Abstract
Background
Matrix metalloproteinases (MMPs) are a family of host-derived proteinases reported to mediate multiple functions associated with periodontal destruction and inflammation. We have previously reported high MMP levels in African-American children with localized aggressive periodontitis (LAP). However, little is known about MMP reductions in gingival crevicular fluid (GCF) after therapy. This study aimed to evaluate MMP levels in the GCF following treatment of LAP and to correlate these levels with clinical response.
Methods
GCF samples were collected from 29 African-American individuals diagnosed with LAP. GCF was collected from one diseased site (pocket depth [PD]>4mm, bleeding on probing [BoP] and clinical attachment level [CAL] ≥2mm) and one healthy site (PD≤3mm, no BoP) from each individual at baseline, 3 and 6 months after periodontal treatment, which consisted of full-mouth SRP and systemic antibiotics. The volume of GCF was controlled using a calibrated gingival fluid meter and levels of MMP-1, 2, 3, 8, 9, 12 and 13 were assessed using fluorometric kits.
Results
MMP-1, 8, 9 12, and 13 levels were reduced significantly up to 6 months, at which point were comparable with healthy sites. Significant correlations were noted between MMP-2, 3, 8, 9, 12 and 13 levels and % of sites with PD>4mm. MMP-3, 12 and 13 levels also correlated with mean pocket depth of affected sites.
Conclusion
Treatment of LAP with SRP and systemic antibiotics was effective in reducing the local levels specific MMPs in African-American individuals, which correlated positively with some clinical parameters.
Keywords: metalloproteinases, aggressive periodontitis, scaling and root planing, antimicrobials
Aggressive periodontitis (AgP) is a relatively rare inflammatory condition, mostly seen in young individuals, characterized by severe and rapid destruction of the periodontium1. It affects individuals with a noncontributory medical history and presents a familial aggregation pattern2–4. When localized (LAP), this disease affects first molars and incisors5. As a result of the specific bacteria that are commonly found subgingivally in these individuals6, 7, a marked immune response is mounted by the host8, 9, resulting in periodontal breakdown.
Connective tissue destruction is essentially controlled by matrix metalloproteinases (MMPs), a family of zinc and calcium-dependent proteolytic enzymes, which are secreted by immune cells including polymorphonuclear leukocytes and fibroblasts10, 11 in response to inflammatory stimuli12, 13. MMPs target extracellular matrix components such as collagen, elastin, fibronectin, laminin and entactin, among others14. They are classified into 28 distinct, but structurally related enzymes, being commonly divided into six groups: collagenases (MMP-1, MMP-8 and MMP-13), gelatinases (MMP-2 and MMP-9), stromelysins (MMP-3), matrilysins, membrane type MMPs and others, such as the macrophage metalloelastase (MMP-12). MMPs also play an important role in physiological events, like morphogenesis, development and tissue repair15, 16. Normally, MMP activity is tightly regulated in order to prevent unnecessary tissue destruction17.
Several studies have shown that MMP levels are increased in chronic periodontitis compared to healthy patients and patients with gingivitis18–20, but little information is available about the levels of various MMPs associated with aggressive periodontitis. In a previous study21, our group found that MMP levels were elevated in diseased sites relative to non-diseased sites in LAP patients, in siblings, and in unrelated controls. In addition, MMPs associated with the diseased sites in LAP patients were generally elevated compared to an adult cohort with chronic periodontitis21.
Considering that the control of LAP and the maintenance of treatment outcomes represent a major challenge for clinicians, monitoring MMP levels after treatment may be an important non-invasive tool to guide clinicians towards early chair-side and point-of-care treatment directions. Thus, the objective of this study was to evaluate MMP levels in the GCF following treatment of patients diagnosed with LAP and to correlate these levels with their clinical response.
