Abstract
Neurodevelopmental disabilities, including autism, attention-deficit hyperactivity disorder, dyslexia, and other cognitive impairments, affect millions of children worldwide, and some diagnoses seem to be increasing in frequency. Industrial chemicals that injure the developing brain are among the known causes for this rise in prevalence. In 2006, we did a systematic review and identified five industrial chemicals as developmental neurotoxicants: lead, methylmercury, polychlorinated biphenyls, arsenic, and toluene. Since 2006, epidemiological studies have documented six additional developmental neurotoxicants—manganese, fluoride, chlorpyrifos, dichlorodiphenyltrichloroethane, tetrachloroethylene, and the polybrominated diphenyl ethers. We postulate that even more neurotoxicants remain undiscovered. To control the pandemic of developmental neurotoxicity, we propose a global prevention strategy. Untested chemicals should not be presumed to be safe to brain development, and chemicals in existing use and all new chemicals must therefore be tested for developmental neurotoxicity. To coordinate these efforts and to accelerate translation of science into prevention, we propose the urgent formation of a new international clearinghouse.
Introduction
Disorders of neurobehavioural development affect 10–15% of all births,1 and prevalence rates of autism spectrum disorder and attention-deficit hyperactivity disorder seem to be increasing worldwide.2 Subclinical decrements in brain function are even more common than these neurobehavioural developmental disorders. All these disabilities can have severe consequences3—they diminish quality of life, reduce academic achievement, and disturb behaviour, with profound consequences for the welfare and productivity of entire societies.4
The root causes of the present global pandemic of neurodevelopmental disorders are only partly understood. Although genetic factors have a role,5 they cannot explain recent increases in reported prevalence, and none of the genes discovered so far seem to be responsible for more than a small proportion of cases.5 Overall, genetic factors seem to account for no more than perhaps 30–40% of all cases of neurodevelopmental disorders. Thus, non-genetic, environmental exposures are involved in causation, in some cases probably by interacting with genetically inherited predispositions.
Strong evidence exists that industrial chemicals widely disseminated in the environment are important contributors to what we have called the global, silent pandemic of neurodevelopmental toxicity.6,7 The developing human brain is uniquely vulnerable to toxic chemical exposures, and major windows of developmental vulnerability occur in utero and during infancy and early childhood.8 During these sensitive life stages, chemicals can cause permanent brain injury at low levels of exposure that would have little or no adverse effect in an adult.
In 2006, we did a systematic review of the published clinical and epidemiological studies into the neurotoxicity of industrial chemicals, with a focus on developmental neurotoxicity.6 We identified five industrial chemicals that could be reliably classified as developmental neurotoxicants: lead, methylmercury, arsenic, polychlorinated biphenyls, and toluene. We also noted 201 chemicals that had been reported to cause injury to the nervous system in adults, mostly in connection with occupational exposures, poisoning incidents, or suicide attempts. Additionally, more than 1000 chemicals have been reported to be neurotoxic in animals in laboratory studies.
We noted that recognition of the risks of industrial chemicals to brain development has historically needed decades of research and scrutiny, as shown in the cases of lead and methylmercury.9,10 In most cases, discovery began with clinical diagnosis of poisoning in workers and episodes of high-dose exposure. More sophisticated epidemiological studies typically began only much later. Results from such studies documented developmental neurotoxicity at much lower exposure levels than had previously been thought to be safe. Thus, recognition of widespread subclinical toxicity often did not occur until decades after the initial evidence of neurotoxicity. A recurring theme was that early warnings of subclinical neurotoxicity were often ignored or even dismissed.11 David P Rall, former Director of the US National Institute of Environmental Health Sciences, once noted that “if thalidomide had caused a ten-point loss of intelligence quotient (IQ) instead of obvious birth defects of the limbs, it would probably still be on the market”.12 Many industrial chemicals marketed at present probably cause IQ deficits of far fewer than ten points and have therefore eluded detection so far, but their combined effects could have enormous consequences.
In our 2006 review,6 we expressed concern that additional developmental neurotoxicants might lurk undiscovered among the 201 chemicals then known to be neurotoxic to adult human beings and among the many thousands of pesticides, solvents, and other industrial chemicals in widespread use that had never been tested for neurodevelopmental toxicity. Since our previous review, new data have emerged about the vulnerability of the developing brain and the neurotoxicity of industrial chemicals. Particularly important new evidence derives from prospective epidemiological birth cohort studies.
In this Review, we consider recent information about the developmental neurotoxicity of industrial chemicals to update our previous report.6 Additionally, we propose strategies to counter this pandemic and to prevent the spread of neurological disease and disability in children worldwide.
Unique vulnerability of the developing brain
The fetus is not well protected against industrial chemicals. The placenta does not block the passage of many environmental toxicants from the maternal to the fetal circulation,13 and more than 200 foreign chemicals have been detected in umbilical cord blood.14 Additionally, many environmental chemicals are transferred to the infant through human breastmilk.13 During fetal life and early infancy, the blood–brain barrier provides only partial protection against the entry of chemicals into the CNS.15
Moreover, the developing human brain is exceptionally sensitive to injury caused by toxic chemicals,6 and several developmental processes have been shown to be highly vulnerable to chemical toxicity. For example, in-vitrostudies suggest that neural stem cells are very sensitive to neurotoxic substances such as methylmercury.16 Some pesticides inhibit cholinesterase function in the developing brain,17 thereby affecting the crucial regulatory role of acetylcholine before synapse formation.18 Early-life epigenetic changes are also known to affect subsequent gene expression in the brain.19 In summary, industrial chemicals known or suspected to be neurotoxic to adults are also likely to present risks to the developing brain.
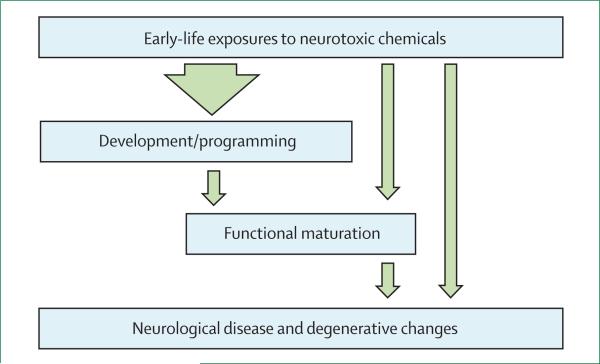
Figure 1 shows the unique vulnerability of the brain during early life and indicates how developmental exposures to toxic chemicals are particularly likely to lead to functional deficits and disease later in life.
Figure 1. Effect of neurotoxicants during early brain development.
Exposures in early life to neurotoxic chemicals can cause a wide range of adverse effects on brain development and maturation that can manifest as functional impairments or disease at any point in the human lifespan, from early infancy to very old age.
