elevated serum CA19-9. (b) CK7 Immunostain on patient with elevated serum CA19-9. (c) CA19-9 Immunostain on patient with normal serum CA19-9. (d) CK7 Immunostain on patient with normal serum CA19-9. (e–f) Immunostain for both CA19-9 and CK7 highlights native bile ducts and reactive ductules, progenitor-like and intermediate hepatobilliary cells within a cirrhotic nodule.
In five patients with low serum CA19-9, CA19-9 positive staining was also observed. Two patients had HCC that was biologically more aggressive and similar to patients with elevated serum CA19-9. One patient had borderline serum CA19-9 (34 U/ml) and two patients had immunostaining performed on liver resections prior to entry of the study that limits the interpretation of their staining.
Radiological examination
CA19-9 was not associated with extrahepatic or intrahepatic biliary dilatation observed on imaging (OR 1.37, P=0.24, and OR 1.1, P=0.29, respectively). Serum CA19-9 correlated with total bilirubin, but not with alkaline phosphatase or common hepatic duct size on imaging (total bilirubin: Pearson's correlation coefficient 0.30, P=0.0003, alkaline phosphatase: 0.07, P=0.41, common hepatic duct size=0.09, P=0.30). No patients were actively infected with cholangitis at time of serum CA19-9 collection, as this would have excluded them from study participation. However, as biliary obstruction could potentially confound our outcome, we included it as a covariate in another multivariable model. CA19-9 remained an independent predictor of mortality even with inclusion of total bilirubin in the model (HR: 1.98; 95% CI: 1.02–3.82, P=0.04). Interestingly, CA19-9 ≥100 was associated with peripheral tumors seen on imaging, but not central tumors (Table 1).
DISCUSSION
Better prognostic serum biomarkers are needed to predict outcomes in those with HCC. Some risk factors associated with worse prognosis, such as poor tumor differentiation and microvascular invasion, are often only available after surgery.19 Elevated AFP levels have been associated with poor prognosis.4, 24 However, AFP can not only be normal in HCC patients but also be elevated in patients without HCC.25, 26
CA19-9 is a readily accessible serum biomarker, which has been used primarily in patients with pancreatic and biliary malignancies. The prognostic value of CA19-9 has not been extensively studied in HCC. In two small retrospective studies, an elevated CA19-9 level has been associated with increased mortality in patients with mixed HCC-CC, specifically a 7.5-fold increase in mixed HCC-CC patients with CA19-9≥80 U/ml.12, 27 More recently, elevated preoperative CA19-9 was shown to be predictive of worse survival in HCC patients who underwent liver transplant or resection.13, 14 Wan et al.13, 28 reported that in patients with either elevated serum CA19-9>100 U/ml or AFP>400 ng/ml, the 5 year post-transplant survival decreased to 40.5%, in contrast to the 75% observed for HCC patients transplanted within Milan criteria. A single elevated marker (either AFP >400 ng/ml or CA19-9 >100 U/ml) was predictive of 2.67-fold increased mortality risk (HR: 2.67; 95% CI 1.65–4.33, P<0.001). However, both of these reports are Asian studies with a predominantly hepatitis B population.
Our data show that a serum CA19-9≥100 U/ml is associated with a 2.6-fold increased mortality risk in a multivariable analysis (HR: 2.58; 95% CI: 1.41–4.72, P=0.002) of a large cohort of unselected HCC patients in the United States. In contrast, our patient cohort has predominantly hepatitis C and underwent various different treatments, suggesting potentially greater generalizability. In the previously mentioned study by Wan et al., only a small percentage of patients (14.2%) had elevated CA19-9 >100 U/ml, and a majority (81.3%) of those patients were outside Milan criteria, which was not adjusted for in the multivariable analysis. We directly adjusted for variables including outside Milan criteria, Child–Pugh class, BCLC stage, AFP, MELD, and various treatment interventions that could potentially confound our outcome in the multivariable analysis.
