Abstract
Both calpain activation and endoplasmic reticulum (ER) stress are implicated in ischemic heart injury. However, the role of calpain in ER stress remains largely elusive. This study investigated whether calpain activation causes ER stress, thereby mediating cardiomyocyte apoptosis in an in vitro model of hypoxia/re-oxygenation (H/R). In neonatal mouse cardiomyocytes and rat cardiomyocyte-like H9c2 cells, up-regulation of calpain-1 sufficiently induced ER stress, c-Jun N-terminal protein kinase1/2 (JNK1/2) activation and apoptosis. Inhibition of ER stress or JNK1/2 prevented apoptosis induced by calpain-1. In an in vitro model of H/R-induced injury in cardiomyocytes, H/R was induced by a 24-hour hypoxia followed by a 24-hour re-oxygenation. H/R activated calpain-1, induced ER stress and JNK1/2 activation, and triggered apoptosis. Inhibition of calpain and ER stress blocked JNK1/2 activation and prevented H/R-induced apoptosis. Furthermore, blockade of JNK1/2 signaling inhibited apoptosis following H/R. The role of calpain in ER stress was also demonstrated in an in vivo model of ischemia/reperfusion using transgenic mice over-expressing calpastatin. In summary, calpain-1 induces ER stress and JNK1/2 activation, thereby mediating apoptosis in cardiomyocytes. Accordingly, inhibition of calpain prevents ER stress, JNK1/2 activation and apoptosis in H/R-induced cardiomyocytes. Thus, ER stress/JNK1/2 activation may represent an important mechanism linking calpain-1 to ischemic injury.
Keywords: Calpain, ER stress, JNK1/2, Apoptosis, Hypoxia/reoxygenation
1. Introduction
Ischemic heart disease is the leading cause of death in most industrialized nations as it causes serious complications such as myocardial infarction (MI). After MI, ischemia-induced myocardial cell death is followed by a progressive remodeling of the heart [1–3], resulting in a severe state of compromised heart function known as heart failure. Heart failure is on the rise as a result of successes in treating acute MI, and up to 40–50% of people with heart failure die within five years of diagnosis [4]. Thus, limiting ischemic injury is extremely important in the prevention of adverse myocardial remodeling and the progression to heart failure.
Calpains are a family of calcium-dependent thiol-proteases [5]. In mammals, fifteen gene products of the calpain family have been reported. Among them, calpain-1 (μ-form) and calpain-2 (m-form) are ubiquitously expressed, and most extensively studied. Calpain-1 and calpain-2 are heterodimers, consisting of a distinct large 80-kDa catalytic subunit encoded by the genes capn1 and capn2, respectively, and a common small 28-kDa regulatory subunit encoded by capn4. They differ in their calcium requirements for activation (~50 μM for calpain-1 and ~1000 μM for calpain-2). Both calpain-1 and calpain-2 are tightly regulated by calpastatin, an endogenous inhibitor that specifically inhibits calpain, but not other cysteine proteases. Over-expression of calpastatin has been widely used to inhibit calpain in a variety of in vitro [6] and in vivo models [7,8]. Calpains participate in cardiac pathophysiology. In cultured cardiomyocytes, we and others demonstrated that calpain-1 is important in promoting cardiomyocyte apoptosis under various pathological conditions [9–11]. In response to hypoxia, calpain is activated and contributes to cardiomyocyte injury [12]. In animal models of ischemia/reperfusion or MI, both pharmacologic and genetic inhibitions of calpain reduce ischemic cardiac injury, attenuate myocardial remodeling and improve myocardial function [13–17]. Furthermore, transgenic over-expression of calpain-1 is sufficient to induce dilated cardiomyopathy and heart failure [7]. These previous studies support an important role of calpain in ischemic heart disease [18]. However, the mechanisms relating calpain activation to ischemic myocardial injury have not been fully addressed.
Endoplasmic reticulum (ER) stress is induced by the accumulation of unfold proteins, triggering the unfolded protein response which activates ER transmembrane sensors to initiate the adaptive responses [19]. These ER transmembrane sensors include protein kinase-like ER kinase (PERK), inositol-requiring kinase 1 (IRE1) and activating transcription factor 6 (ATF6), and their activation results in phosphorylation of eukaryotic translation initiation factor 2α (eIF2α), translation of transcription factor ATF4, XBP1 splicing, and finally the induction of unfolded protein response related genes including chaperones GRP78 and GRP94, XBP1 and CHOP, etc. However, in the case of prolonged or overwhelming ER stress, apoptosis can be induced through CHOP, JNK1/2 and other pathways. Recent animal and human studies have revealed that ER stress-initiated apoptosis is implicated in the pathophysiology of various cardiovascular diseases, including ischemic heart disease and heart failure [20,21]. Thus, ER stress may represent an important therapeutic target for heart diseases [22]. While calpain activation was shown to correlate with ER stress induction in the hypoxic heart [23], calpain has been reported to disturb Ca2+ homeostasis by targeting calcium regulatory proteins such as sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) [24] and calcium channel proteins [25]. As such, they cause the release of ER Ca2+, which may impair protein processing and thereby promote ER stress [26]. Thus, it is possible that calpain may play a role in inducing ER stress in ischemic heart disease.
In this study, we examined whether calpain-1 activation is linked to the induction of ER stress in cardiomyocytes, and investigated whether inhibition of calpain prevents ER stress and reduces cardiomyocyte apoptosis in an in vitro model of hypoxia/re-oxygenation.
