Abstract
Background
This study aimed to determine whether patients with post-traumatic stress disorder (PTSD) show difficulty in recruitment of the regions of the frontal and parietal cortex implicated in top-down attentional control in the presence and absence of emotional distracters.
Method
Unmedicated individuals with PTSD (n=14), and age-, IQ- and gender-matched individuals exposed to trauma (n=15) and healthy controls (n=19) were tested on the affective number Stroop task. In addition, blood oxygen level-dependent responses, as measured via functional magnetic resonance imaging, were recorded.
Results
Patients with PTSD showed disrupted recruitment of lateral regions of the superior and inferior frontal cortex as well as the parietal cortex in the presence of negative distracters. Trauma-comparison individuals showed indications of a heightened ability to recruit fronto-parietal regions implicated in top-down attentional control across distracter conditions.
Conclusions
These results are consistent with suggestions that emotional responsiveness can interfere with the recruitment of regions implicated in top-down attentional control ; the heightened emotional responding of patients with PTSD may lead to the heightened interference in the recruitment of these regions.
Keywords: Anxiety, post-traumatic stress disorder, fMRI, fronto-parietal cortex, top down attention control
Introduction
Hypervigilance, an increased attentional bias to environmental cues associated with threat, is a classic symptom of post-traumatic stress disorder (PTSD). This attentional bias may reinforce the preoccupation with the trauma and thus perpetuate the disorder (Foa et al. 1991 ; Coles & Heimberg, 2002). Increased attention to emotional information can lead to disruption in patients’ goal-directed activity. Indeed, a series of studies have documented increased interference of goal-directed activity by emotional distracters in PTSD (Williams et al. 1996 ; Buckley et al. 2000 ; Bar-Haim et al. 2007).
Considerable functional magnetic resonance imaging (fMRI) work has demonstrated that in healthy individuals emotional attention involves amygdala priming of temporal cortex representations (e.g. Pessoa et al. 2005 ; Blair et al. 2007 ; Mitchell et al. 2007). Further, this work has shown that the priming of task-relevant representations by regions implicated in top-down attentional control leads to reduced amygdala activation to emotional distracters. The suggestion is that increased recruitment of top-down attentional control systems, priming goal-relevant representations, leads to reduced representation of emotional distracters following representational competition (Desimone & Duncan, 1995). Reduced representation of the emotional distracters will result in weaker emotional responding to these distracters (Blair et al. 2007).
Hypervigilance in PTSD might therefore reflect either : (i) increased amygdala responsiveness – the ‘ hyper ’-responsive amygdala elicits heightened priming of emotional representations within temporal cortex making the emotional distracters more likely to win the representational competition ; and/or (ii) dysfunction within regions implicated in top-down attentional control, resulting in weaker priming of task-relevant stimuli and thus reduced competition by representations of these stimuli against emotional distracters ; and/or (iii) an increased amygdala response to emotional stimuli which would interfere with the recruitment of regions implicated in task-related top-down attention control (Pessoa, 2009).
In line with the first hypothesis, considerable work has suggested heightened amygdala responsiveness in PTSD (Rauch et al. 2006 ; Felmingham et al. 2010 ; Shin & Liberzon, 2010). This could underpin findings of increased interference by emotional distracters in the disorder (see Williams et al. 1996 ; Buckley et al. 2000 ; Bar-Haim et al. 2007). Indeed, recent work has shown that the amygdala blood oxygen level-dependent (BOLD) response to threat cues in PTSD patients is inversely associated with their ability to disengage from these cues in attentional-bias paradigms (El Khoury-Malhame et al. 2011).
With respect to the second and third hypotheses, less imaging work has investigated top-down attentional control in PTSD. Three studies employing emotional Stroop-type paradigms indicate that patients with PTSD, relative to trauma controls (TCs), show greater interference in the recruitment of regions implicated in top-down attentional control in the presence of emotional distracters (Shin et al. 2001 ; Bremner et al. 2004 ; Kim et al. 2008). Thus patients with PTSD showed reduced recruitment of the anterior cingulate cortex (ACC) and lateral frontal cortices during task performance in the context of combat relative to general negative words (Shin et al. 2001), negative relative to neutral words (Bremner et al. 2004) and negative relative to neutral faces (Kim et al. 2008). However, data from one of these studies also suggested that patients with PTSD show more generalized difficulties in the recruitment of regions in top-down attentional control. Thus, the PTSD patients in the Bremner et al. (2004) study showed significantly less recruitment of the parietal cortex during non-emotional Stroop conflict trials. Moreover, Pannu Hayes et al. (2009) reported that patients with greater PTSD symptomatology showed attenuated activity in the recruitment of frontal cortices in the context of top-down attention to neutral targets. Further, albeit indirect, support for the suggestion of a more general difficulty in recruiting regions involved in top-down attentional control comes from the literature examining the largely overlapping fronto-parietal system recruited during working memory performance. Patients with PTSD, relative to controls, show reduced activity, and connectivity, within these regions during working memory performance (Moores et al. 2008 ; Daniels et al. 2010). The ability to recruit these systems further is inversely related with level of PTSD symptoms (Morey et al. 2008). In short, it remains currently unclear whether PTSD involves a generalized reduced ability to recruit regions implicated in top-down attentional control (hypothesis 2) and/or particular difficulty in the recruitment of these regions in the context of emotional distracters (hypothesis 3). The primary goal of the present study was to test the predictions from these hypotheses. Specifically, hypothesis 1 predicts significant groupremotion interactions, with patients with PTSD showing heightened amygdala responses to emotional distracters. Hypothesis 2 predicts significant grouprtask interactions within regions involved in top-down attentional control, with patients with PTSD showing reduced responses during task conditions. Hypothesis 3 predicts significant groupremotionrtask interactions within these regions, with the PTSD group showing reduced responses during task conditions in the presence of emotional distracters.