Macrovascular invasion is known to be associated with worse prognosis and was more common in our cohort of patients with high CA19-9 (≥100 U/ml). However, we show that even after adjusting for macrovascular invasion, CA19-9 remained an independent predictor of worse prognosis (HR: 2.16; 95% CI: 1.07–4.34, P=0.03). Interestingly in our analysis, liver transplant was the only treatment regimen that was statistically significant in reducing mortality (HR: 0.15; 95% CI: 0.04–0.55, P=0.004). It was associated with an 85% risk reduction. Loco-regional therapy, hepatic resection, and chemotherapy were associated with decreased mortality, but were not statistically significant. This could be related to small sample sizes, or due to fairly advanced HCC that may have limited response to these treatment interventions.
We had also pursued CA19-9 immunohistochemistry and expected to see CA19-9 staining of the actual tumor tissue, suggesting a mixed cholangio hepatocellular phenotype known to be associated with worse prognosis. Surprisingly, no tumor stained positively for CA19-9. We observed that patients with elevated serum CA19-9 had a striking increase in surrounding ductular proliferative reaction and positive CA19-9 immunostain of progenitor-like cells and intermediate hepatobiliary cells in the non-tumor regions. These cell types also expressed CK7, a marker of biliary epithelium and liver progenitor cells.22, 23 It is possible that stromal progenitor cell activation and ductular proliferation are associated with increased hepatocarcinogenesis, and are contributing to the clinically observed increased mortality. Interestingly, intermediate hepatobiliary cells with CK7 expression have been associated with increased risk of hepatocarcinogenesis in hepatitis C cirrhosis patients, and worse survival and increased intratumoral proliferation in patients with combined HCC-CC.29, 30
It is also possible that CA19-9 immunostaining correlates with an aggressive HCC phenotype that lacks the distinct histopathological features of a combined HCC-CC. Two patients with low serum CA19-9 but with positive CA19-9 immunostaining had HCC that was biologically more aggressive and similar to patients with elevated serum CA19-9. This suggests that a positive CA19-9 immunostain may be more sensitive than serum levels in detecting an aggressive HCC. Assessment of more explants and more detailed molecular characterization of these tumors are needed to clarify this.
A subtype of HCC associated with worse prognosis that shares a gene signature similar to fetal hepatoblasts has been previously described. This subtype shares similar genetic features as hepatic oval cells or hepatic progenitor cells.31 More recently, hepatic progenitor cells transfected with oncogenic viruses were shown to give rise to anaplastic tumors, whereas fetal hepatoblasts treated similarly yielded tumors with a cholangiocarcioma-like phenotype.32 HCCs with a progenitor phenotype correlated more with HBV tumors in this study. In our population, CA19-9≥100 U/ml was not associated with HBV. However, this might be reflective of differences of our respective populations.
Our study has several limitations. It is a single-center study with a relatively short median follow-up (8.3 months) and with a limited number of cases having available histological specimens (due to the advanced stage of many subjects where biopsies were not considered necessary for HCC diagnosis). Mixed HCC-CC is associated with a worse prognosis, and may have been underdiagnosed in our cohort as histology was only available for 62% of our patients. However, mixed HCC-CC is rare, representing only ~1– 6.3% of the histologically proven HCC cases at various centers.33, 34, 35, 36, 37 In a multi-centered Japan Liver Cancer study group manuscript, mixed HCC-CC only represented 1% of the 4765 cases of HCC.37 Any bias in any case would likely be non-differential and should not affect overall findings.
Moreover, HCC patients with Child–Pugh C cirrhosis were excluded in this cohort. Their mortality is high and median survival time is usually <3 months for untreated HCC.38, 39 Liver disease thus becomes more important in predicting survival in many patients than tumor characteristics.38 One study demonstrated that more patients died from liver failure than tumor progression. Independent predictors of survival were Child–Pugh Score (10–15), treatment intervention, AFP>100 ng/ml, and des-γ carboxy prothrombin>300 mAU/ml but not tumor size or number.39 These patients are not included in our cohort and it is difficult to predict how inclusion of this subgroup would affect our analysis. Their high mortality can potentially diminish CA19-9's predictive value. However, despite this, AFP and des-γ carboxy prothrombin remained independent predictors in the previously mentioned study. Thus, future studies should be pursued to study this biomarker's predictive value in this subgroup of patients.