METHODS
Study Population
Healthy male (n=11) and female (n=18) individuals, aged 5–21, all African-Americans, were recruited from august 2009 to august 2012 to participate in this study, which is part of a larger longitudinal clinical trial of LAP in children (Clinicaltrials.gov, #NCT01330719). Only children and young individuals were included, since LAP is known to be an early-onset type of periodontal disease. These patients were recruited in Leon County Health Department, Tallahassee, Florida and in Duval County Health Department, Florida. Patients or their legal authorized representatives (LAR) were asked a series of questions in order to address health conditions and inclusion/exclusion criteria. A complete dental and medical history was taken for each individual. Inclusion criteria were as follows: African-American patients, diagnosed with localized aggressive periodontitis (LAP), defined by ≥2 teeth (incisor or first molar, and no more than two other teeth other than first molars and incisors) presenting pocket depth (PD) ≥5mm with bleeding on probing (BoP), clinical attachment level (CAL) ≥2mm and radiographic bone loss1,2. Exclusion factors included: having any history of systemic disease that could interfere with the clinical characteristics, incidence, or progression of periodontal disease; having taken antibiotics within the previous three months; allergy to penicillin; use of medications that could influence the characteristics of periodontal disease; smokers or pregnant/lactating women. The study protocol was explained and informed consent was obtained from all individuals, or their LAR as required in the study protocol, approved by the University of Florida Institutional Review Board.
Sample Collection
Gingival crevicular fluid (GCF) was collected from one diseased site (PD≥5mm, BoP and CAL ≥2mm) and one healthy site (PD≤3mm with no BoP) from each LAP individual at baseline, 3 and 6 months after treatment using a paper strip§. The same sites were sampled at each timepoint. Each GCF collection site was isolated with cotton gauze, gently air dried and supragingival plaque was removed. The paper strip was then inserted 1–2mm into the sulcus for approximately 10 sec. The volume of fluid collected was measured using a calibrated gingival fluid meter** and placed in a microcentrifuge tube and kept on ice. Samples were stored at −80 °C until processed.
Clinical Measurements and Treatment Protocol
Periodontal clinical parameters were recorded at baseline, 3 and 6 months after treatment, including: pocket depth (PD), clinical attachment level (CAL), bleeding on probing (BoP), and visible plaque (Pl). CAL was calculated by PD + GM (gingival margin position). GM was given a negative value when located coronal to the CEJ (cementum-enamel junction). All measurements were performed with the use of a UNC-15 periodontal probe†† at 6 sites per tooth and were recorded using a computer software‡‡. After completion of baseline measurements, individuals received full mouth debridement with ultrasonic device§§ followed by scaling and root planing (SRP) and oral hygiene instructions, along with oral care products***. Local anesthesia was provided as required. Immediately after treatment, all individuals were prescribed 500 mg amoxicillin and 250 mg metronidazole 3 times daily for 7 days. The antibiotics dosage was adjusted for children under 40kg (20 mg/kg/day of amoxicillin and 10 mg/kg tid 7 days of metronidazole).
The individuals were re-examined at each timepoint and received full mouth debridement with with ultrasonic device and additional therapy (SRP) for residual pockets >4 mm, as required. Antibiotics were only prescribed once at initial therapy. Professional plaque removal and reinforcement of home care procedures were also performed.
GCF Processing and MMP Enzymatic Assay
The GCF absorbed to the strip was eluted with 50 μL of MMP buffer (50 mM Tris-HCL, 0.2 M NaCL, 5 mM CaCl2, pH 7.5) followed by centrifugation to minimize retention of proteins on the filter paper. The activities of MMP-1, 2, 3, 8, 9, 12, and 13 were assessed using commercially available fluorimetric MMP kits specific for each MMP†††, in accordance with the manufacturer’s instructions. The detection limits of these kits are given by the manufacturer as 0.1 to 1.0 ng depending on the particular assay kit. Briefly, each specific MMP substrate (50 μL) was diluted in 5 ml of assay buffer (provided with the kit) and 50 μL of diluted substrate was added to the desired well of a black fluorimetric 96-well microtiter plate‡‡‡. Two μL of the sample was added to each well and mixed by gently shaking for 15 minutes in the dark and then incubated for an additional 45 minutes in the dark. Each of the 7 MMP substrates was arranged on each plate in rows and the samples were applied in columns, with the first two wells of each row serving as controls for background fluorescence. Fluorescence intensity was measured at Ex/Em = 490 nm/520 nm using a fluorimetric reader and associated software§§§. The data, initially expressed as relative fluorescence units, were converted to ng based on standard curves (R2 >0.99) generated with the purified recombinant MMP**** and its specific substrate. This value was then adjusted to concentration (ng/μL) based on the volume of the GCF collected, according to a calibration curve. MMPs were assayed without activating the pro-enzyme MMP form, and the active forms of 7 MMPs (MMP-1, 2, 3, 8, 9, 12 and 13), which are more closely related to progressive periodontal disease, were chosen12.