One limitation of much of the previous work is the absence of a matched healthy comparison/control (HC) group. Typically, PTSD patients are contrasted with individuals who have also experienced trauma but who have not developed PTSD (TCs). A limitation of this approach is that it may not allow the identification of the pathophysiology in the patients. Instead, it may identify reasons for resilience in the TCs. In the context of the recruitment of regions implicated in top-down attentional control, it is interesting to note that patients with PTSD have been reported to show reduced, and TCs enhanced (at least in the context of emotional up-regulation), recruitment of prefrontal regions during emotional reappraisal (New et al. 2009). Our second goal was thus to contrast the ability of patients with PTSD to recruit regions implicated in top-down attentional control with that of both HCs and TCs. Specifically, with respect to the hypotheses above, we wished to determine whether the predicted results for the patients with PTSD were seen both relative to HCs and TCs. Moreover, we wished to determine whether TCs might show superior recruitment of regions involved in top-down attention relative to the other two groups during task performance as suggested by recent data (New et al. 2009).
Our third goal was to investigate the impact of positive distracters. Considerable fMRI work involving PTSD has considered negative stimuli (e.g. Rauch et al. 2000 ; Armony et al. 2005 ; Britton et al. 2005 ; Shin et al. 2005) ; however, little work has investigated positive stimuli (Jatzko et al. 2006 ; Sailer et al. 2008). Behavioral work, including our own (Vythilingam et al. 2007), indicates a reduced impact of positive relative to negative distracters (McNally et al. 1990 ; Bryant & Harvey, 1995). If, however, anxiety pathology relates to reduced recruitment of prefrontal attentional control (see Bishop, 2008), we might expect to see reduced recruitment of these regions in PTSD in the context of both positive and negative distracters. In short, the current study tested whether patients with PTSD would show reduced recruitment of prefrontal and parietal regions implicated in attentional control, relative to HCs and TCs, in the presence of both negative and positive distracters.
Method
Subjects
The participants in the present study were 14 patients with PTSD, 15 TCs and 19 non-traumatized HC individuals. The groups did not significantly differ in age, gender distribution or IQ (Table 1). All patients were free of medication for at least 2 weeks prior to testing (6 weeks if fluoxetine).
Table 1.
Subject characteristics
| Healthy controls (n = 19) |
Trauma controls (n = 15) |
Patients with PTSD (n = 14) |
|
|---|---|---|---|
| Age, years | 32.4 (8.79) | 31.4 (7.94) | 33.9 (9.98) |
| Gender, n | |||
| Female | 17 | 11 | 12 |
| Male | 2 | 4 | 2 |
| Race, n | |||
| Caucasian | 13 | 9 | 10 |
| African-American | 4 | 4 | 3 |
| Other | 2 | 2 | 1 |
| IQ | 117.5 (9.15) | 110.9 (11.62) | 106.0 (18.20) |
| CAPS* | 0 | 4.1 (6.95) | 67.9 (15.91) |
| IDS* | 0.5 (0.77) | 0.4 (0.74) | 16.5 (15.35) |
| SIGH-A* | 0.4 (0.59) | 0.3 (0.83) | 19.8 (17.68) |
PTSD, Post-traumatic stress disorder; IQ, intelligence quotient; CAPS, Clinician Administered PTSD Scale; IDS, Inventory of Depressive Symptomatology; SIGH-A, Hamilton Anxiety Rating Scale.
Data are given as mean (standard deviation) or as number of participants.
Group differences for CAPS, IDS and SIGH-A were all significant (p < 0.001).
All subjects were screened using the Structural Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) Axis I Disorder (SCID ; First et al. 1997). Subjects in the PTSD group met the DSM-IV (1994) criteria for PTSD based on the SCID. In addition, to be included in the study, their severity of PTSD as measured by the Clinician Administered PTSD Scale (CAPS ; Blake et al. 1990, 1995), was ≥50. Subjects in the TC group had to have experienced significant trauma based on the SCID but not meet the criteria for any current psychiatric disorder including PTSD and their CAPS score was ≤16. HCs did not have a past or current history of psychiatric illnesses or trauma according to the SCID. All subjects were in good physical health and were recruited from National Institute of Mental Health (NIMH) Institutional Review Board-approved advertisements.
The subjects with PTSD reported significantly greater depression, as indexed by the Inventory of Depressive Symptomatology (IDS ; Rush et al. 1986), and anxiety, as indexed by the Hamilton Anxiety Rating Scale (Shear et al. 2001), than the TCs and HCs (Table 1). Co-morbid diagnoses for the patients with PTSD included current major depressive disorder (MDD) (n=4), past MDD (n=6), current social phobia (n=1) and alcohol/substance abuse in complete remission (n=1). One of the TC individuals had a past history of bulimia. None of the HCs had present or past psychiatric disorders. The patients with PTSD and the TCs had experienced childhood sexual/ physical abuse (seven PTSD, two TC), sexual assaults in adulthood (three PTSD, three TC) and life-threatening events in adulthood (e.g. armed robbery ; five PTSD, 10 TC).