Another limitation of our study is that only serum was collected during enrollment, and not whole blood that is needed for Lewis blood group antigen typing. Up to 10% of the Caucasian population do not have the Lewis blood group antigen and do not express CA19-9.40 These data were not available for our cohort and we were unable to exclude these patients. In addition, we were also unable to perform time-dependent analysis of CA19-9. However, our study revealed strong statistically significant results in an unselected, diverse prospectively collected cohort of HCC patients.
In conclusion, CA19-9 independently predicted a 2.6-fold increased mortality in a prospectively collected group of HCC patients in a multivariable analyses. In contrast to the two recent studies, the HCC patients in our cohort underwent a broader array of treatments and had different underlying etiologies of liver disease. CA19-9 remained an independent predictor for survival even after adjusting for treatment outcomes, Child–Pugh, AFP, MELD, Milan criteria, and macrovascular invasion. Future studies should be done to validate this biomarker's ability to risk stratify patients and clarify the mechanisms for this possible relationship.
Study Highlights
Guarantor of the article: Abby B. Siegel, MD, MS.
Specific author contributions: Christine C. Hsu was involved in the study concept and design, data acquisition, analysis and interpretation, and manuscript drafting. Abhishek Goyal was involved in data acquisition, biostatistical analysis and interpretation of data, and critical revision of the manuscript for important intellectual content. Alina Iuga performed immunohistochemistry, histology interpretation and review, and critical revision of the manuscript for important intellectual content. Saravanan Krishnamoorthy reviewed CT and MRI imaging and was involved in the interpretation and analysis of imaging data. Valerie Lee, Elizabeth Verna, and Rosa Rodriguez were involved in data acquisition and analysis, and critical revision of manuscript for important intellectual content. Shuang Wang and Fei-Na Chen performed the biostatical analysis and data interpretation. Jean Emond, Paul Berk, Jay Lefkowitch, Lorna Dove, and Robert S. Brown Jr were involved in the data acquisition and analysis and critical revision of the manuscript for intellectual content. Abby B. Siegel created the study concept and design, supervised the study, and was involved in data acquisition, analysis, and interpretation, manuscript drafting, and obtaining funding for the study.
Financial support: NIH K23 CA149084-01A1 and Steven J Levinson Medical Research Foundation (ABS), and an AASLD Advanced/Transplant Fellowship Award (CCH).
Potential competing interests: None.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Sections were presented at Gastrointestinal ASCO, San Francisco; January 24-26, 2013.
Supplementary Material
References
- Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova V, Miraglia R, Maruzzelli L, et al. Predictive factors of downstaging of hepatocellular carcinoma beyond the Milan criteria treated with intra-arterial therapies. Cardiovasc Intervent Radiol. 2013;36:433–439. doi: 10.1007/s00270-012-0458-1. [DOI] [PubMed] [Google Scholar]
- Shao YY, Lin ZZ, Hsu C, et al. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010;116:4590–4596. doi: 10.1002/cncr.25257. [DOI] [PubMed] [Google Scholar]
- Ioannou GN, Perkins JD, Carithers RL., Jr Liver transplantation for hepatocellular carcinoma: impact of the MELD allocation system and predictors of survival. Gastroenterology. 2008;134:1342–1351. doi: 10.1053/j.gastro.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Dvorchik I, Roayaie S, et al. Liver transplantation for hepatocellular carcinoma: extension of indications based on molecular markers. J Hepatol. 2008;49:581–588. doi: 10.1016/j.jhep.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka N, Oka M, Yamada-Okabe H, et al. Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet. 2003;361:923–929. doi: 10.1016/S0140-6736(03)12775-4. [DOI] [PubMed] [Google Scholar]
