Abstract
Conduct disorder is a childhood behaviour disorder that is characterized by persistent aggressive or antisocial behaviour that disrupts the child’s environment and impairs his or her functioning. A proportion of children with conduct disorder have psychopathic traits. Psychopathic traits consist of a callous–unemotional component and an impulsive–antisocial component, which are associated with two core impairments. The first is a reduced empathic response to the distress of other individuals, which primarily reflects reduced amygdala responsiveness to distress cues; the second is deficits in decision making and in reinforcement learning, which reflects dysfunction in the ventromedial prefrontal cortex and striatum. Genetic and prenatal factors contribute to the abnormal development of these neural systems, and social–environmental variables that affect motivation influence the probability that antisocial behaviour will be subsequently displayed.
Aggressive and antisocial behaviours are the leading cause of child and adolescent referrals to mental health clinicians and can lead to a diagnosis of conduct disorder1. However, not all patients receiving this diagnosis show the same pathophysiology. One form of conduct disorder is marked by the presence of psychopathic traits and will be the main focus of this Review. Psychopathic traits have a core callous– unemotional component (for example, lack of guilt and empathy) and an impulsive–antisocial component2. They are detectable early in childhood and persist into adulthood3,4. Clinically, understanding psychopathic traits is important, as their presence can interfere with socialization5 and currently available conduct-disorder treatments6,7.
There has been rapid progress in our understanding of the neurobiology of psychopathic traits, particularly the callous–unemotional component, over the past 5 years. Indeed, partly as a result of neurobiological studies8–10 a form of callous–unemotional specifier (termed ‘limited prosocial emotions’) has been introduced to the conduct disorder diagnosis in the fifth edition of the Diagnostic and Statistical Manual (DSM-5)11. To qualify for this specifier, an individual must have displayed two of four characteristics in the previous 12 months in multiple settings. These characteristics are lack of remorse or guilt; callousness (that is, lack of empathy); lack of concern about performance (for example, at school); and shallow or deficient affect (a lack of expression of feelings to others). A different form of conduct disorder is associated with increased risk of mood and anxiety disorders and emotional lability (BOX 1).
Box 1. Different forms of conduct disorder.
Patients receiving a diagnosis of conduct disorder do not all have the same pathophysiology. One set of neurodevelopmental impairments — decreased amygdala responsiveness to distress cues and decreased striatal and ventromedial prefrontal cortex (vmPFC) sensitivity to reinforcement signals that are critical for successful decision making (FIG. 1) — can lead to a diagnosis of conduct disorder associated with psychopathic traits. Another set of dysfunctions can also lead to a diagnosis of conduct disorder, as explained below.
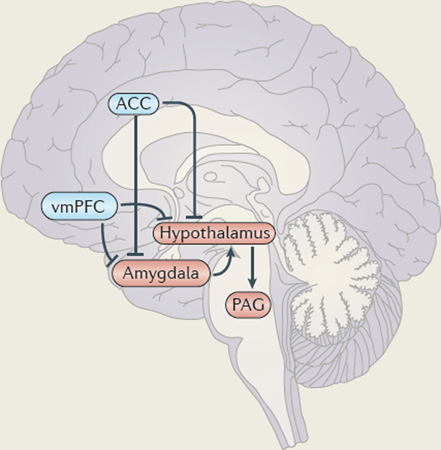
Mammals demonstrate a graded and instinctual response to threat: distant threats induce freezing; as the threats draw closer, they induce flight; and, finally, reactive aggression is induced when they are very close and escape is impossible184. Reactive aggression involves unplanned, enraged attacks on the object perceived to be the source of the threat or frustration. Animal studies have shown that reactive aggression is mediated by a circuit that runs from the medial amygdala, largely via the stria terminalis to the medial hypothalamus, and from there to the dorsal half of the periaqueductal grey (PAG)185–188.
This circuitry is assumed to mediate reactive aggression in humans as well189 (see the figure). Certainly, several recent functional MRI studies have identified these regions to be involved in defensive reactions to threat in humans190–192. This circuitry is assumed to be regulated by frontal cortical regions, particularly the vmPFC and, potentially, regions of the anterior cingulate cortex (ACC).
If the basic threat circuit (amygdala–hypothalamus–PAG) is overly responsive, either because of prior priming or inadequate regulation, the individual is more likely to respond to a threat with reactive aggression than with freezing or flight53. In youths with conduct problems and low callous–unemotional traits, this circuit is overly responsive, as evidenced by, for example, increased amygdala responses to fearful expressions49. Moreover, they are more likely to display higher levels of threat-based and frustration-based reactive aggression193. Such individuals probably represent many of the 40% with conduct disorder who also meet criteria for a mood or anxiety disorder194. Notably, a high rating for psychopathic traits (which characterizes the other form of conduct disorder) is typically associated with a decreased risk for anxiety and mood disorder symptoms, particularly when the relationship between anxiety on the one hand, and antisocial and impulsive behaviour on the other hand is accounted for195–197. This inverse relationship between psychopathic traits and mood and anxiety disorders is unsurprising, as increased amygdala responsiveness is also commonly associated with mood and anxiety disorders198. By contrast, psychopathic traits are associated with decreased amygdala responsiveness8,10,30,48–50,83.
This Review discusses why psychopathic traits in youths are associated with an increased risk of antisocial behaviour and aggression. I use a cognitive neuroscience approach; that is, I consider how specific functional impairments in specific neural systems give rise to the development of psychopathic traits. I then use cognitive neuroscience findings to interpret both data on genetic and environmental risk factors for aggression and data on potential treatment possibilities. Finally, I present an integrative model of psychopathic traits, conduct disorder and aggression more generally.
A cognitive neuroscience approach
Youths with psychopathic traits show two main cognitive impairments. The first is a specific form of empathic dysfunction. Indeed, the clinical literature has long associated psychopathy with empathy impairment12,13. However, the term empathy subsumes two critical processes that are distinct at both the cognitive and the neural level14: cognitive empathy involves the representation of the intentions and thoughts of other individuals (also known as theory of mind)15, whereas emotional empathy involves affective responses to emotional displays of other individuals and to verbal descriptions of the emotional states of other individuals. Psychopathic traits are not associated with reductions in cognitive empathy but they — and particularly the callous–unemotional component — are associated with reductions in specific forms of emotional empathy (in particular, responding to the fear, sadness, pain and happiness of others). This functional impairment is associated with reduced amygdala and ventromedial prefrontal cortex (vmPFC) responsiveness to distress cues (FIG. 1).
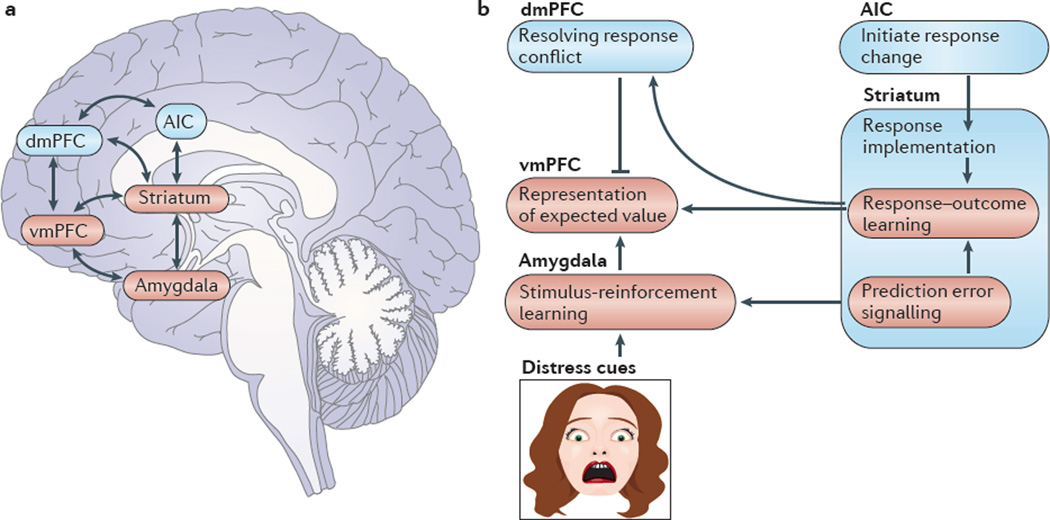
Figure 1. Core regions implicated in, and functions disrupted by, psychopathic traits.
a | Core regions implicated in psychopathic traits: the amygdala, the caudate (which is part of the striatum) and the ventromedial prefrontal cortex (vmPFC). In addition to these core regions, the anterior insular cortex (AIC) and the dorsomedial PFC (dmPFC) may also be implicated. b | Functional impairments associated with psychopathic traits. In individuals with psychopathic traits, impaired processing of distress cues results in impaired learning about actions that harm others (stimulus-reinforcement learning), which involves the amygdala. In addition, impaired prediction error signalling in these individuals, which involves the striatum, causes impairments in both stimulus-reinforcement and response–outcome learning. As a result, the expected value of objects, cues and responses are poorly learnt and represented (in the vmPFC), and decision making is impaired. Response conflict resolution (which involves the dmPFC), initiation of response change (which involves the AIC) and response implementation (caudate) — functions implicated in guiding an individual away from suboptimal behavioural choices — are thought to be generally intact in individuals with psychopathic traits. These regions are also recruited to guide an individual away from suboptimal behavioural choices based on expected value information, that is, because the response about to be made is associated with punishment. Individuals with psychopathic traits show reduced recruitment of these areas on the basis of expected value information.
The second cognitive impairment in youths with psychopathic traits is impairment in aspects of decision making, specifically in reinforcement learning and the representation of reinforcement expectancies. This impairment may relate more to the impulsive–antisocial component of psychopathic traits and is also seen, at least partially, in patients with other externalizing disorders, such as attention-deficit hyperactivity disorder (ADHD), and in those at risk of developing drug addiction. This functional impairment is associated with dysfunction in the vmPFC and striatum (FIG. 1).
Cognitive empathy
It has been known for some time that adults with psychopathic traits show no impairment in cognitive empathy16–18. This finding has been replicated recently in adolescents with psychopathic traits19,20. A recent functional MRI (fMRI) study examined participants who were using information about the intentions and beliefs of other individuals to predict their behaviour21. This study demonstrated that youths with psychopathic traits show normal recruitment of the medial frontal cortex (including the vmPFC), temporal parietal junction, posterior cingulate cortex and temporal pole when engaged in this cognitive empathy task. These are all regions that have been implicated in cognitive empathy in healthy individuals22–24.
Emotional empathy
Empathic reactions can be evoked by facial cues, auditory cues, body postures and even text. Emotional empathy has a communicatory function: the emotional cues of others impart specific information to the observer25,26, and emotional empathy is the observer’s ‘translation’ of this communication. It has been argued that different facial expressions provide different communicatory signals, initiate different forms of reinforcement-based learning and are processed by neural systems that are at least partially distinct25.
The emotional empathy impairment in youths (and adults) with psychopathic traits is selective. For example, they have normal recognition of expressions of anger and disgust (for meta-analytic reviews of the literature, see REFS 27,28), and blood oxygen level-dependent (BOLD) responses to angry expressions are similar to those in typically developing adolescents (the response to disgust expressions has not been tested)8,29,30. By contrast, they display impaired processing of both distress cues (that is, expressions of fear, sadness or pain) and happy expressions; studies quite consistently show impaired recognition of fearful and, to a lesser extent, sad and happy expressions in youths (and adults) with psychopathic traits31–36. These findings have been confirmed in recent meta-analytic reviews of the literature27,28. The impaired recognition of fearfulness and sadness also applies to vocal tones35,37 and body postures38. In addition, youths with psychopathic traits show reduced autonomic responses to fearful and sad expressions and pain in other individuals, as well as atypical electroencephalography responses to pain in others39–43. In line with findings of reduced responsiveness to the distress of others, children with high callous–unemotional traits state that they are less concerned (relative to children with low callous–unemotional traits) that aggressive behaviour will result in suffering in the victim44. Importantly, although youths with psychopathic traits show a reduced response to emotional stimuli (whether indexed by autonomic or amygdala activity), the response is not absent, and increasing the intensity of an emotional stimulus — through morphing31 or by orientating the participant’s attention towards the eyes — reduces or removes group differences in fearful expression recognition34,45.
