Abstract
Signaling via the Akt/mTOR pathway influences CD4+ T cell differentiation; low levels favor Treg induction and high levels favor Th induction. Although the lipid phosphatase PTEN suppresses Akt activity the control of PTEN activity is poorly studied in T cells. Here, we identify multiple mechanisms that regulate PTEN expression. During Th induction, PTEN function is suppressed via lower mRNA levels, lower protein levels and an increase in C terminal phosphorylation. Conversely, during Treg induction, PTEN function is maintained through the stabilization of PTEN mRNA transcription and sustained protein levels. We demonstrate that differential Akt/mTOR signaling regulates PTEN transcription via the FoxO1 transcription factor. A mathematical model that includes multiple modes of PTEN regulation recapitulates our experimental findings and demonstrates how several feedback loops determine differentiation outcomes. Together, this work provides novel mechanistic insights into how differential regulation of PTEN controls alternate CD4+ T cell fate outcomes.
Introduction
Ligand binding to the TCR induces a cascade of signaling events that culminates in gene transcription, T cell proliferation and differentiation. We (1) and others (2, 3) have demonstrated that Foxp3-expressing T regulatory cells (Tregs) are induced when naïve T cells are exposed to low antigen doses, and have shown that Treg expanded following stimulation with low dose antigen prevent autoimmune diabetes in vivo (4, 5). Treg induction is inversely correlated with the degree of signaling via the Akt/mTOR pathway (1, 6, 7).
We developed a mathematical model to determine how TCR signal strength contributes to Treg induction (8). Regulation of the phosphatase and tensin homolog (PTEN) protein emerged as a critical control point in Treg induction (8). Expression and function of PTEN are tightly regulated via transcriptional control and post-translational modifications (PTMs) (9, 10). Naïve T cells express high levels of PTEN and following T cell activation PTEN protein disappears (11), but the mechanism for this down regulation is not known. Treg are known to express higher levels of PTEN (12) and this changes the signaling pathways triggered following IL-2 activation (13).
Here, we identify multiple modes of PTEN regulation that affect CD4+ T cell differentiation. T helper (Th) induction is characterized by reduced PTEN transcription, degradation of PTEN protein, and higher C terminal phosphorylation. However, in Treg induction, PTEN mRNA and protein levels are maintained. Additionally, we identify a loop connecting Akt/mTOR signaling to the regulation of PTEN via FoxO1 as a novel mechanism for regulating CD4+ T cell differentiation. Together, these findings define how TCR signal strength differentially tunes PTEN function to drive Th versus Treg differentiation.
Materials and Methods
Mice
C57BL/6 mice were obtained from Jackson Laboratories. All mice were housed in a specific pathogen-free facility at the University of Pittsburgh and treated under Institutional Animal Care and Use Committee-approved guidelines in accordance with approved protocols.
CD4+ T cell isolation and activation
CD4+ T cells were isolated from C57BL/6 spleens using a CD4+ negative selection kit (Miltenyi Biotech). In some experiments CD25+ T cells were removed using CD25 microbeads. T cell activation assays were performed with low (0.25 μg/mL) or high (1μg/mL) dose plate-bound anti-CD3 monoclonal antibody (mAb) in the presence of soluble anti-CD28 mAb (1μg/mL). For inhibition studies, T cells were treated for one hour with the following drugs prior to stimulation: caspase inhibitor (ZVAD, 80 μM), PTEN inhibitor (SF1670, 10 μM) and Akt inhibitor (Akti1/2, 10 μM).
Flow Cytometry
Activated CD4+ T cells were stained with the following mAb: anti-CD3-APC-eFluor780, anti-CD4-APC, anti-CD25-PE, anti-Foxp3-Pac blue (eBioscience), and anti-pS6 (Ser235)-FITC (Cell Signaling Technology) using buffers from eBioscience. The stained cells were analyzed on a LSR II flow cytometer and data were analyzed with the Flowjo software package.
Western blotting
Western blotting was performed using the following antibodies from Cell Signaling Technology: β-Actin, PTEN (138G6), Phospho-PTEN (Ser380/Thr382/383), FoxO1 (C29H4), Phospho-FoxO1 (Thr24), and ubiquitin.