Statistical Analysis
Statistical analysis was performed using a statistics Software††††. ANOVA on ranks (Kruskal Wallis test) was applied among all time points using concentration (ng/μL) of each MMP and clinical parameters. Dunn’s multiple comparisons test was used to determine differences among time points. Wilcoxon matched-pairs signed rank test was used for comparisons between baseline diseased and healthy sites within LAP patients. In addition, Spearman’s correlations were run between MMP levels and clinical parameters. Statistical significance was considered to be a p-value≤0.05.
RESULTS
A summary of the demographics and clinical aspects of the individuals included in the study is presented in Table 1. Four participants had mixed dentition, and disease was present on primary teeth. Participants reported full compliance with the antibiotic regimen and no side effects were reported. Missed timepoints are indicated in figure 1.
Table 1.
Demographics and clinical characteristics of the population at baseline (n=29).
| Age | Gender | %PD>4mm | %BoP | %Plaque | Mean PD sites/all(mm) | Mean CAL(mm) | PD site (mm) |
|---|---|---|---|---|---|---|---|
| 14.86±3.65 | 11 M/18 F | 17.81±15.19 | 17.67±13.11 | 38.04±21.79 | 5.22±0.75 2.25±0.82 |
2.92±1.30 | 6.00±1.69 |
Values are given by Means ± Standard deviation. M= # of males; F= # of females; Mean PD sites= Mean pocket depth of sites with PD>4mm; Mean PD all= mean PD of all sites; Mean CAL= mean clinical attachment level of affected sites; BoP=bleeding on probing; PD site = mean pocket depth from sampled site.
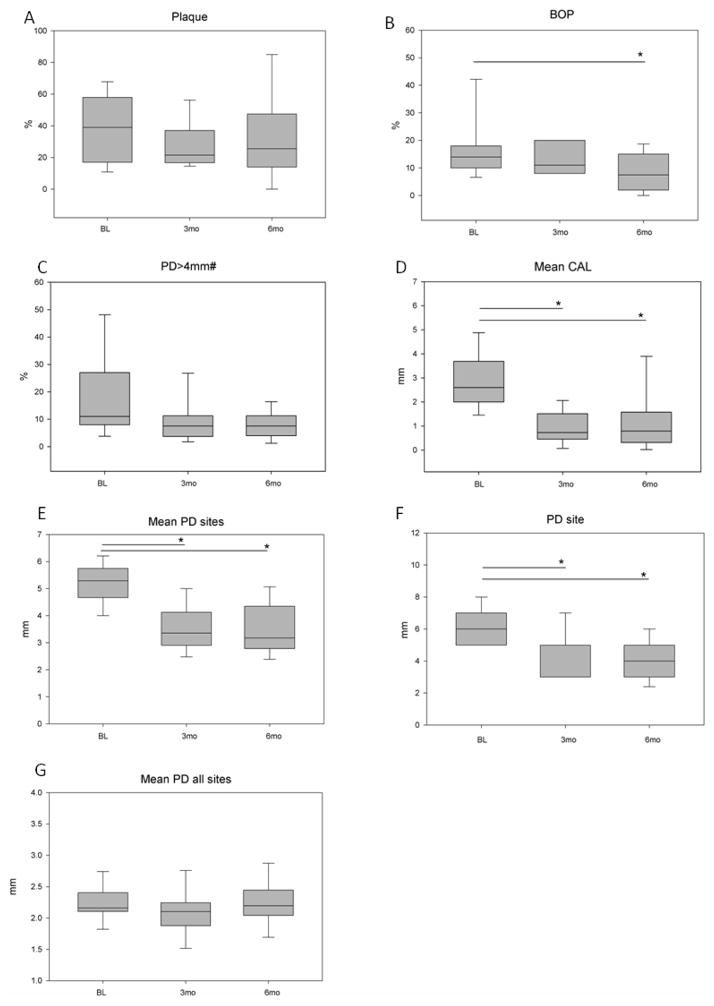
Figure 1.
(A–G). Clinical parameters at baseline and after treatment. PD>4mm = mean % of sites with PD>4mm. Mean PD sites= Mean pocket depth of sites with PD>4mm; Mean PD all= mean PD of all sites; Mean CAL= mean clinical attachment level of affected sites; BoP=bleeding on probing; PD site = mean pocket depth from diseased site sampled for GCF. Values in boxes are represented as Means (horizontal bars) ± Standard deviation (whiskers). BL= baseline (n=29); 3mo 3 months (n=16); 6mo= 6 months (n=22). Statistically significant differences* (One Way ANOVA, p<0.05) are represented as horizontal bars between timepoints by Dunn’s multiple comparisons. # indicates an overall significant difference in ANOVA on ranks.