New findings about known hazards
Recent research on well-documented neurotoxicants has generated important new insights into the neuro-developmental consequences of early exposures to these industrial chemicals.
Joint analyses that gathered data for lead-associated IQ deficits from seven international studies20,21 support the conclusion that no safe level of exposure to lead exists.22 Cognitive deficits in adults who had previously shown lead-associated developmental delays at school age suggest that the effects of lead neurotoxicity are probably permanent.23 Brain imaging of young adults who had raised lead concentrations in their blood during childhood showed exposure-related decreases in brain volume.24 Lead exposure in early childhood is associated with reduced school performance25 and with delinquent behaviour later in life.26,27
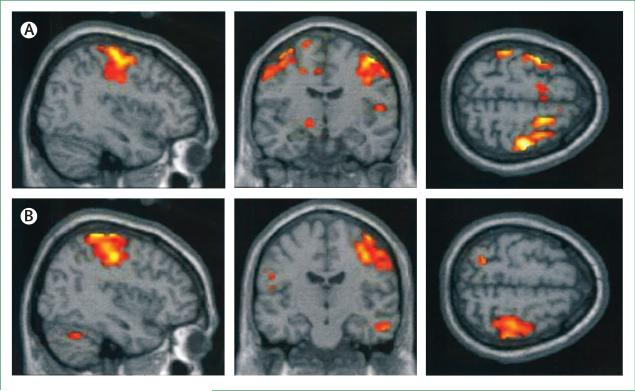
Developmental neurotoxicity due to methylmercury occurs at much lower exposures than the concentrations that affect adult brain function.28 Deficits at 7 years of age that were linked to low-level prenatal exposures to methylmercury were still detectable at the age of 14 years.29 Some common genetic polymorphisms seem to increase the vulnerability of the developing brain to methylmercury toxicity.30 Functional MRI scans of people exposed prenatally to excess amounts of methylmercury showed abnormally expanded activation of brain regions in response to sensory stimulation and motor tasks (figure 2).31 Because some adverse effects might be counterbalanced by essential fatty acids from seafood, statistical adjustment for maternal diet during pregnancy results in stronger methylmercury effects.32,33
Figure 2. Functional MRI scans show abnormal activation in the brain.
Average activation during finger tapping with the left hand in three adolescents with increased prenatal methylmercury exposure (A) and three control adolescents (B). The control participants activate the premotor and motor cortices on the right, whereas participants exposed to methylmercury activate these areas bilaterally.31
Prenatal and early postnatal exposures to inorganic arsenic from drinking water are associated with cognitive deficits that are apparent at school age.34,35 Infants who survived the Morinaga milk arsenic poisoning incident had highly raised risks of neurological disease during adult life.36
The developmental neurotoxicity of polychlorinated biphenyls has been consolidated and strengthened by recent findings.37 Although little new information has been published about the developmental neurotoxicity of toluene, much has been learned about the developmental neurotoxicity of another common solvent, ethanol, through research on fetal alcohol exposure. Maternal consumption of alcohol during pregnancy, even in very small quantities, has been linked to a range of neurobehavioural adverse effects in offspring, including reduced IQ, impaired executive function and social judgment, delinquent behaviour, seizures, other neurological signs, and sensory problems.38
Newly recognised developmental neurotoxicants
Prospective epidemiological birth cohort studies make it possible to measure maternal or fetal exposures in real time during pregnancy as these exposures actually occur, thus generating unbiased information about the degree and timing of prenatal exposures. Children in these prospective studies are followed longitudinally and assessed with age-appropriate tests to show delayed or deranged neurobehavioural development. These powerful epidemiological methods have enabled the discovery of additional developmental neurotoxicants.
Cross-sectional data from Bangladesh show that exposure to manganese from drinking water is associated with reduced mathematics achievement scores in school children.39 A study in Quebec, Canada, showed a strong correlation between manganese concentrations in hair and hyperactivity.40 School-aged children living near manganese mining and processing facilities have shown associations between airborne manganese concentrations and diminished intellectual function41 and with impaired motor skills and reduced olfactory function.42 These results are supported by experimental findings in mice.43
A meta-analysis of 27 cross-sectional studies of children exposed to fluoride in drinking water, mainly from China, suggests an average IQ decrement of about seven points in children exposed to raised fluoride concentrations.44 Confounding from other substances seemed unlikely in most of these studies. Further characterisation of the dose–response association would be desirable.
The occupational health literature45 suggests that solvents can act as neurotoxicants, but the identification of individual responsible compounds is hampered by the complexity of exposures. In a French cohort study of 3000 children, investigators linked maternal occupational solvent exposure during pregnancy to deficits in behavioural assessment at 2 years of age.46 The data showed dose-related increased risks for hyperactivity and aggressive behaviour. One in every five mothers in this cohort reported solvent exposures in common jobs, such as nurse or other hospital employee, chemist, cleaner, hairdresser, and beautician. In Massachusetts, USA, follow-up of a well-defined population with prenatal and early childhood exposure to the solvent tetrachloroethylene (also called perchlor ethylene) in drinking water showed a tendency towards deficient neurological function and increased risk of psychiatric diagnoses.47
Acute pesticide poisoning occurs frequently in children worldwide, and subclinical pesticide toxicity is also widespread. Clinical data suggest that acute pesticide poisoning during childhood might lead to lasting neurobehavioural deficits.48,49 Highly toxic and bioaccumulative pesticides are now banned in high-income nations, but are still used in many low-income and middle-income countries. In particular, the organochlorine compounds dichlorodiphenyltrichloroethane (DDT), its metabolite dichlorodiphenyldichloroethylene (DDE), and chlordecone (Kepone), tend to be highly persistent and remain widespread in the environment and in people's bodies in high-use regions. Recent studies have shown inverse correlations between serum concentrations of DDT or DDE (which indicate accumulated exposures), and neurodevelopmental performance.50,51
Organophosphate pesticides are eliminated from the human body much more rapidly than are organochlorines, and exposure assessment is therefore inherently less precise. Nonetheless, three prospective epidemiological birth cohort studies provide new evidence that prenatal exposure to organophosphate pesticides can cause developmental neurotoxicity. In these studies, prenatal organophosphate exposure was assessed by measurement of maternal urinary excretion of pesticide metabolites during pregnancy. Dose-related correlations were recorded between maternal exposures to chlorpyrifos or other organophosphates and small head circumference at birth—which is an indication of slowed brain growth in utero—and with neurobehavioural deficits that have persisted to at least 7 years of age.52–54 In a subgroup study, MRI of the brain showed that prenatal chlorpyrifos exposure was associated with structural abnormalities that included thinning of the cerebral cortex.55
Herbicides and fungicides might also have neurotoxic potential.56 Propoxur,57 a carbamate pesticide, and permethrine,58 a member of the pyrethroid class of pesticides, have recently been linked to neuro developmental deficits in children.