2. Materials and methods
2.1. Animals
This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). All experimental procedures were approved by the Animal Use Subcommittee at the University of Western Ontario, Canada. Breeding pairs of C57BL/6 mice were purchased from the Jackson Laboratory. Transgenic mice with over-expression of calpastatin (Tg-CAST) were generously provided by Dr. Laurent Baud (the Institut National de la Santé et de la Recherche Médicale, Paris, France) through the European Mouse Mutant Archive. Mice with cardiomyocyte-specific disruption of capn4 (capn4-ko) were generated as described in our recent report [27]. All of the adult mice used in this study, including controls, were littermates of the same generation. A breeding program was implemented to produce neonates at our animal care facilities.
2.2. Ischemia/reperfusion (I/R) protocol
Animals were subjected to ischemia for 30 min followed by reperfusion for 3 h as we previously described [28,29].
2.3. Cell culture, adenoviral infection and siRNA transfection
The neonatal mouse cardiomyocytes were prepared and cultured according to methods we described previously [30].
The rat cardiomyocyte-like H9c2 cells were purchased from the American Type Culture Collection (ATCC) and cultured H9c2 cells were employed within 10 generations.
Cells were infected with adenoviral vectors containing human capn1 gene (Ad-capn1, SignaGen Laboratories), human capn2 gene (Ad-capn2), rat calpastatin gene (Ad-CAST), or beta-gal (Ad-gal, Vector Biolabs) as a control at a multiplicity of infection (MOI) of 100 PFU/cell. Adenovirus-mediated gene transfer was implemented as previously described [10]. All experiments were performed after 24 h of adenoviral infection.
Cells were transfected with siRNA specific for capn1 and capn2 (Santa Cruz Biotechnology, Inc.) using TransMessenger Transfection Reagent (Qiagen) as we previously described [11]. A scrambled siRNA served as a control.
2.4. Hypoxia/re-oxygenation (H/R)
Cardiomyocytes were subjected to a 24-hour period of hypoxia, followed by re-oxygenation for another 24 h. For the induction of hypoxia, we placed the culture dishes in a sealed chamber containing GENbag anaer (bioMérieux) for 24 h at 37 °C. Hypoxia was monitored using anear indicator (bioMérieux). The GENbag anaer rapidly reduces O2 concentration in chamber within 30 min. Re-oxygenation was achieved by changing culture media and returning cells to normal culture conditions. We found that after hypoxia for 3 h the O2 concentration was below 0.1% while pH value in culture media was 7.2 (before hypoxia pH value was 7.4).
2.5. Calpain activity
Calpain activities were determined as described previously [6,10,11].
2.6. Western blot analysis
The protein levels of calpain-1, calpain-2, GRP78, CHOP, ATF6, phosphorylated PERK (pPERK), phosphorylated and total JNK1/2, SERCA2a and GAPDH were determined by western blot analysis as previously described [6,10,11,15].
2.7. Assessment of apoptosis
Caspase-3 activity was determined using a commercial caspase-3 activity assay kit as described in our recent report [11].
DNA fragmentation was measured using a Cellular DNA Fragmentation ELISA kit (Roche Applied Science, Canada) according to the manufacturer’s instructions.
2.8. Statistical analysis
All data were presented as mean ± SD. ANOVA followed by Newman–Keuls test was performed for multi-group comparisons. A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Up-regulation of calpain-1 is sufficient to induce apoptosis, ER stress and JNK1/2 activation in cardiomyocytes
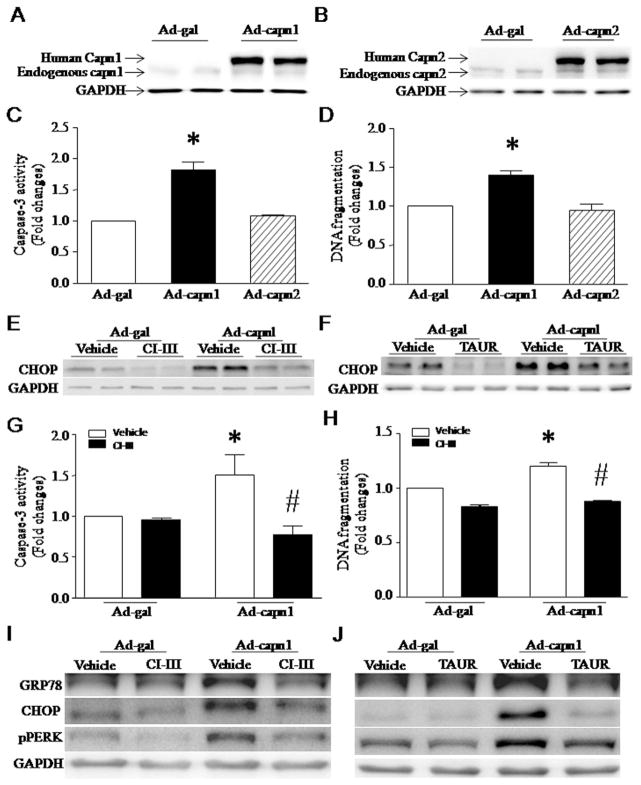
We have recently demonstrated that calpain-1/2 expression and activities are increased in the heart after MI [15]. To examine whether up-regulation of calpain-1/2 is sufficient to induce apoptosis, we infected neonatal mouse cardiomyocytes and rat cardiomyocyte-like H9c2 cells with Ad-capn1, Ad-capn2 or Ad-gal as a control. Twenty-four hours later, infection with Ad-capn1 and Ad-capn2 significantly elevated the protein levels of calpain-1 and calpain-2, respectively (Fig. 1A and B). Up-regulation of calpain-1 induced increases in caspase-3 activation and DNA fragmentation (Fig. 1C, D, G and H), indicative of apoptosis. This effect of calpain-1 was inhibited by co-incubation with calpain inhibitor-III (10 μM) (Fig. 1G and H), suggesting that apoptosis induced by up-regulation of calpain-1 is due to its enzymatic activity rather than its protein accumulation. In contrast, up-regulation of calpain-2 did not induce apoptosis in cardiomyocytes (Fig. 1C and D).