Periodontal treatment resulted in improvement of clinical parameters (Figure 1), specifically in the percentage of sites with PD>4mm, mean PD of affected sites (PD>4mm with attachment loss and radiographic bone loss), mean PD from specific sampled sites, BoP and mean CAL. (One Way ANOVA, p<0.05).
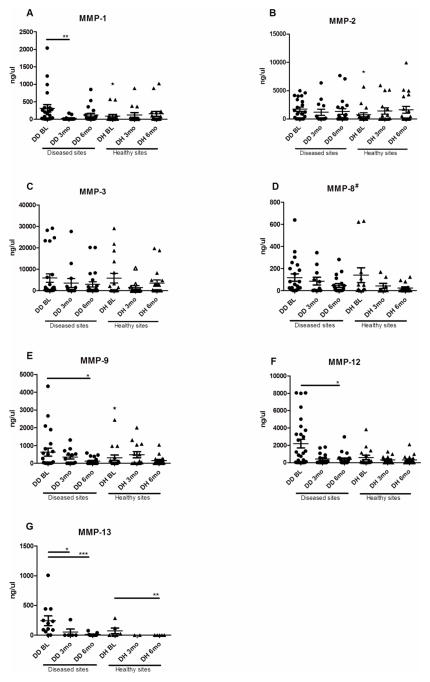
A graphic summary of the MMP levels in different timepoints is presented in Figure 2. There was a marked reduction in MMP-1, 8, 9 12, and 13 levels following periodontal treatment. Following treatment, MMP levels in diseased sites achieved levels comparable to healthy sites. There were no differences in MMP levels among the healthy sites time-points, except for MMP 13, where a significant reduction was also found from baseline to 6 months. There was also greater levels of MMP 1, 2, and 9 in baseline diseased versus healthy sites.
Figure 2.
(A–G). MMP levels at baseline and after treatment. Values (dots: Diseased sites; triangles: Healthy sites) are represented as Means (horizontal bars) ± Standard deviation (whiskers). BL= baseline; 3mo= 3 months; 6mo= 6 months. Statistically significant differences* (Kruskal Wallis test) are represented as horizontal bars between timepoints. Stars above healthy sites represent differences between diseased and healthy sites. P-values are represented as *(p<0.05), **(p<0.01) and ***(p<0.0001). #indicates an overall difference found in ANOVA on ranks (p<0.05).
A coefficient correlation matrix was run to determine whether any positive relationships existed between clinical parameters and MMP levels reductions. A summary of these results are presented in Table 2. %PD>4mm correlated significantly with MMP-2, 3, 8, 9, 12 and 13. Significant correlations were also noted between MMP-3, 12 and 13 with mean PD of affected sites.
Table 2.
Correlations between clinical parameters and MMP reductions after treatment
| Clinical parameters/MMP | MMP-1 | MMP-2 | MMP-3 | MMP-8 | MMP-9 | MMP-12 | MMP-13 |
|---|---|---|---|---|---|---|---|
| PD site | 0.151 | 0.199 | 0.190 | 0.205 | 0.175 | 0.158 | 0.196 |
| BoP | −0.150 | −0.145 | −0.099 | −0.104 | 0.002 | −0.099 | 0.115 |
| Mean CAL | 0.020 | 0.135 | 0.184 | 0.166 | 0.184 | 0.210 | 0.249 |
| %PD>4mm | 0.162 | 0.347 (0.007*) | 0.314 (0.016*) | 0.313 (0.016*) | 0.351 (0.008*) | 0.349 (0.007*) | 0.337 (0.009*) |
| plaque % | 0.136 | 0.198 | 0.056 | 0.108 | 0.181 | 0.219 | 0.231 |
| Mean PD | 0.117 | 0.205 | 0.265 (0.044*) | 0.231 | 0.233 | 0.263 (0.045*) | 0.343 (0.008*) |
Values are represented by correlation coefficient r2 (Spearman Correlation).
P values are presented in parenthesis when statistically significant (p<0.05).
PD site= Mean pocket depth of sampled site; BoP=bleeding on probing; Mean CAL=mean clinical attachment level of affected sites; %PD>4 = mean percent of pockets >4mm; mean PD= mean pocket depth of affected sites.