Behavioral task (affective number Stroop ; Blair et al. 2007)
The individual numerical stimuli consisted of two, three, four, or five 1’s, 2’s, 3’s, 4’s, 5’s or 6’s randomly presented within a nine-point grid of * symbols (for example stimuli, see Fig. 1). The emotional stimuli consisted of 40 positive, 40 negative and 40 neutral pictures selected from the International Affective Picture System (IAPS ; Lang & Greenwald, 1988). The normative mean valence and arousal values on a nine-point scale were, respectively, 2.71 (s.e.=0.11) and 5.85 (s.e.=0.11) for negative pictures, 7.30 (s.e.=0.11) and 5.01 (s.e.=0.10) for positive pictures, and 4.96 (s.e.=0.07) and 2.78 (s.e.=0.08) for neutral pictures.
Fig. 1.
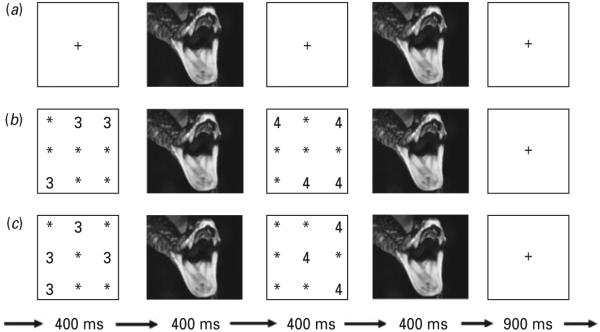
Example trial sequences : (a) negative view trial ; (b) negative congruent trial ; (c) negative incongruent trial.
Each trial began with a fixation point (+). For the numerical trials, the fixation point was then replaced by the first numerical display, followed by the first picture stimulus presented, then the second numerical display, then the second picture display, followed by a blank stimulus (Fig. 1). The subject determined with a button press whether the first or second numerical display contained more numbers. Subjects did not receive feedback on their performance. On congruent trials, the Arabic numeral distracter information was consistent with the numerosity information, i.e. the second (greater numerosity) display also contained Arabic numerals of larger value than the first display (e.g. two 2’s and four 4’s) (Fig. 1 b). On incongruent trials, the Arabic numeral distracter information was inconsistent with the numerosity information, i.e. the second (greater numerosity) display contained numerals of smaller value than the first display (e.g. two 4’s and four 2’s) (Fig. 1 c). For the view trials, there were no numerical displays ; the numerical displays were replaced by fixation points (Fig. 1 a).
There were four runs, each consisting of 10 randomized presentations of each emotion × task condition. Further, 26 fixation points were presented in each run (10 randomly throughout, six at beginning/end).
fMRI parameters
Whole-brain BOLD fMRI data were acquired using a 1.5-T General Electric MRI scanner. Functional T2* weighted images were acquired using an echo-planar single-shot gradient echo pulse sequence with a 64r64 matrix [repetition time (TR)=3000 ms, echo time (TE)=30 ms, field of view (FOV)=240 mm, 3.75 × 3.75 × 4 mm), followed by a high-resolution T1-weighed anatomical image to aid spatial normalization (three-dimensional spoiled gradient-recalled acquisition in the steady state).
fMRI analysis
Imaging data were pre-processed and analysed in Analysis of Functional NeuroImages (AFNI ; http://afni.nimh.nih.gov/afni/). At the individual level, functional images from the first four trials of each run collected before equilibrium magnetization was reached were discarded. Functional images from the four time series were motion corrected and spatially smoothed with a 6 mm full-width half-maximum Gaussian filter. The time series were normalized by dividing the signal intensity of a voxel at each point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients represented a percentage of signal change from the mean. Following this, regressors depicting each of the response types were created by convolving the train of stimulus events with a c-variate hemodynamic response function to account for the slow hemodynamic response. This involved 10 regressors (positive view/congruent/incongruent, negative view/congruent/incongruent, neutral view/congruent/incongruent, error/missed responses). Thus, regressors entered into the analyses of variance (ANOVAs) consisted of correct trials only. Each trial was modeled as a single regressor using an event-related model. Linear regression modeling was performed fitting the BOLD signal to the 10 regressors. A baseline plus linear drift and quadratic trend were modeled in each voxel’s time series to control for voxel-wise correlated drifting. This produced for each voxel and each regressor, a β coefficient and its associated t statistic. Voxel-wise group analyses involved transforming single subject β coefficients into the standard coordinate space of Talairach & Tournoux (1988).
The group analysis of the BOLD data was then performed on regression coefficients from individual subject analyses following the strategy adopted in Blair et al. (2007). Due to concerns about the reduction in statistical power associated with a three-level analysis, separate ANOVAs were again conducted for negative and positive distracters. To test whether negative distracters might have a greater impact on patients with PTSD than positive distracters, a third ANOVA contrasting negative and positive distracters was conducted. Thus, three ANOVAs were performed (see below for details). To correct for multiple comparisons for the whole-brain analysis at p<0.005, we performed a spatial clustering operation using AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/ manual/AlphaSim.pdf) with 1000 Monte Carlo simulations taking into account the entire echo-planing imaging matrix, with a map-wise false-positive probability of p<0.05 corrected for multiple comparisons. Due to the significant group difference in IDS scores, activity within the functional regions of interest identified by the ANOVAs were further analysed by analysis of covariance (ANCOVA) with IDS scores as the covariate to determine whether group differences in this covariate could be determining the group effects. For all the regions reported below, the introduction of the IDS covariate did not remove the significant interaction with group.
