Abstract
For the last several decades, hypoxia has been recognized to be one of the key factors in tumor aggression and an important impediment to local and distant control of malignant tumors. In addition, hypoxia is a major cause of failure of both radiation therapy and chemotherapy. It has been shown that hypoxia is an independent negative prognostic factor for patient outcome in various solid tumors. Clinical studies using polarographic oxygen electrodes, as a tool for measuring hypoxia, were the first to demonstrate the presence of hypoxia in human tumors and its association with poor prognosis. However, this method is invasive and has technical limitations that prevent its routine clinical use. Over the years, imaging as a noninvasive method has attracted a lot of attention and several radiotracers have been developed for noninvasive evaluation of hypoxia. One of the most promising radiotracers is the copper(II) complex of diacetyl-2,3-bis(N4-methyl-3-thiosemicarbazonato) ligand (Cu-ATSM) for imaging with positron emission tomography. In this review, the preclinical evaluation of Cu-ATSM as well as its clinical value in several solid tumors will be discussed.
Keywords: Neoplasms, Tomography, emission computed, Radionuclide imaging
Hypoxia is an important feature of solid tumors resulting from an imbalance between oxygen supply and tissue demand for oxygen. It has been shown that hypoxic tumors are more resistant to radiotherapy and some chemotherapeutic agents, and overall have a poor outcome. Hypoxic tumors are also more likely to be locally aggressive and to metastasize more frequently compared with normoxic tumors.1, 2 Considerable research has focused on developing methods for measuring hypoxia in vivo and on strategies that target hypoxia. Reliable and practical methods for detecting hypoxia are crucial for developing approaches that improve tumor oxygenation or ameliorate the effects of hypoxia. The polarographic oxygen electrodes (Eppendorf GMbH, Hamburg, Germany) are considered the gold standard method for measurement of hypoxia that produced clinically relevant information. Early clinical studies with oxygen electrodes have demonstrated that hypoxic tumors respond poorly to radiation therapy.3–10 However, the oxygen electrode method is invasive, subject to sampling errors, technically demanding, and useful only for studying tumors accessible to electrode placement. A suitable method for routine clinical application needs to be practical, readily available and reliable, and thus the oxygen electrode method is not considered clinically practical.
Recently, there has been a great deal of interest in developing accurate and practical methods for the measurement of hypoxia by using noninvasive imaging methods. In particular, the use of positron emission tomography (PET) has received substantial attention. There are several hypoxic tracers suitable for PET imaging. This review focuses on the preclinical and clinical studies done with the hypoxic tracer, the copper(II) complex of diacetyl-2,3-bis(N4-methyl-3-thiosemicarbazonato) ligand (Cu-ATSM).
Copper radionuclides
Of the metallic radionuclides suitable for use in nuclear medicine and PET imaging, the available copper isotopes offer unparalleled versatility in terms of their favorable emission characteristics and half-lives (t1/2).11 In addition, copper chemistry and its relevance to radiopharmaceutical production including ligand design, complex formation and functionalisation is a mature field of study and has been the subject of several reviews.11–18 Copper-60 (t1/2=23.7 min, β+=93%, EC=7%)19 and copper-64 (t1/2=12.7 h, β+=17.4%, EC=43%)20, 21 are the two most commonly used radionuclides of copper for PET imaging applications. McCarthy et al. have described the production of both 60Cu and 64Cu via the p, n transmutation reactions from isotopically enriched 60Ni and 64Ni, respectively.19, 20 High specific activities in the range 80–300 mCi/μg of 60Cu and 95–310 mCi/μg of 64Cu have been obtained by using a biomedical cyclotron with low beam energy bombardment of solid Ni targets. The range of half-lives available (from minutes to hours) means that Cu radionuclides find application as tracers which require rapid biodistribution and repeated imaging protocols (60Cu) 22, 23 and in systems such as labeled monoclonal antibodies and peptides which require increased circulation times (64Cu).24, 25
Cu-ATSM: a hypoxia-selective radiotracer
The ability to locate and quantify the extent of hypoxia within solid tumors by using non-invasive nuclear imaging would facilitate early diagnosis and help clinicians select the most appropriate treatment for each individual patient. Hypoxia-selective uptake of Cu-ATSM was first discovered in 1997 (Figure 1).26 Further investigations found that Cu-ATSM displays hypoxia-selectivity in in vitro cellular uptake and washout studies,27–30 and shows high uptake/retention in hypoxic tumorous tissues in vivo.29, 31, 32
Figure 1.