PTEN RNA levels
Total RNA was isolated from CD4+ T cells the Aurum Total RNA mini kit (Bio Rad). Quantitative RT-PCR (qPCR) was performed on a Step One Plus real time PCR system using SYBR Green (Applied Biosystems). Primer pair used: 5’ ACACCGCCAAATTTAACTGC 3’ and 5’ TACACCAGTCCGTCCCTTTC 3’.
Chromatin immunoprecipitation (ChIP) of FoxO1
ChIP for FoxO1 was performed using the CHIP-IT high sensitivity kit (Active Motif) and an anti-FoxO1 antibody (Cell Signaling Technology). qPCR was used to quantify the amount of the PTEN 5KB upstream element (primer pair used: 5’ CCTTGCATCAGCCATTAAAGAAA 3’ and 5’ CTGCTGTACCAGGCTATATGTG) associated with FoxO1.
FoxO1 siRNA knockdown
A murine FoxO1 siRNA kit (Origene) was used to knock down FoxO1 expression. The siRNAs were introduced into isolated CD4+ T cells using a standard protocol (Lonzo Nucleofectortm kit for Mouse T cells). Western blot analysis for FoxO1, PTEN and Actin was performed after 48 hours of incubation.
Nuclear/cytoplasmic isolation and localization
Nuclear and cytoplasmic fractions of activated T cells were generated using the NE-PER nuclear and cytoplasmic kit following the manufacturers instructions (Thermo Scientific).
Computational modeling of T cell development
Simulations of PTEN levels in cells destined to become Th or Treg cells were performed as described (8). A minimized version was specified as a Boolean model using the logical rules shown in Supplemental Fig. 2. For the simulations performed here, the logical rules were translated into the BioNetGen rule-based modeling language using an automated tool called Boolean2BNGL included in the BioNetGen package (14). The simulations, performed according to the General Asynchronous update scheme (15), used a modified version of Gillespie’s Direct Stochastic Simulation Algorithm (16).
Statistics
All statistical calculations were performed in the GraphPad Prism 6 software package. Except where indicated all analyses used the two-way ANOVA analysis with Bonferroni post analysis correction. Some analyses utilized the one-way ANOVA or Student t test. For all tests the P values are denoted by: <0.0001= ****, <0.001= ***, <0.01= ** and <0.05= *.
Results and Discussion
PTEN protein levels are suppressed during Th differentiation but maintained during Treg differentiation
Our computational model of Th/Treg differentiation (8) predicted that, in developing Th cells, PTEN rapidly decreased and remained absent, whereas PTEN was only transiently down-modulated in cells destined to become Treg (Fig. 1A). To test this prediction, we performed T cell activation assays with low and high doses of anti-CD3 mAb, which drive Treg and Th differentiation respectively (Supplemental Fig. 1A, B) (1, 4, 17). Western blot analysis of PTEN in activated CD4+ T cells confirmed the model predictions and PTEN levels were markedly and persistently reduced with high dose stimulation, but only transiently reduced with low dose stimulation (Fig. 1B and C). Similar results were obtained with CD25 depleted CD4+ T cells (Supplemental Fig. 1C, D)
FIGURE 1. TCR signal strength regulates PTEN protein levels.
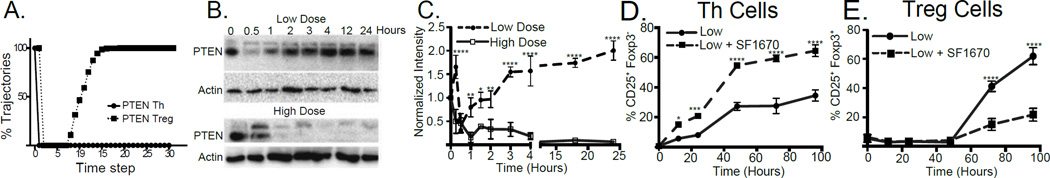
(A) PTEN activity in simulations of CD4 T cell differentiation leading to Th (solid line) and Treg fate (dotted line) reported as percentage of simulation trajectories. (B) PTEN protein levels were tracked by Western blotting of activated CD4+ T cells under low- (upper panel) and high- (bottom panel) dose conditions. (C) Actin-normalized PTEN values were plotted. D and E CD4+ T cells were activated under low dose TCR stimulation in the presence (dashed line) or absence (solid line) of a PTEN inhibitor (SF1670). The development of Th (CD25+ Foxp3−) (D) or Treg (CD25+ Foxp3+) (E) was assessed by flow cytometry on gated CD3+ CD4+ T cells. Results in C, D and E represent the mean ± SEM from 3 independent experiments.
