Abstract
Objective and design
SapC-DOPS is a newly combined compound consisting of saposin C and dioleoylphosphatidylserine (DOPS). Our recent study showed that SapC-DOPS exhibits anti-tumor activity. However, SapC-DOPS has recognition elements of Toll-like receptor (TLR) 2 and TLR4; therefore, we want to know whether SapC-DOPS can induce abnormal immunoreaction via identification TLRs.
Methods
We investigated the capacity of SapC-DOPS to induce cytokines in vivo and in vitro and analyzed the involvement of TLR and NF-kB in these cytokines production.
Results
SapC-DOPS could activate the cytokine production by peripheral macrophages, enhance the expressions of TLR4 and stimulate the NF-κB nuclear translocation. PDTC, an NF-κB inhibitor, could decrease the SapC-DOPS inducible TNF-α and IL-1β production.
Conclusions
SapC-DOPS was similar to LPS in the immune response and may induce the production of cytokines in macrophages via the TLR4 signaling pathway and, at least in part, the alteration of the NF-κB pathway.
Keywords: SapC-DOPS, Raw264.7, TLRs, NF-κB
Introduction
Saposin (Sap) C, along with saposins A, B, and D, is proteolytically processed from a precursor protein, called prosaposin [1–5]. This protein is a small (80 amino acid), multifunctional glycoprotein and has a high affinity to phospholipid membranes containing the negatively charged lipid, dioleoylphosphatidylserine (DOPS) [6]. At acidic pH values, SapC can reorganize and destabilize the phospholipid-containing membranes [7–9], and fuse acidic phospholipid vesicles [10, 11]. Previous studies showed that SapC-DOPS, a protein lipid complex consisting of SapC and DOPS, could improve lysosomal storage diseases with abnormalities in the CNS [12]. Our recent study showed that SapC-DOPS exhibits anti-tumor activity [13]. Thus, we try to develop the combinations to a new anti-tumor drug. However, DOPS is a lipid and SapC is a glycoprotein. Toll-like receptor (TLR) 2 can recognize peptidoglycan and TLR4 can recognize bacterial lipopolysaccharide (LPS) to induce cytokine production, including tumor necrosis factor-alpha (TNF-α), interferon-β (IFN-β) and interleukin-1β (IL-1β) via the NF-κB pathway. But it is unclear whether SapC-DOPS may induce an abnormal immune response via recognition of TLRs.
A macrophage is a type of white blood cell that ingests foreign material. They help destroy bacteria, protozoa, and tumor cells and also release substances that stimulate other cells of the immune system. They are key players in both innate immunity and adaptive immunity. In stimuli, macrophages recognize specific molecular patterns through pattern recognition receptors (PRRs) such as TLR, leading to macrophage activation [14]. After recognition, TLRs trigger intracellular signaling pathways that result in the induction of type I IFN and inflammatory cytokines such as TNF-α and IL-1β, mainly through the activation of the NF-κB and mitogen-activated protein kinases (MAPK) signaling pathway [15]. These events allow this cell to perceive and respond to pathogens and rapidly initiate the host immune response. However, it is unclear whether or how SapC-DOPS induces macrophage activities.
In this study, for the first time, we reported that the SapC-DOPS can affect the cytokines production of macrophages via in vivo and in vitro experiments and found that the mechanism of the effects might be involved in TLR4 and NF-κB signaling pathways.
Materials and methods
Reagents
Lipopolysaccharide (LPS) was purchased from Sigma–Aldrich (E.coli 0111; B4. St., Louis, MO, USA). The reagents for flow cytometry, mouse-TLR4-Alex488, mouse-TLR2-PE, IgG2b-PE Abs, and the mouse TNF-α, IL-1β and IFN-β ELISA kit were obtained from eBioscience (Inc, USA). The anti-NF-κB subunit p65 Ab for Western blot was purchased from Santa Cruz (Inc, USA) and anti-β-actin Ab from Sigma (Inc, USA). SapC-DOPS (the purity of SapC is over 98%. The SapC:DOPS molar ratio is 1:1) was kindly provided by Dr. Xiaoyang Qi (University of Cincinnati College of Medicine, Cincinnati, OH, USA). SapC-DOPS was dissolved in phosphate-buffered saline (PBS) (0.14 M NaCl, 2.6 mM KCl, 8 mM Na2HPO4, and 1.5 mM KH2PO4) for the stock.
The in vivo effect of SapC-DOPS on C57BL/6 mice
Female C57BL/6 mice (4–6 weeks) were purchased from the Model Animal Research Center of Nanjing University used for in vivo study, and housed at 24°C in a humidity-controlled room with a 12 h light/dark photoperiod (light on at 08:00 and off at 20:00), and constant access to water and chow. The mice were allowed to acclimatize for at least 1 week prior to being used in experiments. All procedures were approved by the Institutional Animal Care and Use Committee of Nanjing University.