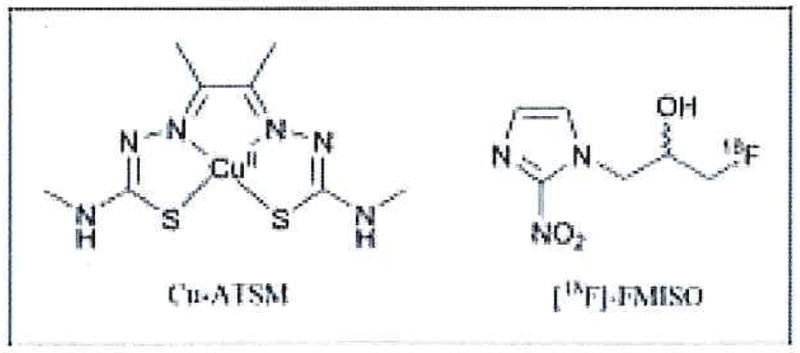
Chemical structures of the hypoxia-selective tumor imaging agents Cu-ATSM and [18F]FMISO.
The 18F-radiolabeled compound, [18F]fluoro-misonidazole ([18F]FMISO) is the most widely used tracer in the clinic for in vivo PET imaging of tumor hypoxia (Figure 1).33 Many studies have demonstrated that [18F]FMISO uptake is indicative of tissue oxygenation and is selective for tissues with pO2 <3 mmHg. However, the unfavorable pharmacokinetics and imaging characteristics of [18F]FMISO have limited its wider application in clinical oncology.33–35 Limitations include low tumor-to-background (T/B) contrast ratios (typically <1.2 for blood and muscle) and slow clearance from background tissue. Optimal contrast requires a 2-h delay before image acquisition, during which time the 18F (t1/2=109.7 min) has decayed by one half-life, which reduces the signal-to-noise. In comparison, Cu-ATSM radiolabeled with either 60Cu or 64Cu can delineate tumor hypoxia in <1 h with T/B contrast ratios typically >2 and uptake has been shown to be predictive of patient response to therapy.
In 1999, the in vitro kinetics of uptake and retention of 64Cu-ATSM in EMT-6 tumor cells and in vivo biodistribution in female BALB/c mice were compared with the established hypoxia marker [18F]FMISO and the non-hypoxia-selective bis(thiosemicarbazonato) complex 64Cu-PTSM.29 This was the first report to demonstrate pO2-depcndent uptake of 64Cu-ATSM in vivo. Copper-64-ATSM showed oxygen dependent uptake and retention, with three-fold higher retention in tissue/cells with pO2 between 0.1–0.5% in comparison to normal oxygen concentrations. Fluorine-18-FMISO was also found to be hypoxia-selective, but only at lower percentages of pO2 than 64Cu-ATSM, and 64Cu-PTSM and showed pO2-independent cellular uptake of between 83–85% after 1 h incubation. The in vivo uptake and washout kinetics of 64Cu-ATSM were found to be superior to those of observed for [18F]FMISO and biodistribution studies indicated that optimal tumor uptake (0.76% injected dose [ID]/organ) occurs within 5 min postintravenous injection.31 The main conclusion was that 64Cu-ATSM is an effective radiopharmaceutical for use in delineating hypoxic tumor tissue from the normoxic background.
Mechanistic studies
Despite numerous articles describing in vitro chemical, biochemical and spectroscopic studies and in vivo work using PET imaging, precise mechanistic details of the pO2-dependence of Cu-ATSM cellular uptake, localization and trapping within normoxic and hypoxic tissue remain uncertain. Each process is likely to be dependent on several factors including the nature of the copper(II) complex, tissue phenotype, and local tissue oxygen tension.
After the initial reports of hypoxia-selectivity of Cu-ATSM, several groups investigated structure-activity relationships and potential mechanisms to explain observed differences in biological behavior for this class of structurally related copper bis(thiosemicarbazonato) complexes.26–28, 36–39 Two plausible mechanisms quickly emerged. The first mechanism was proposed by Fujibayashi et al.26 Experiments showed that Cu-ATSM accumulates in hypoxic myocardium via a bioreductive retention mechanism involving nicotinamide adenine dinucleotide (NADH)-dependent enzymes of the electron transport chain, present in mitochondria.26 These results suggested that upon intracellular reduction, Cu-ATSM becomes trapped irreversibly. Reduction of Cu-ATSM only occurs in hypoxic cells and involves electron transfer from hyper-reduced Complex I (ubiquinone oxidoreductase) using NADH as a two-electron donor. In normoxic cells, Complex I is incapable of reducing Cu-ATSM. In contrast, the non-hypoxia selective complex, Cu-PTSM which has a less negative one-electron reduction potential, may be reduced in all cells by Complex I in its normal state (i.e. not hyper-reduced), leading to irreversible intracellular trapping. In this mechanism, pO2-dependence of the mitochondria-mediated one-electron reduction is the discriminating factor which controls the reversibility of cellular uptake.
In 2001, Obata et al.36 showed that in subcellular fractions of Erhlich ascites tumor cells, Cu-ATSM reduction was mediated by enzymes located in the microsome/cytosol fraction, rather than the mitochondria. Reduction was found to be heat sensitive and was enhanced by the addition of both NADH and reduced nicotinamide adenine dinucleotide phosphate (NADPH) to the medium. Neither NADH nor NADPH were capable of reducing Cu-ATSM alone. Specific reductase inhibitor studies also showed that inhibition of the microsomal enzymes, NADH-dependent cytochrome b5 reductase and NADPH-dependent cytochrome P450 reductase by phenylthiourea and adenosine monophosphate, respectively, caused a 25–50% decrease in [Cu(II)ATSM] reduction, compared with control experiments.