The group of compounds known as polybrominated diphenyl ethers (PBDEs) are widely used as flame retardants and are structurally very similar to the polychlorinated biphenyls. Experimental evidence now suggests that the PBDEs might also be neurotoxic.59 Epidemiological studies in Europe and the USA have shown neurodevelopmental deficits in children with increased prenatal exposures to these compounds.60–62 Thus, the PBDEs should be regarded as hazards to human neurobehavioural development, although attribution of relative toxic potentials to individual PBDE congeners is not yet possible.
Other suspected developmental neurotoxicants
A serious difficulty that complicates many epidemiological studies of neurodevelopmental toxicity in children is the problem of mixed exposures. Most populations are exposed to more than one neurotoxicant at a time, and yet most studies have only a finite amount of power and precision in exposure assessment to discern the possible effects of even single neurotoxicants. A further problem in many epi demiological studies of non-persistent toxicants is that imprecise assessment of exposure tends to obscure associations that might actually be present.63 Guidance from experimental neurotoxicity studies is therefore crucial. In the assessment of potential developmental neurotoxicants, we have used a strength of evidence approach similar to that used by the International Agency for Research on Cancer for assessing epidemiological and experimental studies.
Phthalates and bisphenol A are added to many different types of plastics, cosmetics, and other consumer products. Since they are eliminated rapidly in urine, exposure assessment is complicated, and such imprecision might lead to underestimation of the true risk of neurotoxicity. The best-documented effects of early-life exposure to phthalates are the consequence of disruption of endocrine signalling.64 Thus, prenatal exposures to phthalates have been linked to both neurodevelopmental deficits and to behavioural abnormalities characterised by shortened attention span and impaired social interactions.65 The neurobehavioural toxicity of these compounds seems to affect mainly boys and could therefore relate to endocrine disruption in the developing brain.66 In regard to bisphenol A, a prospective study showed that point estimates of exposure during gestation were linked to abnormalities in behaviour and executive function in children at 3 years of age.67
Exposure to air pollution can cause neuro developmental delays and disorders of behavioural functions.68,69 Of the individual components of air pollution, carbon monoxide is a well-documented neurotoxicant, and indoor exposure to this substance has now been linked to deficient neurobehavioural performance in children.70 Less clear is the reported contribution of nitrogen oxides to neurodevelopmental deficits,71 since these compounds often co-occur with carbon monoxide as part of complex emissions. Tobacco smoke is a complex mixture of hundreds of chemical compounds and is now a well-documented cause of developmental neurotoxicity.72 Infants exposed pre natally to polycyclic aromatic hydrocarbons from traffic exhausts at 5 years of age showed greater cognitive impairment and lower IQ than those exposed to lower levels of these compounds.68
Perfluorinated compounds, such as perfluorooctanoic acid and perfluorooctane sulphonate, are highly persistent in the environment and in the human body, and seem to be neurotoxic.73 Emerging epidemiological evidence suggests that these compounds might indeed impede neurobehavioural development.74
Developmental neurotoxicity and clinical neurology
Exposures in early life to developmental neurotoxicants are now being linked to specific clinical syndromes in children. For example, an increased risk of attention-deficit hyperactivity disorder has been linked to prenatal exposures to manganese, organophosphates,75 and phthalates.76 Phthalates have also been linked to behaviours that resemble components of autism spectrum disorder.77 Prenatal exposure to automotive air pollution in California, USA, has been linked to an increased risk for autism spectrum disorder.78
The persistent decrements in intelligence documented in children, adolescents, and young adults exposed in early life to neurotoxicants could presage the development of neurodegenerative disease later in life. Thus, accumulated exposure to lead is associated with cognitive decline in the elderly.79 Manganese exposure may lead to parkinsonism, and experimental studies have reported Parkinson's disease as a result of developmental exposures to the insecticide rotenone, the herbicides paraquat and maneb, and the solvent trichloroethylene.80 Any environmental exposure that increases the risk of neurodegenerative disorders in later life (figure 1) requires urgent investigation as the world's population continues to age.81
The expanding complement of neurotoxicants
In our 2006 review,6 we expressed concern that additional developmental neurotoxicants might lie undiscovered in the 201 chemicals that were then known to be neurotoxic to human adults, in the roughly 1000 chemicals known to be neurotoxic in animal species, and in the many thousands of industrial chemicals and pesticides that have never been tested for neurotoxicity. Exposure to neurotoxic chemicals is not rare, since almost half of the 201 known human neurotoxicants are regarded as high production volume chemicals.
Our updated literature review shows that since 2006 the list of recognised human neurotoxicants has expanded by 12 chemicals, from 202 (including ethanol) to 214 (table 1 and appendix)—that is, by about two substances per year. Many of these chemicals are widely used and disseminated extensively in the global environment. Of the newly identified neuro developmental toxicants, pesticides constitute the largest group, as was already the case in 2006. In the same 7-year period, the number of known developmental neurotoxicants has doubled from six to 12 (table 2). Although the pace of scientific discovery of new neurodevelopmental hazards is more rapid today than in the past, it is still slower than the identification of adult neurotoxicants.
Table 1.
Industrial chemicals known to be toxic to the human nervous system in 2006 and 2013, according to chemical group
| Number known in 2006 | Number known in 2013 | Identified since 2006 | |
|---|---|---|---|
| Metals and inorganic compounds | 25 | 26 | Hydrogen phosphide82 |
| Organic solvents | 39* | 40 | Ethyl chloride83 |
| Pesticides | 92 | 101 | Acetamiprid,84 amitraz,85 avermectin,86 emamectin,87 fipronil (Termidor),88 glyphosate,89 hexaconazole,90 imidacloprid,91 tetramethylenedisulfotetramine92 |
| Other organic compounds | 46 | 47 | 1,3-butadiene93 |
| Total | 202* | 214 | 12 new substances |
Including ethanol.
Table 2.
Industrial chemicals known to cause developmental neurotoxicity in human beings in 2006 and 2013, according to chemical group
| Known in 2006 | Newly identified | |
|---|---|---|
| Metals and inorganic compounds | Arsenic and arsenic compounds, lead, and methylmercury | Fluoride and manganese |
| Organic solvents | (Ethanol) toluene | Tetrachloroethylene |
| Pesticides | None | Chlorpyrifos and DDT/DDE |
| Other organic compounds | Polychlorinated biphenyls | Brominated diphenyl ethers |
| Total | 6* | 6 |
DDT=dichlorodiphenyltrichloroethane. DDE=dichlorodiphenyldichloroethylene.
including ethanol.