The 48 mice were divided into two groups; that is, 24 mice in each group. The mice of group one were injected with SapC-DOPS (20 mg/kg body weight) intraperitonially (i.p.) once every other day throughout the experimental period, while the mice of group two were injected with PBS in the same way. Six mice in each group were killed at arranged time points (0, 7, 14, 21 or 28 days) after injection. Blood was drawn from each mouse using retro-orbital puncture and stored at 4°C overnight before being centrifuged (1500×g, 10 min, 4°C). The plasma was stored at −80°C until TNF-α, IL-1β and IFN-β were quantified using specific enzyme-linked immunosorbent assays according to the manufacturer’s instructions.
Resident peritoneal cells were harvested from peritoneal cavities of the mice by two peritoneal washes with serum-free DMEM (Gibco BRL, Grand Island, NY, USA) to detect the cytokines production. After centrifugation (10 min, 250×g), cells were resuspended in DMEM supplemented with 10% fetal calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). According to experimental design, cells were plated in six-well plates [16]. Peritoneal cells were incubated for 2 h (37°C, 5% CO2) to allow macrophage adhesion, and then the cells were washed three times to remove non-adherent cells. This protocol resulted routinely in a culture containing 95% macrophages used for analyzing mRNA of TNF-α, IL-1β and IFN-β by RT-PCR.
Cells and culture
A murine macrophage cell line, Raw264.7, purchased from American Type Culture Collection (in 10 passages, Rockville, MD, USA), was used for the following in vitro study. Raw264.7 cells were cultured in complete medium (DMEM supplemented with 10% fetal calf serum, 100 U/ ml penicillin, and 100 μg/ml streptomycin) in 75 cm2 flasks at 37°C in a humidified atmosphere of 5% CO2. The cells were seeded in 24-well plates at 3 × 105 cells/ml overnight before treatment. According to previous experiments, the cells with 60–80% confluence were incubated with three different concentrations of SapC-DOPS (0.2, 1 and 5 μg/ml). The cells treated with PBS and 0.5 μg/ml LPS served as negative and positive controls, respectively.
Annexin V/PI staining
After treatment with SapC-DOPS for 24 h, Raw264.7 cells were centrifuged to remove the medium, washed once with binding buffer (10 mM Hepes, 140 mM NaCl, 2.5 mM CaCl2 in aquadest.) and stained with 5 μl Annexin V-FITC at room temperature for 15 min. An amount of 10 μl of 20 μg/ml propidium iodide (PI) was added at room temperature for 10 min and cells were analyzed by flow cytometry. Viable cells were negative for both PI and Annexin V, apoptotic cells were positive for Annexin V and negative for PI, and late apoptotic dead cells displayed both high annexin V and PI labeling. Non-viable cells which underwent necrosis were positive for PI and negative for Annexin V. The same experiment was carried out in triplicate.
RT-PCR and Real-time PCR
The Raw264.7 cells were collected after treatment with SapC-DOPS for 3, 6, 12 or 24 h, respectively. The mRNA expressions of TNF-α, IL-1β, IFN-β, TLR2 and TLR4 were determined by RT-PCR. Additionally, to detect the effect of NF-κB inhibitor PDTC on cytokine expression by macrophages, we treated the Raw264.7 cells with 5 μg/ml SapC-DOPS with or without 100 μM PDTC. Then the TNF-α, IL-1β and IFN-β mRNA expressions were quantified by real-time PCR. The primers used in semi-quantitative RT-PCR and real-time PCR were synthesized by Invitrogen and their sequences are listed in Table 1.
Table 1.
Primers used in this study
| Primers | Sequence 5′–3′ | Product size (bp) |
|---|---|---|
| GAPDH | F: AACGACCCCTTCATTGAC R TCCACGACATACTCAGCAC |
191 |
| IL-1β | F:CTTCAGGCAGGCAGTAT R: CATCCCATGAGTCACAGA |
149 |
| TNF-α | F:TGGCCTCCCTCTCATCAGTTCTATG R: GTTTGCTACGACGTGGGCTAC |
97 |
| IFN-β | F: CGGACTTCAAGATCCCTAT R: TAGTCTCATTCCACCCAGT |
135 |
| TLR2 | F:TGGAGGTGTTGGATGTTAG R:TAGGAGTTCGCAGGAGCA |
256 |
| TLR4 | F:TGGCATCATCTTCATTG R:TCAGGATTCGAGGCTT |
180 |
Cytokine analysis by ELISA
The culture supernatants were collected after treatment with SapC-DOPS for 24 h, and quantification of IL-1β, TNF-α and IFN-β was performed by ELISA according to the manufacturer’s instructions. The absorbances were read by an automated microtiter plate reader (Bio-Rad Model 550) at 450 nm. A standard curve was obtained from the standard sample provided with the kit. The absolute levels of the three cytokines were calculated by the standard curve. All the above measurements were carried out in triplicate.