Considering correlations among different MMPs, it was noted that MMPs strongly and positively correlated with each other (P<0.05). For instance, the gelatinases correlated strongly with each other (MMP-2 and -9; r2 = 0.887). MMP-3 (stromelysin) and MMP-12 (macrophage elastase) yielded a positive correlation (r2 >0.80) with each of the two gelatinases.
DISCUSSION
The data presented here were obtained from a cohort study originally designed to determine the mechanisms responsible for the rapid periodontal destruction in LAP and to assess the treatment responses in various aspects. Our group has already published interesting results showing relatively high levels of MMPs present in the GCF from the diseased sites of children with AgP relative to non-diseased sites within the same children, their siblings, healthy unrelated children, and individuals with chronic periodontitis21. The most important finding of the present study is the substantial reduction in MMP levels, initially found to be in very high levels, following periodontal treatment.
To our knowledge, this is the first study monitoring a wide range of MMPs in the GCF of patients with LAP overtime. Up to date, a very few studies reported effects of different periodontal treatment approaches for AgP on MMP levels. Recently, Emingil et al22 demonstrated that MMP-8 levels in the GCF significantly decreased, over a 6-month period after periodontal treatment, along with clinical and microbiological improvements. However, the authors utilized either SRP or adjunctive azithromycin therapy, and the population in study consisted of generalized AgP patients. Another study aimed to compare the clinical status and MMP levels after SRP alone or with ozonotherapy in patients with aggressive and chronic periodontitis, up to 2 months after treatment23. Interestingly, the authors reported that SRP with additional ozonotherapy provided an increase in MMP levels in patients with chronic periodontitis and a reduction in MMP levels in patients with aggressive periodontitis. Nevertheless, in this later study, the levels of MMP-1, MMP-8 and MMP-9 were estimated in non-stimulated saliva with an ELISA method. Our findings are congruent with these previous studies, since we also found reductions in these MMP levels after periodontal treatment. The differences in the amount of reductions in MMP levels may be related to different treatment approaches, different methods of detection and disease severity.
It is important to mention that the most marked reduction in MMP levels, overall, occurred in a short-time period (3–6 months after treatment), when the levels of MMP in the diseased achieved levels comparable to healthy sites in these individuals.
The positive correlations found between some clinical parameters and MMP levels strengthen the role of periodontal treatment in controlling inflammation. MMP-2 (named as gelatinase A), mainly cleaving type IV collagen, is believed to play important roles in tissue destruction and remodeling in periodontitis24–27. Elevated MMP-2 levels of tissue or gingival crevicular fluid (GCF) have been observed in inflammatory sites in chronic periodontitis27, 28. Interestigly, in our study, it was correlated with % of deep sites, as expected, as it was present in higher levels in diseased versus healthy sites of these individuals. However, it did not show a significant reduction after treatment, possibly due to continued remodeling activity of these sites10.
MMP-12 is an essential molecule for macrophage migration and macrophage mediated matrix degradation. Although MMP-12 was first believed to be produced solely by macrophages, evidence suggests that osteoclast-derived MMP-12 could cleave bone matrix proteins pivotal for osteoclast–matrix interactions29. In our study, it was correlated with %PD>4mm, as well as mean PD of affected sites, which are related to both disease extent and severity. MMP-13 in GCF has also been suggested to reflect alveolar bone destruction during the course of adult periodontitis30. In our study, MMP-13 levels also correlated with %PD>4mm and mean PD>4mm. Our study shows reduction of both these MMPs after treatment. Interestingly, MMPs 1, 8, 9, 12 and 13 have all been shown to be related to periodontal disease process and they all showed a significant reduction over time after treatment of LAP, as well as a positive correlation with each other9, 12, 24. Reductions of MMPs 8, 9 and 13 after periodontal treatment have also been reported before 22.
Our study represents a contribution aimed at better characterization of the hyperinflammatory response associated with localized sites in individuals with AgP. Especially in a site specific disease, such as LAP, understanding biomarkers for periodontal destruction at a site-level is essential for the understanding of this disease activity during different treatment phases. It should be emphasized that our assay measured the active forms of MMPs, which is more closely related to progressive periodontal disease12.