To determine if PTEN activity regulated CD4+ T cell differentiation, activation assays were performed in the presence of a PTEN inhibitor. PTEN inhibition of T cells stimulated with high dose anti-CD3 resulted in the development of Th cells and enhancement of Akt/mTOR signaling as expected (Supplemental Fig. 1E). However, under low-dose stimulation, PTEN inhibition enhanced Th (Fig. 1D) and suppressed Treg (Fig. 1E) induction. These results are consistent with recent studies using PTEN shRNA, showing decreased Treg induction and increased pS6 in cells with PTEN knockdown (13, 18).
PTEN is ubiquitinated under conditions of Th but not Treg induction
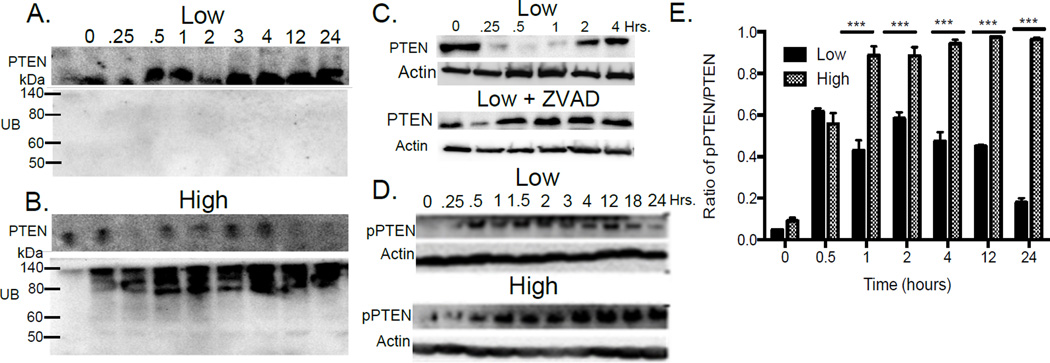
TCR signaling perturbs PTEN protein levels (Fig. 1) (13, 18), but the molecular mechanisms that regulate PTEN protein levels in T cells are unexplored. The rapidity of PTEN degradation in Th and Treg conditions suggest that PTMs contribute to the regulation of PTEN turnover. In other cell types, polyubiquitination of PTEN by the E3 ubiquitin ligase Nedd4 leads to proteosomal degradation (19). In Treg conditions, PTEN was not ubiquitinated (Fig. 2A), whereas in Th conditions, PTEN was extensively ubiquitinated (Fig. 2B).
FIGURE 2. PTEN is regulated by ubiquitination, phosphorylation and caspase degradation.
PTEN was immunoprecipitated (PTEN IP) from CD4+ T cells activated under low (A) or high (B) TCR stimulation and probed for total ubiquitin (α UB). (C) T cells were activated with low dose in the presence or absence of a pan-caspase inhibitor (ZVAD) and PTEN levels determined by Western blot. (D) Phosphorylation of the PTEN C-terminus was assessed by western blotting under low and high dose TCR stimulation (quantitated in panel E). Results represent the mean ± SEM from 3 independent experiments.
During Treg induction, PTEN is degraded soon after T cell activation yet PTEN is not ubiquitinated (Fig. 1 and Fig. 2A), suggesting a different mechanism for the early degradation of PTEN. Multiple caspases, including -3 and -9, are known to cleave PTEN (20) and caspases function in TCR signaling (21, 22). Pan caspase inhibition with the inhibitor ZVAD blocked degradation of PTEN under Treg conditions (Fig. 2C), demonstrating that caspase-mediated degradation of PTEN occurs during CD4+ T cell activation.
TCR activation promotes PTEN phosphorylation
Constitutive phosphorylation of the C terminus of PTEN suppresses its enzymatic activity (23, 24), and the casein kinase 2 (CK2) has been shown to phosphorylate these sites in T cells (25, 26). We explored the phosphorylation state of PTEN during T cell differentiation. PTEN was phosphorylated at a cluster of sites including Ser380, Thr382 and Thr383 during both Treg and Th induction (Fig. 2D), but relative levels of PTEN phosphorylation were significantly higher during Th induction (Fig. 2E). These results are the first demonstration in T cells that levels of PTEN and enzymatic activity are controlled by multiple PTMs, which are differentially regulated by TCR signal strength.
