Summary
Anemia accelerates disease progression and increases mortality among HIV-infected individuals. Few studies have characterized this problem in developing countries. Hemoglobin values of adults presenting to an HIV tertiary care center in India between 1996 and 2007 were collected (n=6996). Multivariate logistic regression analysis was performed to examine associations among anemia, HIV progression, and co-morbidities. Overall anemia prevalence was 41%. Twenty percent of patients with CD4 counts >500 cells/µL were anemic, compared to 64% of those with CD4 counts <100 cells/µL (p<0.001). In multivariate analysis, CD4 count <100 cells/µL (OR:5.0, CI:4.0–6.3), underweight body-mass index (OR:4.8, CI:3.6–6.5), female gender (OR:3.1, CI:2.8–3.6), and tuberculosis (OR:1.6, CI:1.4–1.8) were significantly associated with anemia. In this setting, management of anemia should focus on antiretroviral therapy, nutritional supplementation, and tuberculosis control. The high anemia prevalence among patients meeting criteria for antiretroviral therapy highlights the need for increased access to non-AZT nucleoside reverse transcriptase inhibitors in developing countries.
Keywords: anemia, HIV, tuberculosis, India, malnutrition, resource-limited settings
Introduction
Anemia is the most common hematological complication associated with HIV, with rates increasing with disease progression.1 Anemia is independently associated with decreased quality of life, accelerated disease progression, and increases mortality in HIV-infected individuals.2,3 Survival time in HIV-infected individuals may be improved with recovery from anemia.1 By adversely affecting quality of life, anemia may exacerbate poverty in communities with high HIV prevalence.4
In developing countries such as India, where more than half of women in the general population are anemic,5 the interaction between HIV and anemia may be even more detrimental. Estimates of the prevalence of anemia among HIV-infected individuals in sub-Saharan Africa range from 70–90%,3,6–8 as compared to 20–60% in reports from developed countries. 1,9 Few studies have examined factors associated with anemia in developing country settings. Risk factors for anemia in developing countries may vary from those in developed countries due to endemic malnutrition, helminth infections, tuberculosis (TB), malaria, and a different spectrum of opportunistic infections. Here we report the prevalence of, and factors associated with, anemia among HIV-infected individuals at an HIV/AIDS tertiary care center in South India.
Methods
The Y.R. Gaitonde Centre for AIDS Research and Education (YRG CARE) is an HIV tertiary care clinic in Chennai, India, which has provided medical care to nearly 10,000 patients since 1994. Analysis was done using the YRG CARE HIV Natural History Study Observational Database, which has been previously validated and approved by YRG CARE’s independent institutional review board.10 This database captures demographic and clinical details (including CD4 cell counts, hemoglobin values, opportunistic infections, and HIV-related co-morbidities) from every patient visit. Patients greater than 18 years of age who visited between January 1, 1996 and December 31, 2006 were included. Only baseline hemoglobin values ordered at the time of a patient’s initial enrollment for care were analyzed.
Anemia was classified by the following WHO criteria for both men and women: “non-anemic” (hemoglobin value ≥ 11 g/dL), grade one (9.5–10.9 g/dL), grade two (8–9.4 g/dL), grade three (6.5–7.9 g/dL), and grade four (<6.5 g/dL). For comparison with other studies, anemia was also reclassified as hemoglobin values <12 g/dL for women and <13 g/dL for men as noted in the discussion. Body-mass index (BMI) values, CD4 cell counts, and HIV-associated co-morbidities recorded or diagnosed at the time of enrollment into care were included in the analysis.
Statistical analyses were performed with SPSS (version 10.0.5; Chicago, IL). Normal data were summarized using mean and standard deviation (SD) and non-normal data using median and interquartile range (IQR). Student’s t-test was used to compare the mean hemoglobin values of the various CD4 cell count strata. Trends in anemia grades among the different CD4 cell count strata were tested using χ2 for trend with Epi Info (version 3.3.2, CDC, Atlanta, GA). Univariate and multivariate logistic regression were performed to understand the associations between anemia and the various demographic and clinical characteristics. Statistically significant variables (p<0.05) were included in the multivariate model using the forward stepwise method, and the best reduced model was built based on a 2 log likelihood value.