Phenotype analysis by flow cytometry
The Raw264.7 cells were collected after treatment with SapC-DOPS for 24 h, washed twice in PBS, and then incubated for 30 min at 4°C with Alex488 labeled TLR4 and PE labeled TLR2, respectively, according to the manufacturer’s instructions. The labeled cells were fixed in 1% polyformaldehyde and analyzed on a FACS Calibur (Becton–Dickinson, San Diego, CA, USA). An isotype control was used for setting gates. Three parallel samples were set. Data analysis was performed using the Cell Quest Software (Becton–Dickinson, San Diego, CA, USA).
p65 detection by Western blot
Raw264.7 cells were incubated with or without 5 μg/ml SapC-DOPS for 0.5, 1.5 and 3 h, respectively. PBS and 0.5 μg/ml LPS treatments served as negative and positive controls. Nuclear extract was prepared according to the previously reported methods [17]. Briefly, the cells were lysed in cell lysis buffer (10 mM HEPES; pH 7.5, 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol [DTT], 0.5% Nonidet-40 and 0.5 mM PMSF along with the protease inhibitor cocktail) and allowed to swell on ice for 10 min. Tubes were vortexed to disrupt cell membranes and then centrifuged at 12,000×g at 4°C for 10 min. The supernatant was stored as cytoplasm extract and the nuclei pellets were washed thrice with the cell lysis buffer and resuspended in the nuclear extraction buffer containing 20 mM HEPES (pH 7.5), 400 mM NaCl, 1 mM EDTA, 1 mM DTT, and 1 mM PMSF with protease inhibitor cocktails, and incubated in ice for 30 min. The extracted nuclei were pelleted at 12,000×g for 15 min at 4°C and the supernatant was collected as the nuclear extract. The nuclear and cytoplasmic extracts (200 μg/lane) were subjected to 12% SDS–PAGE and transferred onto polyvinylidene difluoride membranes (Millipore, USA). The membranes were blocked by incubating the membrane in 1% bovine serum albumin (BSA) in PBS at room temperature for 1 h. After washing twice with PBST (PBS with 0.05% Tween-20) buffer, the membrane was incubated with primary antibody (polyclonal anti-NF-κB p65 subunit [1:2000] or anti-β-actin [1:400]) overnight at 4°C. The membrane was then washed with TBST buffer thrice and incubated with HRP-conjugated goat anti-rabbit secondary antibody (1:1000) at room temperature for 1 h. Protein bands were detected by the enhanced chemiluminescene (ECL) reaction (Millipore, USA).
Statistical analysis
Data are presented as mean ± standard deviation (SD). Data were subjected to one way analysis of variance (ANOVA), and the ANOVA post Bonferronites by the software of Graphpad Prism 5.0. A value of P < 0.05 was considered to indicate a statistically significant difference.
Results
The in vivo effects of SapC-DOPS on cytokine expression levels
We examined the levels of plasma cytokines including TNF-α, IL-1β and IFN-β in mice. The results showed that the treatment of 20 mg/kg SapC-DOPS greatly increased the level of plasma TNF-α comparing with PBS group (Fig. 1a), while the plasma IL-1β and IFN-β levels were undetected. Moreover, to evaluate the effects of SapC-DOPS on macrophages, we detected the cytokine mRNA expression from resident peripheral macrophages. The results showed that SapC-DOPS increased the mRNA expression of TNF-α, IL-1β and IFN-β from 14 to 21 days (Fig. 1b, c).
Fig. 1.
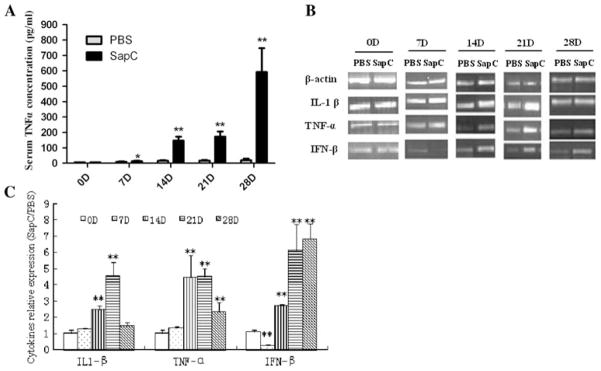
The in vivo effects of SapC-DOPS on cytokine levels. Mice were administered SapC-DOPS or vehicle (PBS) for 0, 7, 14, 21 or 28 days. a After SapC-DOPS injection for 0, 7, 14, 21, 28 days, a serum sample was obtained and then TNF-α was analyzed by ELISA. Results were expressed as means ± SD from three independent experiments, each including pools of six mice per group. At 7 days, the average concentraton of TNF-α was 6 pg/ml (PBS group) and 7.067 pg/ml (SapC treated group). b The effect of SapC-DOPS on mRNA expression of TNF-α, IL-1β and IFN-β in peripheral macrophages were examined by RT-PCR. c Densitometric analysis. The intensity of the band was scanned. The quotients of the SapC-DOPS group/PBS group gene were calculated. The results are shown as mean ± SE from three representative independent experiments. *p < 0.05,**p < 0.01 versus PBS group
SapC-DOPS does not induce apoptosis of macrophages
To investigate the toxic effects of SapC-DOPS on macrophages, apoptosis was assayed by Annexin V/PI staining. The results showed that treatment with different concentrations of SapC-DOPS was not cytotoxic to macrophages (Fig. 2).
Fig. 2.