In healthy individuals, amygdala activation by distress cues leads to both increased arousal (via projections to the brainstem) and increased attention to these cues. This increased attention reflects the reciprocal connections between the amygdala and temporal cortex, such that amygdala activity will stimulate the neurons that represent the emotionally salient features of the eliciting cue, further strengthening the representation of these features and increasing the probability that they will ‘win’ the competition for representation46. In the case of fearful expressions, the eye region is a particularly emotionally salient feature47 and representation of the eyes will thus be particularly strengthened when a healthy individual sees a fearful face. As a result of stimulus-reinforcement learning, an association is formed between the ‘social punishment’ of the fearful or sad facial expression and any representations of objects or actions associated with this expression. Which object is associated will be specified by the expresser’s eye gaze 29.
The deficits in emotional empathy shown by adolescents with psychopathic traits involve amygdala dysfunction31. Indeed, fMRI studies in adolescents with psychopathic traits have consistently shown reduced amygdala responses to images of faces with fearful expressions8,10,30,48,49. Furthermore, youths with conduct disorder who have psychopathic traits show reduced amygdala and rostral medial frontal cortical responses to images of other individuals in pain47,50. The impaired recognition of happy expressions may also relate to amygdala dysfunction, but this has not yet been empirically confirmed. It is the callous–unemotional component of psychopathic traits that seems to be particularly associated with the reduced amygdala response to distress cues10,48.
Appropriate processing of distress cues is critical for socialization. Many studies in humans and animals have shown the role of emotional expressions in the transmission of the value of actions and objects. For example, humans value positively those actions and objects that make care-givers smile and avoid actions and objects for which care-givers show fear51,52. Similarly, individuals approach objects associated with happiness in another individual and avoid objects associated with fear or disgust in another individual. The amygdala allows the association of the stimulus (the object or action towards which the expression was displayed) with the reinforcement (the expression itself), so that the object or action becomes associated with a value53. Indeed, recent animal studies have confirmed a critical role for the amygdala in observational fear54. In individuals with psychopathic traits, reduced processing of distress cues by the amygdala would lead to reduced aversion for actions that harm others, so that the individual is more likely to commit actions that harm others to achieve their goals (BOX 2).
Box 2. Care-based moral judgements.
The deficits in emotional empathy and reinforcement-based decision making in adolescents with psychopathic traits affect social cognition in these individuals, particularly the formation of appropriate care-based moral judgements (that is, judgements about transgressions that result in harm to another individual (for example, one person hitting another), known as care-based transgressions). It has been argued that emotion has an important role in care-based moral judgments199–202. Specifically, care-based judgments rely on the amygdala associating the aversive emotional response to the victim’s distress with the representation of the action that caused this distress and on the ventromedial prefrontal cortex (vmPFC) representing the value of the transgression53. Adults with psychopathic traits show reduced amygdala and vmPFC activity in response to care-based transgressions and a weaker correlation between their amygdala response and their rating of the severity of care-based transgressions compared with control participants203,204. Similarly, youths with psychopathic traits show reduced amygdala activity and reduced amygdala–vmPFC functional connectivity when making moral judgements83. There is also behavioural evidence that youths (and adults) with psychopathic traits show impaired care-based moral reasoning69,199. In addition, a recent study showed that adolescents with psychopathic traits give less to charities when such giving comes at a personal cost205.
Perhaps the most interesting finding regarding the altered moral reasoning in individuals with psychopathic traits is that it is selective for care-based transgressions. Indeed, their moral judgement of conventional transgressions (which concern social normative behaviour and are heavily reinforced by the anger of hierarchy figures (for example, not talking during class)) and disgust-based transgressions (transgressions that may elicit disgust reactions from observers — often against forms of sexual activity)69,199,206–208 is normal69,199,209,210. In addition, adults with psychopathic traits (adolescents have not yet been tested) judge care-based transgressions less seriously than control individuals, whereas there are no differences in how they judge the severity of conventional and disgust-based transgressions209–211.
It has been argued that the ability to respond to emotional reactions of others is critically important in socialization (including in judging social transgressions). As partially independent emotional learning systems are thought to allow the learning of valence information provided by different emotional expressions25,53, a selective impairment in processing care-based transgressions may be due to impairment in a particular emotional learning system. Specifically, distress cues (for example, from people subjected to care-based transgressions) are processed by the amygdala; the inferior frontal cortex is involved in processing angry expressions (which occur during conventional transgressions; for a meta-analytic review of the expression literature, see REF. 212); and the insula is important for processing disgust expressions (which occur during disgust-based transgressions)200. Thus, the selective impairment in recognizing distress cues but not disgusted or angry expressions in individuals with psychopathic traits (for meta-analytic reviews of the literature, see REFS 27,28) is in agreement with the finding that such individuals show impaired processing of care-based transgressions but normal processing of conventional and disgust-based transgressions69,199,209,210.
Emotional empathy also has a communicatory function: it is critical for learning the social value of actions and objects and important for appropriate decision making. In healthy individuals, the strength of the representation of the distress of the victim is inversely related to the probability of an aggressive response55. Brain regions that are important for representing the valence of objects and actions and for using this information to guide choices towards or away from these objects and actions include the vmPFC and anterior insular cortex (see below)56–59. Furthermore, there is considerable evidence, including from lesion studies, that the vmPFC and insular cortex are critical for empathic responding60–63. Youths with conduct disorder show reduced rostral vmPFC activation in response to observing pain in other individuals50, as well as reduced insula responses when using emotional reactions from other individuals to predict the subsequent behaviour of these individuals21. In both cases, the activity reduction in these areas was correlated with the severity of psychopathic50 or callous–unemotional21 traits.
In summary, these findings suggest that adolescents with psychopathic traits not only form weaker associations between representations of actions that harm others and the aversive consequences of these actions to other individuals but also apply this information less during decision making. Such individuals are more likely to commit actions that will harm other individuals, because they are less likely to be deterred from committing such actions by any expectations regarding the distress the action would cause in the victims.
Emotional learning and decision making
Youths with psychopathic traits show pronounced impairments in emotional learning and decision making. This reflects not only reduced responsiveness to the social reinforcers (emotional expressions) considered above but also deficits in the processes underlying aversive conditioning, passive avoidance learning64, operant extinction2,65,66 and reversal learning67,68. These impairments manifest when making moral judgements69 and in other decision-making paradigms70. It should be noted that reduced aversive conditioning has so far been assessed and found only in adult individuals with psychopathic traits71,72. However, youths with conduct disorder have also been reported to show reduced aversive conditioning73, and a propensity for aversive conditioning at the age of 3 years predicts future antisocial behaviour74.
Youths with psychopathic traits show deficits in the capacity to link outcomes (rewards or punishments) with stimuli or responses, and this is due to dysfunction within the amygdala, striatum (caudate and nucleus accumbens) and vmPFC75. In addition, there is evidence that the use of outcome information by the anterior insula, inferior frontal cortex and dorsomedial PFC (dmPFC) to guide the individual away from suboptimal behavioural choices58,59 is disrupted in adolescents with psychopathic traits76. Clinically, this impairment may manifest in many destructive behaviours: for example, deciding to mug people on the street corner on which the individual was almost arrested the night before or to fight someone in response to mild provocation (in other words, to ‘be a man’), despite repeated negative consequences of such behaviours. The effective use of reinforcement outcome information during decision making requires two components. The first is the appropriate representation of the reward or punishment received when an action has been performed. Prediction error signalling is critical for this56,77. Prediction error signals are thought to spur reinforcement learning (mediated by both the amygdala and striatum): the greater the prediction error, the greater the change in the reinforcement associated with the stimulus78. The second component is the appropriate representation of the expected value when considering whether to perform an action.
The vmPFC is involved in encoding received rewards56,57. Youths with psychopathic tendencies64 and conduct disorder79, or with increased levels of antisocial behaviour80, show reduced vmPFC response to the receipt of a reward (although there is one report that youths with externalizing disorders show heightened responsiveness to rewards81). This is thought to reflect disrupted prediction error signalling9,64,76. Many studies in healthy adults have shown that the size of the positive prediction error (when a reward is larger than expected) is correlated with activity within the striatum56,57. This positive association between positive prediction errors and activity within the striatum also exists in healthy youths, but it is less strong in adolescents with psychopathic traits76. Given the importance of prediction error signalling for initiating reinforcement learning78, reduced prediction error signalling should result in poorer, and slower, learning of the reinforcements associated with objects and actions.
One particularly interesting feature of prediction error signalling in youths with psychopathic traits concerns their response to punishment. Punishments that are worse than expected are typically associated with a reduction in striatal activity in healthy adults and youths56,57. By contrast, adolescents with psychopathic traits showed a positive relationship between prediction errors to punishment and activity within the striatum76. This is in agreement with earlier studies that showed increased responses to unexpected punishment within the vmPFC and striatum in youths with psychopathic traits and in antisocial youths more generally64,80. The reason for these increased, as opposed to decreased, striatal and vmPFC responses to unexpected punishment remains unclear, but it probably contributes to their decision-making impairment.
The representation of expected value is also disrupted in youths with psychopathic traits. Studies have shown a positive correlation between the expected value associated with a response or a stimulus and activity within the vmPFC in healthy adults and adolescents56,57. Thus, as the expected value associated with a stimulus increases (that is, the stimulus is an increasingly good predictor of reward), the individual is more likely to respond to that stimulus. By contrast, in a recent model-based fMRI study, youths with psychopathic traits showed a weaker association between expected value and both choice behaviour and vmPFC activity during choice than healthy youths76. Thus, youths with psychopathic traits are poorer at using and representing expected value information, and this may impair their decision making.
Interestingly, healthy adolescents also show a stronger correlation between expected value and activity within the anterior insula, inferior frontal cortex and dmPFC than adolescents with psychopathic tendencies when avoiding stimuli that it would have been better to approach (because appropriate representation of expected values would predict that responding would engender reward)76. The anterior insula, inferior frontal cortex and dmPFC have been implicated in guiding the individual away from suboptimal choices58,59. Although one study suggested that the functioning of the anterior insula and dmPFC is disrupted in individuals with psychopathic traits82, this disruption is only partial. The recruitment of both regions is comparable to that seen in healthy youths in tests in which they are altering their behaviour in immediate response to punishment cues or during response conflict9,83. As such, the reduced activity may reflect problems in the use of expected value information rather than disruption in these regions perse.
Notably, although youths with conduct disorder and psychopathic traits show the above deficits in decision making and its computational underpinnings, there are no clear indications that these deficits are related to the severity of psychopathic traits specifically. This is in contrast to the empathy dysfunction discussed above: repeated findings have shown that weaker responses in the amygdala, vmPFC and anterior insula to empathy cues are associated with higher severity of psychopathic traits8,21,48,50. Decision-making deficits may not be specific to psychopathic traits but may also occur in individuals showing high levels of externalizing behaviour. For example, both individuals with ADHD84–86 and children of alcoholics, who are at risk of developing various externalizing problems, including drug addiction and conduct disorder87,88, show reduced striatal activity in anticipation of rewards89,90. This shared dysfunction may underpin the high comorbidity of conduct disorder with ADHD91 and substance dependence92.
In summary, adolescents with psychopathic traits show reduced representation of reward outcomes and expected value in the vmPFC, as well as reduced reward prediction error signalling and potentially highly atypical punishment prediction error signalling in the striatum. These computational impairments probably underlie the severe decision-making impairments seen in this population. However, these deficits (at least the reduced striatal response to reward) are partially shared by other populations that show increased externalizing behaviour.
Structural and endocrinological findings
Structural imaging studies
Given that fMRI studies consistently show reduced activity in the amygdala in response to fearful expressions in youths with psychopathic traits, as well as aberrant striatal and vmPFC activity in youths with conduct disorder and psychopathic traits, it is worth considering whether structural abnormalities are also seen within these regions. Unfortunately, most structural imaging studies performed so far involved groups of patients with conduct disorder more generally rather than patients with psychopathic traits specifically. Nevertheless, these studies have relatively consistently reported reduced amygdala volumes93–97, although two did not98,99. Moreover, temporal cortex volume93,95,100 and thickness101 are reduced in youths with conduct disorder. Findings regarding the vmPFC have been mixed, with reductions in volume93,97, cortical thickness102 or folding101 in this area reported in some studies, but not in others94–96,98,99. Reduced caudate volume has only been reported three times95–97, but the relative absence of such reports may reflect a lack of investigations targeting this area. In addition, and critically, a study involving over 200 incarcerated adolescents in a maximum-security facility confirmed reductions in volume, which were associated with the emotion dysfunction component of psychopathy in particular, within a large brain region that centred on the vmPFC and included the amygdala, temporal cortex and caudate97.