However, this first mechanism was not fully consistent with observed cellular uptake and washout studies,29 which led to the proposal of a second mechanism by Dearling et al.27,28 and Maurer et al.37 They postulated that Cu-ATSM reduction is reversible and occurs in both hypoxic and normoxic cells, generating an unstable, anionic copper(I) complex, [Cu(I)ATSM]. It was suggested that this species may dissociate slowly in cells with low oxygen concentration leading to irreversible trapping of the copper(I) ion. In the presence of normal oxygen tensions, the [Cu(I)ATSM] anion may be oxidized by molecular oxygen to give the neutral Cu-ATSM complex, which could then diffuse back out of the cell. In this mechanism, the relative stability of the reduced copper(I) anion towards ligand dissociation or reoxidation is the discriminating factor between Cu-ATSM and the non-hypoxia-selective blood perfusion tracer, Cu-PTSM.40
Recent experimental and computational work provided the first experimental evidence directly probing the reduction, reoxidation and pH-mediated ligand dissociation reactions (Figure 2).39 These studies used advanced spectroelectrochemical techniques to generate the [Cu(I)ATSM] in situ and monitor its stability with respect to oxidation by dioxygen and proton-mediated ligand dissociation. The work led to the proposal of a revised mechanism of hypoxia-selectivity which builds upon previous work and indicates that hypoxia-selectivity of Cu-ATSM arises due to a delicate equilibrium in which the rates of reduction (most likely enzyme-mediated), reoxidation and protonation are fast relative to the rate of pH-mediated ligand dissociation. Kinetic simulations of in vitro cellular uptake data from Lewis et al.29 support the validity of the revised mechanism.39 Figure 2 shows a schematic representation of the proposed mechanism and simulated rate constants based on a fit between proposed mechanism and experimental cellular uptake/washout data.
Figure 2.
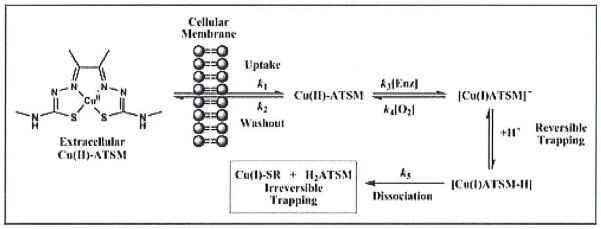
A mechanistic scheme showing the proposed pathways involved in the hypoxia-selective uptake and retention of Cu-ATSM and related copper bis(thiosemicarbazonato) complexes.39 Estimated rate constants k1-k5 were obtained based on a simulations between the proposed mechanism and experimental cellular uptake and washout data reported by Lewis et al.29 For cellular uptake and washout, k1=9.8(±0.59)×10−4 s−1 and k2=2.9(±0.17)×10−3 s−1, respectively. For intracellular reduction k3=5.2(±0.31)×10−2 s−1, reoxidation k4=2.2(±0.13) mol−1 dm3 s−1 and for proton-mediated ligand dissociation k5=9(±0.54)×10−5 s−1. NB: The rate of protonation/deprotonation was assumed to be fast.
Clinical studies
Copper-60-ATSM and, to lesser extent, 64Cu-ATSM have been used to study various human solid tumors. The estimated human dosimetry for both 60Cu-ATSM and 64Cu-ATSM based on biodistribution data in mature female Sprague-Dawley rats as well as human biodistribution demonstrated favorable Cu-ATSM dosimetry for human use.41 The clinical protocol has no specific patient preparation; in particular, no fasting is needed. The current simplified imaging protocol consists of a 30-min emission scan at the level of the tumor approximately 30 min after administration of Cu-ATSM. The attenuation scan can be performed using CT or a conventional attenuation scan using a positron emitting source. Initial kinetic analysis of the images along with arterial blood sampling demonstrated that muscle activity was essentially constant after the first 10 min of the 60-min PET scan and paralleled the blood activity of 60Cu-ATSM. Thus, no blood sampling is required for image analysis. The images are evaluated visually and semiquantitatively by tumor-to-muscle activity ratio (T/M) using maximum-pixel value for the tumor and the average value for muscle activity.
Several human solid tumors have been studied with 60Cu-ATSM-PET with particular attention to assessment of the relationship between Cu-ATSM uptake and response to therapy and/or outcome in these tumors.