Results
In this analysis, 6996 patients who were over 18 years of age and had a hemoglobin value recorded at the time of enrollment into care were included, of whom 70% were male and 96% had acquired HIV through heterosexual transmission. Those without a hemoglobin value recorded at the time of enrollment into care, 2648 patients (27.5%), were excluded from all analyses. Male-female ratio, mean age, and mean BMI between the included and excluded groups were similar. Specifically, patients included in the analysis had a 70% male predominance, a mean age of 33.6 years (SD 8.3), and a mean BMI of 20.1 (SD 2.8); this compares to a 63% male predominance, a mean age of 32.2 year (SD 8.0), and a mean BMI of 20.0 (SD 3.9) in the group excluded from the analysis.
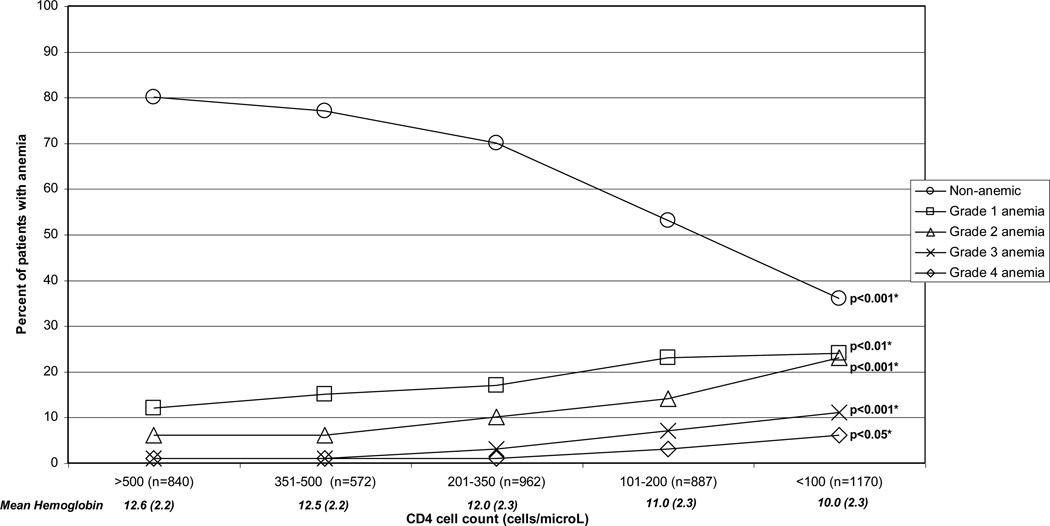
The mean hemoglobin value for the entire cohort was 11.4 g/dL (SD:2.5). Using the WHO definition of anemia, 41% of the cohort was anemic, with 19% having grade one, 13% having grade two, 6% having grade three, and 3% having grade four anemia. Among the 4431 patients with CD4 cell count values available, the mean hemoglobin value declined from 12.6 g/dL at CD4 cell counts >500 cells/µL to 10.0 g/dL at CD4 cell counts <100 cells/µL (p-value for trend <0.001, Figure 1). The percent of non-anemic individuals decreased from 80% at CD4 cell counts >500 cells/µL to 36% at CD4 cell counts <100 cells/µL (p<0.001, Figure 1). There was a corresponding statistically significant increase in the percentage of patients with all grades of anemia with declining CD4 cell count (Figure 1). Using the alternative definition of anemia as any hemoglobin value <12 g/dL for women and <13 g/dL for men, 69% of the entire cohort (72% of women and 67% of men) and 89% of patients with a CD4 cell count <100 cell/µL were anemic.
Figure 1.
Influence of HIV disease progression on anemia and mean hemoglobin
* p-value of χ2 for trend
In the multivariate model, CD4 cell count <100 cells/µL and underweight BMI were both associated with an approximately five times increased adjusted odds of anemia (Table 1). Female gender, extrapulmonary TB, and pulmonary TB also had strong independent associations with anemia. Age >31 years, oral and esophageal candidiasis, and generalized lymphadenopathy had milder independent associations with anemia.
Table 1.