The strong fMRI evidence — as well as the rather weaker structural findings — for amygdala and vmPFC abnormalities suggests a possible disruption in the uncinate fasciculus, the white-matter tract that connects the amygdala to the frontal lobe. Certainly, adults with psychopathic traits show reduced functional anisotropy of this white-matter tract103–105. However, one diffusion tensor imaging study reported no fractional anisotropy difference in the uncinate fasciculus between adolescents with psychopathic traits and control youths106, whereas two other studies reported an increase in fractional anisotropy in youths with conduct disorder107,108. These diverging findings may reflect the development of this disorder, sample differences (for example, less severe cases in one study than in another) or the effect of past lifestyle choices in the adult samples (for example, opiate use is associated with reduced fractional anisotropy within the uncinate fasciculus109).
As noted above, fMRI studies have shown that the ability of the anterior insula and dmPFC to use expected value information to guide behaviour may be compromised in adolescents with psychopathic traits76, even if these regions respond normally to cues for response change or to response conflict9,83. Structural MRI (sMRI) studies have relatively consistently reported reductions in the volume94,95, thickness102 or folding101 of the insular cortex in youths with conduct disorder. However, it should be noted that no relationship of these reductions with psychopathic traits has been reported97,98. The literature is considerably more mixed with respect to the dmPFC, with some studies95,101,102 but not others93,94,97,99 showing structural reductions in youths with conduct disorder.
Given the potential neurodevelopmental nature of psychopathic traits, it is worth noting findings on the cavum septum pellucidum (CSP) (also see BOX 3). A large CSP is a marker of abnormal brain development, particularly with regard to midline structures110,111. The rapid development of the alvei of the amygdala, hippocampus, septal nuclei, fornix, corpus callosum and other midline structures is attributed to fusion of the CSP111. Disruption in the development of these limbic structures interrupts this posterior-to-anterior fusion and leads to the preservation of the CSP. Two recent studies have reported that youths and adults with conduct problems are more likely to have a large CSP relative to that of comparison individuals112,113. However, the youths sampled in this study were not selected specifically for psychopathic traits but had conduct problems more generally112. These data indicate that brain maldevelopment occurs very early in (at least a substantial minority of) patients with conduct problems. However, it is important to note that an increased incidence of a large CSP is also found in patients with post-traumatic stress disorder (PTSD)114, schizophrenia115 and bipolar disorder116. Thus, different forms of psychopathology may be associated with an increased CSP. Alternatively, there may be a common form (or cause) of early brain maldevelopment that puts an individual at risk of a wide range of psychiatric conditions, and other environmental or genetic factors may determine which condition develops. For example, fetal exposure to alcohol and other narcotics increases the risk not only of enlarged CSP117 but also of aggression118,119 and schizophrenia120.
Box 3. The development of psychopathic traits.
There are considerable genetic influences on the development of the systems considered here (the amygdala, caudate and ventromedial prefrontal cortex (vmPFC)) and their interconnections213–215. It is assumed that the genetic contribution to psychopathic traits results in a disruption of this development, but this remains to be empirically confirmed. Environmental variables (such as enrichment, diet and parental deprivation) also influence the development of these structures216–218 and some (for example, alcohol abuse) may induce modulation of gene expression219. As such, they may have a role in the development of psychopathic traits, although this also remains to be empirically confirmed (there is some evidence that parental warmth may have an impact160,161).
Dysfunction in these structures will give rise to reduced anxiety and to decision-making deficits. However, it is argued that, by itself, this dysfunction will not give rise to core features of psychopathic traits, such as decreased guilt and conscience and increased antisocial behaviour and instrumental aggression53. Rather, these are thought to be developmental consequences of the dysfunction in these brain structures that disrupt successful socialization. Socialization requires the individual to develop an association between the aversive reinforcement of the victim’s distress cues and the representation of the antisocial actions that induced this distress53. Future representation of this action will thus engender the negative expected value (the ‘badness’) of this action. The negative expected value will guide the individual away from the antisocial action and make the individual feel bad should they commit the action (guilt will occur if the individual represents their own causal role in the antisocial behaviour). In line with this, increased levels of the temperamental trait ‘fearfulness’ are associated with increased conscience development and guilt220,221. The argument is not that socialization occurs through fear but rather that fearfulness is a measure for the integrity of the amygdala. The amygdala is critical for the development of the associations that are the basis of socialization.
It is worth considering the influence of adolescence on psychopathic traits. Mid-adolescence is associated with a period of enhanced responsiveness to threat and reward222–225, as well as gradual maturation of systems implicated in top-down attention and response control (that is, the dorsomedial, superior and lateral frontal cortices)226,227. The increased reward sensitivity in mid-adolescence may increase antisocial behaviour in youths with psychopathic traits. Such an individual may be more likely to seek means — including antisocial means if these have been learnt — to achieve their goals and may remain relatively insensitive to the negative actions of their behaviour for others. The mid-adolescence increase in functional integrity of frontal systems engaged in top-down control has not yet been associated with any effect on the severity of psychopathic traits.
Finally, the developmental impact of substance abuse on the pathophysiology of psychopathic traits should be considered, as psychopathic traits are a risk factor for substance abuse228, and substance abuse usually commences in mid-adolescence. Importantly, substance use has been associated with atrophy in, among others, the amygdala and vmPFC229–231 and thus is likely to further disrupt the functional integrity of neural systems that are already dysfunctional in youths with psychopathic traits.
In summary, sMRI findings are consistent with the fMRI findings about the amygdala and caudate in individuals with conduct disorder but rather less consistent with fMRI findings about the vmPFC. The sMRI literature also supports the idea that conduct disorder may be associated with insula dysfunction. Moreover, structural volumes of the amygdala, caudate and insula were inversely correlated with severity of psychopathic traits in a sample (N = 296) of incarcerated adults121. Such structural abnormalities in individuals with psychopathic traits may be common from adolescence into adulthood. By contrast, white-matter connections between the amygdala and PFC may be disturbed in adults with psychopathic traits, but this is not consistently seen in youths with psychopathic traits.
Endocrinological findings
An aberrant cortisol response in childhood has long been associated with an increased risk of antisocial behaviour122. However, some studies have reported increased, and others reduced, cortisol responses in antisocial populations123,124. Cortisol is a peripheral marker of hypothalamus–pituitary–adrenal (HPA) axis activity — that is, of the stress response. The amygdala facilitates the activation of the HPA axis125. Given that youths with psychopathic traits (possibly callous–unemotional traits in particular) show abnormal amygdala activity, it could be expected that antisocial adolescents with psychopathic traits show a reduced cortisol response126. However, this prediction requires empirical investigation.
Genetic and environmental factors
Genetic factors
On the basis of the findings discussed above (and in BOX 1), one could argue that genetic variants leading to reduced amygdala responsiveness to distress cues, as well as to reduced caudate and vmPFC responses to prediction error and expected value, should be associated with increased risk of psychopathic traits, whereas genetic variants leading to increased amygdala responsiveness to threat should be associated with an increased risk of reactive aggression127. Indeed, findings from twin studies indicate a genetic contribution to aggression128, and callous–unemotional traits are clearly heritable129. However, only preliminary molecular genetic data are available. For example, one genome-wide association study generated a list of single-nucleotide polymorphisms (SNPs) that might be associated with psychopathic traits, but none of these SNPs reached genome-wide statistical significance130. This was probably due to the relatively small sample sizes in this study (300 each of the high and low psychopathic traits groups)130. Given the small sample sizes of most SNP studies and the lack of replications, the few results that have been obtained should be considered with caution.
Some data suggest that specific genetic polymorphisms are associated with increased amygdala responsiveness to threat. These include variants of the monoamine oxidase type A (MAOA) gene, a functional polymorphism in the promoter region of the serotonin transporter gene (5-HTTLPR; also known as SLC6A4) that is associated with reduced gene expression, and the Met158 variant of the catechol-O-methyltransferase (COMT) gene131–133. These polymorphisms are also associated with an increased risk of aggression134–138. However, these studies did not assess whether this concerns reactive (as opposed to instrumental) aggression specifically; the reasoning above (and in BOX 1) would predict this to be the case.
It is possible that variants of MAOA, 5-HTTLPR and COMT that are associated with relatively decreased amygdala responsiveness to threat might be associated with increased risk of psychopathic traits, but few studies have investigated this. One study reported no relationship between rs4680 (Val158Met) COMT polymorphisms and callous–unemotional traits, although there were trend relationships between two other COMT SNPs and callous-unemotional traits — rs6269 (COMT promoter) and rs4818 (Leu136Leu)139. Another study reported that the high-expressing genotypic variant of 5-HTTLPR, which is associated with reduced amygdala response to threat140, is also associated with increased callous–unemotional traits, but only in individuals with low family socioeconomic backgrounds138. A recent report showed that functional SNPs of the genes encoding serotonin receptors 1B and 2A and various polymorphisms of the oxytocin receptor gene are associated with callous–emotional traits141,142. However, whether these genetic variants are also associated with a reduced amygdala response to fearful expressions has yet to be determined.
In summary, although there is a genetic contribution to callous-unemotional traits, specific gene variants associated with both decreased amygdala responsiveness (that is, the neurobiological characteristic that may underpin psychopathic traits) and a generally increased risk of aggression have not yet been identified. By contrast, certain variants of COMT, MAOA and 5-HTTLPR are associated with increased amygdala responsiveness and an increased risk of aggression (which, according to my model, would be specific for reactive aggression).
Environmental factors
The data above suggest that, similarly to genetic factors, environmental factors that lead to reduced amygdala responsiveness to distress cues should be associated with increased psychopathic traits, and that environmental factors that lead to increased amygdala responsiveness to threat should be associated with an increased risk of threat-based reactive aggression. In agreement with this, exposure to high threat levels (in the context of abuse or family violence) and/ or to neglect leads to heightened amygdala responses to threat143–145 and increased risk of reactive aggression146.
No specific environmental factors that decrease amygdala responsiveness have yet been identified. Indeed, it has been reported that environmental factors play a smaller part than genetic factors in the high levels of aggression exhibited by youths who show callous–unemotional traits147. Nevertheless, certain environmental (in particular prenatal) factors may have a role, as maternal substance abuse during pregnancy is associated within an increased likelihood of callous– unemotional traits in the child (also see BOX 3)148. It is also possible that some environmental factors — interacting with specific genetic variants — result in reduced rather than increased amygdala responsiveness to emotional stimuli.
Even if environmental factors play only a small part in the pathophysiology of psychopathic traits, they clearly affect the expression of these traits. Deficits in responding to the distress of others (and in prediction error and expected value signalling) described above would give rise to an individual who is less concerned by the distress of others and makes poorer decisions. However, such deficits would not by themselves increase an individual’s motivation to offend; environmental factors such as reduced socioeconomic status may do so, and exposure to criminal environments may provide the individual with behavioural repertoires. Thus, a pathophysiology such as altered amygdala responsiveness does not necessarily manifest as offending behaviour; it may only do so given certain environmental backgrounds.
Treatment implications
Conduct disorder is regarded as difficult to treat. However, there are findings that ‘social and emotional learning’ prevention strategies that foster the development of emotional regulation, relationship skills and responsible decision making can prevent or reduce the development of conduct problems149. Similarly, psychosocial treatments such as Multidimensional Treatment Foster Care150 and Multisystemic Therapy151 have been shown to be effective in the treatment of conduct disorder152.
However, as discussed in this Review, there are two types of conduct disorder — one associated with psychopathic traits and one associated with reactive aggression as well as mood and anxiety disorders — and they probably require different treatments. Indeed, parenting strategies that reduce conduct problems in many youths have been found to be less effective in youths with conduct problems and high levels of callous–unemotional traits relative to youths with conduct problems and low levels of callous–unemotional traits5,153 (but see also REF. 154). Moreover, children with callous–unemotional traits have been found to be more resistant to psychosocial intervention than other aggressive children6,7,155–157.
Given that reduced amygdala responsiveness to distress cues is associated with an increased risk of psychopathic traits, whereas increased amygdala responsiveness to threat is associated with an increased risk of threat-based reactive aggression (BOX 1), some patients may require interventions that increase amygdala responsiveness (and increase appropriate prediction error and expected value signalling during decision making), whereas others may require interventions that decrease amygdala responsiveness. Psychosocial prevention and intervention strategies can be notably effective in reducing threat sensitivity in the context of anxiety disorders. Interestingly, such treatments have been shown to reduce the heightened amygdala response to threat in patients with PTSD158. Many adolescents with conduct disorder have experienced maltreatment, and co-morbidity with PTSD is high159 — presumably in the subgroup that shows heightened threat sensitivity. It is thus plausible, although it remains to be formally tested, that the youths with conduct problems principally benefiting from current psychosocial interventions are those with heightened threat responsiveness and that treatment works by reducing amygdala responsiveness to threat.