Fig. 1.
Apoptosis and ER stress induced by infection with Ad-capn1. (A–F) Cultured neonatal mouse cardiomyocytes were infected with Ad-capn1, Ad-capn2 or Ad-gal as a control, and then incubated with calpain inhibitor-III (CI-III), TAUR or vehicle. Twenty-four hours later, western blot was performed to analyze capn1, capn2, CHOP and GAPDH proteins, and apoptosis was assessed by measuring caspase-3 activity and DNA fragmentation. (A) A representative western blot for capn1 protein from 3 different experiments. (B) A representative western blot for capn2 protein from 3 different experiments. (C) Caspase-3 activity. (D) DNA fragmentation. (E and F) Representative western blots from 3 different experiments for CHOP and GAPDH proteins. (G and H) H9c2 cells were infected with Ad-capn1 or Ad-gal as a control, and then incubated with CI-III, TAUR or vehicle. Twenty-four hours later, apoptosis was assessed by caspase-3 activity (G) and DNA fragmentation (H). (I and J) Representative western blots from 3 different experiments for GRP78, CHOP, pPERK and GAPDH. Data are mean ± SD from 3 different experiments. *P < 0.05 vs Ad-gal or Ad-gal + Vehicle and #P < 0.05 vs Ad-capn1 + Vehicle.
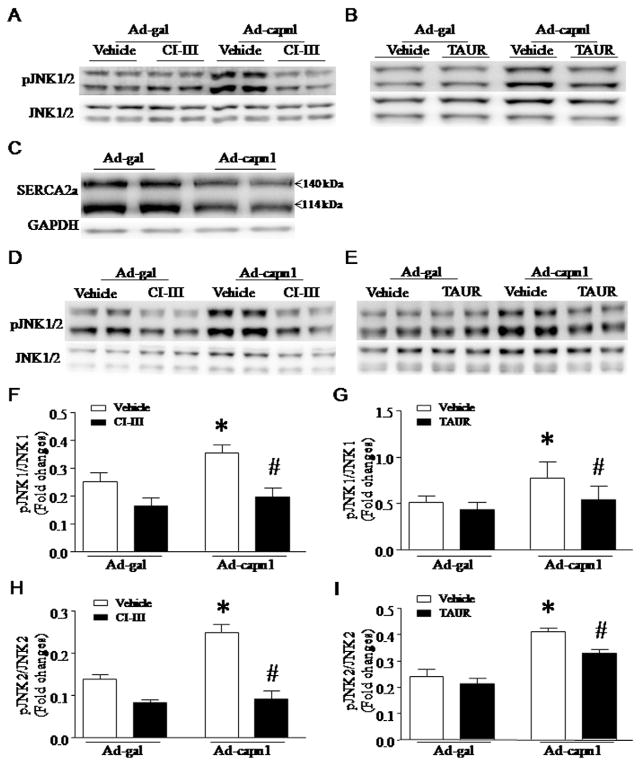
To examine the induction of ER stress, we analyzed the protein levels of ER stress markers including GRP78, CHOP and pPERK. Up-regulation of calpain-1 increased the protein levels of CHOP, GRP78 and pPERK in neonatal mouse cardiomyocytes and H9c2 cells, which were significantly decreased by co-incubation with calpain inhibitor-III (10 μmol/L, Fig. 1E and I) or ER stress inhibitor, tauroursodeoxycholate (TAUR, 100 μmol/L, Fig. 1F and J). Calpain-1 also increased JNK1/2 phosphorylation in neonatal mouse cardiomyocytes and H9c2 cells, which was prevented by calpain inhibitor-III (Fig. 2A, D, F and H) or TAUR (Fig. 2B, E, G and I). Over-expression of calpain-1 dramatically reduced the protein levels of SERCA2a in both neonatal mouse cardiomyocytes (Fig. 2C). However, up-regulation of calpain-2 did not induce ER stress and JNK1/2 activation (data not shown). Thus, these results provide direct evidence that calpain-1 sufficiently induces ER stress, which in turn activates JNK1/2 in cardiomyocytes. Since both neonatal mouse cardiomyocytes and H9c2 cells responded to up-regulation of calpain-1 in a similar way, we used cardiomyocyte-like H9c2 cells for most of the following studies.
Fig. 2.