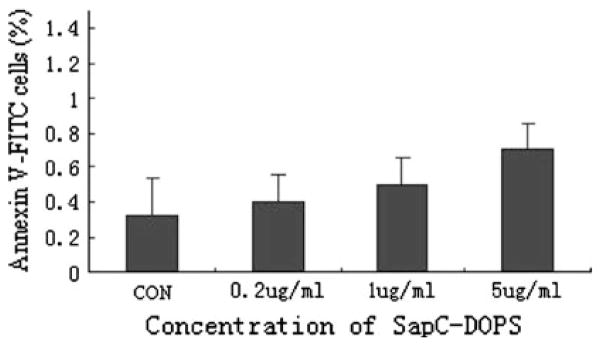
Effects of SapC-DOPS on apoptosis of Raw264.7 cells. Raw264.7 cells were administered with different concentrations of SapC-DOPS (0.2, 1 and 5 μg/ml) for 24 h and then the cells were determined by Annexin V/PI double stain. Graph shows the percentages of apoptotic population (Annexin V +/PI-) population. Results were expressed as means ± SD from three independent experiments
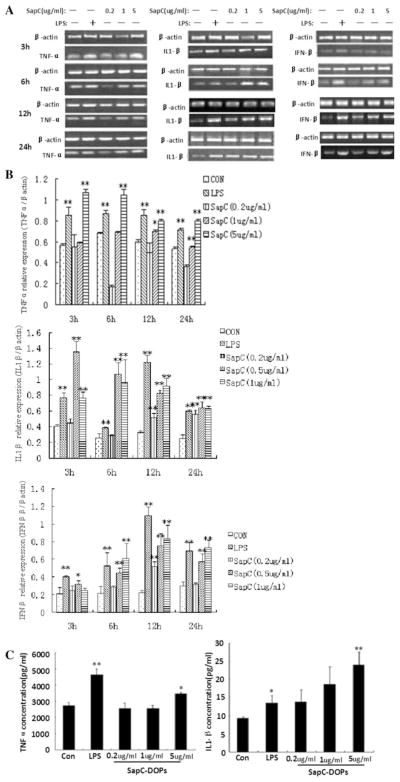
SapC-DOPS enhances the TNF-α and IL-1β production in Raw264.7 cells
To determine the effects of SapC-DOPS on TNF-α, IL-1β and IFN-β, we used RT-PCR analysis to detect the mRNA expression of these inflammatory cytokines in Raw264.7 cells. The β-actin gene was kept as the housekeeping control. The results showed that SapC-DOPS administration upregulated expressions of TNF-α, IL-1β and IFN-β mRNA, especially 5 μg/ml SapC-DOPS (Fig. 3a, b). TNF-α and IL-1β protein levels were quantified by ELISA. The results showed that TNF-α (from 2650 to 3476 pg/ml) and IL-1β (from 8.43 to 22.17 pg/ml) were upregulated significantly in the 5 μg/ml SapC-DOPS treatment group, whereas 0.2 and 1 μg/ml SapC-DOPS had no significant effect on the TNF-α and IL-1β expressions. Although the concentration of IL-1β was gradually increased following the incubation of Raw264,7 cells with SapC-DOPS (Fig. 3c), IFN-β was undetected.
Fig. 3.
Effects of SapC-DOPS on cytokines production. Raw264.7 cells were treated with PBS or the indicated different concentrations of SapC-DOPS, with 5 μg/ml LPS as positive control. a After incubation with SapC-DOPS (0.2, 1 and 5 μg/ml) or LPS for 3, 6, 12 or 24 h, Raw264.7 cells were collected and TNF-α, IL-1β and IFN-β mRNA expression were analyzed by RT-PCR. The representative agarose gel electrophoresis of three independent experiments is shown. b Densitometric analysis. The intensity of the band was scanned. The quotients of three genes/β-actin gene were calculated. The results are shown as mean ± SE from three representative independent experiments. c After incubation with SapC-DOPS (0.2, 1 and 5 μg/ml) or LPS for 24 h, the TNF-α and IL-1β level in cell culture supernatant were determined by ELISA. Results were expressed as means ± SEM from three independent experiments. *p < 0.05, **p <0.01 versus control
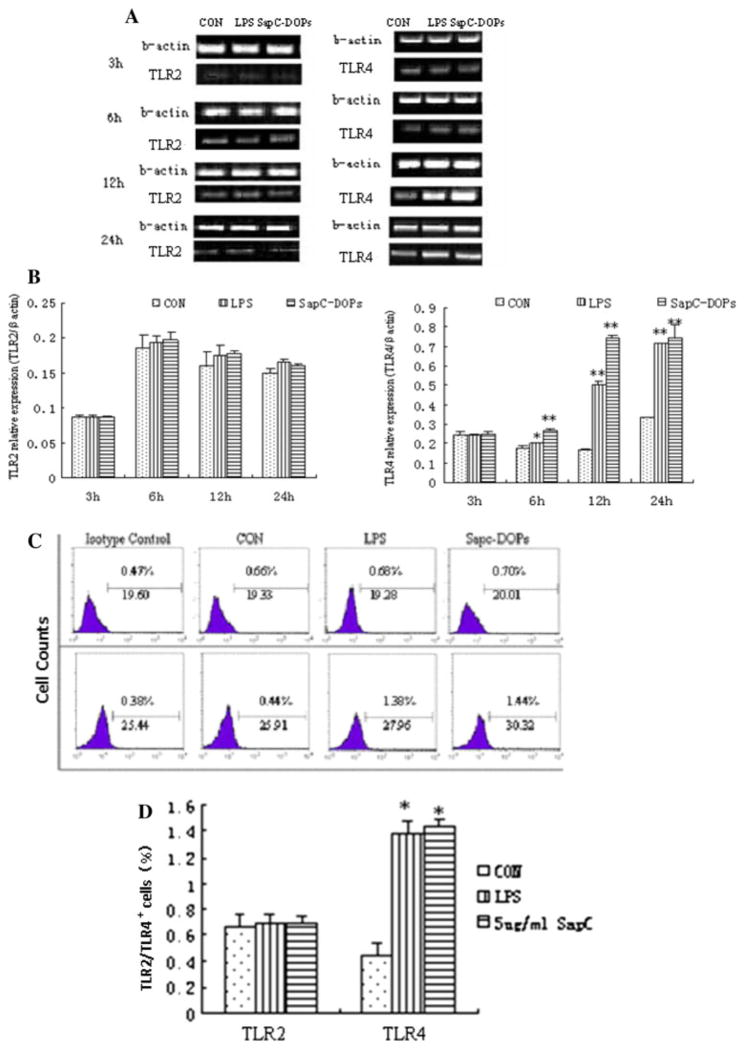
Effect of SapC-DOPS on TLR4 expression in Raw264.7
TLR2 and TLR4 expressions in macrophages were examined with administration of 5 μg/ml SapC-DOPS for 3, 6, 12 and 24 h. The results showed that the TLR2 mRNA and protein expression did not significantly change compared with the control group (Fig. 4). The TLR4 mRNA expression was significantly enhanced at 6, 12 and 24 h (Fig. 4a, b), while its protein level was just significantly enhanced at 24 h (Fig. 4c). The TLR4 expression levels in both mRNA and protein were similar between the treatment of SapC-DOPS and LPS groups (Fig. 4).
Fig. 4.