Although it has been demonstrated that psychosocial interventions reduce the increased amygdala responsiveness to threat in patients with PTSD158, there have been no findings that such interventions can increase a reduced amygdala responsiveness to distress cues, although this may be possible. There have been some reports that psychosocial interventions can reduce levels of callous–unemotional traits160, particularly in adolescents from families with high parental warmth160,161. Of course, in the absence of fMRI studies of treatment efficacy, it is also possible that these more successful interventions may alter only the behavioural manifestation of the psychopathic traits in specific social contexts rather than the pathophysiology underlying the psychopathic traits itself.
There is evidence that atypical antipsychotic drugs have some efficacy in the treatment of aggression in children162,163. Certainly, their usage is common; it is estimated that in the USA, over 70% of youths with disruptive behaviour disorders are given antipsychotics164. The atypical antipsychotic aripiprazole is a partial agonist at dopamine D2 and serotonin 1A receptors165,166, and the antipsychotic risperidone has been shown to markedly increase extracellular levels of dopamine, serotonin, noradrenaline and acetylcholine in the rat medial PFC167. Studies have shown that some of the dysfunctions seen in youths with psychopathic traits can be mimicked through manipulation of the seroton-ergic and dopaminergic systems. For example, serotonin depletion disrupts the recognition of fearful expressions and impairs performance on reinforcement-based decision-making tasks (passive avoidance learning and reversal learning)168–170 — tasks in which adolescents with psychopathic traits show impairment. The neuro-transmitter dopamine is important for reinforcement signalling56,171,172, and dopamine depletion has been shown to disrupt performance on reinforcement-based decision-making tasks173. Dopamine antagonists reduce the amygdala responsiveness to threat stimuli174, and dopamine agonists increase the amygdala response to fearful expressions175. Thus, neuroscience might provide a computational underpinning for the idea that the atypical antipsychotics are beneficial for adolescents with conduct disorder and psychopathic traits. However, it should be noted that atypical antipsychotics have considerable side effects176, including weight gain177 and type 2 diabetes mellitus178. As such, future studies should addresses whether these compounds do indeed normalize the patient’s pathophysiology.
Conclusions and future directions
Psychopathic traits are characterized by core impairments in empathy, particularly in the processing of distress cues, and core impairments in decision making, specifically in prediction error signalling and the representation of reward outcomes and expected value. These impairments are associated with dysfunction in the amygdala, vmPFC and striatum, although other brain areas may also be involved (FIG. 2). These impairments, with some exceptions, are also seen in adults with psychopathic traits (BOX 4). Studies in animals are increasing our understanding of these computational impairments.
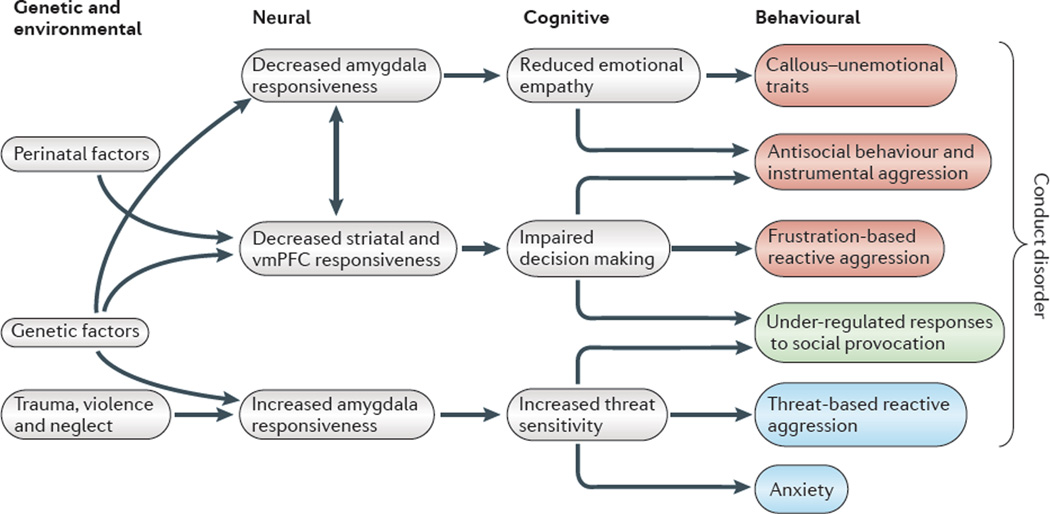
Figure 2. A framework for understanding conduct disorder.
This model shows the aetiological (genetic and environmental), neural, cognitive and behavioural aspects of conduct disorder. Genetic factors reduce amygdala activation, specifically in response to distress cues, and consequently reduce emotional empathy. Genetic factors may also influence striatal and ventromedial prefrontal cortex (vmPFC) responsiveness to prediction error and expected value information and thereby lead to impaired decision making, but this has yet to be empirically demonstrated. Owing to the extensive interconnections between the amygdala, striatum and vmPFC, early dysfunction in one area is likely to be associated with dysfunction in the others. Perinatal factors, such as maternal substance abuse during pregnancy, can affect the functional integrity of these regions. All of these factors may lead to similar dysfunction at the cognitive level and may result in callous–unemotional traits and in increased antisocial behaviour and instrumental aggression. Impairments in decision making increase the risk that these individuals fail to achieve their goals, become frustrated and demonstrate frustration-based reactive aggression. Specific genetic polymorphisms as well as exposure to trauma, violence and neglect can result in increased amygdala responsiveness, specifically to threat cues. Such increased responsiveness increases threat sensitivity and the likelihood that a threat triggers reactive aggression (as opposed to freezing or escape behaviour). Increased amygdala responsiveness is also associated with an increased risk for anxiety disorders. Thus, patients meeting criteria for conduct disorder can have callous–unemotional traits or high levels of anxiety: callous–unemotional traits are associated with reduced amygdala responses to threat, whereas anxiety is associated with increased amygdala responses to threat. This suggests that there are at least two forms of conduct disorder. The first is referred to here as ‘conduct disorder with psychopathic traits’ and includes behaviours marked in red. The second is known as ‘conduct disorder associated with anxiety and emotional lability’ and includes the behaviours marked in blue (also see BOX 1). Both forms are likely to show under-regulated responses to social provocation (marked in green).
Box 4. Comparing youths and adults with psychopathic traits.
Cognitive neuroscience studies have shown similarities and differences between youths and adults with psychopathic traits (see the table; following recent methodological criticisms about some work on adults with psychopathic traits232, this comparison includes only clinical populations that are matched for IQ or for whom IQ differences are statistically considered).
Youths with psychopathic traits and adults with psychopathic traits are notably similar in terms of their functional impairments. Both show reduced psychophysiological responsiveness to the distress of others and impaired recognition of emotional (particularly fearful and sad) expressions, extinction, reversal learning and care-based moral judgement. Adults with psychopathic traits also show impairment on aversive conditioning tasks, but such studies have not yet been conducted in younger individuals.
A comparison of structural MRI (sMRI) studies shows that amygdala volume is reduced in both youths and adults with psychopathic traits. Findings regarding the integrity of the uncinate fasciculus in these groups are inconsistent. In addition, adults with psychopathic traits seem to have reduced structural integrity of the ventromedial prefrontal cortex (vmPFC), but there are no consistent findings in younger patients.
Functional MRI (fMRI) studies have shown reduced functional connectivity between the amygdala and vmPFC in both youths and adults with psychopathic traits. Findings of reduced amygdala responses to emotional provocation in adults with psychopathic traits are similar to reduced amygdala responses to fearful expressions found in youths with psychopathic traits. But studies that specifically examined the response to fearful expressions in adults with psychopathic traits have typically not shown reduced amygdala responses233–235 (but see REF. 236). However, it is difficult to draw conclusions from these studies, as only the study that reported group differences actually observed an amygdala response to fearful expressions in the comparison individuals236.
fMRI studies have shown that youths with psychopathic traits have reduced responsiveness to reward within the vmPFC64,76. However, reward responsiveness has not been investigated in adults with psychopathic traits. Of the two fMRI studies in adults with psychopathic traits that have reported vmPFC dysfunction, one study reported reduced vmPFC differential responsiveness to moral versus non-moral images204, whereas the second study reported increased vmPFC activity in individuals identifying another person’s emotional responsiveness237. Given the differences in task between these two studies and the lack of specific investigations of reward responsiveness, it is not currently possible to conclude whether there is consistency between youths and adults with respect to vmPFC functioning.
In short, cognitive neuroscience findings in youths and adult with psychopathic traits are relatively similar — this is perhaps not surprising and indicates that the underlying pathophysiology is similar. It is likely that future studies will identify differences. For example, long-term drug abuse, which is increased in individuals with psychopathic traits228, may have progressively deleterious effects on brain structure and function, and may exacerbate pre-existing pathophysiology and cause additional dysfunctions.
| Data | Youths | Adults |
|---|---|---|
| Functional impairments (neurocognitive testing) | ||
| Psychophysiological responsiveness to the distress of others |
Reduced (as measured by skin conductance responses)39 |
Reduced (as measured by skin conductance responses)42,238,239 |
| Expression recognition | Impaired27,28 | Impaired27,28 |
| Aversive conditioning | Unclear | Impaired71 |
| Extinction | Impaired65 | Impaired240 |
| Reversal learning | Impaired68 | Impaired241 |
| Care-based moral judgement | Impaired69 | Impaired199,242,243 |
| sMRI findings | ||
| Amygdala | Reduced93–97 | Reduced121,244 |
| vmPFC | Inconsistent findings | Reduced121,245,246 |
| Uncinate fasciculus | Inconsistent findings | Reduced connectivity103–105 |
| fMRI findings | ||
| Amygdala-vmPFC functional connectivity | Reduced8,83,106 | Reduced104 |
| Amygdala responsiveness to emotional cues | Reduced8,10,30,48,49 | Reduced203,204,247 |
| vmPFC responsiveness | Reduced to reward64,76 | Inconsistent findings |
A molecular neuroscience-level understanding of this disorder is crucial for the development and refinement of treatments, but this is currently only at an early stage. Importantly, it is now possible to model aspects of the empathy and decision-making impairments in animals. For example, mice show observational learning from the emotional displays of other mice54, and rats can perform a task that is very similar to the passive avoidance task used to study individuals with psychopathic traits179,180. Such animal models allow us to target brain areas for molecular investigation that cognitive neuroscience studies of psychopathic traits have shown to be relevant to the disorder.
Perhaps the most important promise of neurobiological studies into psychopathic traits is that they may identify biomarkers that can provide differential diagnoses and predict long-term prognosis and treatment efficacy. Although differential diagnoses can be provided on the basis of an individual’s overt behaviour and their self-report of impairment, they are prone to environmental influences on behaviour, inaccuracies in self-report and clinician biases. It can be argued that, at least in the future, diagnosis by identifying pathophysiology is more likely to be relevant for treatment decisions181. Currently, we only have putative fMRI and neurocognitive biomarkers of psychopathic traits8,76. Studies will need to be conducted to determine whether they predict long-term prognosis and treatment efficacy. With respect to prognosis, some preliminary findings show that reduced amygdala volume, reduced aversive conditioning and lower error-related brain activity predict future offending74,182,183. These will need to be confirmed. Currently, we have no data on whether the putative fMRI and neurocognitive biomarkers of psychopathic traits predict treatment response. Moreover, we have no data on whether current treatments alter the pathophysiology of psychopathic traits. But fMRI studies will allow us the possibility of determining whether current (and novel) treatments address the underlying pathophysiology rather than the immediate behavioural manifestation of this pathophysiology.
There has been rapid development in our understanding of the cognitive neuroscience of psychopathic traits over the past 5 years — the first fMRI studies into the neural correlates of psychopathic traits in youths only appeared in 2008 (REFS 8,9). The collection of data is accelerating and new avenues of research, such as modelling the functional impairments in animals and molecular neuroscience approaches, are becoming available. It is perhaps time to believe that we will soon be able to more effectively help adolescents with psychopathic traits.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health, USA, under grant number 1-ZIA-MH002860-08.