Factors Associated with Anemia in Univariate and Multivariate Analyses
| Risk factor | Non-anemic N (%) |
Anemic N (%) |
Univariate Odds Ratio (CI) |
Multivariate Odds Ratio (CI) |
|---|---|---|---|---|
| Gender | ||||
| Male | 3105 (75) | 1820 (64) | 1.0 | 1.0 |
| Female | 1052 (25) | 1019 (36) | 1.7 (1.5–1.8) | 3.1 (2.8–3.6) |
| Age | ||||
| 18–30 | 1764 (42) | 1088 (38) | 1.0 | 1.0 |
| 31–50 | 2231 (54) | 1624 (57) | 1.2 (1.1–1.3) | 1.1 (1.02–1.3) |
| >50 | 162 (4) | 127 (5) | 1.3 (1.0–1.6) | 1.4 (1.07–1.9) |
| Body Mass Index (kg/m2) | ||||
| >25 | 409 (10) | 63 (2) | 1.0 | 1.0 |
| 18.5–24.9 | 1534 (37) | 704 (25) | 3.0 (2.3–3.9) | 2.5 (1.9–3.4) |
| <18.5 | 933 (22) | 972 (34) | 6.8 (5.1–8.9) | 4.8 (3.6–6.5) |
| Not available | 1281 (31) | 1100 (39) | -- | -- |
| CD4 cell count (cells/µL) | ||||
| >500 | 673 (16) | 167 (6) | 1.0 | 1.0 |
| 351–500 | 440 (11) | 132 (5) | 1.2 (0.9–1.6) | 1.1 (0.9–1.5) |
| 201–350 | 665 (16) | 297 (11) | 1.8 (1.4–2.2) | 1.6 (1.3–2.1) |
| 101–200 | 469 (11) | 418 (15) | 3.6 (2.9–4.5) | 2.7 (2.1–3.4) |
| 0–100 | 421 (10) | 749 (26) | 7.2 (5.8–8.8) | 5.0 (4.0–6.3) |
| Not available | 1489 (36) | 1076 (38) | -- | |
| Pulmonary tuberculosis | ||||
| No | 3338 (80) | 1784 (63) | 1.0 | 1.0 |
| Yes | 819 (20) | 1055 (37) | 2.4 (2.2–2.7) | 1.6 (1.4–1.8) |
| Extrapulmonary TB | ||||
| No | 4023 (97) | 2528 (89) | 1.0 | 1.0 |
| Yes | 134 (3) | 311 (11) | 3.7 (3.0–4.5) | 2.5 (2.0–3.1) |
| Candidiasis (Oral and Esophageal) | ||||
| No | 3109 (75) | 1594 (56) | 1.0 | 1.0 |
| Yes | 1048 (25) | 1245 (44) | 2.3 (2.1–2.6) | 1.5 (1.3–1.7) |
| PCP | ||||
| No | 4071 (98) | 2695 (95) | 1.0 | 1.0 |
| Yes | 86 (2) | 144 (5) | 2.5 (1.9–3.3) | 1.3 (0.97–1.7) |
| Cryptococcal meningitis | ||||
| No | 4121 (99) | 2801 (99) | 1.0 | |
| Yes | 36 (1) | 38 (1) | 1.6 (0.98–2.46) | |
| Cytomegalovirus retinitis | ||||
| No | 4140 (99.5) | 2812 (99) | 1.0 | 1.0 |
| Yes | 17 (0.4) | 27 (1) | 2.3 (1.3–4.3) | 1.4 (0.7–2.7) |
| Oral hairy leukoplakia | ||||
| No | 4072 (98) | 2776 (98) | 1.0 | |
| Yes | 85 (2) | 63 (2) | 1.1 (0.8–1.5) | |
| Lymphadenopathy | ||||
| No | 3891 (94) | 2492 (88) | 1.0 | 1.0 |
| Yes | 266 (6) | 347 (12) | 2.0 (1.7–2.4) | 1.6 (1.3–1.9) |
| Herpes zoster | ||||
| No | 3610 (87) | 2474 (87) | 1.0 | |
| Yes | 547 (13) | 365 (13) | 1.0 (0.8–1.1) | |
| Herpes simplex | ||||
| No | 3973 (96) | 2694 (95) | 1.0 | |
| Yes | 184 (4) | 145 (5) | 1.2 (0.9–1.5) | |
| Chronic diarrhea | ||||
| No | 4014 (97) | 2671 (94) | 1.0 | 1.0 |
| Yes | 143 (3) | 168 (6) | 1.8 (1.4–2.2) | 1.3 (1.0–1.7) |
| Bacterial infections of the skin | ||||
| No | 4144 (99.7) | 2829 (99.6) | 1.0 | |
| Yes | 13 (0.3) | 10 (0.4) | 1.1 (0.5–2.6) | |
| Fungal Skin Infections | ||||
| No | 4069 (98) | 2789 (98) | 1.0 | |
| Yes | 88 (2) | 50 (2) | 0.8 (0.6–1.2) | |
| Ascites | ||||
| No | 4153 (99.9) | 2824 (99.5) | 1.0 | 1.0 |
| Yes | 4 (0.1) | 15 (0.5) | 5.5 (1.8–16.5) | 2.4 (0.7–8.0) |
| Renal Disease | ||||
| No | 4120 (99) | 2807 (99) | 1.0 | |
| Yes | 37 (1) | 32 (1) | 1.3 (0.8–2.0) | |
| Syphilis | ||||
| No | 4150 (99.8) | 2838 (100) | 1.0 | |
| Yes | 7 (0.2) | 1 (0) | 0.2 (0.03–1.7) | |
| Malaria | ||||
| No | 4150 (99.8) | 2830 (99.7) | 1.0 | |
| Yes | 7 (0.2) | 9 (0.3) | 1.9 (0.7–5.1) |
Discussion
This study highlights the high prevalence of anemia in HIV-infected individuals in south India, especially among immunosuppressed patients. Using a definition of anemia as any hemoglobin value <12 g/dL for females and <13 g/dL for males, other developing country studies found a 70–90% anemia prevalence among HIV-infected individuals, which is similar to the 69% found in our study.3,6–8 The 72% prevalence of anemia among women in this study is significantly higher than the 50–57% prevalence in large-scale surveys of women from the general population of the two south Indian states from which our clinic derives its patient population.5 Most developing country studies have focused specifically on HIV-infected groups at higher risk for anemia, such as pregnant or TB co-infected patients. Since this study has patients of both genders distributed across all CD4 cell count strata, it may more accurately reflect the prevalence of anemia among HIV-infected individuals in India.