In 12 patients with T2–T4 head and neck cancer, T/M >4 for tumor uptake of 60Cu-ATSM represented a poorer prognosis compared with T/M ≤4 (unpublished data). In this study, 60Cu-ATSM-PET images were co-registered with CT images using the thermoplastic immobilization head mask to create a hypoxia imaging-guided IMRT treatment plan for radiation therapy of patients with head and neck cancer.42 A study of patients with suspected or proven stage II–IV non-small cell lung cancer demonstrated that 60Cu-ATSM uptake was predictive of response to therapy with either ionizing radiation and/or chemotherapy. The mean T/M for 60Cu-ATSM was significantly lower in responders (1.5±0.4) than in non-responders (3.4±0.8) (P=0.002). An T/M threshold of 3 was found to discriminate between those patients likely to respond to therapy and non-responders: all responders had a T/M <3 and all non-responders had a T/M ≥3. These patients also underwent clinical PET imaging with [18F]fluorodeoxyglucose ([18F]FDG). Standardized uptake values of [18F]FDG in tumors were not significantly different in responders and non-responders (P=0.7) and did not correlate with 60Cu-ATSM uptake (r=0.04; P=0.9). Thus, it is likely that 60Cu-ATSM reveals clinically unique information about tumor oxygenation that is predictive of tumor response to therapy.43
In patients with T2–T4 rectal carcinoma who were studied with 60Cu-ATSM prior to neoadjuvant chemoradiotherapy, 60Cu-ATSM uptake predicted prognosis. The median T/M ratio of 2.6 discriminated those with worse prognosis from those with better prognosis. Both overall and progression-free survivals were worse with hypoxic tumors (T/M>2.6) than with non-hypoxic tumors (T/M ≤2.6) (both P<0.05). Again in these patients who underwent clinical [18F]FDG-PET, tumor [18F]FDG uptake did not correlate with 60Cu-ATSM uptake (r=0.4; P=0.9) and there was no significant difference in mean tumor [18F]FDG uptake between patients with hypoxic tumors and those with normoxic tumors (P=0.3).44
Cervical cancer has been studied most extensively with 60Cu-ATSM-PET and it was found to be predictive of outcome. Patients with locally advanced cervical cancer were studied prior to initiation of definitive therapy. T/M threshold of 3.5 was found to be a statistically significant cutoff value that accurately distinguished patients whose cancer did not recur from those who developed a recurrence after completing therapy. In these patients, progression-free survival and cause-specific survival were significantly better with a T/M for 60Cu-ATSM of ≤3.5 (P=0.006 and P=0.04, respectively). The 3-year progression-free survival of patients with normoxic tumors (T/M of ≤3.5, Figure 3) was 71%, and that of patients with hypoxic tumors (T/M of >3.5, Figure 4) was 28% (P=0.01). In addition, the corresponding cause-specific survival estimates were 74% and 49%, respectively (P=0.05). No significant difference was found in the frequency of lymph node involvement between patients with a T/M of >3.5 and patients with a T/M of ≤3.5. Tumor uptake of 60Cu-ATSM had no significant correlation with disease stage.45,46
Figure 3.
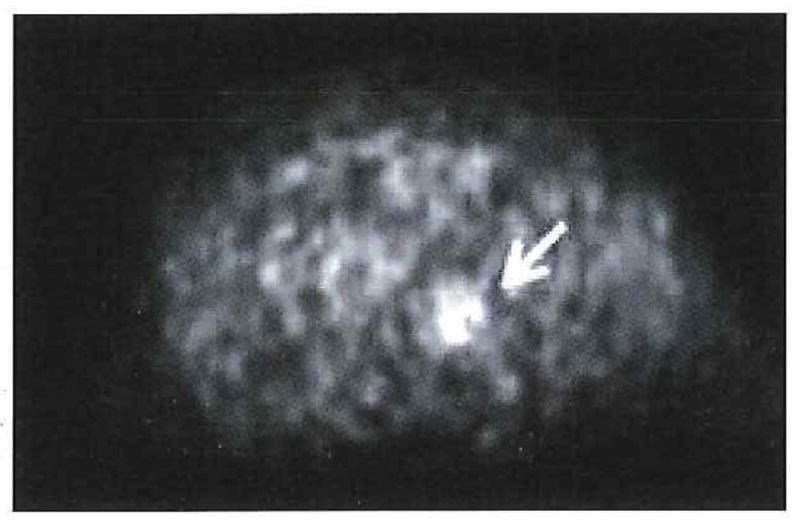
Normoxic tumor. Transaxial 60Cu-ATSM-PET image of the pelvis demonstrates mildly increased uptake (arrow) within the primary cervical cancer (T/M=2).
Figure 4.