PTEN transcription is regulated by FoxO1
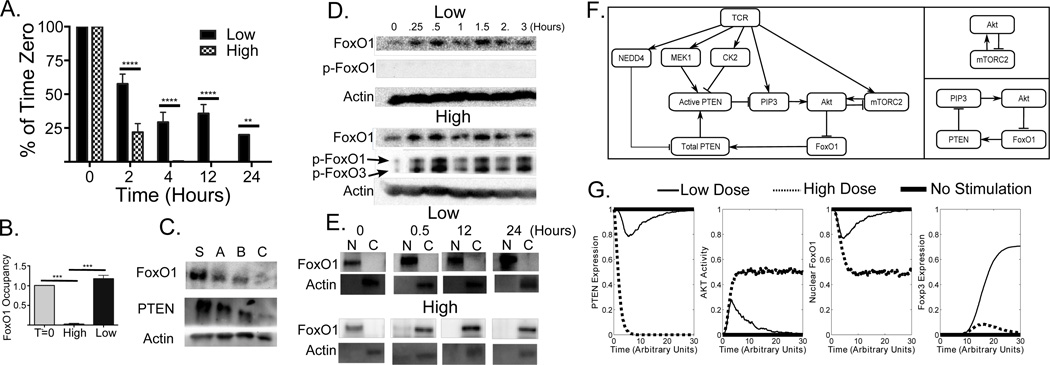
High levels of TCR signaling have been correlated with a reduction in PTEN mRNA (13, 18). Therefore, we followed the expression of PTEN mRNA during Th and Treg induction. PTEN mRNA decreased in both Treg and Th activation conditions (Fig. 3A), but, whereas about 20% of PTEN mRNA remained under Treg conditions, little PTEN mRNA was detected under Th conditions.
FIGURE 3. FoxO1 regulates PTEN transcription.
(A) PTEN mRNA levels were measured during Treg (black bars) and Th (hashed bars) Standard deviations were determined from three independent experiments. (B) ChIP experiments were performed to measure the association of FoxO1 and a 5 KB region upstream of the PTEN promoter at time=0 (T=0) and after 12 hrs of stimulation under Th (High) and Treg (Low) conditions. Results were normalized to unstimulated T cells (T=0). A one-way ANOVA was performed. (C) Western blot analysis of PTEN, FoxO1 and actin protein levels in CD4 T cells treated with 3 FoxO1 siRNAs (A, B, C) and scrambled control (S) for 48 hours (D) Total levels of FoxO1 and Phospho-Thr24 FoxO1 were monitored by Western blotting. (E) Cellular localization of FoxO1 during low and high dose TCR stimulation determined by biochemically isolating nuclear, N, and cytoplasmic, C, fractions and Western blotting for FoxO1. Anti-actin antibody identifies the cytoplasmic fraction. (F) A reduced logical model of PTEN and Akt dynamics was developed (left panel). Two key feedback loops can be identified: negative feedback, in which Akt inhibits mTORC2 activity, (top right panel) and positive feedback, in which Akt inactivates FoxO1 (bottom right panel). (G) Average levels of total PTEN (far left panel), active Akt (left panel), and nuclear FoxO1 (right panel) from the reduced model, and average levels of Foxp3 (far right panel) from the full model with inclusion of new regulatory elements from the reduced model for no TCR stimulation (thick solid line), low TCR stimulation (thin solid line) and high stimulation (dashed line) as a function of simulated time computed from 10,000 simulated trajectories.
The mechanisms used to maintain PTEN mRNA levels under Treg induction conditions are not known. In our T cell differentiation model (8), the recovery of PTEN in Treg conditions arises from a link coupling Foxp3 to PTEN. However, recent data implicates the transcription factor FoxO1 in the induction of Treg, and phosphorylation of FoxO1 by Akt was established as a mechanism by which Akt/mTOR signaling inhibits Treg differentiation (27).
Previous chromatin immunoprecipitation sequencing (ChIP-Seq) data identified a FoxO1 binding site 5 kb upstream of the PTEN promoter (28), and we hypothesized that FoxO1 might be responsible for maintenance of PTEN transcription in Treg conditions. We performed ChIP assays to determine if FoxO1 bound to this region. In both resting CD4+ T cells and under Treg conditions, FoxO1 bound the 5 kb upstream element, but did not exhibit significant binding under Th conditions (Fig. 3B). siRNA knock down of FoxO1 resulted in a concomitant decrease in PTEN protein levels, consistent with a positive role for FoxO1 in the regulation of PTEN levels (Fig. 3C)..