Role of calpain-1 and ER stress in JNK1/2 activation. (A–C) Cultured neonatal mouse cardiomyocytes were infected with Ad-capn1, Ad-capn2 or Ad-gal as a control, and then incubated with calpain inhibitor-III (CI-III), TAUR or vehicle for 24 h. (A and B) Representative western blots for JNK1/2 and phosphorylated JNK1/2 from 3 different experiments. (C) Representative western blots for SERCA2a from 3 different cultures show that infection with Ad-capn1 decreases the protein levels of SERCA2a. (D–I) H9c2 cells were infected with Ad-capn1 or Ad-gal as a control, and then incubated with CI-III, TAUR or vehicle for 24 h. (D and E) Representative western blots from 3 different experiments for JNK1/2 and phosphorylated JNK1/2. (F and I) Quantification for the ratio of phosphorylated JNK1/2 over JNK1/2. Data are mean ± SD from 3 different experiments. *P < 0.05 vs Ad-gal or Ad-gal + Vehicle and #P < 0.05 vs Ad-capn1 + Vehicle.
3.2. Inhibition of ER stress and JNK1/2 prevents calpain-1-induced apoptosis in cardiomyocytes
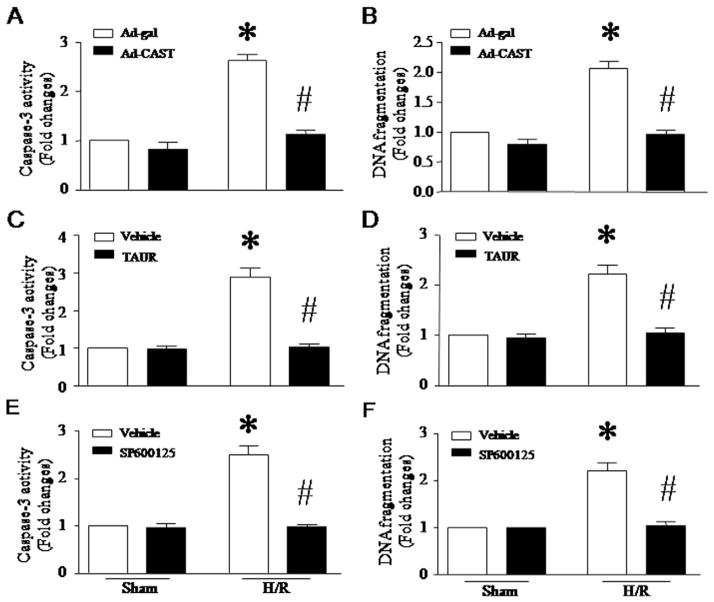
It is well-known that ER stress promotes apoptosis. We reasoned that calpain-1-induced apoptosis is mediated through the induction of ER stress. To examine this hypothesis, we infected H9c2 cells with Ad-capn1 or Ad-gal, and then incubated them with TAUR (100 μM). Inhibition of ER stress prevented caspase-3 activation and DNA fragmentation induced by calpain-1 up-regulation in H9c2 cells (Fig. 3A and B). This result supports our hypothesis that induction of ER stress represents an important mechanism by which calpain-1 induces apoptosis in cardiomyocytes.
Fig. 3.
Effects of TAUR and SP600125 on apoptosis in H9c2 cells. H9c2 cells were infected with Ad-capn1 or Ad-gal as a control, and then incubated with TAUR, SP600125 or vehicle. Twenty-four hours later, apoptosis was assessed by caspase-3 activity (A and C) and DNA fragmentation (B and D). Data are mean ± SD from 3 different experiments. *P < 0.05 vs Ad-gal + Vehicle and #P < 0.05 vs Ad-capn1 + Vehicle.
Having shown that inhibition of ER stress prevents JNK1/2 activation, we hypothesized that JNK1/2 contributed to apoptosis induced by calpain-1 in cardiomyocytes. To address this hypothesis, we incubated H9c2 cells with JNK1/2 inhibitor SP600125 (10 μmol/L) after infection with Ad-capn1 or Ad-gal. SP600125 significantly attenuated caspase-3 activity and reduced DNA fragmentation in Ad-capn1 infected H9c2 cells (Fig. 3C and D). Taken together, these results support the view that calpain-induced apoptosis is mediated through the ER stress/JNK1/2 pathway in H9c2 cells.