Results
Behavioral data
Following Blair et al. (2007), we first examined the effect of negative emotion and task on reaction times (RTs) and the percentage of correct responses using two 3 (group : PTSD, TC, HC) × 2 (emotion : negative, neutral) × 2 (task : congruent, incongruent) ANOVAs (Table 2). In terms of RTs, there was a significant main effect of emotion [F(1,45)=13.50, p<0.005], with slower responses to trials involving negative relative to neutral pictures [M(negative)=882.01, s.e.=27.13 ; M(neutral)=849.29, s.e.=25.00]. There was also a significant main effect for task [F(1, 45)=20.10, p<0.001], with responses slower to incongruent relative to congruent trials [M(incongruent)=881.32, s.e.=26.41 ; M(congruent)=849.98, s.e.=25.46]. The emotion × group interaction and group main effect did not reach significance (F=0.12 and 1.16, n.s.). In terms of response rates, there were no significant effects (F<1, n.s.).
Table 2.
RTs and percentage correct responses by the three groups
| Healthy controls |
Trauma controls |
Patients with PTSD |
||||
|---|---|---|---|---|---|---|
| Condition | RT, ms | % correct | RT, ms | % correct | RT, ms | % correct |
| Positive congruent | 824.47 (222.09) | 81.05 (14.20) | 893.39 (163.75) | 76.76 (12.16) | 860.04 (177.94) | 77.14 (16.47) |
| Positive incongruent | 837.47 (208.87) | 81.20 (17.41) | 933.05 (171.73) | 78.29 (12.21) | 879.11 (195.74) | 81.74 (15.43) |
| Negative congruent | 824.32 (213.44) | 81.65 (18.38) | 907.20 (142.89) | 75.43 (11.77) | 877.44 (194.70) | 75.10 (18.87) |
| Negative incongruent | 844.47 (206.53) | 81.20 (15.83) | 934.35 (144.06) | 75.61 (10.85) | 904.28 (199.65) | 75.10 (18.46) |
| Neutral congruent | 792.68 (191.33) | 81.65 (13.93) | 872.70 (143.87) | 76.19 (10.73) | 825.53 (169.35) | 75.51 (16.72) |
| Neutral incongruent | 804.24 (196.07) | 79.85 (15.89) | 915.83 (144.09) | 74.86 (9.55) | 884.75 (190.94) | 76.73 (17.43) |
RT, Reaction time; PTSD, post-traumatic stress disorder.
Data are given as mean (standard deviation).
We next examined the effect of positive emotions on RTs and response rates (Table 2). In terms of RTs, there was a significant main effect of emotion [F(1, 45)=5.63, p<0.05], with slower responses to positive relative to neutral trials [M(positive)=871.23, s.e.=257.88 ; M(neutral)=849.29, s.e.=25.00]. There was also a significant main effect for task [F(1, 45)=26.55, p<0.001], with responses slower to incongruent relative to congruent trials [M(incongruent)=875.73, s.e.=26.85 ; M(congruent)=844.80, s.e.=25.62]. The emotion × group interaction and group main effect were not significant (F<1 for both, n.s.). In terms of response rates, there was a trend towards more correct responses to the positive relative to the neutral trials (F=3.43, p<0.05 one-tailed). None of the other effects was significant (F<1, n.s.).
Third, we examined the effect of negative versus positive emotion on RTs and response rates (Table 2). There was a significant main effect of emotion [F(1, 45)=3.10, p<0.05 one-tailed], with slower responses to negative relative to positive trials. There was also a significant main effect for task [F(1, 45)= 19.76, p<0.001], with slower responses to incongruent relative to congruent trials. The emotion × group interaction and group main effect did not reach significance (F<1 for both, n.s.). In terms of correct response rates, there again was a trend towards more correct responses to positive relative to negative trials (F=3.77, p<0.10) ; however, none of the other effects was significant (F<1, n.s.).
fMRI data
Our initial analysis focused on negative distracters and involved a 3 (group : PTSD, TC, HC) × 2 (emotion : negative, neutral) × 3 (task : view, congruent, incongruent) ANOVA. The main interactions with respect to our predictions (group × emotion × task and group × emotion) are described, providing a test of our a priori hypotheses.
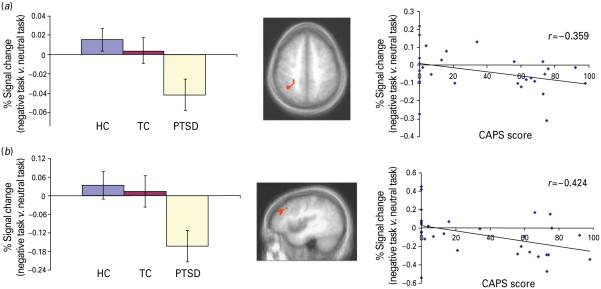
Our principal goal was to determine whether patients with PTSD (and TCs) would show increased (or reduced) disruption of the recruitment of the dorsal ACC, lateral superior frontal and parietal cortex in the presence of negative distracters. This goal was examined with reference to the group × emotion × task interaction, which identified the right inferior parietal cortex [Brodmann area (BA) 40] ; see Table 3 and Fig. 2. Within this region, follow-up contrasts demonstrated that patients with PTSD showed significantly greater reduction in activity during task (but not view) trials in the presence of negative relative to neutral distracters than both TCs and HCs (p<0.05 in both cases). Notably, also, the group × emotion interactions identified the right lateral superior frontal cortex (BA 9). Within this region, follow-up contrasts demonstrated that patients with PTSD showed significantly greater reduction in activity during task (and view) trials in the presence of negative relative to neutral distracters than both the TCs and HCs (at least p<0.05 in all cases).