Phosphorylation and nuclear localization of FoxO1 depend on TCR signal strength
Previous work demonstrated that unphosphorylated FoxO1 is localized in the nucleus and that Akt-mediated phosphorylation of Thr24 drives FoxO1 into the cytoplasm (29). We found that FoxO1 remained unphosphorylated under Treg conditions, but was rapidly phosphorylated under Th conditions (Fig. 3D). As expected, FoxO1 remained in the nucleus in resting CD4+ T cells and under Treg conditions, but was largely in the cytoplasm following high dose stimulation (Fig. 3E), consistent with our observations on regulation of PTEN transcription.
Logical model that includes FoxO1 shows how positive and negative feedback loops determine T cell fate
Using components and interactions from our previous model of T cell differentiation (8), we developed a more limited model focusing on the regulation of PTEN, Akt, and FoxO1 (Fig. 3F, left). Simulations exhibited rapid and sustained loss of PTEN (Fig. 3G) at high antigen dose, as well as transient and incomplete loss of PTEN at low dose, in agreement with experimental findings. Under both conditions there was a rapid initial increase in Akt activity (Fig. 3G), sustained by positive feedback involving the loss of nuclear FoxO1 (Fig. 3F bottom). At high dose, the activation of CK2 blocked PTEN activity and sustained Akt activation. At low dose, PTEN activity rebounded as negative feedback through mTORC2 (Fig. 3F, top right) decreased Akt activity, increasing nuclear FoxO1 and, consequently, PTEN transcription. PTEN activity then further suppressed Akt, permitting full restoration of PTEN expression. Insertion of our reduced model back into our previous model of T cell differentiation resulted in sustained induction of Foxp3 in about 70% of cells at low dose and only transient induction of Foxp3 at high dose (Fig. 3G), in agreement with results from the previous model and experiments (8).
Transient suppression of PTEN at low dose arises from the inclusion of two regulatory mechanisms in the model: the requirement for MEK1 to activate PTEN, (30) and inhibition of mTORC2 by Akt (31). Prior to MEK1 activation by TCR (32), PTEN is inactive, allowing the transient induction of Akt, even at low dose, which may be necessary for T cell activation and proliferation regardless of differentiation outcome. Subsequent activation of PTEN by MEK1 then inhibits Akt in concert with the negative feedback provided by indirect Akt inhibition of mTORC2 (31).
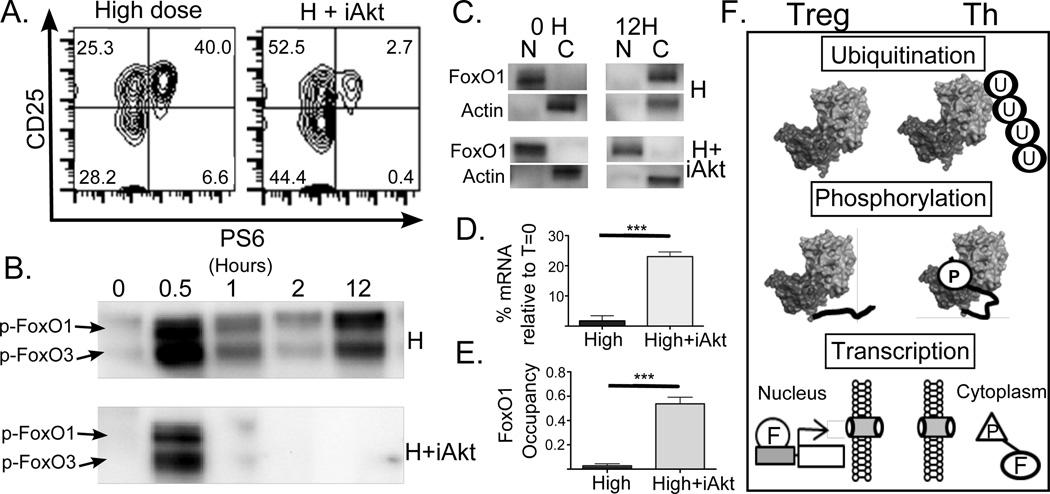
Akt regulates FoxO1 nuclear localization and binding to PTEN promoter
In order to further elucidate the role of Akt in the regulation of FoxO1, we treated CD4+ T cells with an Akt inhibitor (Akti1/2) simultaneously with activation by a high dose of stimulation. The efficacy of the Akt inhibitor was confirmed by a reduction in pS6 expression (Fig. 4A). Under high TCR stimulation alone, we observed sustained phosphorylation of FoxO1 (Fig. 4B) and cytoplasmic localization of FoxO1 at 12h (Fig. 4C). We also observed loss of FoxO1 binding to the 5 kb element upstream of the PTEN promoter (Fig. 4D) and loss of PTEN mRNA expression (Fig. 4E). Addition of the Akt inhibitor, on the other hand, resulted in only transient phosphorylation of FoxO1 (Fig. 4B), which remained in the nucleus at 12h (Fig. 4C) and was accompanied by sustained binding of FoxO1 upstream of the PTEN promoter and significantly higher PTEN mRNA expression. These results show that Akt is an important regulator of PTEN expression, acting through the transcription factor FoxO1.