3.3. Hypoxia/re-oxygenation (H/R) induces calpain-1 activation, ER stress and JNK1/2 activation in cardiomyocytes
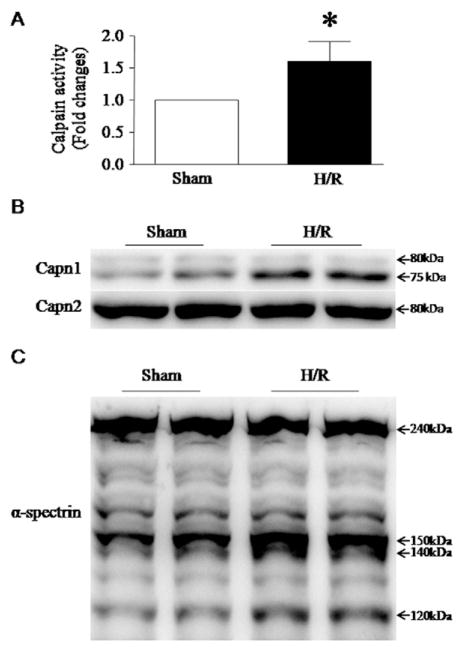
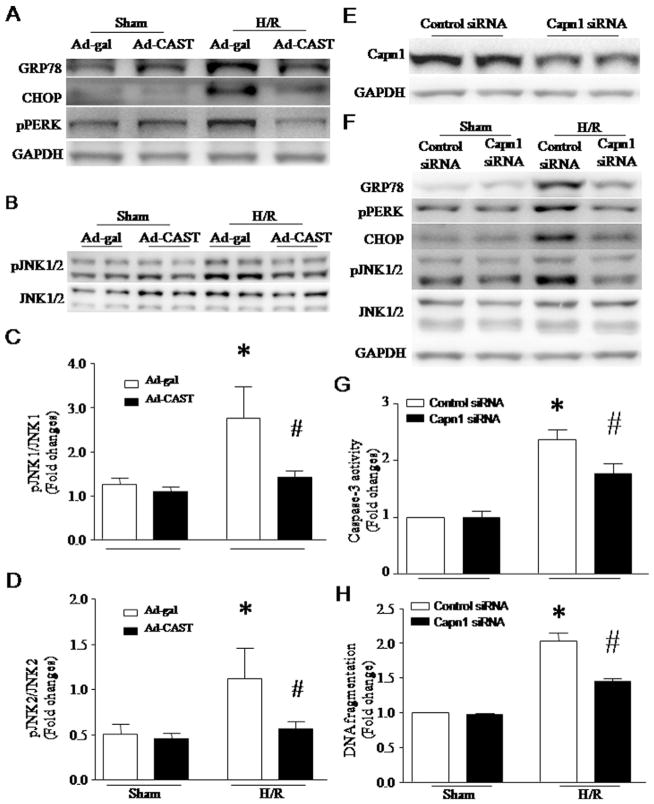
Both calpain activation and ER stress were induced in the ischemic heart [15,31]. To determine whether calpain activation plays a role in ER stress, we infected H9c2 cells with Ad-CAST or Ad-gal as a control, and then induced H/R on these cells. H/R induced calpain-1 activation as evidenced by increases in calpain enzymatic activity, active calpain-1 fragment (75 kDa) and cleaved fragment of α-spectrin (140 kDa) (Fig. 4A–C). Calpain-1 activation correlated with increases in the protein levels of CHOP, GRP78 and pPERK, indicative of ER stress (Fig. 5A and F), and JNK1/2 phosphorylation (Fig. 5B–D). These effects of H/R on ER stress and JNK1/2 activation were significantly attenuated by over-expression of calpastatin (Fig. 5A–D), suggesting an important role of calpain in ER stress and JNK1/2 activation. However, H/R did not induce the proteolysis of ATF6 (data not shown). To substantiate the role of calpain-1 in ER stress and apoptosis, we silenced calpain-1 and calpain-2 in H9c2 cells using their respective siRNA. Silencing calpain-1 attenuated H/R-induced ER stress and apoptosis (Fig. 5E–H). In contrast, silencing calpain-2 had no effects on ER stress and apoptosis (data not shown).
Fig. 4.
Measurement of calpain activation in H9c2 cells following H/R. H9c2 cells were subjected to a 24-hour hypoxia followed by a 24-hour re-oxygenation. (A) Calpain enzymatic activity was measured in H9c2 cells. Data are mean ± SD from 3 different experiments. *P < 0.05. (B) is a representative western blot for a 75 kDa active fragment of calpain-1 and (C) for the cleavage of α-spectrin (140 kDa), a natural substrate of calpain-1.
Fig. 5.
(A–D) Role of calpain in ER stress and JNK1/2 activation following H/R. H9c2 cells were infected with Ad-CAST or Ad-gal, and then subjected to a 24-hour hypoxia followed by a 24-hour re-oxygenation. (A) Representative western blots from 3 different experiments for GRP78, CHOP, pPERK and GAPDH. (B) Representative western blots from 3 different experiments for phosphorylated JNK1/2 and JNK1/2. (C and D) Quantification for the ratio of phosphorylated JNK1/2 over JNK1/2. Data are mean ± SD from 3 different experiments. *P < 0.05 vs Ad-gal + Sham and #P < 0.05 vs Ad-gal + H/R. (E–H) Effects of calpain-1 knockdown on ER stress and apoptosis in H9c2 cells following H/R. H9c2 cells were transfected with siRNA for calpain-1 or control siRNA. Twenty-four hours after transfection, the cells were subjected to H/R. (E) Representative western blot for capn1 and GAPDH proteins from 3 different cultures. (F) Representative western blots for ER stress markers (GRP78, phosphorylated PERK (pPERK), CHOP, phosphorylated JNK1/2, and JNK1/2) from 3 different cultures show that silencing calpain-1 reduces H/R-induced ER stress. (G) Caspase-3 activity and (H) DNA fragmentation. Data are mean ± SD from 3 different experiments. *P < 0.05 vs Sham + Control siRNA and #P < 0.05 vs H/R + Control siRNA.