The gap that exists between the number of substances known to be toxic to the adult brain and the smaller number known to be toxic to the much more vulnerable developing brain is unlikely to close in the near future. This discrepancy is attributable to the fact that toxicity to the adult brain is usually discovered as a result of acute poisoning incidents, typically with a clear and immediate association between causative exposure and adverse effects, as occurs for workplace exposures or suicide attempts. By contrast, the recognition of developmental neurotoxicity relies on two sets of evidence collected at two different points in time: exposure data (often obtained from the mother during pregnancy), and data for the child's postnatal neurobehavioural development (often obtained 5–10 years later). Because brain functions develop sequentially, the full effects of early neurotoxic damage might not become apparent until school age or beyond. The most reliable evidence of developmental neurotoxicity is obtained through prospective studies that include real-time recording of information about exposure in early life followed by serial clinical assessments of the child. Such research is inherently slow and is hampered by the difficulty of reliable assessment of exposures to individual toxicants in complex mixtures.
Consequences of developmental neurotoxicity
Developmental neurotoxicity causes brain damage that is too often untreatable and frequently permanent. The consequence of such brain damage is impaired CNS function that lasts a lifetime and might result in reduced intelligence, as expressed in terms of lost IQ points, or disruption in behaviour. A recent study compared the estimated total IQ losses from major paediatric causes and showed that the magnitude of losses attributable to lead, pesticides, and other neurotoxicants was in the same range as, or even greater than, the losses associated with medical events such as preterm birth, traumatic brain injury, brain tumours, and congenital heart disease (table 3).94
Table 3.
Total losses of IQ points in US children 0–5 years of age associated with major risk factors, including developmental exposure to industrial chemicals that cause neurotoxicity
| Number of IQ points lost | |
|---|---|
| Major medical and neurodevelopmental disorders | |
| Preterm birth | 34 031 025 |
| Autism spectrum disorders | 7 109 899 |
| Paediatric bipolar disorder | 8 164 080 |
| Attention-deficit hyperactivity disorder | 16 799 400 |
| Postnatal traumatic brain injury | 5 827 300 |
| Environmental chemical exposures | |
| Lead | 22 947 450 |
| Methylmercury | 1 590 000* |
| Organophosphate pesticides | 16 899 488 |
| Other neurotoxicants | Unknown |
Loss of cognitive skills reduces children's academic and economic attainments and has substantial long-term economic effects on societies.4 Thus, each loss of one IQ point has been estimated to decrease average lifetime earnings capacity by about €12 000 or US$18 000 in 2008 currencies.96 The most recent estimates from the USA indicate that the annual costs of childhood lead poisoning are about US$50 billion and that the annual costs of methylmercury toxicity are roughly US$5 billion.97 In the European Union, methylmercury exposure is estimated to cause a loss of about 600 000 IQ points every year, corresponding to an annual economic loss of close to €10 billion. In France alone, lead exposure is associated with IQ losses that correspond to annual costs that might exceed €20 billion.98 Since IQ losses represent only one aspect of developmental neurotoxicity, the total costs are surely even higher.
Evidence from worldwide sources indicates that average national IQ scores are associated with gross domestic product (GDP)—a correlation that might be causal in both directions.99 Thus, poverty can cause low IQ, but the opposite is also true. In view of the widespread exposures to lead, pesticides, and other neurotoxicants in developing countries, where chemical controls might be ineffective compared with those in more developed countries,100,101 developmental exposures to industrial chemicals could contribute substantially to the recorded correlation between IQ and GDP. If this theory is true, developing countries could take decades to emerge from poverty. Consequently, pollution abatement might then be delayed, and a vicious circle can result.
The antisocial behaviour, criminal behaviour, violence, and substance abuse that seem to result from early-life exposures to some neurotoxic chemicals result in increased needs for special educational services, institutionalisation, and even incarceration. In the USA, the murder rate fell sharply 20 years after the removal of lead from petrol,102 a finding consistent with the idea that exposure to lead in early life is a powerful determinant of behaviour decades later. Although poorly quantified, such behavioural and social consequences of neuro-developmental toxicity are potentially very costly.76
Prevention of developmental neurotoxicity caused by industrial chemicals is highly cost effective. A study that quantified the gains resulting from the phase-out of lead additives from petrol reported that in the USA alone, the introduction of lead-free petrol has generated an economic benefit of $200 billion in each annual birth cohort since 1980,103 an aggregate benefit in the past 30 years of over $3 trillion. This success has since been repeated in more than 150 countries, resulting in vast additional savings. Every US$1 spent to reduce lead hazards is estimated to produce a benefit of US$17–220, which represents a cost-benefit ratio that is even better than that for vaccines.4 Furthermore, the costs associated with the late-life consequences of developmental neurotoxicity are enormous, and the benefits from prevention of degenerative brain disorders could be very substantial.
New methods to identify developmental neurotoxicants
New toxicological methods now allow a rational strategy for the identification of developmental neurotoxicants based on a multidisciplinary approach.104 A new guideline has been approved as a standardised approach for the identification of developmental neurotoxicants.105 However, completion of such tests is expensive and requires the use of many laboratory animals, and reliance on mammals for chemicals testing purposes needs to be reduced.106 US governmental agencies have established the National Center for Computational Toxicology and an initiative— the Tox 21 Program—to promote the evolution of toxicology from a mainly observational science to a predominantly predictive science.107
In-vitro methods have now reached a level of predictive validity that means they can be applied to neurotoxicity testing.108 Some of these tests are based on neural stem cells. Although these cell systems do not have a blood– brain barrier and particular metabolising enzymes, these approaches are highly promising. As a further option, data for protein links and protein–protein interactions can now be used to explore potential neurotoxicity in silico,109 thus showing that existing computational methods might predict potential toxic effects.110
In summary, use of the whole range of approaches along with clinical and epidemiological evidence, when available, should enable the integration of information for use in at least a tentative risk assessment. With these methods, we anticipate that the pace of scientific discovery in developmental neurotoxicology will accelerate further in the years ahead.
Conclusions and recommendations
The updated findings presented in this Review confirm and extend our 2006 conclusions.6 During the 7 years since our previous report, the number of industrial chemicals recognised to be developmental neurotoxicants has doubled. Exposures to these industrial chemicals in the environment contribute to the pandemic of developmental neurotoxicity.
Two major obstacles impede efforts to control the global pandemic of developmental neurotoxicity. These barriers, which we noted in our previous review6 and were recently underlined by the US National Research Council,111 are: large gaps in the testing of chemicals for developmental neurotoxicity, which results in a paucity of systematic data to guide prevention; and the huge amount of proof needed for regulation. Thus, very few chemicals have been regulated as a result of developmental neurotoxicity.