Effects of SapC-DOPS on TLR2 and TLR4 expression. Raw264.7 cells were treated with PBS or 5 μg/ml SapC-DOPS, LPS (0.5 μg/ml) and PBS as positive control. a After incubation with SapC-DOPS or LPS for 3, 6, 12 or 24 h, Raw264.7 cells were collected and TLR2 or TLR4 mRNA were determined by RT-PCR. b Densitometric analysis. The intensity of the band was scanned. The quotients of TLR2 and TLR4/β-actin gene were calculated. The results are shown as mean ± SE from three representative independent experiments. c, d The TLR2 or TLR4 protein level in macrophages after incubating with 5 μg/ml SapC-DOPS or 0.5 μg/ml LPS were measured with flow cytometry analysis. Results were expressed as means ± SD from three independent experiments. *p < 0.05, **p <0.01 versus control
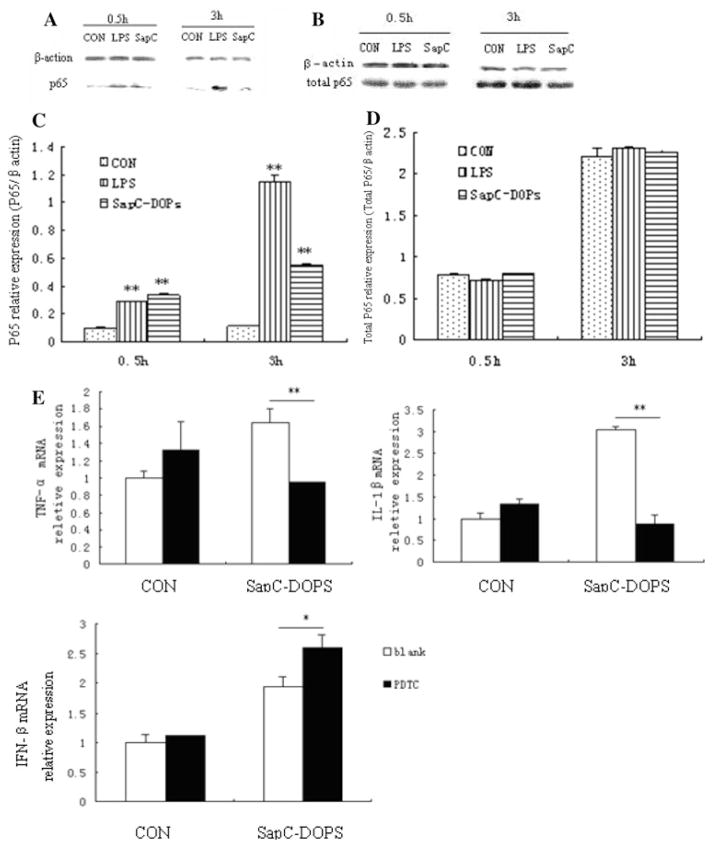
Role of NF-κB in SapC-DOPS-inducible cytokines in Raw264.7 cells
To clarify whether the enhanced production of cytokines induced by SapC-DOPS is related to the activation of NF-κB, we examined NF-κB p65 nuclear translocation in Raw264.7 cells in response to SapC-DOPS incubation for 0.5 and 3 h. The results showed that the treatment with SapC-DOPS for 0.5 and 3 h significantly enhanced NF-κB p65 nuclear translocation (Fig. 5a, c). We next assessed whether the NF-κB p65 nuclear translocation in the cells was a consequence of an increased expression of the total NF-κB p65. As shown in Fig. 5b, d, the expression of total NF-κB p65 was not altered by SapC-DOPS treatment, suggesting that NF-κB p65 activity might be directly enhanced by SapC-DOPS administration. To further elucidate NF-κB p65 requirement for the production of proinflammatory cytokines in response to SapC-DOPS, the cells were incubated in the presence of the inhibitor of NF-κB PDTC [18] 1 h before and during SapC-DOPS stimulation. SapC-DOPS treatment alone increased TNF-α and IL-1β secretion as expected, while this effect was significantly decreased by PDTC (Fig. 5e). In contrast to the TNF-α and IL-1β, the SapC-DOPS-induced IFN-β was significantly enhanced by the PDTC pretreatment. Altogether, these data suggest that the effect of SapC-DOPS on the NF-κB pathway, at least in part, contributes to the increased production of proinflammatory cytokines.
Fig. 5.
Effects of SapC-DOPS on NF-κB activation. Raw264.7 cells were treated with 5 μg/ml SapC-DOPS. LPS and PBS at 0.5 μg/ml served as positive and negative controls, respectively. a After 0.5 or 3 h, nuclear extracts were isolated from cells and Western blot analysis of NF-κB was performed using polyclonal antibodies against p65 subunit. b the total NF-κB p65 is detected by Western blot analysis. c Densitometric analysis. The intensity of the band was scanned. The quotients of p65/β-actin protein were calculated. The results are shown as mean ± SE from three representative independent experiments. d Densitometric analysis. The intensity of the band was scanned. The quotients of total p65/β-actin protein were calculated. The results are shown as mean ± SE from three representative independent experiments. e Effects of NF-κB on SapC-DOPS inducible TNF-α, IL-1β and IFN-β. Raw264.7 cells were incubated in the presence of 100 μM PDTC 1 h before and then during the 24 h stimulation by SapC-DOPS. TNF-α, IL-1β and IFN-β expression were measured with real-time PCR analysis. Results were expressed as means ± SD from three independent experiments. *p < 0.05, **p <0.01 versus control