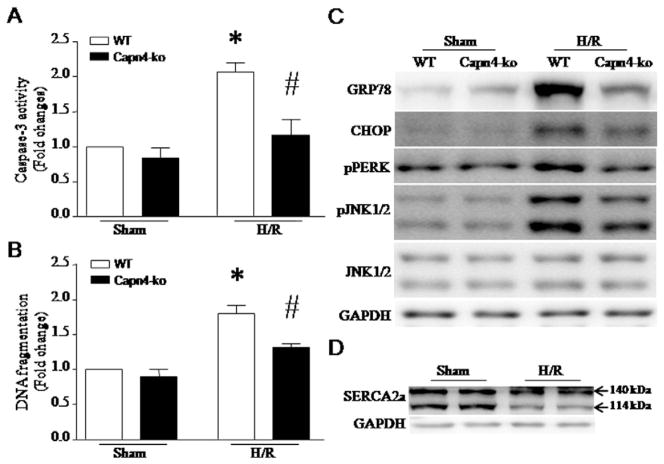
To reproduce the role of calpain in primary neonatal cardiomyocytes, we isolated and cultured neonatal cardiomyocytes from capn4-ko mice and their wild-type littermates. After hypoxia for 12 h followed by re-oxygenation for 6 h, apoptosis and ER stress were induced in wild-type cardiomyocytes; however, deletion of capn4 significantly reduced apoptosis and ER stress in capn4-ko cardiomyocytes following H/R (Fig. 6A–C). H/R also reduced the protein levels of SERCA2a (Fig. 6D).
Fig. 6.
ER stress and apoptosis in capn4-ko cardiomyocytes following H/R. Neonatal cardiomyocytes were isolated and cultured from capn4-ko mice and their littermates. Twenty-four hours after isolation, the cells were subjected to H/R (12-hour hypoxia followed by 6-hour re-oxygenation). Apoptosis was determined by caspase-3 activity (A) and DNA fragmentation (B). Data are mean ± SD from 3 different experiments. *P < 0.05 vs Sham + WT and #P < 0.05 vs H/R + WT. (C) Representative western blots for ER stress markers from 3 different cultures show that deletion of capn4 attenuates ER stress in cardiomyocytes following H/R. (D) A representative western blot for SERCA2a from 3 different cultures shows a reduction in SERCA2a protein after H/R.
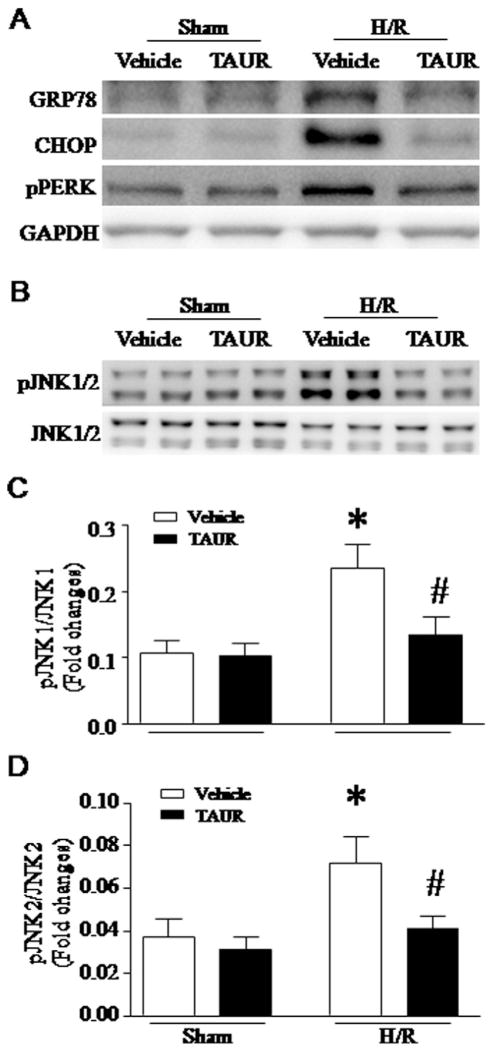
To study whether ER stress induced JNK1/2 activation, we incubated H9c2 cells with TAUR (100 μmol/L), an inhibitor of ER stress or vehicle. After H/R, incubation with TAUR prevented ER stress and JNK1/2 phosphorylation (Fig. 7A–D). Thus, these results demonstrate that calpain-1 activation mediates H/R-induced ER stress and subsequent JNK1/2 signaling in cardiomyocytes.
Fig. 7.
Role of ER stress in JNK1/2 activation following H/R. H9c2 cells were incubated with TAUR or vehicle and then subjected to a 24-hour hypoxia followed by a 24-hour re-oxygenation. (A) Representative western blots from 3 different experiments for GRP78, CHOP, pPERK and GAPDH. (B) Representative western blots from 3 different experiments for phosphorylated JNK1/2 and JNK1/2. (C and D) Quantification for the ratio of phosphorylated JNK1/2 over JNK1/2. Data are mean ± SD from 3 different experiments. *P < 0.05 vs Vehicle + Sham and #P < 0.05 vs Vehicle + H/R.
3.4. Inhibition of calpain, ER stress and JNK1/2 prevents apoptosis induced by H/R
To investigate the role of calpain/ER stress/JNK1/2 signaling in apoptosis, we infected H9c2 cells with Ad-CAST or Ad-gal, or incubated them with TAUR (100 μmol/L), JNK1/2 inhibitor SP600125 (10 μmol/L) or vehicle. After H/R, apoptosis was determined. As shown in Fig. 8, H/R significantly increased caspase-3 activity and DNA fragmentation, whereas inhibition of calpain (Fig. 8A and B), ER stress (Fig. 8C and D) or JNK1/2 (Fig. 8E and F) prevented such effects. These results demonstrate that calpain, ER stress and JNK1/2 mediate H/R-induced apoptosis in cardiomyocytes.