LAP is a rare form of periodontal disease, with prevalence rates <1% reported in North American populations. However, from our preliminary report so far, it is clear that the improvement in clinical parameters is accompanied by the reductions in MMP levels. Future studies including more participants and longer follow-up during maintenance phase should confirm these findings and give us better insight into the role of MMPs in LAP disease activity.
CONCLUSION
Treatment of LAP with SRP and systemic antibiotics was effective in reducing the local levels of specific MMPs in African-American individuals, which correlated positively with reductions in clinical parameters related to disease severity.
Acknowledgments
The authors acknowledge the following: financial support from NIH/NIDCR (R01DE019456) and CNPq/Brazil (PF Gonçalves postdoctoral fellowship); Dr. Hiba Al-Kassab and the doctors/staff from Leon County Dental Clinic, for the assistance in coordinating our visits and our work with patients.
Sources of Funding Statement
This study was supported by NIH/NIDCR R01DE019456. Clinical trial registration: #NCT01330719. PF Gonçalves postdoctoral fellowship was supported by the National Council of Technological and Scientific Development, (CNPq), Brazil.
Footnotes
Periopaper, Proflow, Amityville, NY
Periotron 8000, Oraflow Inc, Plainview, NY
Hu-Friedy, Chicago, IL
Florida Probe, Gainesville, FL
Cavitron Jet Plus, Dentsply, York, PA
Sonicare Crest, Philips, Eindhoven, Netherlands
Sensolyte 520; AnaSpec, Fremont, CA
Fisher Scientific, Ocala, FL
Synergy HT: BioTek, Winooski, VT
AnaSpec, San Jose, CA
GraphPad Prism, version 5.0, GraphPad Software Inc, La Jolla, CA
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this manuscript.
Conflict of Interest:
The authors state that they have no conflict of interests, financial or commercial relationships to declare.
References
- 1.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Lang NP, Bartold M, Cullinan M, et al. Consensus Report: Aggressive Periodontitis. Ann Periodontol. 1999;4:53. [Google Scholar]
- 3.Albandar JM, Tinoco EM. Global epidemiology of periodontal diseases in children and young persons. Periodontol 2000. 2002;29:153–176. doi: 10.1034/j.1600-0757.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- 4.Tonetti MS, Mombelli A. Early-onset periodontitis. Ann Periodontol. 1999;4:39–53. doi: 10.1902/annals.1999.4.1.39. [DOI] [PubMed] [Google Scholar]
- 5.Lang N, Bartold M, Cullinan M, et al. Consensus Report: Aggressive Periodontitis. Annals of Periodontology. 1999;4:53. [Google Scholar]
- 6.Haubek D, Ennibi OK, Poulsen K, Vaeth M, Poulsen S, Kilian M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet. 2008;371:237–242. doi: 10.1016/S0140-6736(08)60135-X. [DOI] [PubMed] [Google Scholar]
- 7.Shaddox LM, Huang H, Lin T, et al. Microbiological Characterization in Children with Aggressive Periodontitis. J Dent Res. 2012 doi: 10.1177/0022034512456039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaddox L, Wiedey J, Bimstein E, et al. Hyper-responsive phenotype in localized aggressive periodontitis. J Dent Res. 2010;89:143–148. doi: 10.1177/0022034509353397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaddox LM, Wiedey J, Calderon NL, et al. Local inflammatory markers and systemic endotoxin in aggressive periodontitis. J Dent Res. 2011;90:1140–1144. doi: 10.1177/0022034511413928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sodek J, Overall CM. Matrix metalloproteinases in periodontal tissue remodelling. Matrix Suppl. 1992;1:352–362. [PubMed] [Google Scholar]
- 11.Birkedal-Hansen H. Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 1993;64:474–84. doi: 10.1902/jop.1993.64.5s.474. [DOI] [PubMed] [Google Scholar]
- 12.Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral Dis. 2004;10:311–318. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- 13.Uitto VJ, Overall CM, McCulloch C. Proteolytic host cell enzymes in gingival crevice fluid. Periodontol 2000. 2003;31:77–104. doi: 10.1034/j.1600-0757.2003.03106.x. [DOI] [PubMed] [Google Scholar]
- 14.Narayanan AS, Page RC. Connective tissues of the periodontium: a summary of current work. Coll Relat Res. 1983;3:33–64. [PubMed] [Google Scholar]