- Llovet JM. Clinical and molecular classification of hepatocellular carcinoma. Liver Transpl. 2007;13:S13–S16. doi: 10.1002/lt.21325. [DOI] [PubMed] [Google Scholar]
- Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol. 2012;3:105–119. doi: 10.3978/j.issn.2078-6891.2011.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli D, Caruso S, Pedrazzani C, et al. CA19-9 serum levels in obstructive jaundice: clinical value in benign and malignant conditions. Am J Surg. 2009;198:333–339. doi: 10.1016/j.amjsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Basso D, Meggiato T, Fabris C, et al. Extra-hepatic cholestasis determines a reversible increase of glycoproteic tumour markers in benign and malignant diseases. Eur J Clin Invest. 1992;22:800–804. doi: 10.1111/j.1365-2362.1992.tb01449.x. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Chen Z, Ma LL, et al. Factors influencing the prognosis of patients with intrahepatic cholangiocarcinoma. Acta Gastroenterol Belg. 2012;75:215–218. [PubMed] [Google Scholar]
- Kim KH, Lee SG, Park EH, et al. Surgical treatments and prognoses of patients with combined hepatocellular carcinoma and cholangiocarcinoma. Ann Surg Oncol. 2009;16:623–629. doi: 10.1245/s10434-008-0278-3. [DOI] [PubMed] [Google Scholar]
- Wan P, Zhang J, Long X, et al. Serum levels of preoperative alpha-fetoprotein and CA19-9 predict survival of hepatic carcinoma patients after liver transplantation. Eur J Gastroenterol Hepatol. 2014;26:553–561. doi: 10.1097/MEG.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Chen CH, Hu RH, et al. Elevated preoperative serum CA19-9 levels in patients with hepatocellular carcinoma is associated with poor prognosis after resection. Scientific WorldJournal. 2013;2013:380797. doi: 10.1155/2013/380797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader TR, Beavers KL, Semelka RC. MR imaging features of primary sclerosing cholangitis: patterns of cirrhosis in relationship to clinical severity of disease. Radiology. 2003;226:675–685. doi: 10.1148/radiol.2263011623. [DOI] [PubMed] [Google Scholar]
- Cooperberg PL, Li D, Wong P, et al. Accuracy of common hapatic duct size in the evaluation of extrahepatic biliary obstruction. Radiology. 1980;135:141–144. doi: 10.1148/radiology.135.1.7360952. [DOI] [PubMed] [Google Scholar]
- Hakansson K, Ekberg O, Hakansson HO, et al. MR characteristics of acute cholangitis. Acta Radiol. 2002;43:175–179. doi: 10.1080/028418502127347718. [DOI] [PubMed] [Google Scholar]
- Collazos J, Genolla J, Ruibal A. CA 19-9 in non-neoplastic liver diseases. A clinical and laboratory study. Clin Chim Acta. 1992;210:145–151. doi: 10.1016/0009-8981(92)90053-s. [DOI] [PubMed] [Google Scholar]
- Koschny R, Schmidt J, Ganten TM. Beyond Milan criteria—chances and risks of expanding transplantation criteria for HCC patients with liver cirrhosis. Clin Transplant. 2009;23:49–60. doi: 10.1111/j.1399-0012.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- Hakeem AR, Young RS, Marangoni G, et al. Systematic review: the prognostic role of alpha-fetoprotein following liver transplant for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:987–999. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- Tyson GL, Duan Z, Kramer JR, et al. Level of alpha-fetoprotein predicts mortality among patients with hepatitis C-related hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:989–994. doi: 10.1016/j.cgh.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libbrecht L, Desmet V, Van Damme B, et al. The immunohistochemical phenotype of dysplastic foci in human liver: correlation with putative progenitor cells. J Hepatol. 2000;33:76–84. doi: 10.1016/s0168-8278(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Mishra L, Banker T, Murray J, et al. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318–329. doi: 10.1002/hep.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Q, Avolio AW, Manzia TM, et al. Role of alpha-fetoprotein in selection of patients with hepatocellular carcinoma waiting for liver transplantation: must we reconsider it. Int J Biol Markers. 2011;26:153–159. doi: 10.5301/JBM.2011.8557. [DOI] [PubMed] [Google Scholar]