Abbreviations
- Observational fear
The phenomenon that an infant’s avoidance responses to a previously novel object are modified by the mother’s apparent emotional reaction to this object. Typically, infants avoid objects associated with maternal fear
- Transgressions
Actions that violate norms
- Passive avoidance learning
An experimental paradigm in which the individual learns to approach or passively avoid (by not responding to) objects that elicit either reward or punishment (for example, money gain or loss)
- Operant extinction
An experimental paradigm in which the individual learns that responding to an object is rewarding but then, after a change of reinforcement contingency, should extinguish this response as responding comes to be associated with punishment
- Reversal learning
An experimental paradigm in which the individual initially learns to make a response towards one of a paired set of stimuli to gain reward but then, after a change of reinforcement contingency, should reverse their behaviour towards the second object as the first object comes to be associated with punishment
- Prediction error
The difference between the amount of reward or punishment received and the amount expected
- Expected value
The expected reward or punishment following the commission of a specific response
- Functional anisotropy
A parameter in diffusion tensor imaging, which images brain structures by measuring the diffusion properties of water molecules. It provides information about the microstructural integrity of white-matter tracts
Footnotes
Competing interests statement
The author declares no competing financial interests.
References
- 1.Kazdin AE, Whitley M, Marciano PL. Child- therapist and parent-therapist alliance and therapeutic change in the treatment of children referred for oppositional, aggressive, and antisocial behavior. J. Child Psychol. Psychiatry. 2006;47:436–445. doi: 10.1111/j.1469-7610.2005.01475.x. [DOI] [PubMed] [Google Scholar]
- 2.Barry CT, et al. The importance of callous-unemotional traits for extending the concept of psychopathy to children. J. Abnorm. Psychol. 2000;109:335–340. doi: 10.1037/0021-843X.109.2.335. [DOI] [PubMed] [Google Scholar]
- 3.Lynam DR, Caspi A, Moffitt TE, Loeber R, Stouthamer-Loeber M. Longitudinal evidence that psychopathy scores in early adolescence predict adult psychopathy. J. Abnorm. Psychol. 2007;116:155–165. doi: 10.1037/0021-843X.116.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke JD, Loeber R, Lahey BB. Adolescent conduct disorder and interpersonal callousness as predictors of psychopathy in young adults. J. Clin. Child Adolesc. Psychol. 2007;36:334–346. doi: 10.1080/15374410701444223. [DOI] [PubMed] [Google Scholar]
- 5.Wootton JM, Frick PJ, Shelton KK, Silverthorn P. Ineffective parenting and childhood conduct problems: the moderating role of callous- unemotional traits. J. Consult. Clin. Psychol. 1997;65:301–308. doi: 10.1037/0022-006x.65.2.292.b. The first study to report that the type of parenting has less of an impact on the behaviour of youths with high levels of callous-unemotional traits relative to youths with low levels of callous- unemotional traits; that is, the study shows that the pathophysiology of callous-unemotional traits interferes with socialization.
- 6.Hawes DJ, Dadds MR. The treatment of conduct problems in children with callous-unemotional traits. J. Consult. Clin. Psychol. 2005;73:737–741. doi: 10.1037/0022-006X.73.4.737. [DOI] [PubMed] [Google Scholar]
- 7.Waschbusch DA, Carrey NJ, Willoughby MT, King S, Andrade BF. Effects of methylphenidate and behavior modification on the social and academic behavior of children with disruptive behavior disorders: the moderating role of callous/unemotional traits. J. Clin. Child Adolesc. Psychol. 2007;36:629–644. doi: 10.1080/15374410701662766. A good example of a paper showing that psychosocial techniques have less of an impact on the behaviour of youths with high levels of callous- unemotional traits than youths with low levels of callous-unemotional traits. This paper is of particular interest as it also suggests that methylphenidate administration may be helpful in youths with high callous-unemotional traits.
- 8.Marsh AA, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am. J. Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. The first study to document that youths with psychopathic traits show reduced amygdala responses to the fearful expressions of other individuals.
- 9.Finger EC, et al. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch. General Psychiatry. 2008;65:586–594. doi: 10.1001/archpsyc.65.5.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am. J. Psychiatry. 2009;166:95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- 11.Pardini DA, Frick PJ, Moffitt TE. Building an evidence base for DSM-5 conceptualizations of oppositional defiant disorder and conduct disorder: introduction to the special section. J. Abnorm. Psychol. 2010;119:683–688. doi: 10.1037/a0021441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hare RD. A research scale for the assessment of psychopathy in criminal populations. Pers. Indiv. Differ. 1980;1:111–119. [Google Scholar]
- 13.Frick PJ. Callous-unemotional traits and conduct problems: a two-factor model of psychopathy in children. Issues Criminal. Legal Psychol. 1995;24:47–51. [Google Scholar]
- 14.Blair RJR. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious. Cogn. 2005;14:698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Frith U. Autism: Explaining the Enigma. Blackwell; 1989. [Google Scholar]
- 16.Blair RJR, et al. Theory of mind in the psychopath. J. Forens. Psychiatry. 1996;7:15–25. [Google Scholar]
- 17.Richell RA, et al. Theory of mind and psychopathy: can psychopathic individuals read the ‘language of the eyes’? Neuropsychologia. 2003;41:523–526. doi: 10.1016/s0028-3932(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 18.Dolan M, Fullam R. Theory of mind and mentalizing ability in antisocial personality disorders with and without psychopathy. Psychol. Med. 2004;34:1093–1102. doi: 10.1017/s0033291704002028. [DOI] [PubMed] [Google Scholar]
- 19.Jones AP, Happe FG, Gilbert F, Burnett S, Viding E. Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. J. Child Psychol. Psychiatry. 2010;51:1188–1197. doi: 10.1111/j.1469-7610.2010.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anastassiou-Hadjicharalambous X, Warden D. Cognitive and affective perspective-taking in conduct-disordered children high and low on callous-unemotional traits. Child Adolesc. Psychiatry Ment. Health. 2008;2:16. doi: 10.1186/1753-2000-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastian CL, et al. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch. Gen. Psychiatry. 2012;69:814–822. doi: 10.1001/archgenpsychiatry.2011.2070. [DOI] [PubMed] [Google Scholar]
- 22.Lombardo MV, et al. Shared neural circuits for mentalizing about the self and others. J. Cogn. Neurosci. 2010;22:1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- 23.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Rev. Neurosci. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 24.Saxe R, Baron-Cohen S. The neuroscience of theory of mind. Soc. Neurosci. 2006;1:1–9. doi: 10.1080/17470910601117463. [DOI] [PubMed] [Google Scholar]
- 25.Blair RJR. Facial expressions, their communicatory functions and neuro-cognitive substrates. Phil. Trans. R. Soc. Lond. B. 2003;358:561–572. doi: 10.1098/rstb.2002.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fridlund Ain. In: International Review of Studies on Emotion. Strongman KT, editor. Vol. 2. Wiley-Blackwell; 1992. pp. 117–137. [Google Scholar]
- 27.Marsh AA, Blair RJ. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neurosci. Biobehav. Rev. 2007;32:454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dawel A, O'Kearney R, McKone E, Palermo R. Not just fear and sadness: meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neurosci. Biobehav. Rev. 2012;36:2288–2304. doi: 10.1016/j.neubiorev.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 29.White SF, et al. Reduced activity within the dorsal endogenous orienting of attention network to fearful expressions in youth with disruptive behavior disorders and psychopathic traits. Dev. Psychopathol. 2012;24:1105–1116. doi: 10.1017/S0954579412000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carre JM, Hyde LW, Neumann CS, Viding E, Hariri AR. The neural signatures of distinct psychopathic traits. Soc. Neurosci. 2013;8:122–135. doi: 10.1080/17470919.2012.703623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blair RJR, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. J. Abnorm. Child Psychol. 2001;29:491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- 32.Blair RJR, et al. Reduced sensitivity to other’s fearful expressions in psychopathic individuals. Pers. Indiv. Differ. 2004;37:1111–1121. [Google Scholar]
- 33.Dolan M, Fullam R. Face affect recognition deficits in personality-disordered offenders: association with psychopathy. Psychol. Med. 2006;36:1563–1569. doi: 10.1017/S0033291706008634. [DOI] [PubMed] [Google Scholar]
- 34.Dadds MR, et al. Attention to the eyes and fear-recognition deficits in child psychopathy. Br. J. Psychiatry. 2006;189:280–281. doi: 10.1192/bjp.bp.105.018150. An important study documenting that the impairment in the recognition of fearful expressions seen in youths with callous- unemotional traits is significantly reduced when the participant’s attention is directed to the eye region of the face. This improvement is also seen in patients with amygdala lesions.
- 35.Stevens D, Charman T, Blair RJR. Recognition of emotion in facial expressions and vocal tones in children with psychopathic tendencies. J. Genet. Psychol. 2001;162:201–211. doi: 10.1080/00221320109597961. [DOI] [PubMed] [Google Scholar]
- 36.Woodworth M, Waschbusch D. Emotional processing in children with conduct problems and callous/unemotional traits. Child Care Health Dev. 2008;34:234–244. doi: 10.1111/j.1365-2214.2007.00792.x. [DOI] [PubMed] [Google Scholar]
- 37.Blair RJR, Budhani S, Colledge E, Scott S. Deafness to fear in boys with psychopathic tendencies. J. Child Psychol. Psychiatry. 2005;46:327–336. doi: 10.1111/j.1469-7610.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- 38.Munoz L. Callous-unemotional traits are related to combined deficits in recognizing afraid faces and body poses. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:554–562. doi: 10.1097/CHI.0b013e31819c2419. [DOI] [PubMed] [Google Scholar]
- 39.Blair RJR. Responsiveness to distress cues in the child with psychopathic tendencies. Pers. Indiv. Differ. 1999;27:135–145. [Google Scholar]
- 40.de Wied M, van Boxtel A, Matthys W, Meeus W. Verbal, facial and autonomic responses to empathy-eliciting film clips by disruptive male adolescents with high versus low callous-unemotional traits. J. Abnorm. Child Psychol. 2012;40:211–223. doi: 10.1007/s10802-011-9557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anastassiou-Hadjicharalambous X, Warden D. Physiologically-indexed and self-perceived affective empathy in conduct-disordered children high and low on callous-unemotional traits. Child Psychiatry Hum. Dev. 2008;39:503–517. doi: 10.1007/s10578-008-0104-y. [DOI] [PubMed] [Google Scholar]
- 42.Aniskiewicz AS. Autonomic components of vicarious conditioning and psychopathy. J. Clin. Psychol. 1979;35:60–67. doi: 10.1002/1097-4679(197901)35:1<60::aid-jclp2270350106>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 43.Cheng Y, Hung AY, Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Dev. Psychopathol. 2012;24:623–636. doi: 10.1017/S095457941200020X. [DOI] [PubMed] [Google Scholar]
- 44.Pardini DA, Byrd AL. Perceptions of aggressive conflicts and others’ distress in children with callous-unemotional traits: ‘I’ll show you who’s boss, even if you suffer and I get in trouble'. J. Child Psychol. Psychiatry. 2012;53:283–291. doi: 10.1111/j.1469-7610.2011.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dadds MR, El Masry Y, Wimalaweera S, Guastella AJ. Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:455–463. doi: 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- 46.Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 47.Adolphs R, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 48.White SF, et al. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. Am. J. Psychiatry. 2012;169:750–758. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Viding E, et al. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. Am. J. Psychiatry. 2012;169:1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- 50.Marsh AA, et al. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. J. Child Psychol. Psychiatry. 2013;54:900–910. doi: 10.1111/jcpp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klinnert MD, Emde RN, Butterfield P, Campos JJ. Social referencing: the infant’s use of emotional signals from a friendly adult with mother present. Annu. Prog. Child Psychiatry Child Dev. 1987;22:427–432. [Google Scholar]
- 52.Mineka S, Cook M. Mechanisms involved in the observational conditioning of fear. J. Exp. Psychol. Gen. 1993;122:23–38. doi: 10.1037//0096-3445.122.1.23. [DOI] [PubMed] [Google Scholar]
- 53.Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn. Sci. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Jeon D, et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nature Neurosci. 2010;13:482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cushman F, Gray K, Gaffrey A, Mendes WB. Simulating murder: the aversion to harmful action. Emotion. 2012;12:2–7. doi: 10.1037/a0025071. [DOI] [PubMed] [Google Scholar]
- 56.O’Doherty JP. Beyond simple reinforcement learning: the computational neurobiology of reward-learning and valuation. Eur. J. Neurosci. 2012;35:987–990. doi: 10.1111/j.1460-9568.2012.08074.x. [DOI] [PubMed] [Google Scholar]