The presumption that new chemicals and technologies are safe until proven otherwise is a fundamental problem.111 Classic examples of new chemicals that were introduced because they conveyed certain benefits, but were later shown to cause great harm, include several neurotoxicants, asbestos, thalidomide, diethylstilboestrol, and the chlorofluorocarbons.112 A recurring theme in each of these cases was that commercial introduction and wide dissemination of the chemicals preceded any systematic effort to assess potential toxicity. Particularly absent were advance efforts to study possible effects on children's health or the potential of exposures in early life to disrupt early development. Similar challenges have been confronted in other public health disasters, such as those caused by tobacco smoking, alcohol use, and refined foods. These problems have been recently termed industrial epidemics.113
To control the pandemic of developmental neurotoxicity, we propose a coordinated international strategy (panel). Mandatory and transparent assessment of evidence for neurotoxicity is the foundation of this strategy. Assessment of toxicity must be followed by governmental regulation and market intervention. Voluntary controls seem to be of little value.11
The three pillars of our proposed strategy are: legally mandated testing of existing industrial chemicals and pesticides already in commerce, with prioritisation of those with the most widespread use, and incorporation of new assessment technologies; legally mandated premarket evaluation of new chemicals before they enter markets, with use of precautionary approaches for chemical testing that recognise the unique vulnerability of the developing brain; and the formation of a new clearinghouse for neurotoxicity as a parallel to the International Agency for Research on Cancer. This new agency will assess industrial chemicals for developmental neurotoxicity with a precautionary approach that emphasises prevention and does not require absolute proof of toxicity. It will facilitate and coordinate epidemiological and toxicological studies and will lead the urgently needed global programmes for prevention.
These new approaches must reverse the dangerous presumption that new chemicals and technologies are safe until proven otherwise. They must also overcome the existing requirement to produce absolute proof of toxicity before action can be started to protect children against neurotoxic substances. Precautionary interpretation of data about developmental neurotoxicity should take into account the very large individual and societal costs that result from failure to act on available documentation to prevent disease in children.114 Academic research has often favoured scepticism and required extensive replication before acceptance of a hypothesis,114 thereby adding to the inertia in toxicology and environmental health research and the consequent disregard of many other potential neurotoxicants.115 Additionally, the strength of evidence that is needed to constitute “proof” should be analysed in a societal perspective, so that the implications of ignoring a developmental neurotoxicant and of failing to act on the basis of available data are also taken into account.
Finally, we emphasise that the total number of neurotoxic substances now recognised almost certainly represents an underestimate of the true number of developmental neurotoxicants that have been released into the global environment. Our very great concern is that children worldwide are being exposed to unrecognised toxic chemicals that are silently eroding intelligence, disrupting behaviours, truncating future achievements, and damaging societies, perhaps most seriously in developing countries. A new framework of action is needed.
Supplementary Material
Panel: Recommendations for an international clearinghouse on neurotoxicity.
The main purpose of this agency would be to promote optimum brain health, not just avoidance of neurological disease, by inspiring, facilitating, and coordinating research and public policies that aim to protect brain development during the most sensitive life stages. The main efforts would aim to:
Screen industrial chemicals present in human exposures for neurotoxic effects so that hazardous substances can be identified for tighter control
Stimulate and coordinate new research to understand how toxic chemicals interfere with brain development and how best to prevent long-term dysfunctions and deficits
Function as a clearinghouse for research data and strategies by gathering and assessing documentation about brain toxicity and stimulating international collaboration on research and prevention
Promote policy development aimed at protecting vulnerable populations against chemicals that are toxic to the brain without needing unrealistic amounts of scientific proof
Search strategy and selection criteria.
We identified studies published since 2006 on the neurotoxic effects of industrial chemicals in human beings by using the search terms “neurotoxicity syndromes”[MeSH], “neurotoxic”, “neurologic”, or “neuro*”, combined with “exposure” and “poisoning” in PubMed, from 2006 to the end of 2012. For developmental neurotoxicity, the search terms were “prenatal exposure delayed effects”[MeSH], “maternal exposure” or “maternal fetal exchange”, “developmental disabilities/ chemically induced” and “neurotoxins”, all of which were searched for with the limiters “All Child: 0–18 years, Human”. We also used references cited in the publications retrieved.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute for Environmental Health Sciences (ES09584, ES09797, and ES11687). The funding source had no role in the literature review, interpretation of data, writing of this Review, or in the decision to submit for publication. The contents of this paper are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health. We thank Mary S Wolff(Icahn School of Medicine at Mount Sinai, New York, NY, USA) and Linda S Birnbaum (US National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA) for their critical reading of the report.
Footnotes
Contributors
Both authors did the literature review, wrote and revised the report, and approved the final version.
Conflicts of interest
PG has provided paid expert testimony about mercury toxicology for the US Department of Justice. PJL has provided paid expert testimony in cases of childhood lead poisoning. We declare that we have no other conflicts of interest.