In our study, the influence of HIV disease on anemia starts well before patients are eligible for HAART, with a significant increase in anemia evident even at CD4 cell counts of 201–350 cells/µL. Similar to prior findings, a low CD4 cell count has a strong independent association with anemia even after controlling for opportunistic infections and malnutrition.1,11 This association may represent anemia caused by the HIV virus itself, which may inhibit hematopoiesis directly through infection of progenitor cells or upregulation of cytokines.8 Studies from developed countries suggest that HAART resolves anemia in many patients, which is consistent with our finding that immunosuppression makes a strong independent contribution to anemia.9,12 While a preliminary analysis from our clinic suggests a similar trend of anemia resolution with HAART,13 further prospective data is needed to answer this important question in developing countries.
The association of anemia with low BMI is of specific relevance to India, where the rate of chronic malnutrition is among the highest in the world.14 Interestingly, even individuals with a normal BMI had a two and a half times increased odds of anemia as compared to those who were overweight. Low BMI is associated with many nutrient deficiencies—including iron, folate, and B12—that contribute directly to anemia. The increased independent odds of anemia with candida infection may also reflect micronutrient deficiency, since both oral and esophageal infection decrease oral intake.
As TB is the second most common opportunistic infection in India (after oral candidiasis),15 it may greatly exacerbate the burden of anemia in this population. The etiology of anemia in TB is multifactorial, resulting from a combination of anemia of chronic disease and deficiencies of nutrients such as iron, vitamin A, and selenium.6 The increased odds of anemia in females most likely reflects the high rate of iron deficiency in Indian women due to menstrual blood loss, poor nutritional status, and pregnancy.5 Three of the strongest factors associated with anemia— TB , immunosuppression, and malnutrition—exacerbate each other in synergistic manner. The net result is a vicious cycle placing HIV-infected patients in TB-endemic countries at very high risk for developing anemia.6,7 Therefore, in addition to roll-out of HAART, nutritional support and aggressive TB control should be the cornerstones of anemia management for HIV-infected individuals in India.
The main limitation of this analysis is its cross-sectional design, which precludes definite determination of the temporal relationships between anemia and its associated factors. Also, our database does not capture laboratory findings (i.e., mean corpuscular volume, iron studies, etc.) that could clarify the frequency of anemia due to particular nutrient deficiencies.
Finally, the high prevalence of anemia in patients with CD4 cell counts <200 cells/µL has implications for the choice of initial HAART regimen in developing countries. Since anemia is an adverse effect of zidovudine (AZT), use of this drug is contraindicated in patients with preexisting anemia.16,17 Therefore, more than half of patients who meet criteria for HAART in this setting would not be eligible for initiation on regimen that includes AZT. This highlights the need for increased access to NRTIs with different toxicity profiles, such as tenofovir and abacavir, in resource-limited settings.
Acknowledgements
The authors are grateful to Rashmi, Glory, the other research nurses, the clinical staff at YRG CARE, and the data management team for their generous facilitation of this study. Anitha Cecelia provided help with statistical analysis. Drs. Padmanesan Narasimhan and Pradeep Ambrose provided useful feedback on study design. This study was partially supported by the AIDS International Research and Training Program of the Fogarty International Center of the National Institutes of Health (D43TW00237). Ramnath Subbaraman was funded by a Fogarty-Ellison Overseas Fellowship in Global Health and Clinical Research (3 D43 TW000237-13S1) and by a Yale University School of Medicine Short-term Research Grant.