- 57.Balleine BW, O’Doherty JP. Human and rodent homologues in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Budhani S, Marsh AA, Pine DS, Blair RJR. Neural correlates of response reversal: considering acquisition. Neuroimage. 2007;34:1754–1765. doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 59.Kuhnen CM, Knutson B. The neural basis of financial risk-taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Driscoll DM, Dal Monte O, Solomon J, Krueger F, Grafman J. Empathic deficits in combat veterans with traumatic brain injury: a voxel-based lesion-symptom mapping study. Cogn. Behav. Neurol. 2012;25:160–166. doi: 10.1097/WNN.0b013e318280cf4e. [DOI] [PubMed] [Google Scholar]
- 61.Engen HG, Singer T. Empathy circuits. Curr. Opin. Neurobiol. 2012;23:275–282. doi: 10.1016/j.conb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Janowski V, Camerer C, Rangel A. Empathic choice involves vmPFC value signals that are modulated by social processing implemented in IPL. Soc. Cogn. Affect. Neurosci. 2013;8:201–208. doi: 10.1093/scan/nsr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leopold A, et al. Damage to the left ventromedial prefrontal cortex impacts affective theory of mind. Soc. Cogn. Affect. Neurosci. 2012;7:871–880. doi: 10.1093/scan/nsr071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Finger EC, et al. Disrupted reinforcement signaling in the orbital frontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am. J. Psychiatry. 2011;168:834–841. doi: 10.1176/appi.ajp.2010.10010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher L, Blair RJR. Cognitive impairment and its relationship to psychopathic tendencies in children with emotional and behavioural difficulties. J. Abnorm. Child Psychol. 1998;26:511–519. doi: 10.1023/a:1022655919743. [DOI] [PubMed] [Google Scholar]
- 66.O’Brien BS, Frick PJ. Reward dominance: associations with anxiety, conduct problems, and psychopathy in children. J. Abnorm. Child Psychol. 1996;24:223–240. doi: 10.1007/BF01441486. [DOI] [PubMed] [Google Scholar]
- 67.Blair RJR, Colledge E, Mitchell DG. Somatic markers and response reversal: is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? J. Abnorm. Child Psychol. 2001;29:499–511. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- 68.Budhani S, Blair RJR. Response reversal and children with psychopathic tendencies: success is a function of salience of contingency change. J. Child Psychol. Psychiatry. 2005;46:972–981. doi: 10.1111/j.1469-7610.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 69.Blair RJR. Moral reasoning in the child with psychopathic tendencies. Pers. Indiv. Differ. 1997;22:731–739. [Google Scholar]
- 70.Fairchild G, et al. Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biol. Psychiatry. 2009;66:162–168. doi: 10.1016/j.biopsych.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rothemund Y, et al. Fear conditioning in psychopaths: event-related potentials and peripheral measures. Biol. Psychol. 2012;90:50–59. doi: 10.1016/j.biopsycho.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 72.Birbaumer N, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch. Gen. Psychiatry. 2005;62:799–805. doi: 10.1001/archpsyc.62.7.799. [DOI] [PubMed] [Google Scholar]
- 73.Fairchild G, Van Goozen SH, Stollery SJ, Goodyer IM. Fear conditioning and affective modulation of the startle reflect in male adolescents with early-onset of adolescence-onset conduct disorder and healthy control subjects. Biol. Psychiatry. 2008;63:279–285. doi: 10.1016/j.biopsych.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 74.Gao Y, Raine A, Venables PH, Dawson ME, Mednick SA. Association of poor childhood fear conditioning and adult crime. Am. J. Psychiatry. 2010;167:56–60. doi: 10.1176/appi.ajp.2009.09040499. [DOI] [PubMed] [Google Scholar]
- 75.Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Phil. Trans. R. Soc. B. 2008;363:2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.White SF, et al. Disrupted expected value and prediction error signaling in youth with disruptive behavior disorders during a passive avoidance task. Am. J. Psychiatry. 2013;170:315–323. doi: 10.1176/appi.ajp.2012.12060840. The first model-based fMRI study of the reinforcement-based decision-making impairment in youths with disruptive behaviour disorders and its relationship with psychopathic traits. This was the first study to report specific computational impairments in this population in prediction error and expected value signalling within the caudate and vmPFC, respectively.
- 77.Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- 78.Rescorla RA, Wagner AR. In: Classical Conditioning II. Black AH, Prokasy WF, editors. Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 79.Rubia K, et al. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. Am. J. Psychiatry. 2009;166:83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- 80.Crowley TJ, et al. Risky decisions and their consequences: neural processing by boys with antisocial substance disorder. PLoS ONE. 2010;5:e12835. doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bjork JM, Chen G, Smith AR, Hommer DW. Incentive-elicited mesolimbic activation and externalizing symptomatology in adolescents. J. Child Psychol. Psychiatry. 2010;51:827–837. doi: 10.1111/j.1469-7610.2009.02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anderson NE, Kiehl KA. The psychopath magnetized: insights from brain imaging. Trends Cogn. Sci. 2012;16:52–60. doi: 10.1016/j.tics.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marsh AA, et al. Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Res. 2011;194:279–286. doi: 10.1016/j.pscychresns.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Strohle A, et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39:966–972. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- 85.Plichta MM, et al. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/ hyperactivity disorder. Biol. Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 86.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/ hyperactivity disorder. Biol. Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 87.Chassin L, Pitts SC, DeLucia C, Todd M. A longitudinal study of children of alcoholics: predicting young adult substance use disorders, anxiety, and depression. J. Abnorm. Psychol. 1999;108:106–119. doi: 10.1037//0021-843x.108.1.106. [DOI] [PubMed] [Google Scholar]
- 88.Serec M, et al. Health-related lifestyle, physical and mental health in children of alcoholic parents. Drug Alcohol Rev. 2012;31:861–870. doi: 10.1111/j.1465-3362.2012.00424.x. [DOI] [PubMed] [Google Scholar]
- 89.Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol. Clin. Exp. Res. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yau WY, et al. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J. Neurosci. 2012;32:2544–2551. doi: 10.1523/JNEUROSCI.1390-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jensen PS, et al. ADHD comorbidity findings from the MTA study: comparing comorbid subgroups. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 92.Armstrong TD, Costello EJ. Community studies on adolescent substance use, abuse, or dependence and psychiatric comorbidity. J. Consult. Clin. Psychol. 2002;70:1224–1239. doi: 10.1037//0022-006x.70.6.1224. [DOI] [PubMed] [Google Scholar]
- 93.Huebner B, et al. Morphometric brain abnormalities in boys with conduct disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:540–547. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- 94.Sterzer P, Stadler C, Poustka F, Kleinschmidt A. A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage. 2007;37:335–342. doi: 10.1016/j.neuroimage.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 95.Fairchild G, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. Am. J. Psychiatry. 2011;168:624–633. doi: 10.1176/appi.ajp.2010.10081184. [DOI] [PubMed] [Google Scholar]
- 96.Fairchild G, et al. Brain structure abnormalities in adolescent girls with conduct disorder. J. Child Psychol. Psychiatry. 2013;54:86–95. doi: 10.1111/j.1469-7610.2012.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in incarcerated male adolescents with psychopathic traits. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:94–103. doi: 10.1016/j.jaac.2012.10.013. One of the few structural imaging studies of youths with psychopathic traits. It is particularly important because of the large number of participants assessed.
- 98.De Brito SA, et al. Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- 99.Dalwani M, et al. Reduced cortical gray matter volume in male adolescents with substance and conduct problems. Drug Alcohol Depend. 2011;118:295–305. doi: 10.1016/j.drugalcdep.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krusei MJP, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 101.Hyatt CJ, Haney-Caron E, Stevens MC. Cortical thickness and folding deficits in conduct-disordered adolescents. Biol. Psychiatry. 2011;72:207–214. doi: 10.1016/j.biopsych.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fahim C, et al. Neuroanatomy of childhood disruptive behavior disorders. Aggress. Behav. 2011;37:326–337. doi: 10.1002/ab.20396. [DOI] [PubMed] [Google Scholar]
- 103.Craig MC, et al. Altered connections on the road to psychopathy. Mol. Psychiatry. 2009;14:946–953. doi: 10.1038/mp.2009.40. [DOI] [PubMed] [Google Scholar]
- 104.Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. J. Neurosci. 2011;31:17348–17357. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sundram F, et al. White matter microstructural abnormalities in the frontal lobe of adults with antisocial personality disorder. Cortex. 2012;48:216–229. doi: 10.1016/j.cortex.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 106.Finger EC, et al. Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Res. 2012;202:239–244. doi: 10.1016/j.pscychresns.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarkar S, et al. Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: a diffusion tensor imaging study. Psychol. Med. 2013;43:401–411. doi: 10.1017/S003329171200116X. [DOI] [PubMed] [Google Scholar]
- 108.Passamonti L, et al. Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS ONE. 2012;7:e48789. doi: 10.1371/journal.pone.0048789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Upadhyay J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bodensteiner JB, Schaefer GB. Wide cavum septum pellucidum: a marker of disturbed brain development. Pediatr. Neurol. 1990;6:391–394. doi: 10.1016/0887-8994(90)90007-n. [DOI] [PubMed] [Google Scholar]
- 111.Sarwar M. The septum pellucidum: normal and abnormal. Am. J. Neuroradiol. 1989;10:989–1005. [PMC free article] [PubMed] [Google Scholar]
- 112.White SF, et al. The relationship between large cavum septum pellucidum and antisocial behavior, callous- unemotional traits and psychopathy in adolescents. J. Child Psychol. Psychiatry. 2012;54:575–581. doi: 10.1111/j.1469-7610.2012.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Raine A, Lee L, Yang Y, Colletti P. Neurodevelopmental marker for limbic maldevelopment in antisocial personality disorder and psychopathy. Br. J. Psychiatry. 2010;197:186–192. doi: 10.1192/bjp.bp.110.078485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.May FS, Chen QC, Gilbertson MW, Shenton ME, Pitman RK. Cavum septum pellucidum in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. Biol. Psychiatry. 2004;55:656–658. doi: 10.1016/j.biopsych.2003.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nopoulos P, Krie A, Andreasen NC. Enlarged cavum septi pellucidi in patients with schizophrenia: clinical and cognitive correlates. J. Neuropsychiatry Clin. Neurosci. 2000;12:344–349. doi: 10.1176/jnp.12.3.344. [DOI] [PubMed] [Google Scholar]
- 116.Kim MJ, et al. The occurrence of cavum septipellucidi enlargement is increased in bipolar disorder patients. Bipolar Disord. 2007;9:274–280. doi: 10.1111/j.1399-5618.2007.00442.x. [DOI] [PubMed] [Google Scholar]
- 117.Swayze V, et al. Magnestic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–240. doi: 10.1542/peds.99.2.232. [DOI] [PubMed] [Google Scholar]
- 118.Streissguth AP, et al. Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. J. Dev. Behav. Pediatr. 2004;25:228–238. doi: 10.1097/00004703-200408000-00002. [DOI] [PubMed] [Google Scholar]
- 119.Wakschlag LS, et al. Interaction of prenatal exposure to cigarettes and MAOA genotype in pathyways to youth antisocial behavior. Mol. Psychiatry. 2013;15:928–937. doi: 10.1038/mp.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schlotz W, Phillips DI. Fetal origins of mental health: evidence and mechanisms. Brain Behav. Immunol. 2009;23:905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 121.Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. J. Abnorm. Psychol. 2012;121:649–658. doi: 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van Goozen SH, Matthys W, Cohen-Kettenis PT, Thijssen JH, van Engeland H. Adrenal androgens and aggression in conduct disorder prepubertal boys and normal controls. Biol. Psychiatry. 1998;43:156–158. doi: 10.1016/S0006-3223(98)00360-6. [DOI] [PubMed] [Google Scholar]
- 123.Fairchild G, et al. Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biol. Psychiatry. 2008;64:599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lopez-Duran NL, Olson SL, Hajal NJ, Felt BT, Vazquez DM. Hypothalamic pituitary adrenal axis functioning in reactive and proactive aggression in children. J. Abnorm. Child Psychol. 2009;37:169–182. doi: 10.1007/s10802-008-9263-3. [DOI] [PubMed] [Google Scholar]
- 125.LeDoux JE. The amygdala. Curr. Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 126.Hawes DJ, Brennan J, Dadds MR. Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Curr. Opin. Psychiatry. 2009;22:357–362. doi: 10.1097/YCO.0b013e32832bfa6d. [DOI] [PubMed] [Google Scholar]
- 127.Blair RJR, Peschardt KS, Budhani S, Mitchell DG, Pine DS. The development of psychopathy. J. Child Psychol. Psychiatry. 2006;47:262–276. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- 128.Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol. Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- 129.Viding E, Blair RJR, Moffitt TE, Plomin R. Evidence for substantial genetic risk for psychopathy in 7-year-olds. J. Child Psychol. Psychiatry. 2005;46:592–597. doi: 10.1111/j.1469-7610.2004.00393.x. One of the first studies to document the high heritability of callous-unemotional traits in youths.