FIGURE 4. Akt regulates FoxO1 phosphorylation and localization.
T cells were stimulated under high dose conditions in the presence or absence of an Akt inhibitor (Akti1/2). (A) Phosphorylation of S6 (pS6) was monitored by flow cytometry. An example of untreated (left panel) and Akt inhibitor treated (right panel) T cells stimulated for 12 hours are depicted. (B) Phosphorylation of Thr24 on FoxO1 was monitored by Western blotting at the indicated time points. (C) Nuclear and cytoplasmic fractions were Western blotted for total FoxO1 protein. (D) PTEN mRNA levels were determined by qPCR for T cells stimulated for 12 hrs with high dose (High dose) and high dose treated with Akt inhibitor (H+iAkt). (E) ChIP experiments measured the association of FoxO1 and the 5KB upstream of the PTEN promoter under Th (H) and Th with Akt inhibitor (H+iAkt) conditions. A t test was performed in D and E. The multiple mechanisms identified that differentially regulate PTEN under Treg and Th conditions are illustrated in F.
Conclusion
We have demonstrated that differential regulation of PTEN determines CD4+ T cell fate. In Th differentiation, stimulated by high TCR strength, multiple mechanisms suppress PTEN activity by lowering protein levels and enzymatic activity (Fig. 4F). Low TCR signal strength, resulting in Treg induction, also involves early downregulation of PTEN, but requires that PTEN activity is re-established at longer times because inhibition of PTEN under these conditions reduces Treg and increases Th induction. Additionally, we identify a critical feedback loop driven by differential Akt/mTOR signaling that regulates PTEN transcription via the FoxO1 transcription factor. By combining biochemical and computational modeling approaches, our work defines how differential regulation of PTEN can produce alternate CD4+ T cell fate outcomes.
Supplementary Material
Acknowledgements
We thank Leonard Harris for help with the Boolean2BNGL package.
Footnotes
JRF and NMZ acknowledge funding from NIH (P41 GM103712) and NSF (0926181). LPK acknowledges funding from NIH (GM080398 and AI095730). WFH and RPS acknowledge support from NIH for post- and predoctoral training from grants T32 AI089443 and T32 EB009403 respectively.