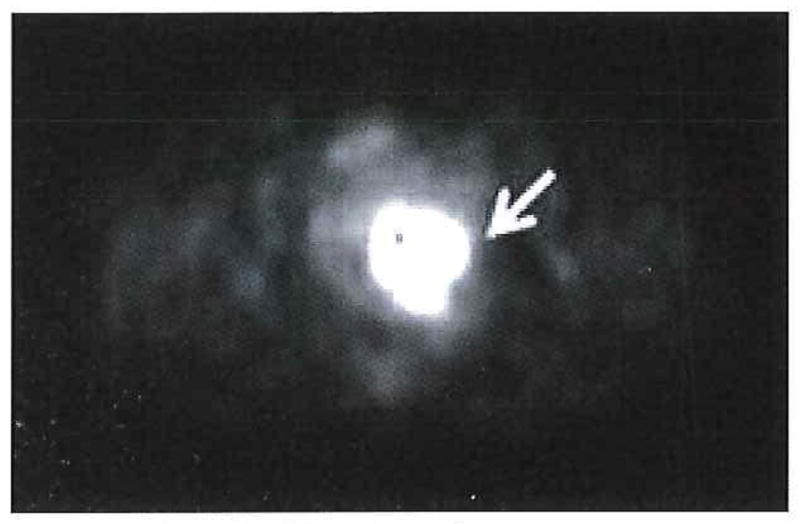
Hypoxic tumor. Transaxial 60Cu-ATSM-PET image of the pelvis demonstrates intense uptake (arrow) within the primary cervical cancer (T/M=5.4).
The results of clinical studies suggest that 60Cu-ATSM, a hypoxia-targeting radiopharmaceutical, is an important clinical biomarker of prognosis in several human cancers. One of the limitations of 60Cu-ATSM for widespread clinical use is related to its short radioactive half-life (t1/2=23.7 min). However, another radionuclide of copper, namely 64Cu, which has a much longer half-life (t1/2=12.7 hr) is an optimal candidate to substitute for 60Cu-ATSM. The longer half-lives of 64Cu allow for distribution to PET facilities without an in-house cyclotron in a fashion similar to that for 18F-labeled radiopharmaceuticals. A comparison of image quality of 60Cu-ATSM with 64Cu-ATSM that was performed under a United States Food and Drug Administration Investigational New Drug application (IND 62,675) 47 in patients with locally advanced cervical cancer demonstrated that the image quality of these two radiopharmaceuticals was comparable; although generally, the images with 64Cu-ATSM had a slightly better target-to-background ratio and tumors were delineated more clearly by comparison with the 60Cu-ATSM images. Moreover, the pattern of uptake was similar on the images obtained with the tracers during two different imaging sessions 1 to 9 days apart, indicating that the macroscopic distribution of hypoxia did not change greatly over this interval. This is an important finding given that the treatment of tumor hypoxia typically targets the chronic form of hypoxia. In addition, the T/M for 60Cu-ATSM processed with cascade subtraction (60Cu-ATSM decays by positron decay with the concurrent emission of numerous cascade γ-photons and thus, fortuitous cascade coincidences were removed by convolution of the cascade γ-ray kernel) was similar to that of 64Cu-ATSM (7.3±1.8 vs 7.4±1.9).
Copper-ATSM has several well-established advantages over other radiopharmaceuticals used for PET imaging of hypoxia, including a more facile method for synthesis, a faster clearance rate from normoxic tissue allowing a short time between injection and imaging, and a simpler method for quantification. All these qualities of 64Cu-ATSM make it an attractive tracer for clinical imaging of tumor hypoxia. This method can be used to identify patients for clinical trials of treatment strategies designed to overcome hypoxia.
Second generation agents
Quantitative imaging of tumor hypoxia is vital for both improving the treatment received by cancer patients and in the design of ever more selective hypoxia-targeted therapies. Nitroimidazoles were originally studied as hypoxia-selective radiosensitizers.33 However, with the failure to yield significant improvements to treatment regimes, the pathway is open for the development of new tracers for PET imaging and radiotherapy.1,2 Recent advances in synthetic strategies based on functionalizing the exocyclic nitrogen of the Cu-ATSM complex have been reported (Figure 5).48–50 Preliminary in vitro and in vivo studies indicate that improved hypoxia-selective uptake and retention may be achieved by careful control of properties such as redox potentials, lipophilicity and molecular size.51 In addition, novel methods for the facile production of high specific activity Cu-ATSM and functionalized copper bis(thiosemicarbazonato) complexes have been developed.52
Figure 5.
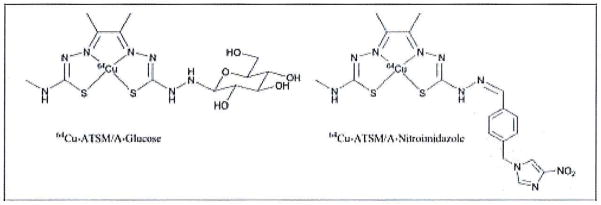
Second generation imaging agents derived from the Cu-ATSM structural motif.48–50
Conclusions
Pretherapy information on the oxygenation status of a tumor could play an important role in treatment selection. Studies of human solid tumors have shown that 60Cu-ATSM-PET is a promising method for assessment of tumor hypoxia and has the potential to be clinically useful in the management of oncologic patients. Although the information about tumor oxygenation and thus outcome can be obtained with the oxygen electrode method, imaging with 60Cu-ATSM-PET offers several important advantages including the ability to perform repeated, noninvasive assessment of hypoxia within the entire tumor regardless of the tumor’s location. Future clinical studies with 64Cu-ATSM would be of great importance in establishing this tracer as a clinical imaging method for selecting hypoxic tumors to be treated with hypoxia-targeted therapies and monitoring the effectiveness of such therapies. This method can be used to identify patients for clinical trials of treatment strategies designed to overcome hypoxia.