Discussion
The present study was undertaken to investigate the capacity of SapC-DOPS to induce cytokines in vivo and in vitro and analyze the involvement of TLR and NF-kB in these cytokine productions. Our data showed that SapC-DOPS could activate the cytokine production by peripheral macrophages, enhance the expressions of TLR4 and stimulate the NF-κB nuclear translocation. PDTC, an NF-κB inhibitor, could decrease the SapC-DOPS inducible TNF-α and IL-1β production. We concluded that SapC-DOPS was similar to LPS in the immune response and may induce the production of cytokines in macrophages via the TLR4 signaling pathway and, at least in part, the alteration of the NF-κB pathway.
Macrophages play an important role in the host immune response. They can secrete many cytokines, such as TNF-α, IL-1β, IFN-β. These cytokines involve the innate immune response and inhibit tumorigenesis [19, 20]. The present study demonstrated that the treatment of 20 mg/kg SapC-DOPS greatly increased the level of plasma TNF-α compared with the PBS group, while the plasma IL-1β and IFN-β levels were undetected. Moreover, SapC-DOPS increased the mRNA expression of TNF-α, IL-1β and IFN-β from 14 to 21 days. SapC-DOPS administration up-regulated expressions of TNF-α, IL-1β and IFN-β mRNA in Raw264.7 cells. TNF-α and IL-1β protein levels were also up-regulated significantly in the 5 μg/ml SapC-DOPS treatment group. These results indicated that SapC-DOPS might influence the macrophages function to produce cytokines, and subsequently stimulate the whole immune system. However, in our experiments, IL-1β and IFN-β could not be detected in serum. IFN-β was also undetectable in supernatant. This may be attributed to the differences of signal transduction pathways of in vivo and in vitro immune cells, especially macrophages, in the response to SapC-DOPS.
SapC-DOPS was similar to LPS in interaction with TLR4 in Raw264.7. The present study indicated that SapC-DOPS treatment in vitro modulated the membrane receptor TLR4 expression of Raw264.7 cells. The TLR4 expression levels in both mRNA and protein were similar between the treatment of SapC-DOPS and LPS groups. We suppose that SapC-DOPS may mimic the bacterial polysaccharide interaction with TLR4, and subsequently activate intracellular signaling pathways, including the NF-κB pathway. After activation of TLR4, there are two intracellular cascades that regulate signal transduction processes, gene expression, and production of proinflammatory mediators. One of these cascades requires a specific intracellular adaptor protein called MyD88, while the other cascade utilizes the TRIF adaptor protein. Moreover, based on the results of recent studies, it is clear that LPS can induce cellular activation through TLR4 [21, 22]. It is well established that TNF-α secretion is a prototypical measure for activation of the MyD88-dependent pathway, whereas secretion of IFN-β is commonly used as an indicator of TRIF-dependent cellular activation. Thus, this suggests that SapC-DOPS may induce different cytokine productions via two different intracellular cascades.