References
- 1.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2009. Vital Health Stat 2010. 10:1–82. [PubMed] [Google Scholar]
- 2.Landrigan PJ, Lambertini L, Birnbaum LS. A research strategy to discover the environmental causes of autism and neurodevelopmental disabilities. Environ Health Perspect. 2012;120:a258–60. doi: 10.1289/ehp.1104285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellinger DC. Interpreting epidemiologic studies of developmental neurotoxicity: conceptual and analytic issues. Neurotoxicol Teratol. 2009;31:267–74. doi: 10.1016/j.ntt.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Gould E. Childhood lead poisoning: conservative estimates of the social and economic benefits of lead hazard control. Environ Health Perspect. 2009;117:1162–67. doi: 10.1289/ehp.0800408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Research Council . Scientific frontiers in developmental toxicology and risk assessment. National Academies Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- 6.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–78. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 7.Grandjean P. How environmental pollution impairs brain development – and how to protect the brains of the next generation. Oxford University Press; New York: 2013. Only one chance. [Google Scholar]
- 8.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Needleman HL. The removal of lead from gasoline: historical and personal reflections. Environ Res. 2000;84:20–35. doi: 10.1006/enrs.2000.4069. [DOI] [PubMed] [Google Scholar]
- 10.Grandjean P, Satoh H, Murata K, Eto K. Adverse effects of methylmercury: environmental health research implications. Environ Health Perspect. 2010;118:1137–45. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landrigan PJ, Goldman LR. Children's vulnerability to toxic chemicals: a challenge and opportunity to strengthen health and environmental policy. Health Aff. 2011;30:842–50. doi: 10.1377/hlthaff.2011.0151. [DOI] [PubMed] [Google Scholar]
- 12.Weiss B. Food additives and environmental chemicals as sources of childhood behavior disorders. J Am Acad Child Psychiatry. 1982;21:144–52. doi: 10.1016/s0002-7138(09)60913-4. [DOI] [PubMed] [Google Scholar]
- 13.Needham LL, Grandjean P, Heinzow B, et al. Partition of environmental chemicals between maternal and fetal blood and tissues. Environ Sci Technol. 2011;45:1121–26. doi: 10.1021/es1019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Environmental Working Group . Body burden—the pollution in newborns. Environmental Working Group; Washington, DC: 2005. [Google Scholar]
- 15.Zheng W, Aschner M, Ghersi-Egea JF. Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol. 2003;192:1–11. doi: 10.1016/s0041-008x(03)00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bose R, Onishchenko N, Edoff K, Janson Lang AM, Ceccatelli S. Inherited effects of low-dose exposure to methylmercury in neural stem cells. Toxicol Sci. 2012;130:383–90. doi: 10.1093/toxsci/kfs257. [DOI] [PubMed] [Google Scholar]
- 17.Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Augusti-Tocco G, Biagioni S, Tata AM. Acetylcholine and regulation of gene expression in developing systems. J Mol Neurosci. 2006;30:45–48. doi: 10.1385/JMN:30:1:45. [DOI] [PubMed] [Google Scholar]
- 19.Roth TL. Epigenetics of neurobiology and behavior during development and adulthood. Dev Psychobiol. 2012;54:590–97. doi: 10.1002/dev.20550. [DOI] [PubMed] [Google Scholar]
- 20.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children's intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–99. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Budtz-Jorgensen E, Bellinger D, Lanphear B, Grandjean P. An international pooled analysis for obtaining a benchmark dose for environmental lead exposure in children. Risk Anal. 2013;33:450–61. doi: 10.1111/j.1539-6924.2012.01882.x. [DOI] [PubMed] [Google Scholar]
- 22.Grandjean P. Even low-dose lead exposure is hazardous. Lancet. 2010;376:855–56. doi: 10.1016/S0140-6736(10)60745-3. [DOI] [PubMed] [Google Scholar]
- 23.Mazumdar M, Bellinger DC, Gregas M, Abanilla K, Bacic J, Needleman HL. Low-level environmental lead exposure in childhood and adult intellectual function: a follow-up study. Environ Health. 2011;10:24. doi: 10.1186/1476-069X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cecil KM, Brubaker CJ, Adler CM, et al. Decreased brain volume in adults with childhood lead exposure. PLoS Med. 2008;5:e112. doi: 10.1371/journal.pmed.0050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang N, Baker HW, Tufts M, Raymond RE, Salihu H, Elliott MR. Early childhood lead exposure and academic achievement: evidence from Detroit public schools, 2008–2010. Am J Public Health 2013. 103:e72–77. doi: 10.2105/AJPH.2012.301164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fergusson DM, Boden JM, Horwood LJ. Dentine lead levels in childhood and criminal behaviour in late adolescence and early adulthood. J Epidemiol Community Health. 2008;62:1045–50. doi: 10.1136/jech.2007.072827. [DOI] [PubMed] [Google Scholar]
- 27.Wright JP, Dietrich KN, Ris MD, et al. Association of prenatal and childhood blood lead concentrations with criminal arrests in early adulthood. PLoS Med. 2008;5:e101. doi: 10.1371/journal.pmed.0050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oken E, Bellinger DC. Fish consumption, methylmercury and child neurodevelopment. Curr Opin Pediatr. 2008;20:178–83. doi: 10.1097/MOP.0b013e3282f5614c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28:536–47. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Julvez J, Smith GD, Golding J, et al. Genetic predisposition to cognitive deficit at age 8 years associated with prenatal methylmercury exposure. Epidemiology. 2013;24:643–50. doi: 10.1097/EDE.0b013e31829d5c93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White RF, Palumbo CL, Yurgelun-Todd DA, et al. Functional MRI approach to developmental methylmercury and polychlorinated biphenyl neurotoxicity. Neurotoxicology. 2011;32:975–80. doi: 10.1016/j.neuro.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budtz-Jorgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environ Health Perspect. 2007;115:323–27. doi: 10.1289/ehp.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strain JJ, Davidson PW, Bonham MP, et al. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology. 2008;29:776–82. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wasserman GA, Liu X, Parvez F, et al. Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environ Health Perspect. 2007;115:285–89. doi: 10.1289/ehp.9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamadani JD, Tofail F, Nermell B, et al. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol. 2011;40:1593–604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Tsukuma H, Oshima A. Long-term prospective study of 6104 survivors of arsenic poisoning during infancy due to contaminated milk powder in 1955. J Epidemiol 2010. 20:439–45. doi: 10.2188/jea.JE20090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel SM, Wolff MS. Causal inference considerations for endocrine disruptor research in children's health. Annu Rev Public Health. 2013;34:139–58. doi: 10.1146/annurev-publhealth-031811-124556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neurospychol Rev. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan K, Wasserman GA, Liu X, et al. Manganese exposure from drinking water and children's academic achievement. Neurotoxicology. 2012;33:91–97. doi: 10.1016/j.neuro.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115:122–27. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riojas-Rodriguez H, Solis-Vivanco R, Schilmann A, et al. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect. 2010;118:1465–70. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucchini RG, Guazzetti S, Zoni S, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012;33:687–96. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreno JA, Yeomans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB. Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicol Sci. 2009;112:394–404. doi: 10.1093/toxsci/kfp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi AL, Sun G, Zhang Y, Grandjean P. Developmental fluoride neurotoxicity: a systematic review and meta-analysis. Environ Health Perspect. 2012;120:1362–68. doi: 10.1289/ehp.1104912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Julvez J, Grandjean P. Neurodevelopmental toxicity risks due to occupational exposure to industrial chemicals during pregnancy. Ind Health. 2009;47:459–68. doi: 10.2486/indhealth.47.459. [DOI] [PubMed] [Google Scholar]