Acknowledgments
Fundings.—This work was supported by NIH Grant CA81525 and DOE Grant DE-FG02-87ER60512..
References
- 1.Brown JM. The hypoxic cell: a target for selective cancer therapy—eighteenth Bruce F. Cain Memorial Award lecture Cancer Res. 1999;59:5863–70. [PubMed] [Google Scholar]
- 2.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–16. [PubMed] [Google Scholar]
- 3.Höckel M, Knoop C, Schlenger K, Vorndran B, Baussmann E, Mitze M, et al. Intratnmoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol. 1993;26:45–50. doi: 10.1016/0167-8140(93)90025-4. [DOI] [PubMed] [Google Scholar]
- 4.Höckel M, Knoop C, Schlenger K, Vorndran B, Knapstein PG, Vaupel P. Intratumoral pO2 histography as predictive assay in advanced cancer of the uterine cervix. Adv Exp Med Biol. 1994;345:445–50. doi: 10.1007/978-1-4615-2468-7_59. [DOI] [PubMed] [Google Scholar]
- 5.Höckel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–15. [PubMed] [Google Scholar]
- 6.Höckel M, Schlenger K, Höckel S, Aral B, Schaffer U, Vaupel P. Tumor hypoxia in pelvic recurrences of cervical cancer. Int J Cancer. 1998;79:365–9. doi: 10.1002/(sici)1097-0215(19980821)79:4<365::aid-ijc10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Höckel M, Schlenger K, Knoop C, Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res. 1991;51:6098–102. [PubMed] [Google Scholar]
- 8.Rofstad EK, Sundfor K, Lyng H, Trope CG. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br J Cancer. 2000;83:354–9. doi: 10.1054/bjoc.2000.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pitson G, Fyles A, Milosevic M, Wylie J, Pintilie M, Hill R. Tumor size and oxygenation are independent predictors of nodal diseases in patients with cervix cancer. Int J Radiat Oncol Biol Phys. 2001;51:699–703. doi: 10.1016/s0360-3016(01)01662-5. [DOI] [PubMed] [Google Scholar]
- 10.Isa AY, Ward TH, West CM, Slevin NJ, Homer JJ. Hypoxia in head and neck cancer. Br J Radiol. 2006;79:791–8. doi: 10.1259/bjr/17904358. [DOI] [PubMed] [Google Scholar]
- 11.Blower PJ, Lewis JS, Zweit J. Copper radionuclides and radiopharmaceuticals in nuclear medicine. Nucl Med Biol. 1996;23:957–80. doi: 10.1016/s0969-8051(96)00130-8. [DOI] [PubMed] [Google Scholar]
- 12.Anderson CJ, Green MA, Fujibayashi Y. Chemistry of copper radionuclides and radiopharmaceutical products. In: Welch WJ, Redvanly C, editors. Handbook of Radiopharmaceuticals: radiochemistry and applications. West. Sussex, England: John Wiley & Sons, Ltd; 2003. pp. 401–22. [Google Scholar]
- 13.Anderson CJ, Welch MJ. Radiometal-labeled agents (non-technetium) for diagnostic imaging. Chem Rev. 1999;99:2219–34. doi: 10.1021/cr980451q. [DOI] [PubMed] [Google Scholar]
- 14.Novak-Hofer I, Schubiger PA. Copper-67 as a therapeutic nuclide for radioimmunotherapy. Eur J Nucl Med Mol Imaging. 2002;29:821–30. doi: 10.1007/s00259-001-0724-y. [DOI] [PubMed] [Google Scholar]
- 15.Smith SV. Molecular imaging with copper-64. J Inorg Biochem. 2004;98:1874–901. doi: 10.1016/j.jinorgbio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Reichert DE, Lewis J, Anderson CJ. Metal complexes as diagnostic tools. Coord Chem Rev. 1999;184:3–66. [Google Scholar]
- 17.Vvere AL, Lewis JS. Cu-ATSM: a radiopharmaceutical for the PET imaging of hypoxia. Dalton Trans. 2007:4893–902. doi: 10.1039/b705989b. [DOI] [PubMed] [Google Scholar]
- 18.McQuade P, McCarthy DW, Welch MJ. Metal radionuclides for PET imaging. In: Valk PE, Bailey DL, Townsend DW, Maisey MN, editors. Positron emission tomography: basic science and clinical practice. London: Springer-Verlag London Ltd; 2003. pp. 237–64. [Google Scholar]
- 19.McCarthy DW, Bass LA, Cutler PD, Shefer RE, Klinkowstein RE, Herrero P, et al. High purity production and potential applications of copper-60 and copper-61. Nucl Med Biol. 1999;26:351–8. doi: 10.1016/s0969-8051(98)00113-9. [DOI] [PubMed] [Google Scholar]