Fig. 8.
Determination of apoptosis in H9c2 cells after H/R. H9c2 cells were infected with Ad-CAST or Ad-gal, or incubated with TAUR, SP600125 or vehicle, and then subjected to a 24-hour hypoxia followed by a 24-hour re-oxygenation. Apoptosis was assessed by measuring caspase-3 activity (A, C, E) and DNA fragmentation (B, D, F). Data are mean ± SD from 3 different experiments. *P < 0.05 vs Sham + Ad-gal or Sham + Vehicle and #P < 0.05 vs H/R + Ad-gal or H/R + Vehicle.
3.5. Role of calpain in ER stress in an in vivo model of I/R
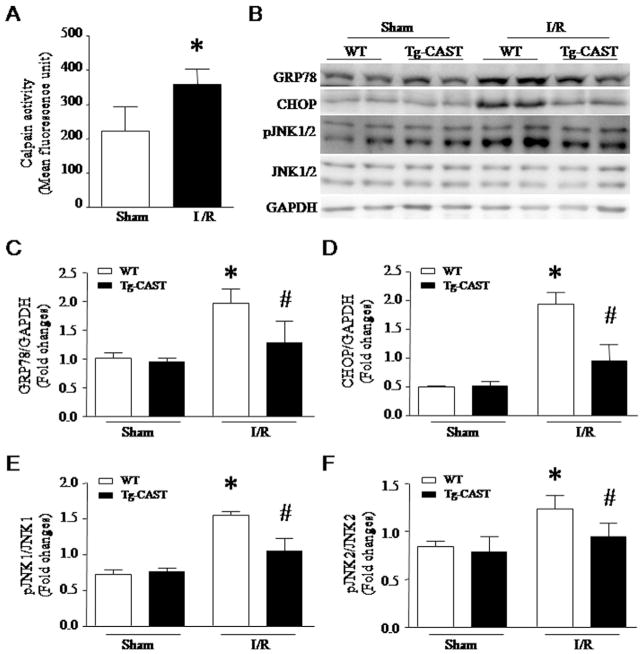
To examine the role of calpain in ER stress in vivo, I/R protocol was performed in Tg-CAST mice and their wild-type littermates. I/R resulted in an increase in calpain activity (Fig. 9A) and induced ER stress in wild-type mice as determined by increases in GRP78, CHOP and phosphorylated JNK1/2; however, the induction of ER stress was significantly attenuated in Tg-CAST mice (Fig. 9B–F). These results confirm that calpain plays an important role in ER stress in a mouse model of I/R injury in vivo.
Fig. 9.
Effects of calpastatin over-expression on ER stress in an in vivo mouse model of ischemia/reperfusion (I/R). Adult transgenic mice with calpastatin over-expression (Tg-CAST) and their wild-type littermates (WT) were subjected to an I/R procedure. (A) Calpain activity was measured in heart tissue lysates. Data are mean ± SD, n = 6. *P < 0.05 vs Sham. (B) Representative western blots for ER stress markers from 2 out of 6 hearts in each group. (C–F) Quantifications for GRP78 (C), CHOP (D), phosphorylated JNK1/JNK1 (E) and phosphor-ylated JNK2/JNK2 (F). Data are mean ± SD, n = 6. *P < 0.05 vs Sham + WT and #P < 0.05 vs I/R + WT.
4. Discussion
The major findings of this study are that up-regulation of calpain-1 is sufficient to induce ER stress and apoptosis in cardiomyocytes, and that inhibition of calpain-1 prevents ER stress and apoptotic cell death in H/R-stimulated cardiomyocytes. Calpain-1/ER stress mediates JNK1/2 activation in H/R-stimulated cardiomyocytes. Furthermore, inhibition of ER stress and JNK1/2 abrogates calpain-1 or H/R-induced apoptosis. The role of calpain in ER stress is also demonstrated in a mouse model of I/R-injury. These findings describe a new mechanism connecting calpain-1 activation to ischemic injury in cardiomyocytes.
Numerous studies have reported increases in calpain-1/2 expression and activities in cardiomyocytes following ischemia/re-oxygenation [32] that contribute to cardiomyocyte apoptosis [9–11]. The up-regulation of calpain has also been observed in ischemic hearts [15,17, 33]. Both pharmacological calpain inhibitors and genetic inhibition of calpain reduce acute myocardial injury and improve myocardial function in animal models of ischemia/reperfusion and MI [13–17]. Our recent study demonstrated that cardiac-specific deletion of capn4 attenuates myocardial remodeling and heart failure in mice after MI [15]. These previous studies have indicated the pathophysiological significance of calpain activation in ischemic heart disease. The present study further confirms the detrimental role of calpain-1 in ischemic cardiomyocytes in vitro. These data suggest that up-regulation of calpain-1 may directly contribute to the development of heart failure following ischemia/reperfusion. Indeed, cardiac-specific over-expression of calpain-1 was sufficient to induce heart failure in transgenic mice [7].