NF-κB activation was involved in the enhanced cytokine production in Raw264.7 cells induced by SapC-DOPS. It has been reported that LPS can be recognized by TLR4, and so activates inflammatory gene expression through NF-κB signaling [23]. A number of studies have shown that the signal-dependent activation of NF-κB requires both IκB-α degradation and NF-κB phosphorylation, and these two steps can be uncoupled [24]. The phosphorylation of NF-κB leads to its nuclear translocation, and binding to enhancers or promoters of target genes. In the present study, SapC-DOPS administration significantly enhanced the nuclear translocation of NF-κB. The NF-κB inhibitor PDTC, inhibiting the NF-κB activation by inhibition the phosphorylation of IκB [25], decreased the SapC-DOPS inducible TNF-α and IL-1β, but enhanced the SapC-DOPS inducible IFN-β. These data indicated that SapC-DOPS might influence cytokine production in macrophages by directly or indirectly regulating TLR4 expressions. Furthermore, IFN-β can be induced by macrophages in a manner independent of MyD88 [26]. Previous reports showed the transcription of IFN-β gene was cooperative controlled by several transcription factors, including NF-κB, interferon regulatory factor (IRF) 3 and IRF7 [27]. Therefore, we speculated that SapC-DOPS might also intact with some other membrane receptors, which in turn induces the activation of NF-κB, IRF3 or/ and IRF7, which eventually initiate the transcription of IFN-β.
In conclusion, SapC-DOPS can enhance the production of TNF-α, IL-1β or IFN-β in macrophages. Its effects on these cytokines production by macrophages were involved in the TLR4 signaling pathway and NF-κB pathway. SapC-DOPS was similar to LPS in the immune response.
Acknowledgments
This work supported by grants from the National Natural Science Foundation (project number 30771959), Natural Science Research Foundation of Jiangsu Province (project number BK2006119) and Technology Research for Advanced Medicine of Jiangsu province (project number BG2007604).
Contributor Information
Kaihua Lu, Immunology and Reproductive Biology Lab of Medical School and State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210093, People’s Republic of China.
Guangfeng Zhao, Immunology and Reproductive Biology Lab of Medical School and State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210093, People’s Republic of China.
Hongna Lu, Immunology and Reproductive Biology Lab of Medical School and State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210093, People’s Republic of China.
Shuli Zhao, Immunology and Reproductive Biology Lab of Medical School and State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210093, People’s Republic of China.
Yuxian Song, Immunology and Reproductive Biology Lab of Medical School and State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210093, People’s Republic of China.
Xiaoyang Qi, Jiangsu Changzhou Changji Biotechnology Development Co., Ltd, Changzhou 213000, People’s Republic of China.
Yayi Hou, Email: yayihou@nju.edu.cn, Immunology and Reproductive Biology Lab of Medical School and State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210093, People’s Republic of China. Jiangsu Key Laboratory of Molecular Medicine, Nanjing University, Nanjing 210093, People’s Republic of China.
References
- 1.Nakano T, Sandhoff K, Stümper J, Christomanou H, Suzuki K. Structure of full-length cDNA coding for sulfatide activator, a co-beta-glucosidase and two other homologous proteins: two alternate forms of the sulfatide activator. J Biochem. 1989;105:152–4. doi: 10.1093/oxfordjournals.jbchem.a122629. [DOI] [PubMed] [Google Scholar]
- 2.Rorman EG, Grabowski GA. Molecular cloning of a human co-betaglucosidase cDNA: evidence that four sphingolipid hydrolase activator proteins are encoded by single genes in humans and rats. Genomics. 1989;5:486–92. doi: 10.1016/0888-7543(89)90014-1. [DOI] [PubMed] [Google Scholar]
- 3.Fujibayashi S, Wenger DA. Synthesis and processing of sphingolipid activator protein-2 (SAP-2) in cultured human fibroblasts. J Biol Chem. 1986;261:15339–43. [PubMed] [Google Scholar]
- 4.Fujibayashi S, Wenger DA. Biosynthesis of the sulfatide/GM1 activator protein (SAP-1) in control and mutant cultured skin fibroblasts. Biochim Biophys Acta. 1986;875:554–62. doi: 10.1016/0005-2760(86)90077-9. [DOI] [PubMed] [Google Scholar]
- 5.Leonova T, Qi X, Bencosme A, Ponce E, Sun Y, Grabowski GA. Proteolytic processing patterns of prosaposin in insect and mammalian cells. J Biol Chem. 1996;271:17312–20. doi: 10.1074/jbc.271.29.17312. [DOI] [PubMed] [Google Scholar]