References
- 1.Turner MS, Kane LP, Morel PA. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J. Immunol. 2009;183:4895–4903. doi: 10.4049/jimmunol.0901459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J. Exp. Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat. Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 4.Turner MS, Isse K, Fischer DK, Turnquist HR, Morel PA. Low TCR signal strength induces combined expansion of Th2 and regulatory T cell populations that protect mice from the development of type 1 diabetes. Diabetologia. 2014;57:1428–1436. doi: 10.1007/s00125-014-3233-9. [DOI] [PubMed] [Google Scholar]
- 5.Daniel C, Weigmann B, Bronson R, von Boehmer H. Prevention of type 1 diabetes in mice by tolerogenic vaccination with a strong agonist insulin mimetope. J. Exp. Med. 2011;208:1501–1510. doi: 10.1084/jem.20110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O'Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc. Natl. Acad. Sci. U S A. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miskov-Zivanov N, Turner MS, Kane LP, Morel PA, Faeder JR. The duration of T cell stimulation is a critical determinant of cell fate and plasticity. Sci. Signal. 2013;6:ra97. doi: 10.1126/scisignal.2004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Paluch BE, Wang X, Jiang X. PTEN at a glance. J. Cell. Sci. 2012;125:4687–4692. doi: 10.1242/jcs.093765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nature reviews. Mol. Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 11.Newton RH, Turka LA. Regulation of T cell homeostasis and responses by pten. Front. Immunol. 2012;3:151. doi: 10.3389/fimmu.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, Thompson CB, Burchill MA, Farrar MA, Turka LA. Distinct IL-2 Receptor Signaling Pattern in CD4+CD25+ Regulatory T Cells. J. Immunol. 2004;172:5287–5296. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faeder JR, Blinov ML, Hlavacek WS. Rule-based modeling of biochemical systems with BioNetGen. Meth. Mol. Biol. 2009;500:113–167. doi: 10.1007/978-1-59745-525-1_5. [DOI] [PubMed] [Google Scholar]
- 15.Saadatpour A, Albert I, Albert R. Attractor analysis of asynchronous Boolean models of signal transduction networks. J. Theor. Biol. 2010;266:641–656. doi: 10.1016/j.jtbi.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Gillespie D. A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J. Comput. Phys. 1976;22:403–434. [Google Scholar]
- 17.Gabrysova L, Christensen JR, Wu X, Kissenpfennig A, Malissen B, O'Garra A. Integrated T-cell receptor and costimulatory signals determine TGF-beta-dependent differentiation and maintenance of Foxp3+ regulatory T cells. Eur. J. Immunol. 2011;41:1242–1248. doi: 10.1002/eji.201041073. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Rodriguez J, Wohlfert EA, Handon R, Meylan F, Wu JZ, Anderson SM, Kirby MR, Belkaid Y, Schwartzberg PL. Itk-mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. J. Exp. Med. 2014;211:529–543. doi: 10.1084/jem.20131459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres J, Rodriguez J, Myers MP, Valiente M, Graves JD, Tonks NK, Pulido R. Phosphorylation-regulated Cleavage of the Tumor Suppressor PTEN by Caspase-3: Implications for the control of proetina stability and PTEN-protein interactions. J. Biol. Chem. 2003;278:30652–30660. doi: 10.1074/jbc.M212610200. [DOI] [PubMed] [Google Scholar]
- 21.Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 22.Puga I, Rao A, Macian F. Targeted cleavage of signaling proteins by caspase 3 inhibits T cell receptor signaling in anergic T cells. Immunity. 2008;29:193–204. doi: 10.1016/j.immuni.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolduc D, Rahdar M, Tu-Sekine B, Sivakumaren SC, Raben D, Amzel LM, Devreotes P, Gabelli SB, Cole P. Phosphorylation-mediated PTEN conformational closure and deactivation revealed with protein semisynthesis. eLife. 2013;2:e00691. doi: 10.7554/eLife.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez F, Ramaswamy S, Nakamura N, Sellers WR. Phosphorylation of the PTEN tail regulates protein stability and function. Mol. Cell. Biol. 2000;20:5010–5018. doi: 10.1128/mcb.20.14.5010-5018.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller SJ, Lou DY, Seldin DC, Lane WS, Neel BG. Direct identification of PTEN phosphorylation sites. FEBS Lett. 2002;528:145–153. doi: 10.1016/s0014-5793(02)03274-x. [DOI] [PubMed] [Google Scholar]
- 26.Sestero CM, McGuire DJ, De Sarno P, Brantley EC, Soldevila G, Axtell RC, Raman C. CD5-Dependent CK2 Activation Pathway Regulates Threshold for T Cell Anergy. J. Immunol. 2012;189:2918–2930. doi: 10.4049/jimmunol.1200065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch'en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, Meijer D, Zhao K, Rudensky AY, Atwal G, Zhang MQ, Li MO. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 30.Zmajkovicova K, Jesenberger V, Catalanotti F, Baumgartner C, Reyes G, Baccarini M. MEK1 Is Required for PTEN Membrane Recruitment, AKT Regulation, and the Maintenance of Peripheral Tolerance. Mol. Cell. 2013;50:43–55. doi: 10.1016/j.molcel.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine 1135 and regulates mTORC2 signaling. Mol. Cell. Biol. 2010;30:908–921. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.