- 15.Birkedal-Hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 16.Ingman T, Tervahartiala T, Ding Y, et al. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. J Clin Periodontol. 1996;23:1127–1132. doi: 10.1111/j.1600-051x.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- 17.Leppert D, Lindberg RL, Kappos L, Leib SL. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res Brain Res Rev. 2001;36:249–257. doi: 10.1016/s0165-0173(01)00101-1. [DOI] [PubMed] [Google Scholar]
- 18.Villela B, Cogen RB, Bartolucci AA, Birkedal-Hansen H. Collagenolytic activity in crevicular fluid from patients with chronic adult periodontitis, localized juvenile periodontitis and gingivitis, and from healthy control individuals. J Periodontal Res. 1987;22:381–389. doi: 10.1111/j.1600-0765.1987.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 19.Larivee J, Sodek J, Ferrier JM. Collagenase and collagenase inhibitor activities in crevicular fluid of patients receiving treatment for localized juvenile periodontitis. J Periodontal Res. 1986;21:702–715. doi: 10.1111/j.1600-0765.1986.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 20.Kryshtalskyj E, Sodek J, Ferrier JM. Correlation of collagenolytic enzymes and inhibitors in gingival crevicular fluid with clinical and microscopic changes in experimental periodontitis in the dog. Arch Oral Biol. 1986;31:21–31. doi: 10.1016/0003-9969(86)90109-3. [DOI] [PubMed] [Google Scholar]
- 21.Alfant B, Shaddox LM, Tobler J, Magnusson I, Aukhil I, Walker C. Matrix metalloproteinase levels in children with aggressive periodontitis. J Periodontol. 2008;79:819–826. doi: 10.1902/jop.2008.070513. [DOI] [PubMed] [Google Scholar]
- 22.Emingil G, Han B, Ozdemir G, et al. Effect of azithromycin, as an adjunct to nonsurgical periodontal treatment, on microbiological parameters and gingival crevicular fluid biomarkers in generalized aggressive periodontitis. J Periodontal Res. 2012;47:729–739. doi: 10.1111/j.1600-0765.2012.01488.x. [DOI] [PubMed] [Google Scholar]
- 23.Skurska A, Pietruska MD, Paniczko-Drężek A, et al. Evaluation of the influence of ozonotherapy on the clinical parameters and MMP levels in patients with chronic and aggressive periodontitis. Adv Med Sci. 2010;55:297–307. doi: 10.2478/v10039-010-0048-x. [DOI] [PubMed] [Google Scholar]
- 24.Ingman T, Sorsa T, Lindy O, Koski H, Konttinen YT. Multiple forms of gelatinases/type IV collagenases in saliva and gingival crevicular fluid of periodontitis patients. J Clin Periodontol. 1994;21:26–31. doi: 10.1111/j.1600-051x.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 25.Mäkelä M, Salo T, Uitto VJ, Larjava H. Matrix metalloproteinases (MMP-2 and MMP-9) of the oral cavity: cellular origin and relationship to periodontal status. J Dent Res. 1994;73:1397–1406. doi: 10.1177/00220345940730080201. [DOI] [PubMed] [Google Scholar]
- 26.Korostoff JM, Wang JF, Sarment DP, Stewart JC, Feldman RS, Billings PC. Analysis of in situ protease activity in chronic adult periodontitis patients: expression of activated MMP-2 and a 40 kDa serine protease. J Periodontol. 2000;71:353–360. doi: 10.1902/jop.2000.71.3.353. [DOI] [PubMed] [Google Scholar]
- 27.Ejeil AL, Igondjo-Tchen S, Ghomrasseni S, Pellat B, Godeau G, Gogly B. Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. J Periodontol. 2003;74:188–195. doi: 10.1902/jop.2003.74.2.188. [DOI] [PubMed] [Google Scholar]
- 28.Pozo P, Valenzuela MA, Melej C, et al. Longitudinal analysis of metalloproteinases, tissue inhibitors of metalloproteinases and clinical parameters in gingival crevicular fluid from periodontitis-affected patients. J Periodontal Res. 2005;40:199–207. doi: 10.1111/j.1600-0765.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 29.Hannas AR, Pereira JC, Granjeiro JM, Tjäderhane L. The role of matrix metalloproteinases in the oral environment. Acta Odontol Scand. 2007;65:1–13. doi: 10.1080/00016350600963640. [DOI] [PubMed] [Google Scholar]
- 30.Golub LM, Lee HM, Greenwald RA, et al. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm Res. 1997;46:310–319. doi: 10.1007/s000110050193. [DOI] [PubMed] [Google Scholar]