- Hsu SX, Siegel AB, Berk PD. A 70-year-old woman with 10 years of markedly elevated alpha-fetoprotein measurements. Semin Liver Dis. 2010;30:99–106. doi: 10.1055/s-0030-1247136. [DOI] [PubMed] [Google Scholar]
- Shim JH, Yoon DL, Han S, et al. Is serum alpha-fetoprotein useful for predicting recurrence and mortality specific to hepatocellular carcinoma after hepatectomy? A test based on propensity scores and competing risks analysis. Ann Surg Oncol. 2012;19:3687–3696. doi: 10.1245/s10434-012-2416-1. [DOI] [PubMed] [Google Scholar]
- Chantajitr S, Wilasrusmee C, Lertsitichai P, et al. Combined hepatocellular and cholangiocarcinoma: clinical features and prognostic study in a Thai population. J Hepatobiliary Pancreat Surg. 2006;13:537–542. doi: 10.1007/s00534-006-1117-1. [DOI] [PubMed] [Google Scholar]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- Ziol M, Nault JC, Aout M, et al. Intermediate hepatobiliary cells predict an increased risk of hepatocarcinogenesis in patients with hepatitis C virus-related cirrhosis. Gastroenterology. 2010;139:335–343. doi: 10.1053/j.gastro.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Cai X, Zhai J, Kaplan DE, et al. Background progenitor activation is associated with recurrence after hepatectomy of combined hepatocellular-cholangiocarcinoma. Hepatology. 2012;56:1804–1816. doi: 10.1002/hep.25874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Heo J, Libbrecht L, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- Holczbauer A, Factor VM, Andersen JB, et al. Modeling pathogenesis of primary liver cancer in lineage-specific mouse cell types. Gastroenterology. 2013;145:221–231. doi: 10.1053/j.gastro.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi J, Nakashima O, Tanaka M, et al. A clinicopathological study on combined hepatocellular and cholangiocarcinoma. J Gastroenterol Hepatol. 1996;11:758–764. doi: 10.1111/j.1440-1746.1996.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Maeda T, Adachi E, Kajiyama K, et al. Combined hepatocellular and cholangiocarcinoma: proposed criteria according to cytokeratin expression and analysis of clinicopathologic features. Hum Pathol. 1995;26:956–964. doi: 10.1016/0046-8177(95)90084-5. [DOI] [PubMed] [Google Scholar]
- Sapisochin G, de Lope CR, Gastaca M, et al. Intrahepatic cholangiocarcinoma or mixed hepatocellular-cholangiocarcinoma in patients undergoing liver transplantation: a Spanish matched cohort multicenter study. Ann Surg. 2014;259:944–952. doi: 10.1097/SLA.0000000000000494. [DOI] [PubMed] [Google Scholar]
- Yin X, Zhang BH, Qiu SJ, et al. Combined hepatocellular carcinoma and cholangiocarcinoma: clinical features, treatment modalities, and prognosis. Ann Surg Oncol. 2012;19:2869–2876. doi: 10.1245/s10434-012-2328-0. [DOI] [PubMed] [Google Scholar]
- Liver Cancer Study Group of Japan Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- Toyoda H, Kumada T, Kiriyama S, et al. Impact of tumor factors on the prognosis of patients with advanced cirrhosis (Child-Pugh class C) and hepatocellular carcinoma. J Gastroenterol Hepatol. 2005;20:963–965. doi: 10.1111/j.1440-1746.2005.03841.x. [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Kita R, Kimura T, et al. Clinical efficacy of non-transplant therapies in patients with hepatocellular carcinoma with Child-Pugh C liver cirrhosis. Anticancer Res. 2014;34:3039–3044. [PubMed] [Google Scholar]
- Kawai S, Suzuki K, Nishio K, et al. Smoking and serum CA19-9 levels according to Lewis and secretor genotypes. Int J Cancer. 2008;123:2880–2884. doi: 10.1002/ijc.23907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.