- 46.Pele F, Muckle G, Costet N, et al. Occupational solvent exposure during pregnancy and child behaviour at age 2. Occup Environ Med. 2013;70:114–19. doi: 10.1136/oemed-2012-100892. [DOI] [PubMed] [Google Scholar]
- 47.Janulewicz PA, White RF, Martin BM, et al. Adult neuropsychological performance following prenatal and early postnatal exposure to tetrachloroethylene (PCE)-contaminated drinking water. Neurotoxicol Teratol. 2012;34:350–59. doi: 10.1016/j.ntt.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kofman O, Berger A, Massarwa A, Friedman A, Jaffar AA. Motor inhibition and learning impairments in school-aged children following exposure to organophosphate pesticides in infancy. Pediatr Res. 2006;60:88–92. doi: 10.1203/01.pdr.0000219467.47013.35. [DOI] [PubMed] [Google Scholar]
- 49.London L, Beseler C, Bouchard MF, et al. Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicology. 2012;33:887–96. doi: 10.1016/j.neuro.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres-Sanchez L, Schnaas L, Rothenberg SJ, et al. Prenatal p,p'-DDE exposure and neurodevelopment among children 3.5–5 years of age. Environ Health Perspect. 2013;121:263–68. doi: 10.1289/ehp.1205034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boucher O, Simard MN, Muckle G, et al. Exposure to an organochlorine pesticide (chlordecone) and development of 18-month-old infants. Neurotoxicology. 2013;35:162–68. doi: 10.1016/j.neuro.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 52.Rauh V, Arunajadai S, Horton M, et al. 7-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119:1196–201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouchard MF, Chevrier J, Harley KG, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year old children. Environ Health Perspect. 2011;119:1189–95. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engel SM, Wetmur J, Chen J, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119:1182–88. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauh VA, Perera FP, Horton MK, et al. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proc Natl Acad Sci USA. 2012;109:7871–76. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bjorling-Poulsen M, Andersen HR, Grandjean P. Potential developmental neurotoxicity of pesticides used in Europe. Environ Health. 2008;7:50. doi: 10.1186/1476-069X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ostrea EM, Jr, Reyes A, Villanueva-Uy E, et al. Fetal exposure to propoxur and abnormal child neurodevelopment at 2 years of age. Neurotoxicology. 2012;33:669–75. doi: 10.1016/j.neuro.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Horton MK, Rundle A, Camann DE, Boyd Barr D, Rauh VA, Whyatt RM. Impact of prenatal exposure to piperonyl butoxide and permethrin on 36-month neurodevelopment. Pediatrics. 2011;127:e699–706. doi: 10.1542/peds.2010-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dingemans MM, van den Berg M, Westerink RH. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environ Health Perspect. 2011;119:900–07. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–58. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herbstman JB, Sjodin A, Kurzon M, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–19. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eskenazi B, Chevrier J, Rauch SA, et al. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121:257–62. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grandjean P, Budtz-Jorgensen E. An ignored risk factor in toxicology: the total imprecision of exposure assessment. Pure Appl Chem. 2010;82:383–91. doi: 10.1351/PAC-CON-09-05-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vandenberg LN, Colborn T, Hayes TB, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Engel SM, Miodovnik A, Canfield RL, et al. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ Health Perspect. 2010;118:565–71. doi: 10.1289/ehp.0901470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swan SH, Liu F, Hines M, et al. Prenatal phthalate exposure and reduced masculine play in boys. Int J Androl. 2010;33:259–69. doi: 10.1111/j.1365-2605.2009.01019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Braun JM, Kalkbrenner AE, Calafat AM, et al. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128:873–82. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perera FP, Li Z, Whyatt R, et al. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics. 2009;124:e195–202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn. 2008;68:117–27. doi: 10.1016/j.bandc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Dix-Cooper L, Eskenazi B, Romero C, Balmes J, Smith KR. Neurodevelopmental performance among school age children in rural Guatemala is associated with prenatal and postnatal exposure to carbon monoxide, a marker for exposure to woodsmoke. Neurotoxicology. 2012;33:246–54. doi: 10.1016/j.neuro.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 71.Vrijheid M, Martinez D, Aguilera I, et al. Indoor air pollution from gas cooking and infant neurodevelopment. Epidemiology. 2012;23:23–32. doi: 10.1097/EDE.0b013e31823a4023. [DOI] [PubMed] [Google Scholar]
- 72.Hernandez-Martinez C, Arija Val V, Escribano Subias J, Canals Sans J. A longitudinal study on the effects of maternal smoking and secondhand smoke exposure during pregnancy on neonatal neurobehavior. Early Hum Dev. 2012;88:403–08. doi: 10.1016/j.earlhumdev.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 73.Mariussen E. Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance. Arch Toxicol. 2012;86:1349–67. doi: 10.1007/s00204-012-0822-6. [DOI] [PubMed] [Google Scholar]
- 74.Gump BB, Wu Q, Dumas AK, Kannan K. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environ Sci Technol. 2011;45:8151–59. doi: 10.1021/es103712g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Froehlich TE, Anixt JS, Loe IM, Chirdkiatgumchai V, Kuan L, Gilman RC. Update on environmental risk factors for attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2011;13:333–44. doi: 10.1007/s11920-011-0221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carpenter DO, Nevin R. Environmental causes of violence. Physiol Behav. 2010;99:260–68. doi: 10.1016/j.physbeh.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 77.Miodovnik A, Engel SM, Zhu C, et al. Endocrine disruptors and childhood social impairment. Neurotoxicology. 2011;32:261–67. doi: 10.1016/j.neuro.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry. 2013;70:71–77. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bandeen-Roche K, Glass TA, Bolla KI, Todd AC, Schwartz BS. Cumulative lead dose and cognitive function in older adults. Epidemiology. 2009;20:831–39. doi: 10.1097/EDE.0b013e3181b5f100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lock EA, Zhang J, Checkoway H. Solvents and Parkinson disease: a systematic review of toxicological and epidemiological evidence. Toxicol Appl Pharmacol. 2013;266:345–55. doi: 10.1016/j.taap.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environ Health Perspect. 2005;113:1230–33. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lauterbach M, Solak E, Kaes J, Wiechelt J, Von Mach MA, Weilemann LS. Epidemiology of hydrogen phosphide exposures in humans reported to the poison center in Mainz, Germany, 1983–2003. Clin Toxicol 2005. 43:575–81. doi: 10.1081/clt-200068847. [DOI] [PubMed] [Google Scholar]