- 130.Viding E, et al. In search of genes associated with risk for psychopathic tendencies in children: a two-stage genome-wide association study of pooled DNA. J. Child Psychol. Psychiatry. 2010;51:780–788. doi: 10.1111/j.1469-7610.2010.02236.x. [DOI] [PubMed] [Google Scholar]
- 131.Smolka MN, et al. Catechol-O-methyltransferase Val158Met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J. Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heinz AJ, Beck A, Meyer-Lindenberg A, Sterzer P, Heinz A. Cognitive and neurobiological mechanisms of alcohol-related aggression. Nature Rev. Neurosci. 2011;12:400–413. doi: 10.1038/nrn3042. [DOI] [PubMed] [Google Scholar]
- 133.Meyer-Lindenberg A, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl Acad. Sci. USA. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rujescu D, Giegling I, Gietl A, Hartmann AM, Moller HJ. A functional single nucleotide polymorphism (V158M) in the COMT gene is associated with aggressive personality traits. Biol. Psychiatry. 2003;54:34–39. doi: 10.1016/s0006-3223(02)01831-0. [DOI] [PubMed] [Google Scholar]
- 135.Caspi A, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 136.Beitchman JH, et al. Serotonin transporter polymorphisms and persistent, pervasive childhood aggression. Am. J. Psychiatry. 2006;164:1103–1105. doi: 10.1176/ajp.2006.163.6.1103. [DOI] [PubMed] [Google Scholar]
- 137.Zai C, et al. Dopaminergic system genes in childhood aggression: possible role for DRD2. World J. Biol. Psychiatry. 2012;13:65–74. doi: 10.3109/15622975.2010.543431. [DOI] [PubMed] [Google Scholar]
- 138.Sadeh N, et al. Serotonin transporter gene associations with psychopathic traits in youth vary as a function of socioeconomic resources. J. Abnorm. Psychol. 2010;119:604–609. doi: 10.1037/a0019709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hirata Y, Zai CC, Nowrouzi B, Beitchman JH, Kennedy JL. Study of the catechol-O-methyltransferase (COMT) gene with high aggression in children. Aggress. Behav. 2013;39:45–51. doi: 10.1002/ab.21448. [DOI] [PubMed] [Google Scholar]
- 140.Hariri AR, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch. Gen. Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 141.Moul C, Dobson-Stone C, Brennan J, Hawes D, Dadds M. An exploration of the serotonin system in antisocial boys with high levels of callous-unemotional traits. PLoS ONE. 2013;8:e56619. doi: 10.1371/journal.pone.0056619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beitchman JH, et al. Childhood aggression, callous-unemotional traits and oxytocin genes. Eur. Child Adolesc. Psychiatry. 2012;21:125–132. doi: 10.1007/s00787-012-0240-6. [DOI] [PubMed] [Google Scholar]
- 143.McCrory EJ, et al. Heightened neural reactivity to threat in child victims of family violence. Curr. Biol. 2011;21:R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 144.Tottenham N, et al. Elevated amygdala response to faces following early deprivation. Dev. Sci. 2011;14:190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am. J. Psychiatry. 2012;169:515–522. doi: 10.1176/appi.ajp.2011.11060855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dodge KA, Pettit GS, Bates JE, Valente E. Social information-processing patterns partially mediate the effect of early physical abuse on later conduct problems. J. Abnorm. Psychol. 1995;104:632–643. doi: 10.1037//0021-843x.104.4.632. A classic study demonstrating the impact that physical abuse has on the development of hostile attribution biases and the implications of this for the development of reactive aggression.
- 147.Fontaine NM, Rijsdijk FV, McCrory EJ, Viding E. Etiology of different developmental trajectories of callous-unemotional traits. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49:656–664. doi: 10.1016/j.jaac.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 148.Barker ED, Oliver BR, Viding E, Salekin RT, Maughan B. The impact of prenatal maternal risk, fearless temperament and early parenting on adolescent callous-unemotional traits: a 14-year longitudinal investigation. J. Child Psychol. Psychiatry. 2011;52:878–888. doi: 10.1111/j.1469-7610.2011.02397.x. [DOI] [PubMed] [Google Scholar]
- 149.Durlak JA, Weissberg RP, Dymnicki AB, Taylor RD, Schellinger KB. The impact of enhancing students’ social and emotional learning: a meta-analysis of school-based universal interventions. Child Dev. 2011;82:405–432. doi: 10.1111/j.1467-8624.2010.01564.x. [DOI] [PubMed] [Google Scholar]
- 150.Chamberlain P, Smith DK. In: Evidence-Based Psychotherapies for Children and Adolescents. Kazdin AE, Weisz JR, editors. Guilford Press; 2003. pp. 282–300. [Google Scholar]
- 151.Henggeler SW, Lee T. In: Evidence-Based Psychotherapies for Children and Adolescents. Kazdin AE, Weisz JR, editors. Guilford Press; 2003. pp. 301–322. [Google Scholar]
- 152.Eyberg SM, Nelson MM, Boggs SR. Evidence-based psychosocial treatments for children and adolescents with disruptive behavior. J. Clin. Child Adolesc. Psychol. 2008;37:215–237. doi: 10.1080/15374410701820117. [DOI] [PubMed] [Google Scholar]
- 153.Oxford M, Cavell TA, Hughes JN. Callous/ unemotional traits moderate the relation between ineffective parenting and child externalizing problems: a partial replication and extension. J. Clin. Child Adolesc. Psychol. 2003;32:577–585. doi: 10.1207/S15374424JCCP3204_10. [DOI] [PubMed] [Google Scholar]
- 154.Pasalich DS, Dadds MR, Hawes DJ, Brennan J. Do callous-unemotional traits moderate the relative importance of parental coercion versus warmth in child conduct problems? An observational study. J. Child Psychol. Psychiatry. 2011;52:1308–1315. doi: 10.1111/j.1469-7610.2011.02435.x. [DOI] [PubMed] [Google Scholar]
- 155.Haas SM, et al. Treatment response in CP/ADHD children with callous/unemotional traits. J. Abnorm. Child Psychol. 2011;39:541–552. doi: 10.1007/s10802-010-9480-4. [DOI] [PubMed] [Google Scholar]
- 156.Masi G, et al. Predictors of nonresponse to psychosocial treatment in children and adolescents with disruptive behavior disorders. J. Child Adolesc. Psychopharmacol. 2011;21:51–55. doi: 10.1089/cap.2010.0039. [DOI] [PubMed] [Google Scholar]
- 157.Manders WA, Dekovic M, Asscher JJ, van der Laan PH, Prins PJ. Psychopathy as predictor and moderator of multisystemic therapy outcomes among adolescents treated for antisocial behavior. J. Abnorm. Child Psychol. 2013;41:1121–1132. doi: 10.1007/s10802-013-9749-5. [DOI] [PubMed] [Google Scholar]
- 158.Felmingham K, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of post traumatic stress disorder. Psychol. Sci. 2007;18:127–129. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 159.Afifi TO, McMillan KA, Asmundson GJ, Pietrzak RH, Sareen J. An examination of the relation between conduct disorder, childhood and adulthood traumatic events, and posttraumatic stress disorder in a nationally representative sample. J. Psychiatr. Res. 2011;45:1564–1572. doi: 10.1016/j.jpsychires.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 160.Hawes DJ, Dadds MR. Stability and malleability of callous-unemotional traits during treatment for childhood conduct problems. J. Clin. Child Adolesc. Psychol. 2007;36:347–355. doi: 10.1080/15374410701444298. [DOI] [PubMed] [Google Scholar]
- 161.Pardini DA, Lochman JE, Powell N. The development of callous-unemotional traits and antisocial behavior in children: are there shared and/ or unique predictors? J. Clin. Child Adolesc. Psychol. 2007;36:319–333. doi: 10.1080/15374410701444215. [DOI] [PubMed] [Google Scholar]
- 162.Greenaway M, Elbe D. Focus on aripiprazole: a review of its use in child and adolescent psychiatry. J. Can. Acad. Child Adolesc. Psychiatry. 2009;18:250–260. [PMC free article] [PubMed] [Google Scholar]
- 163.Findling RL. Atypical antipsychotic treatment of disruptive behavior disorders in children and adolescents. J. Clin. Psychiatry. 2008;69(Suppl. 4):9–14. [PubMed] [Google Scholar]
- 164.Zito JM, et al. Psychotropic medication patterns among youth in foster care. Pediatrics. 2008;121:e157–e163. doi: 10.1542/peds.2007-0212. [DOI] [PubMed] [Google Scholar]
- 165.Burris KD, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J. Pharmacol. Exp. Ther. 2002;302:381–389. doi: 10.1124/jpet.102.033175. [DOI] [PubMed] [Google Scholar]
- 166.Taylor DM. Aripiprazole: a review of its pharmacology and clinical use. Int. J. Clin. Pract. 2003;57:49–54. [PubMed] [Google Scholar]
- 167.Huang M, Ichiwaka J, Li Z, Dai J, Meltzer HY. Augmentation by citalopram of risperidone-induced monoamine release in rat prefrontal cortex. Psychopharmacol. (Berl.) 2006;185:274–281. doi: 10.1007/s00213-005-0206-1. [DOI] [PubMed] [Google Scholar]
- 168.Blair KS, et al. The role of 5-HTTLPR in choosing the lesser of two evils, the better of two goods: examining the impact of 5-HTTLPR genotype and tryptophan depletion in object choice. Psychopharmacology. 2008;196:29–38. doi: 10.1007/s00213-007-0920-y. [DOI] [PubMed] [Google Scholar]
- 169.Finger EC, et al. The impact of tryptophan depletion and 5-HTTLPR genotype on passive avoidance and response reversal instrumental learning tasks. Neuropsychopharmacology. 2007;32:206–215. doi: 10.1038/sj.npp.1301182. [DOI] [PubMed] [Google Scholar]
- 170.Marsh AA, et al. Impaired recognition of fear facial expressions in 5-HTTLPR S-polymorphism carriers following tryptophan depletion. Psychopharmacology (Berl.) 2006;189:387–394. doi: 10.1007/s00213-006-0581-2. [DOI] [PubMed] [Google Scholar]
- 171.Schultz W. Multiple functions of dopamine neurons. F1000 Biol. Rep. 2010;2:2. doi: 10.3410/B2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dayan P. Instrumental vigour in punishment and reward. Eur. J. Neurosci. 2012;35:1152–1168. doi: 10.1111/j.1460-9568.2012.08026.x. [DOI] [PubMed] [Google Scholar]
- 173.Hasler G, Mondillo K, Drevets WC, Blair RJR. Impairments of probabilistic response reversal and passive avoidance following catecholamine depletion. Neuropsychopharmacology. 2009;34:2691–2698. doi: 10.1038/npp.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Takahashi H, et al. Effects of dopaminergic and serotonergic manipulation on emotional processing: a pharmacological fMRI study. Neuroimage. 2005;27:991–1001. doi: 10.1016/j.neuroimage.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 175.Hariri AR, et al. Dexroamphetamine modulates the response of the human amygdala. Neuropsychopharmacology. 2002;27:1036–1040. doi: 10.1016/S0893-133X(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 176.Rugino TA, Janvier YM. Aripiprazole in children and adolescents: clinical experience. J. Child Neurol. 2005;20:603–610. doi: 10.1177/08830738050200071301. [DOI] [PubMed] [Google Scholar]
- 177.Allison DB, Casey DE. Antipsychotic-induced weight gain: a review of the literature. J. Clin. Psychiatry. 2001;62:22–31. [PubMed] [Google Scholar]
- 178.Lambert MT, Copeland LA, Sampson N, Duffy SA. New-onset type-2 diabetes associated with atypical antipsychotic medications. Biol. Psychiatry. 2006;30:919–923. doi: 10.1016/j.pnpbp.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 179.Schoenbaum G, Roesch M. Orbitofrontal cortex, associative learning, and expectancies. Neuron. 2005;47:633–636. doi: 10.1016/j.neuron.2005.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nature Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 181.Insel T, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 182.Aharoni E, et al. Neuroprediction of future rearrest. Proc. Natl Acad. Sci. USA. 2013;110:6223–6228. doi: 10.1073/pnas.1219302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pardini DA, Erickson K, Loeber R, Raine A. Lower amygdala volume in men is associated with childhood aggression, early psychopathic traits, and future violence. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.04.003. http://dx.doi.org/10.1016/j.biopsych.2013.04.003. [DOI] [PMC free article] [PubMed]
- 184.Blanchard RJ, Blanchard DC, Takahashi LK. Attack and defensive behaviour in the albino rat. Animal Behav. 1977;25:197–224. doi: 10.1016/0003-3472(77)90113-0. [DOI] [PubMed] [Google Scholar]
- 185.Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. Oxford Univ. Press; 1998. [Google Scholar]
- 186.Gregg TR, Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:91–140. doi: 10.1016/s0278-5846(00)00150-0. [DOI] [PubMed] [Google Scholar]
- 187.Lin D, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226. doi: 10.1038/nature09736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nature Rev. Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- 189.Blair RJR. Neuro-cognitive models of aggression, the antisocial personality disorders and psychopathy. J. Neurol. Neurosurg. Psychiatry. 2001;71:727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mobbs D, et al. When fear is near: threat imminence elicits prefrontal-periacqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mobbs D, et al. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc. Natl Acad. Sci. USA. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mobbs D, et al. From threat to fear: the neural organization of defensive fear systems in humans. J. Neurosci. 2009;29:12236–12243. doi: 10.1523/JNEUROSCI.2378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dodge KA, Lochman JE, Harnish JD, Bates JE, Pettit GS. Reactive and proactive aggression in school children and psychiatrically impaired chronically assaultive youth. J. Abnorm. Psychol. 1997;106:37–51. doi: 10.1037//0021-843x.106.1.37. [DOI] [PubMed] [Google Scholar]
- 194.Lahey BB, Loeber R, Burke J, Rathouz PJ, McBurnett K. Waxing and waning in concert: dynamic comorbidity of conduct disorder with other disruptive and emotional problems over 7 years among clinic-referred boys. J. Abnorm. Psychol. 2002;111:556–567. doi: 10.1037//0021-843x.111.4.556. [DOI] [PubMed] [Google Scholar]
- 195.Frick PJ, Ray JV, Thornton LC, Kahn RE. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychol. Bull. 2013 doi: 10.1037/a0033076. http://dx.doi.org/10.1037/a0033076. An important recent review on diagnostic considerations with respect to conduct disorder and callous-unemotional traits.