Table 3.
Significant interaction areas of activation for group and negative emotiona
| Region | BA | mm3 | x | y | z | F |
|---|---|---|---|---|---|---|
| Group × emotion × task interaction | ||||||
| Right inferior parietal lobule | 40 | 513 | 33 | −48 | 45 | 4.31 |
| Left fusiform cortex | 20 | 1593 | −45 | −24 | −21 | 5.24 |
| Group × emotion interaction | ||||||
| Right superior frontal gyrus* | 9 | 350 | 42 | 36 | 27 | 6.58 |
| Right inferior frontal cortex | 47 | 513 | 18 | 21 | −18 | 7.58 |
| Group × task interaction | ||||||
| Left dorsolateral frontal cortex | 46 | 2187 | −57 | 33 | 18 | 6.96 |
| Right superior frontal cortex | 6 | 945 | 51 | 0 | 48 | 6.11 |
| Left superior frontal cortex | 9/6 | 891 | −54 | 6 | 45 | 5.81 |
| Right inferior frontal cortex | 47 | 459 | 36 | 36 | −6 | 5.20 |
| Left superior parietal cortex | 7 | 540 | −30 | −63 | 57 | 5.24 |
BA, Brodmann area.
All activations are effects observed in whole-brain analyses (significant at p < 0.005) corrected for multiple comparisons (significant at p <0.05) except
significant at p < 0.05.
Fig. 2.
Regions related to task performance showing reduced activity in the presence of negative distracters in the patients with post-traumatic stress disorder (PTSD). Mean (standard deviation) blood oxygen level-dependent (BOLD) responses in healthy control (HC), trauma control (TC) and PTSD participants and relationship between degree of disruption by negative distracters and Clinician Administered PTSD Scale (CAPS) scores for the PTSD and HC individuals within : (a) right inferior parietal lobule, Brodmann area 40 (x, y, z=33, −48, 45) ; (b) right superior frontal cortex, Brodmann area 9 (x, y, z=42, 36, 27).
In addition, we wished to determine whether patients with PTSD show generalized difficulties in the recruitment of regions implicated in top-down attentional control. This was examined via the group × task interaction, which identified both the left dorsolateral prefrontal cortex and superior frontal cortex (Fig. 3). However, within both regions, the PTSD and HC groups did not differ. Instead, the TCs showed significantly greater activity to incongruent trials relative to both the PTSD and HC groups (p<0.05 in both cases ; Fig. 3).
Fig. 3.
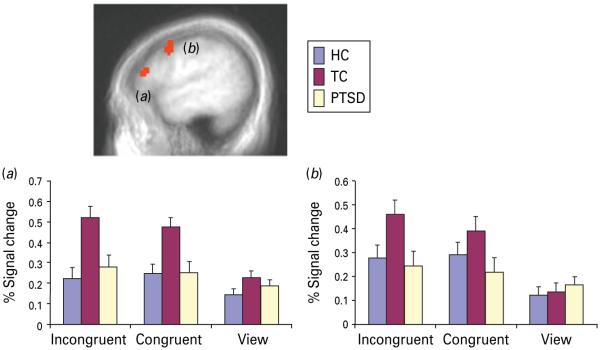
Regions related to task performance showing heightened activity in the trauma controls relative to both the patients with post-traumatic stress disorder and the healthy controls. Mean (standard deviation) blood oxygen level-dependent (BOLD) responses within : (a) left dorsolateral frontal cortex, Brodmann area 46 (x, y, z=−57, 33, 18) ; (b) left superior frontal cortex, Brodmann area 9/6 (x, y, z=−54, 6, 45).
The impact of positive distracters was examined through a second 3 (group : PTSD, TC, HC) × 2 (emotion : positive, neutral) × 3 (task : view, congruent, incongruent) ANOVA. This revealed no regions showing a significant group × emotion × task interaction. However, the group × emotion interaction identified bilateral regions of the medial and inferior frontal cortex and bilateral parietal cortex. Within all these regions, follow-up contrasts demonstrated that patients with PTSD showed significantly greater reduction in activity during task (and view) trials in the presence of positive relative to neutral distracters than both TCs and HCs (at least p<0.05 in all cases ; Table 4).
Table 4.