References
- 1.Sullivan PS, Hanson DL, Chu SY, et al. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: Results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91:301–308. [PubMed] [Google Scholar]
- 2.Semba RD, Martin BK, Kempen JH, et al. The impact of anemia on energy and physical functioning in individuals with AIDS. Arch Intern Med. 2005;165:2229–2236. doi: 10.1001/archinte.165.19.2229. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien ME, Kupka R, Msamanga GI, et al. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr. 2005;40:219–225. doi: 10.1097/01.qai.0000166374.16222.a2. [DOI] [PubMed] [Google Scholar]
- 4.Semba RD. Iron-deficiency anemia and the cycle of poverty among human immunodeficiency virus-infected women in the inner city. Clin Infect Dis. 2003;37(Suppl 2):S105–S111. doi: 10.1086/375892. [DOI] [PubMed] [Google Scholar]
- 5.International Institute for Population Sciences (IIPS), ORC Macro. National family health survey of India (NFHS-2), 1998–1999. Mumbai, India: IIPS; 2000. [Google Scholar]
- 6.van Lettow M, West CE, van der Meer JW, et al. Low plasma selenium concentrations, high plasma human immunodeficiency virus load and high interleukin-6 concentrations are risk factors associated with anemia in adults presenting with pulmonary tuberculosis in Zomba district, Malawi. Eur J Clin Nutr. 2005;59:526–532. doi: 10.1038/sj.ejcn.1602116. [DOI] [PubMed] [Google Scholar]
- 7.Shah S, Whalen C, Kotler DP, et al. Severity of human immunodeficiency virus infection is associated with decreased phase angle, fat mass and body cell mass in adults with pulmonary tuberculosis infection in Uganda. J Nutr. 2001;131:2843–2847. doi: 10.1093/jn/131.11.2843. [DOI] [PubMed] [Google Scholar]
- 8.Semba RD, Gray GE. Pathogenesis of anemia during human immunodeficiency virus infection. J Investig Med. 2001;49:225–239. doi: 10.2310/6650.2001.33967. [DOI] [PubMed] [Google Scholar]
- 9.Mocroft A, Kirk O, Barton SE, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. EuroSIDA study group. AIDS. 1999;13:943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 10.Cecelia AJ, Christybai P, Anand S, et al. Usefulness of an observational database to assess antiretroviral treatment trends in India. Natl Med J India. 2006;19:14–17. [PubMed] [Google Scholar]
- 11.Semba RD, Shah N, Klein RS, et al. Prevalence and cumulative incidence of and risk factors for anemia in a multicenter cohort study of human immunodeficiency virus-infected and -uninfected women. Clin Infect Dis. 2002;34:260–266. doi: 10.1086/338151. [DOI] [PubMed] [Google Scholar]
- 12.Moore RD, Forney D. Anemia in HIV-infected patients receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;29:54–57. doi: 10.1097/00042560-200201010-00007. [DOI] [PubMed] [Google Scholar]
- 13.Subbaraman R, Singh S, Cecelia AJ, et al. Resolution of anemia with use of highly active antiretroviral therapy among HIV-infected patients in southern India [THPE0113]. Presented at 16th International AIDS Conference; 2006 August 13–18; Toronto, Canada. [Google Scholar]
- 14.Gragnolati M, Shekar M, Das Gupta M, et al. India's undernourished children: a call for reform and action. Washington, D.C.: The World Bank; 2005. [Google Scholar]
- 15.Kumarasamy N, Solomon S, Flanigan T, et al. Natural history of human immunodeficiency virus disease in southern India. Clin Infect Dis. 2003;36:79–85. doi: 10.1086/344756. [DOI] [PubMed] [Google Scholar]
- 16.Kumarasamy N, Venkatesh KK, Cecelia AJ, et al. Spectrum of adverse events after generic HAART in southern Indian HIV-infected patients. AIDS Patient Care STDs. 2008;22:337–344. doi: 10.1089/apc.2007.0093. [DOI] [PubMed] [Google Scholar]
- 17.Kumarasamy N, Vallabhaneni S, Cecelia AJ, et al. Reasons for modification of generic highly active antiretroviral therapeutic regimens among patients in southern India. J Acquir Immune Defic Syndr. 2006;41:53–58. doi: 10.1097/01.qai.0000188123.15493.43. [DOI] [PubMed] [Google Scholar]