- 6.Qi X, Grabowski GA. Differential membrane interactions of saposin A and C: implications for the functional specificity. J Biol Chem. 2001;276:27010–7. doi: 10.1074/jbc.M101075200. [DOI] [PubMed] [Google Scholar]
- 7.You HX, Yu L, Qi X. Phospholipid membrane restructuring induced by saposin C: a topographic study using atomic force microscopy. FEBS Lett. 2001;503:97–102. doi: 10.1016/s0014-5793(01)02700-4. [DOI] [PubMed] [Google Scholar]
- 8.You HX, Qi X, Grabowski GA, Yu L. Phospholipid membrane interactions of saposin C: in situ atomic force microscopic study. Biophys J. 2003;84:2043–57. doi: 10.1016/S0006-3495(03)75012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaccaro AM, Tatti M, Ciaffoni F, Salvioli R, Serafino A, Barca A. Saposin C induces pH-dependent destabilization and fusion of phosphatidylserine-containing vesicles. FEBS Lett. 1994;349:181–6. doi: 10.1016/0014-5793(94)00659-8. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Grabowski GA, Qi X. Phospholipid vesicle fusion induced by saposin C. Arch Biochem Biophys. 2003;415:43–53. doi: 10.1016/s0003-9861(03)00219-4. [DOI] [PubMed] [Google Scholar]
- 11.Qi X, Chu Z. Fusogenic domain and lysines in saposin C. Arch Biochem Biophys. 2004;424:210–8. doi: 10.1016/j.abb.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 12.Chu Z, Sun Y, Kuan CY, Grabowski GA, Qi X. Saposin C: neuronal Effect and CNS Delivery by Liposomes. Ann N Y Acad Sci. 2005;1053:237–46. doi: 10.1196/annals.1344.021. [DOI] [PubMed] [Google Scholar]
- 13.Qi X, Chu Z, Mahller YY, Stringer KF, Witte DP, Cripe TP. Cancer-selective targeting and cytotoxicity by liposomal-coupled lysosomal saposin C protein. Clin Cancer Res. 2009;15(18):5840–51. doi: 10.1158/1078-0432.CCR-08-3285. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 15.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–25. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, Zhao XY, Liu Y, Shi Q, Hua ZC, Shen PP. Analysis of immunomodulating nitric oxide, iNOS and cytokines mRNA in mouse macrophages induced by microcystin-LR. Toxicology. 2004;197:67–77. doi: 10.1016/j.tox.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber E, Harshman K, Kemler I, Malipiero U, Schaffner W, Fontana A, et al. Astrocytes and glioblastoma cells express novel octamer-DNA binding proteins distinct from the ubiquitous Oct-1 and B cell type Oct-2 proteins. Nucleic Acids Res. 1990;18:5495–503. doi: 10.1093/nar/18.18.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemay S, Lebedeva TV, Singh AK. Inhibition of cytokine gene expression by sodium salicylate in a macrophage cell line through an NFkappaB-independent mechanism. Clin Diagn Lab Immunol. 1999;6:567–72. doi: 10.1128/cdli.6.4.567-572.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–64. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 20.Barros VED, Ferreira BR, Livonesi M, Figueiredo LTM. Cytokine and nitric oxide production by mouse macrophages infected with brazilian flaviviruses. Rev Inst Med Trop Sao Paulo. 2009;51(3):141–7. doi: 10.1590/s0036-46652009000300004. [DOI] [PubMed] [Google Scholar]
- 21.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–51. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Cuschieri J, Billigren J, Maier RV. Endotoxin tolerance attenuates LPS-induced TLR4 mobilization to lipid rafts: a condition reversed by PKC activation. J Leukoc Biol. 2006;80:1289–97. doi: 10.1189/jlb.0106053. [DOI] [PubMed] [Google Scholar]
- 23.Bochkov VN, Kadl A, Huber J, Gruber F, Binder BR, Leitinger N. Protective role of phospholipids oxidation products in endotoxin-induced tissue damage. Nature. 2002;419:77–81. doi: 10.1038/nature01023. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz ML, Bacher S, Kracht M. IκB-independent control of NF-κB activity by modulatory phosphorylations. Trends Biochem Sci. 2001;26:186–90. doi: 10.1016/s0968-0004(00)01753-9. [DOI] [PubMed] [Google Scholar]
- 25.Young HA, Bream JH. IFN-γ: recent advances in understanding regulation of expression, biological functions, and clinical applications. Curr Top Microbiol Immunol. 2007;316:97–117. doi: 10.1007/978-3-540-71329-6_6. [DOI] [PubMed] [Google Scholar]
- 26.Sawada H, Mitani Y, Maruyama J, Jiang BH, Ikeyama Y, Dida FA, et al. a nuclear factor-kappaB inhibitor pyrrolidine dithiocarbamate ameliorates pulmonary hypertension in rats. Orig Res. 2007;132:1265–74. doi: 10.1378/chest.06-2243. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Takeuchi O, Fujita T, Inoue J, Mühlradt PF, Sato S, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–94. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]