Significant interaction areas of activation for group and positive emotiona
| Region | BA | mm3 | x | y | z | F |
|---|---|---|---|---|---|---|
| Group × emotion interaction | ||||||
| Left lateral frontal gyrus | 46 | 513 | −33 | 24 | 21 | 7.70 |
| Right lateral frontal gyrus* | 9 | 243 | 39 | 30 | 30 | 6.77 |
| Right inferior frontal cortex | 47 | 2241 | 51 | 24 | −3 | 10.73 |
| Right inferior parietal lobule | 40 | 513 | 57 | −48 | 33 | 7.37 |
| Left superior parietal lobule | 7 | 405 | −27 | −63 | 48 | 8.16 |
| Group × task interaction | ||||||
| Right precentral gyrus | 6 | 486 | 48 | 0 | 48 | 4.99 |
| Left lingual gyrus | 18 | 2349 | −9 | −69 | 0 | 7.51 |
| Left superior temporal sulcus | 41 | 540 | −39 | −39 | 6 | 5.24 |
| Left cerebellum | 1350 | −30 | −33 | −33 | 4.83 | |
| Left inferior temporal gyrus | 20 | 1593 | −51 | −21 | −18 | 5.99 |
| Right superior temporal gyrus | 38 | 810 | 42 | 12 | −18 | 5.76 |
| Left amygdala/hippocampus* | - | 378 | −24 | −12 | −15 | 5.00 |
| Right amygdala/hippocampus* | - | 108 | 21 | −12 | −12 | 4.41 |
BA, Brodmann area.
All activations are effects observed in whole-brain analyses (significant at p < 0.005) corrected for multiple comparisons (significant at p <0.05) except
significant at p < 0.05.
A third 3 (group : PTSD, TC, HC) × 2 (emotion : positive, negative) × 3 (task : view, congruent, incongruent) ANOVA examined potential differential interactions of positive and negative distracters across groups. This revealed a significant grouprtaskr emotion interaction within the superior frontal cortex (x, y, z=15, 54, 30 ; BA 9). Patients with PTSD showed trends for reduced activity relative to both comparison groups within this region during task trials in the context of negative distracters [v. HCs : t(31)=1.72, p<0.1 ; v. TCs : t(27)=1.79, p<0.01]. They also showed a trend for reduced activity within this region during task trials in the context of positive distracters but only with respect to TCs [t(27)=1.87, p<0.1 ; v. HCs : t(31)=1.18, n.s.].
Discussion
The present study investigated the ability of patients with PTSD to recruit regions of the frontal and parietal cortex implicated in top-down attentional control in the presence and absence of emotional distracters. Our first main goal was to test the prediction that the increased interference by negative distracters observed in PTSD relates to disruption in the recruitment of frontal (lateral superior and inferior frontal cortex) and parietal regions implicated in top-down control and to determine whether this occurred irrespective of, or only in, the presence of emotional distracters. Our secondary goals were to determine : (i) whether TCs also show difficulties with the recruitment of these regions, albeit to a lesser degree than patients with PTSD, or actually enhanced recruitment as suggested by the findings of New et al. (2009) ; and (ii) whether the presence of positive distracters might also disrupt the recruitment of regions implicated in top-down attentional control.
Previous work, using the emotional Stroop paradigm, suggested that patients with PTSD, relative to TCs, show reduced recruitment of the ACC and lateral frontal cortices during task performance in the context of emotional relative to neutral distracters (Shin et al. 2001 ; Bremner et al. 2004). However, it has been uncertain whether this reflects specific interference by emotional distracters on the recruitment of regions implicated in top-down attentional control or more general recruitment difficulties. The results of the current study, via the groupremotionrtask interaction, suggest that emotional distracters can disrupt the recruitment of the parietal cortex during task performance more severely in patients with PTSD relative to HCs. Moreover, the groupremotion interactions seen for both the negative and positive ANOVAs suggest that the presence of emotional distracters disrupts activity within the frontal regions implicated in top-down attentional control irrespective of task performance (this disruption was seen during view as well as task trials). This is consistent with the suggestions of Pessoa, who has argued that high-threat stimuli can disrupt the recruitment of regions implicated in executive functioning (Pessoa, 2009). The suggestion is that in PTSD this impact of threat stimuli on the recruitment of these regions is exacerbated because of the heightened response to the threat stimuli.
It is interesting to note that the disruption in the recruitment of regions implicated in top-down attentional control in the presence of emotional information was not seen in the TCs who did not differ from HCs. As noted above, little of the previous fMRI work examining attention in PTSD has contrasted patients with both TCs and HCs (Shin et al. 2001 ; Bremner et al. 2004 ; Pannu Hayes et al. 2009). However, the current data suggest that the recruitment of both control groups may be important (see also New et al. 2009). In the present study, the TCs showed superior recruitment of regions implicated in top-down control in the context of task performance (relative to view trials) compared with the both the HC and PTSD groups, which did not differ. These results echo those of New et al. (2009) who found that TCs also showed increased activity in attention-relevant regions of the prefrontal cortex during the up-regulation of emotional responding relative to both the HC and PTSD groups.
It is tempting to consider that this enhanced ability to recruit regions implicated in top-down attentional control protected the TCs from the development of PTSD (see also New et al. 2009). Indeed, increased working memory-related activity within a fronto-parietal network has been linked with decreased PTSD symptom levels in combat veterans (Morey et al. 2008). These regions of the lateral superior and inferior frontal cortices and parietal cortex are repeatedly implicated in emotional regulation (Ochsner et al. 2002 ; Ochsner & Gross, 2005). Indeed, their activation in the context of reappraisal (Ochsner et al. 2002) or emotion attention tasks (Pessoa et al. 2002 ; Blair et al. 2007 ; Mitchell et al. 2007) is related to reduced activity within regions mediating the emotional response such as the amygdala. However, in the absence of longitudinal data this speculation is premature. It is alternatively possible, for example, that the increased ability to recruit these regions simply stems from trauma experience that does not result in PTSD.