- 20.McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, et al. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol. 1997;24:35–43. doi: 10.1016/s0969-8051(96)00157-6. [DOI] [PubMed] [Google Scholar]
- 21.Obata A, Kasamatsu S, McCarthy DW, Welch MJ, Saji H, Yonekura Y, et al. Production of therapeutic quantities of (64)Cu using a 12 MeV cyclotron. Nucl Med Biol. 2003;30:535–9. doi: 10.1016/s0969-8051(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 22.Fujibayashi Y, Wada K, Taniuchi H, Yonekura Y, Konishi J, Yokoyama A. Mitochondria-selective reduction of 62Cu-pyru-valdehyde bis(N4-methylthiosemicarbazone) (62Cu-PTSM) in the murine brain; a novel radiopharmaceutical for brain positron emission tomography (PET) imaging. Biol Pharm Bull. 1993;16:146–9. doi: 10.1248/bpb.16.146. [DOI] [PubMed] [Google Scholar]
- 23.Wong TZ, Lacy JL, Petry NA, Hawk TC, Sporn TA, Dewhirst MW, et al. PET of hypoxia and perfusion with 62Cu-ATSM and 62-Cu-PTSM using a 62Zn/62Cu generator. AJR Am J Roentgenol. 2008;190:427–32. doi: 10.2214/AJR.07.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson CJ, Connett JM, Schwarz SW, Rocque PA, Guo LW, Philpott GW, et al. Copper-64-labeled antibodies for PET imaging. J Nucl Med. 1992;33:1685–91. [PubMed] [Google Scholar]
- 25.Lewis MR, Boswell CA, Laforest R, Buettner TL, Ye D, Connett JM, et al. Conjugation of monoclonal antibodies with TETA using activated esters: biological comparison of 64Cu-TETA-1A3 with 64Cu-BAT-2IT-1A3. Cancer Biother Radiopharm. 2001;16:483–94. doi: 10.1089/10849780152752083. [DOI] [PubMed] [Google Scholar]
- 26.Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med. 1997;38:1155–60. [PubMed] [Google Scholar]
- 27.Dearling JLJ, Lewis JS, McCarthy DW, Welch MJ, Blower PJ. Redox-active metal complexes for imaging hypoxic tissues: structure-activity relationships in cooper(II)bis(thiosemicarbazone) complexes. Chem Commun (Camb) 1998;22:2531–2. [Google Scholar]
- 28.Dearling JL, Lewis JS, Mullen GE, Welch MJ, Blower PJ. Copper bis(thiosemicarbazone) complexes as hypoxia imaging agents: structure-activity relationships. J Biol Inorg Chem. 2002;7:249–59. doi: 10.1007/s007750100291. [DOI] [PubMed] [Google Scholar]
- 29.Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med. 1999;40:177–83. [PubMed] [Google Scholar]
- 30.Burgman P, O’Donoghue JA, Lewis JS, Welch MJ, Humm JL, Ling CC. Cell line-dependent differences in uptake and retention of the hypoxia-selective nuclear imaging agent Cu-ATSM. Nucl Med Biol. 2005;32:623–30. doi: 10.1016/j.nucmedbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Lewis JS, Sharp TL, Laforest R, Fujibayashi Y, Welch MJ. Tumor uptake of copper-diacetyl-bis(N(4)-methylthiosemicarbazone): effect of changes in tissue oxygenation. J Nucl Med. 2001;42:655–61. [PubMed] [Google Scholar]
- 32.Lewis JS, Laforest R, Dehdashti F, Grigsby P, Welch MJ, Siegel BA. An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J Nucl Med. 2008;49:1177–82. doi: 10.2967/jnumed.108.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunn A, Linder K, Strauss HW. Nitroimidazoles and imaging hypoxia. Eur J Nucl Med. 1995;22:265–80. doi: 10.1007/BF01081524. [DOI] [PubMed] [Google Scholar]
- 34.Rajendran JG, Hendrickson KR, Spence AM, Muzi M, Krohn KA, Mankoff DA. Hypoxia imaging-directed radiation treatment planning. Eur J Nucl Med Mol Imaging. 2006;33 (Suppl 1):44–53. doi: 10.1007/s00259-006-0135-1. [DOI] [PubMed] [Google Scholar]
- 35.Rajendran JG, Wilson DC, Conrad EU, Peterson LM, Bruckner JD, Rasey JS, et al. [(18)F]FMISO and [(18)F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. doi: 10.1007/s00259-002-1096-7. [DOI] [PubMed] [Google Scholar]
- 36.Obata A, Yoshimi E, Waki A, Lewis JS, Oyama N, Welch MJ, et al. Retention mechanism of hypoxia selective nuclear imaging/radiotherapeutic agent cu-diacetyl-bis(N4-methylthiosemicarbazone) (Cu-ATSM) in tumor cells. Ann Nucl Med. 2001;15:499–504. doi: 10.1007/BF02988502. [DOI] [PubMed] [Google Scholar]