- 83.Demarest C, Torgovnick J, Sethi NK, Arsura E, Sethi PK. Acute reversible neurotoxicity associated with inhalation of ethyl chloride: a case report. Clin Neurol Neurosurg. 2011;113:909–10. doi: 10.1016/j.clineuro.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Imamura T, Yanagawa Y, Nishikawa K, Matsumoto N, Sakamoto T. Two cases of acute poisoning with acetamiprid in humans. Clin Toxicol. 2010;48:851–53. doi: 10.3109/15563650.2010.517207. [DOI] [PubMed] [Google Scholar]
- 85.Veale DJ, Wium CA, Muller GJ. Amitraz poisoning in South Africa: a two year survey (2008–2009). Clin Toxicol 2011. 49:40–44. doi: 10.3109/15563650.2010.542159. [DOI] [PubMed] [Google Scholar]
- 86.Sung YF, Huang CT, Fan CK, Lin CH, Lin SP. Avermectin intoxication with coma, myoclonus, and polyneuropathy. Clin Toxicol. 2009;47:686–88. doi: 10.1080/15563650903070901. [DOI] [PubMed] [Google Scholar]
- 87.Yang CC. Acute human toxicity of macrocyclic lactones. Curr Pharm Biotechnol. 2012;13:999–1003. doi: 10.2174/138920112800399059. [DOI] [PubMed] [Google Scholar]
- 88.Lee SJ, Mulay P, Diebolt-Brown B, et al. Acute illnesses associated with exposure to fipronil—surveillance data from 11 states in the United States, 2001–2007. Clin Toxicol 2010. 48:737–44. doi: 10.3109/15563650.2010.507548. [DOI] [PubMed] [Google Scholar]
- 89.Malhotra RC, Ghia DK, Cordato DJ, Beran RG. Glyphosatesurfactant herbicide-induced reversible encephalopathy. J Clin Neurosci. 2010;17:1472–73. doi: 10.1016/j.jocn.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 90.David D, Prabhakar A, Peter JV, Pichamuthu K. Human poisoning with hexastar: a hexaconazole-containing agrochemical fungicide. Clin Toxicol. 2008;46:692–93. doi: 10.1080/15563650701447012. [DOI] [PubMed] [Google Scholar]
- 91.Shadnia S, Moghaddam HH. Fatal intoxication with imidacloprid insecticide. Am J Emerg Med. 2008;26:634, e1–4. doi: 10.1016/j.ajem.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 92.Deng X, Li G, Mei R, Sun S. Long term effects of tetramine poisoning: an observational study. Clin Toxicol. 2012;50:172–75. doi: 10.3109/15563650.2012.657758. [DOI] [PubMed] [Google Scholar]
- 93.Khalil M, Abudiab M, Ahmed AE. Clinical evaluation of 1,3-butadiene neurotoxicity in humans. Toxicol Ind Health. 2007;23:141–46. doi: 10.1177/0748233707078773. [DOI] [PubMed] [Google Scholar]
- 94.Bellinger DC. A strategy for comparing the contributions of environmental chemicals and other risk factors to neurodevelopment of children. Environ Health Perspect. 2012;120:501–07. doi: 10.1289/ehp.1104170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grandjean P, Pichery C, Bellanger M, Budtz-Jorgensen E. Calculation of mercury's effects on neurodevelopment. Environ Health Perspect. 2012;120:A452. doi: 10.1289/ehp.1206033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bellanger M, Pichery C, Aerts D, et al. Economic benefits of methylmercury exposure control in Europe: monetary value of neurotoxicity prevention. Environ Health. 2013;12:3. doi: 10.1186/1476-069X-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trasande L, Liu Y. Reducing the staggering costs of environmental disease in children, estimated at $76.6 billion in 2008. Health Aff 2011. 30:863–70. doi: 10.1377/hlthaff.2010.1239. [DOI] [PubMed] [Google Scholar]
- 98.Pichery C, Bellanger M, Zmirou-Navier D, Glorennec P, Hartemann P, Grandjean P. Childhood lead exposure in France: benefit estimation and partial cost-benefit analysis of lead hazard control. Environ Health. 2011;10:44. doi: 10.1186/1476-069X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lynn R, Vanhanen T. IQ and the wealth of nations. Praeger; Westport: 2002. [Google Scholar]
- 100.Blacksmith Institute. The world's worst pollution problems: assessing health risks at hazardous waste sites. New York: Blacksmith Institute. 2012 [Google Scholar]
- 101.Trasande L, Massey RI, DiGangi J, Geiser K, Olanipekun AI, Gallagher L. How developing nations can protect children from hazardous chemical exposures while sustaining economic growth. Health Aff. 2011;30:2400–09. doi: 10.1377/hlthaff.2010.1217. [DOI] [PubMed] [Google Scholar]
- 102.Nevin R. Understanding international crime trends: the legacy of preschool lead exposure. Environ Res. 2007;104:315–36. doi: 10.1016/j.envres.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 103.Schwartz J. Societal benefits of reducing lead exposure. Environ Res. 1994;66:105–24. doi: 10.1006/enrs.1994.1048. [DOI] [PubMed] [Google Scholar]
- 104.National Research Council . Toxicity testing in the 21st century: a vision and a strategy. National Academies Press; Washington, DC: 2007. [Google Scholar]
- 105.Makris SL, Raffaele K, Allen S, et al. A retrospective performance assessment of the developmental neurotoxicity study in support of OECD test guideline 426. Environ Health Perspect. 2009;117:17–25. doi: 10.1289/ehp.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rovida C, Longo F, Rabbit RR. How are reproductive toxicity and developmental toxicity addressed in REACH dossiers? Altex. 2011;28:273–94. doi: 10.14573/altex.2011.4.273. [DOI] [PubMed] [Google Scholar]
- 107.Collins FS, Gray GM, Bucher JR. Toxicology. Transforming environmental health protection. Science. 2008;319:906–07. doi: 10.1126/science.1154619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crofton KM, Mundy WR, Lein PJ, et al. Developmental neurotoxicity testing: recommendations for developing alternative methods for the screening and prioritization of chemicals. Altex. 2011;28:9–15. [PubMed] [Google Scholar]
- 109.Audouze K, Grandjean P. Application of computational systems biology to explore environmental toxicity hazards. Environ Health Perspect. 2011;119:1754–59. doi: 10.1289/ehp.1103533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Willighagen EL, Jeliazkova N, Hardy B, Grafstrom RC, Spjuth O. Computational toxicology using the OpenTox application programming interface and Bioclipse. BMC Res Notes. 2011;4:487. doi: 10.1186/1756-0500-4-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.National Research Council . Science and decisions: advancing risk assessment. National Academies Press; Washington, DC: 2009. [PubMed] [Google Scholar]
- 112.Late lessons from early warnings: science, precaution, innovation. European Environment Agency; Copenhagen: 2013. [Google Scholar]
- 113.Moodie R, Stuckler D, Monteiro C, et al. Profits and pandemics: prevention of harmful effects of tobacco, alcohol, and ultra-processed food and drink industries. Lancet. 2013;381:670–79. doi: 10.1016/S0140-6736(12)62089-3. [DOI] [PubMed] [Google Scholar]
- 114.Grandjean P. Seven deadly sins of environmental epidemiology and the virtues of precaution. Epidemiology. 2008;19:158–62. doi: 10.1097/EDE.0b013e31815be031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grandjean P, Eriksen ML, Ellegaard O, Wallin JA. The Matthew effect in environmental science publication: a bibliometric analysis of chemical substances in journal articles. Environ Health. 2011;10:96. doi: 10.1186/1476-069X-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.