Notably, the presence of positive distracters also had impact on the recruitment of regions implicated in top-down attentional control. Relatively little previous work has investigated the response of patients with PTSD to positive stimuli (Jatzko et al. 2006 ; Sailer et al. 2008). However, behavioral work indicates a reduced impact of positive relative to negative distracters (McNally et al. 1990 ; Bryant & Harvey, 1995 ; Vythilingam et al. 2007). In line with this, the current study revealed significantly greater disruption in the task-related recruitment of the superior frontal cortex in the presence of negative relative to positive distracters relative to HCs.
Four caveats should be considered with respect to the current data. First, the patients with PTSD differed from both comparison groups in depression symptom levels. Moreover, four presented with current MDD and a further six had past MDD. High co-morbidity of PTSD and MDD is frequently reported (Holman et al. 2000). Given this, it could be argued that the current results are partially attributed to pathophysiology relating to depression. While this is possible, it should be noted that all follow-up analyses involved ANCOVAs with depression severity as covariate. All the reported results were strongly significant even when the influence of severity of depressive symptoms was removed. As such, although the relationship between PTSD and MDD remains a critical question, we argue that the current results reflect the pathophysiology of PTSD.
Second, the behavioral performance of the three groups did not differ. This contrasts with previous behavioral work with this task (Vythilingam et al. 2007) as well as the related emotional Stroop task (Bar-Haim et al. 2007). The reason for this failure to replicate is unclear and may relate to non-specific features of the fMRI environment. Indeed, both fMRI studies using the emotional Stroop also failed to observe group × condition interactions in RT or error rate (Shin et al. 2001 ; Bremner et al. 2004) despite the relatively robust appearances of such interactions in the behavioral literature (Bar-Haim et al. 2007). However, findings of dysfunction in the recruitment of regions implicated in top-down attentional control in patients with PTSD, despite comparable task performance, suggest that group differences in these regions stem from neural abnormalities rather than performance differences.
Third and related to the above, given the absence of behavioural group differences it could be argued that patients with PTSD actually require less recruitment of top-down attention control systems to sufficiently prime task-relevant representations. However, there are two reasons to be cautious about this possibility. First, by this logic we would have to conclude that TCs required more recruitment of top-down attentional control systems than the other groups for successful task performance. While possible, it appears unlikely that a group potentially resilient to PTSD is associated with weaker top-down attention control. Second, previous work with this task showed greater interference by emotional distracters in patients with PTSD (Vythilingam et al. 2007). In short, while we believe that this suggestion is unlikely, it cannot be ruled out when only considering the current data.
Fourth, normative rather than the subjects’ own ratings were used to categorize the images as negative, positive and neutral. Previous studies have indicated that patients with PTSD show heightened arousal and negative valence ratings for negative, but not positive, IAPS images relative to comparison individuals (Wolf et al. 2009). It is plausible that the disruption in the recruitment of lateral regions of the superior and inferior frontal cortex in PTSD reflects a heightened emotional response to the negative items rather than a specific problem with the recruitment of these areas in the presence of emotional distracters. In other words, a sufficiently strong negative distracter for healthy participants might have a similar disrupting effect.
In conclusion, the current study investigated the ability of patients with PTSD to recruit regions of the frontal and parietal cortex implicated in top-down attentional control in the presence and absence of emotional distracters. Patients with PTSD showed disrupted recruitment of these regions in the presence of negative and, to a lesser extent, positive distracters. The patients with PTSD did not, however, show indications of a general inability to recruit these regions during task performance. Instead, TCs showed indications of enhanced task-related recruitment relative to both the PTSD and HC groups. These data are consistent with suggestions that emotional responsiveness can interfere with the recruitment of regions implicated in top-down attentional control (Pessoa, 2009) ; patients with PTSD showing heightened emotional responding may show consequent heightened interference with the recruitment of these regions. These data also indicate the importance of both trauma and healthy controls in work on PTSD.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health, NIMH.
Footnotes
Declaration of Interest
D.S.C. has consulted for AstraZeneca, Bristol-Myers-Squibb, Cyberonics, Neurogen, Neuroscience Education Institute, Norvartis Pharmaceuticals Corporation, Orexigen and Unilever UK Central Resources Limited. M.S. has been an employee at Wyeth Research.