- 196.Patrick CJ. Emotion and psychopathy: startling new insights. Psychophysiology. 1994;31:319–330. doi: 10.1111/j.1469-8986.1994.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 197.Verona E, Patrick CJ, Joiner TE. Psychopathy, antisocial personality, and suicide risk. J. Abnorm. Psychol. 2001;110:462–470. doi: 10.1037//0021-843x.110.3.462. [DOI] [PubMed] [Google Scholar]
- 198.Feder A, Nestler EJ, Charney DS. Psychobiology and molecular genetics of resilience. Nature Rev. Neurosci. 2009;10:446–457. doi: 10.1038/nrn2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Blair RJR. A cognitive developmental approach to morality: investigating the psychopath. Cognition. 1995;57:1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- 200.Haidt J. The emotional dog and its rational tail: a social intuitionist approach to moral judgment. Psychol. Rev. 2001;108:814–834. doi: 10.1037/0033-295x.108.4.814. [DOI] [PubMed] [Google Scholar]
- 201.Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:1971–1972. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- 202.Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: the neural basis of human moral cognition. Nature Rev. Neurosci. 2005;6:799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- 203.Glenn AL, Raine A, Schug RA. The neural correlates of moral decision-making in psychopathy. Mol. Psychiatry. 2008;14:5–6. doi: 10.1038/mp.2008.104. [DOI] [PubMed] [Google Scholar]
- 204.Harenski CL, Harenski KA, Shane MS, Kiehl KA. Aberrant neural processing of moral violations in criminal psychopaths. J. Abnorm. Psychol. 2010;119:863–874. doi: 10.1037/a0020979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sakai JT, Dalwani MS, Gelhorn HL, Mikulich-Gilbertson SK, Crowley TJA. Behavioral test of accepting benefits that cost others: associations with conduct problems and callous-unemotionality. PLoS ONE. 2012;7:e36158. doi: 10.1371/journal.pone.0036158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Smetana JG. Preschool children’s conceptions of moral and social rules. Child Dev. 1981;52:1333–1336. [Google Scholar]
- 207.Smetana JG. In: The Child as Psychologist: An Introduction to the Development of Social Cognition. Bennett M, editor. Harvester Wheatsheaf; 1993. pp. 111–141. [Google Scholar]
- 208.Haidt J. The new synthesis in moral psychology. Science. 2007;316:998–1002. doi: 10.1126/science.1137651. [DOI] [PubMed] [Google Scholar]
- 209.Glenn AL, Iyer R, Graham J, Koleva S, Haidt J. Are all types of morality compromised in psychopathy. J. Personal. Disord. 2009;23:384–398. doi: 10.1521/pedi.2009.23.4.384. [DOI] [PubMed] [Google Scholar]
- 210.Aharoni E, Antonenko O, Kiehl KA. Disparities in the moral intuitions of criminal offenders: the role of psychopathy. J. Res. Pers. 2011;45:322–327. doi: 10.1016/j.jrp.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Blair RJR, Cipolotti L. Impaired social response reversal: a case of “acquired sociopathy”. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 212.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 213.Martel G, et al. Murine GRPR and stathmin control in opposite directions both cued fear extinction and neural activities of the amygdala and prefrontal cortex. PLoS ONE. 2012;7:e30942. doi: 10.1371/journal.pone.0030942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Klucken T, et al. The 5-HTTLPR polymorphism is associated with altered hemodynamic responses during appetitive conditioning. Hum. Brain Mapp. 2012;34:2549–2560. doi: 10.1002/hbm.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stein JL, et al. Discovery and replication of dopamine-related gene effects on caudate volume in young and elderly populations (N = 1198) using genome-wide search. Mol. Psychiatry. 2011;16:927–937. doi: 10.1038/mp.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Workman JL, Fonken LK, Gusfa J, Kassouf KM, Nelson RJ. Post-weaning environmental enrichment alters affective responses and interacts with behavioral testing to alter nNOS immunoreactivity. Pharmacol. Biochem. Behav. 2011;100:25–32. doi: 10.1016/j.pbb.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 217.Isaacs EB, et al. The effect of early human diet on caudate volumes and IQ. Pediatr. Res. 2008;63:308–314. doi: 10.1203/PDR.0b013e318163a271. [DOI] [PubMed] [Google Scholar]
- 218.Seidel K, Poeggel G, Holetschka R, Helmeke C, Braun K. Paternal deprivation affects the development of corticotrophin-releasing factor-expressing neurones in prefrontal cortex, amygdala and hippocampus of the biparental octodon degus. J. Neuroendocrinol. 2011;23:1166–1176. doi: 10.1111/j.1365-2826.2011.02208.x. [DOI] [PubMed] [Google Scholar]
- 219.D'Addario C, et al. Ethanol induces epigenetic modulation of prodynorphin and pronociceptin gene expression in the rat amygdala complex. J. Mol. Neurosci. 2013;49:312–319. doi: 10.1007/s12031-012-9829-y. [DOI] [PubMed] [Google Scholar]
- 220.Kochanska G, Gross JN, Lin MH, Nichols KE. Guilt in young children: development, determinants, and relations with a broader system of standards. Child Dev. 2002;73:461–482. doi: 10.1111/1467-8624.00418. [DOI] [PubMed] [Google Scholar]
- 221.Kochanska G. Multiple pathways to conscience for children with different temperaments: from toddlerhood to age 5. Dev Psychol. 1997;33:228–240. doi: 10.1037//0012-1649.33.2.228. [DOI] [PubMed] [Google Scholar]
- 222.Ernst M, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 223.Hare TA, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol. Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Galvan A, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: effects on the psychophysiology of defensive and appetitive motivation. Dev. Psychopathol. 2009;21:27–45. doi: 10.1017/S0954579409000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wendelken C, Baym CL, Gazzaley A, Bunge SA. Neural indices of improved attentional modulation over middle childhood. Dev. Cogn. Neurosci. 2011;1:175–186. doi: 10.1016/j.dcn.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Velanova K, Wheeler ME, Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. Neurosci. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wymbs BT, et al. Callous-unemotional traits as unique prospective risk factors for substance use in early adolescent boys and girls. J. Abnorm. Child Psychol. 2012;40:1099–1110. doi: 10.1007/s10802-012-9628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Alia-Klein N, et al. Gene × disease interaction on orbitofrontal gray matter in cocaine addiction. Arch. Gen. Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Kasanetz F, et al. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol. Psychiatry. 2013;18:729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- 231.Yucel M, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch. Gen. Psychiatry. 2008;65:694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 232.Koenigs M, Baskin-Sommers A, Zeier J, Newman JP. Investigating the neural correlates of psychopathy: a critical review. Mol. Psychiatry. 2011;16:792–799. doi: 10.1038/mp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pardini DA, Phillips M. Neural responses to emotional and neutral facial expressions in chronically violent men. J. Psychiatry Neurosci. 2010;35:390–398. doi: 10.1503/jpn.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Deeley Q, et al. Facial emotion processing in criminal psychopathy. Preliminary functional magnetic resonance imaging study. Br. J. Psychiatry. 2006;189:533–539. doi: 10.1192/bjp.bp.106.021410. [DOI] [PubMed] [Google Scholar]
- 235.Contreras-Rodriguez O, et al. Disrupted neural processing of emotional faces in psychopathy. Soc. Cogn. Affect. Neurosci. 2013 doi: 10.1093/scan/nst014. http://dx.doi.org/10.1093/scan/nst014. [DOI] [PMC free article] [PubMed]
- 236.Dolan MC, Fullam RS. Psychopathy and functional magnetic responance imaging blood oxygenation level-dependent respones to emotional faces in violence patients with schizophrenia. Biol. Psychiatry. 2009;66:570–577. doi: 10.1016/j.biopsych.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 237.Sommer M, et al. In psychopathic patients emotion attribution modulates activity in outcome-related brain areas. Psychiatry Res. 2010;182:88–95. doi: 10.1016/j.pscychresns.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 238.Blair RJR, Jones L, Clark F, Smith M. The psychopathic individual: a lack of responsiveness to distress cues? Psychophysiology. 1997;34:192–198. doi: 10.1111/j.1469-8986.1997.tb02131.x. [DOI] [PubMed] [Google Scholar]
- 239.House TH, Milligan WL. Autonomic responses to modeled distress in prison psychopaths. J. Personal. Social Psychol. 1976;34:556–560. doi: 10.1037//0022-3514.34.4.556. [DOI] [PubMed] [Google Scholar]
- 240.Newman JP, Patterson CM, Kosson DS. Response perseveration in psychopaths. J. Abnorm. Psychol. 1987;96:145–148. doi: 10.1037//0021-843x.96.2.145. [DOI] [PubMed] [Google Scholar]
- 241.Budhani S, Richell RA, Blair RJ. Impaired reversal but intact acquisition: probabilistic response reversal deficits in adult individuals with psychopathy. J. Abnorm. Psychol. 2006;115:552–558. doi: 10.1037/0021-843X.115.3.552. [DOI] [PubMed] [Google Scholar]
- 242.Young L, Koenigs M, Kruepke M, Newman JP. Psychopathy increases perceived moral permissibility of accidents. J. Abnorm. Psychol. 2012;121:659–667. doi: 10.1037/a0027489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Koenigs M, Kruepke M, Zeier J, Newman JP. Utilitarian moral judgment in psychopathy. Soc. Cogn. Affect. Neurosci. 2011;7:708–714. doi: 10.1093/scan/nsr048. An interesting paper documenting the impairment in moral judgements seen in individuals with psychopathy.
- 244.Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. J. Abnorm. Psychol. 2010;119:546–554. doi: 10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- 245.Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Mol. Psychiatry. 2009;14:561–562. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
- 246.Ly M, et al. Cortical thinning in psychopathy. Am. J. Psychiatry. 2012;169:743–749. doi: 10.1176/appi.ajp.2012.11111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kiehl KA, et al. Limbic abnormalities in affective processing by criminal psychopaths as revealed by functional magnetic resonance imaging. Biol. Psychiatry. 2001;50:677–684. doi: 10.1016/s0006-3223(01)01222-7. [DOI] [PubMed] [Google Scholar]