- 37.Maurer RI, Blower PJ, Dilworth JR, Reynolds CA, Zheng Y, Mullen GE. Studies on the mechanism of hypoxic selectivity in copper bis(thiosemicarbazone) radiopharmaceuticals. J Med Chem. 2002;45:1420–31. doi: 10.1021/jm0104217. [DOI] [PubMed] [Google Scholar]
- 38.Holland JP, Green JC, Dilworth JR. Probing the mechanism of hypoxia selectivity of copper bis(thiosemicarbazonato) complexes: DFT calculation of redox potentials and absolute acidities in solution. Dalton Trans. 2006:783–94. doi: 10.1039/b512656h. [DOI] [PubMed] [Google Scholar]
- 39.Holland JP, Barnard PJ, Collison D, Dilworth JR, Edge R, Green JC, et al. Spectroelectrochemical and computational studies on the mechanism of hypoxia selectivity of copper radiopharmaceuticals. Chemistry. 2008;14:5890–907. doi: 10.1002/chem.200800539. [DOI] [PubMed] [Google Scholar]
- 40.Okazawa H, Yonekura Y, Fujibayashi Y, Nishizawa S, Magata Y, Ishizu K, et al. Clinical application and quantitative evaluation of generator-produced copper-62-PTSM as a brain perfusion tracer for PET. J Nucl Med. 1994;35:1910–5. [PubMed] [Google Scholar]
- 41.Laforest R, Dehdashti F, Lewis JS, Schwarz SW. Dosimetry of 60/61/62/64Cu-ATSM: a hypoxia imaging agent for PET. Eur J Nucl Med Mol Imaging. 2005;32:764–70. doi: 10.1007/s00259-004-1756-x. [DOI] [PubMed] [Google Scholar]
- 42.Chao KS, Bosch WR, Mutic S, Lewis JS, Dehdashti F, Mintun MA, et al. A novel approach to overcome hypoxic tumor resistance: Cu-ATSM-guided intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2001;49:1171–82. doi: 10.1016/s0360-3016(00)01433-4. [DOI] [PubMed] [Google Scholar]
- 43.Dehdashti F, Mintun MA, Lewis JS, Bradley J, Govindan R, Laforest R, et al. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging. 2003;30:844–50. doi: 10.1007/s00259-003-1130-4. [DOI] [PubMed] [Google Scholar]
- 44.Dietz DW, Dehdashti F, Grigsby PW, Malyapa RS, Myerson RJ, Picus J, et al. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study. Dis Colon Rectum. 2008;51:1641–8. doi: 10.1007/s10350-008-9420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dehdashti F, Grigsby PW, Mintun MA, Lewis JS, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: relationship to therapeutic response: a preliminary report. Int J Radiat Oncol Biol Phys. 2003;55:1233–8. doi: 10.1016/s0360-3016(02)04477-2. [DOI] [PubMed] [Google Scholar]
- 46.Dehdashti F, Grigsby PW, Lewis JS, Laforest R, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone) J Nucl Med. 2008;49:201–5. doi: 10.2967/jnumed.107.048520. [DOI] [PubMed] [Google Scholar]
- 47.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 48.Holland JP, Aigbirhio FI, Betts HM, Bonnitcha PD, Burke P, Christlieb M, et al. Functionalized bis(thiosemicarbazonato) complexes of zinc and copper: synthetic platforms toward site-specific radiopharmaceuticals. Inorg Chem. 2007;46:465–85. doi: 10.1021/ic0615628. [DOI] [PubMed] [Google Scholar]
- 49.Bonnitcha PD, Vavere AL, Lewis JS, Dilworth JR. In vitro and in vivo evaluation of bifunctional bisthiosemicarbazone 64Cu-complexes for the position emission tomography imaging of hypoxia. J Med Chem. 2008;51:2985–91. doi: 10.1021/jm800031x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holland JP, Barnard PJ, Bayly SR, Betts HM, Churchill GC, Dilworth JR, et al. Synthesis, radiolabeling and confocal fluorescence microscopy of styrene-derivatised bis(thiosemicarbazonato)zinc and -copper complexes. Eur J Inorg Chem. 2008;12:1985–93. [Google Scholar]
- 51.Bayly SR, King RC, Honess DJ, Barnard PJ, Betts HM, Holland JP, et al. In vitro and in vivo evaluations of a hydrophilic 64Cu-bis(thiosemicarbazonato)-glucose conjugate for hypoxia imaging. J Nucl Med. 2008;49:1862–8. doi: 10.2967/jnumed.108.054015. [DOI] [PubMed] [Google Scholar]
- 52.Betts HM, Barnard PJ, Bayly SR, Dilworth JR, Gee AD, Holland JP. Controlled axial coordination: solid-phase synthesis and purification of metallo-radiopharmaceuticals. Angew Chem Int Ed Engl. 2008;47:8416–9. doi: 10.1002/anie.200801936. [DOI] [PubMed] [Google Scholar]