References
- Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. American Journal of Psychiatry. 2005;162:1961–1963. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals : a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2008;12:92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, Fridberg D, Zametkin A, Sturman D, Nelson EE, Drevets WC, Pine DS, Martin A, Blair RJ. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Kiaumnizer G, Charney DS. A clinician rating scale for assessing current and lifetime PTSD : the CAPS-1. Behavior Therapist. 1990;13:187–188. [Google Scholar]
- Bremner JD, Vermetten E, Vythilingam M, Afzal N, Schmahl C, Elzinga B, Charney DS. Neural correlates of the classic color and emotional Stroop in women with abuse-related posttraumatic stress disorder. Biologial Psychiatry. 2004;55:612–620. doi: 10.1016/j.biopsych.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biological Psychiatry. 2005;57:832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Harvey AG. Processing threatening information in posttraumatic stress disorder. Journal of Abnormal Psychology. 1995;104:537–541. doi: 10.1037//0021-843x.104.3.537. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Blanchard EB, Neill WT. Information processing and PTSD : a review of the empirical literature. Clinical Psychology Review. 2000;20:1041–1065. doi: 10.1016/s0272-7358(99)00030-6. [DOI] [PubMed] [Google Scholar]
- Coles ME, Heimberg RG. Memory biases in the anxiety disorders : current status. Clinical Psychology Review. 2002;22:587–627. doi: 10.1016/s0272-7358(01)00113-1. [DOI] [PubMed] [Google Scholar]
- Daniels JK, McFarlane AC, Bluhm RL, Moores KA, Clark CR, Shaw ME, Williamson PC, Densmore M, Lanius RA. Switching between executive and default mode networks in posttraumatic stress disorder : alterations in functional connectivity. Journal of Psychiatry and Neuroscience. 2010;35:258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- El Khoury-Malhame M, Reynaud E, Soriano A, Michael K, Salgado-Pineda P, Zendjidjian X, Gellato C, Eric F, Lefebvre MN, Rouby F, Samuelian JC, Anton JL, Blin O, Khalfa S. Amygdala activity correlates with attentional bias in PTSD. Neuropsychologia. 2011;49:1969–1973. doi: 10.1016/j.neuropsychologia.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R. Neural responses to masked fear faces : sex differences and trauma exposure in posttraumatic stress disorder. Journal of Abnormal Psychology. 2010;119:241–247. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Foa EB, Feske U, Murdock TB, Kozak MJ, McCarthy PR. Processing of threat-related information in rape victims. Journal of Abnormal Psychology. 1991;100:156–162. doi: 10.1037//0021-843x.100.2.156. [DOI] [PubMed] [Google Scholar]
- Holman EA, Silver RC, Waitzkin H. Traumatic life events in primary care patients : a study in an ethnically diverse sample. Archives of Family Medicine. 2000;9:802–810. doi: 10.1001/archfami.9.9.802. [DOI] [PubMed] [Google Scholar]
- Jatzko A, Schmitt A, Demirakca T, Weimer E, Braus DF. Disturbance in the neural circuitry underlying positive emotional processing in post-traumatic stress disorder (PTSD). An fMRI study. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:112–114. doi: 10.1007/s00406-005-0617-3. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kim M, Chey J, Chung A, Bae S, Khang H, Ham B, Yoon S, Jeong D, Lyoo K. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. Journal of Psychiatry Research. 2008;42:268–277. doi: 10.1016/j.jpsychires.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK. The International Affective Picture System Standardization Procedure and Initial Group Results for Affective Judgements : Technical Reports 1A & 1B. Center for Research in Psychophysiology, University of Florida; Gainesville: 1988. [Google Scholar]
- McNally RJ, Kaspi SP, Riemann BC, Zeitlin SB. Selective processing of threat cues in posttraumatic stress disorder. Journal of Abnormal Psychology. 1990;99:398–402. doi: 10.1037//0021-843x.99.4.398. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJ. The impact of processing load on emotion. Neuroimage. 2007;34:1299–1309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moores KA, Clark CR, McFarlane AC, Brown GC, Puce A, Taylor DJ. Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Research. 2008;163:156–170. doi: 10.1016/j.pscychresns.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Cooper DA, Labar KS, McCarthy G. Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Research : Neuroimaging. 2008;162:59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Newmark RE, Zhang J, Triebwasser J, Meyerson D, Lazarus S, Trisdorfer R, Goldstein KE, Goodman M, Koenigsberg HW, Flory JD, Siever LJ, Buchsbaum MS. Laboratory induced aggression : a positron emission tomography study of aggressive individuals with borderline personality disorder. Biological Psychiatry. 2009;66:1107–1114. doi: 10.1016/j.biopsych.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings : an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Pannu Hayes J, Labar KS, Petty CM, McCarthy G, Morey RA. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Research. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control ? Trends in Cognitive Science. 2009;13:160–166. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences USA. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Padmala S, Morland T. Fate of unattended fearful faces in the amygdala is determined by both attentional resources and cognitive modulation. Neuroimage. 2005;28:249–255. doi: 10.1016/j.neuroimage.2005.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction : human neuroimaging research – past, present, and future. Biological Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder : a functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. The Inventory for Depressive Symptomatology (IDS) : preliminary findings. Psychiatry Research. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- Sailer U, Robinson S, Fischmeister FP, Konig D, Oppenauer C, Lueger-Schuster B, Moser E, Kryspin-Exner I, Bauer H. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia. 2008;46:2836–2844. doi: 10.1016/j.neuropsychologia.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Shear MK, Vander Bilt J, Rucci P, Endicott J, Lydiard B, Otto NW, Pollack MH, Chandler L, Williams J, Ali A, Frank DM. Reliability and validity of a structured interview guide for the Hamilton Anxiety Rating Scale (SIGH-A) Depression and Anxiety. 2001;13:166–178. [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; New York, NY: 1988. [Google Scholar]
- Vythilingam M, Blair KS, McCaffrey D, Scaramozza M, Jones M, Nakic M, Mondillo K, Hadd K, Bonne O, Mitchell DG, Pine DS, Charney DS, Blair RJ. Biased emotional attention in post-traumatic stress disorder : a help as well as a hindrance ? Psychological Medicine. 2007;37:1445–1455. doi: 10.1017/S003329170700092X. [DOI] [PubMed] [Google Scholar]
- Williams JM, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Miller MW, McKinney AE. Emotional processing in PTSD : heightened negative emotionality to unpleasant photographic stimuli. Journal of Nervous and Mental Disease. 2009;197:419–426. doi: 10.1097/NMD.0b013e3181a61c68. [DOI] [PMC free article] [PubMed] [Google Scholar]