The mechanisms linking calpain activation to ischemic heart injury and subsequent heart failure are incompletely understood, but appear to be multifactorial. Studies using pharmacologic calpain inhibitors have suggested that the role of calpain in ischemic heart disease may be associated with impairments of Ca2+ handling proteins [24,25], cleavages of contractile proteins such as troponin-T and -I [12,34], disruption of Na+/K+-ATPase activity [33], and release of apoptosis inducing factor and subsequent apoptosis [35]. Calpain is also involved in the calcineurin/NFAT pathways in response to angiotensin-II [36], which contributes to myocardial hypertrophy and leads to heart failure after MI [37]. Recent studies using both calpain-1 transgenic and knockout mice have shown that calpain-1 mediates proteolytic processing of PKCα in ischemic myocardium and releases a persistent and constitutively active free catalytic fragment, PKCα-CT. PKCα-CT localizes to nuclei and directly promotes nucleo-cytoplasmic shuttling of HDAC5, which induces expression of apoptosis and other deleterious genes, leading to heart failure [14,38]. More recently, using cardiac-specific capn4 knockout mice we have reported that up-regulation of calpain induces a reduction of iκB protein and subsequent NF-κB activation. The NF-κB signaling promotes the pro-inflammatory response and infiltration of inflammatory cells in infarcted myocardium, which contribute to myocardial remodeling and heart failure after MI [15]. In the present study, we provide new evidence in support of the view that calpain-1 induces ER stress in mediating ischemic injury in cardiomyocytes. First, up-regulation of calpain-1 is sufficient to induce ER stress and apoptosis in cardiomyocytes. Second, calpain-1 activation correlates with the induction of ER stress in H/R-stimulated cardiomyocytes. Third, inhibition of calpain prevents ER stress induced by H/R in cardiomyocytes and by I/R in hearts in vivo. Fourth, inhibition of ER stress protects cardiomyocytes against H/R- or calpain-1-induced apoptotic cell death. Furthermore, calpain-1 appeared to activate the PERK and IRE1 branches of the ER stress response in cardiomyocytes. Thus, ER stress may be an additional mechanism connecting calpain-1 to ischemic heart injury. It is worthwhile to mention that the present study took a combination of calpain-1/2 siRNA, capn4 knockout and calpastatin over-expression approaches to examine the role of calpain-1 in ER stress and apoptosis in H/R and I/R-induced injury in cardiomyocytes. The utilities of capn4 knockout and calpastatin over-expression provided convincing evidence in support of the involvement of calpain-1/2; however, these approaches could not identify which isoforms is important. Direct knockdown of calpain-1/2 confirmed calpain-1 but not calpain-2 as a specific isoform to trigger ER stress in cardiomyocytes following H/R.
The role of calpain in ER stress has also been suggested in non-cardiomyocytes. For example, calpain-2 was involved in ER stress in SH-SY5Y cells in response to hypoxia [39] and in renal cell death following exposure to reactive chemical toxicants [40]. Pharmacological inhibition of calpain prevented ER stress in macrophages induced by Mycobacterium kansasii type strain [41]. However, in the present study up-regulation of calpain-2 was unable to induce ER stress in cardiomyocytes. Consistently, silencing calpain-2 did not inhibit ER stress and apoptosis induced by H/R in cardiomyocytes. Thus, calpain-2 is not involved in ER stress in cardiomyocytes following H/R. It is most likely that cardiomyocytes may respond differently from other cell types upon calpain-2 activation. Yet, what causes this discrepancy remains unknown.
The IRE1 branch of the ER stress response induces JNK1/2 activation [19]. Consistently, we show that inhibition of ER stress blocks JNK1/2 signaling in both calpain-1 and H/R-stimulated cardiomyocytes. Importantly, we demonstrate that ER stress/JNK1/2 signaling mediates apoptosis in cardiomyocytes following H/R. Our observations support a model whereby calpain-1 activation induces ER stress, which in turn promotes JNK1/2 signaling, leading to apoptosis in cardiomyocytes suffering ischemic injury. Calpain-1 has also been shown to induce cleavage of caspase-12 [42], though whether caspase-12 activation is also operative downstream of calpain-1/ER stress signaling in ischemia-initiated apoptosis requires further investigation.
It is well-known that ischemia, oxidative stress and disturbance of calcium homeostasis usually occur in the heart under stresses, resulting in the accumulation of unfold proteins. ER stress has been demonstrated in the ischemic heart [31]; however, it is currently unknown how calpain-1 mediates ER stress in cardiomyocytes following H/R. The ER is the main organelle in which free Ca2+ is stored [43]. Disturbance of Ca2+ homeostasis in the ER lumen inhibits chaperone function and creates ER stress. The SERCA family resides in the sarcoplasmic reticulum (SR) within muscle cells. Its main function is the reuptake of Ca2+ from the cytosol into the ER/SR lumen [44]. Thus, inhibition of SERCA with thapsigargin blocks reuptake of Ca2+ from the cytoplasm, leading to ER stress [45]. Cardiomyocytes mainly express SERCA2a [44]. In is-chemic and failing hearts, the protein levels of SERCA2a are significantly reduced [46], which correlate with an increase in calpain activity. Early studies have suggested that SERCA2a may be a substrate of calpain-1 [24]. Thus, it is possible that up-regulation of calpain-1 activity may induce the proteolytic cleavage of SERCA2a and thereby block reuptake of Ca2+ from the cytoplasm, leading to ER stress. In fact, our data shows that both up-regulation of calpain-1 and H/R decrease the protein levels of SERCA2a in cardiomyocytes. However, we did not detect calpain-1 protein in isolated SR/ER from cardiomyocytes, which is consistent with a previous report in lung adenocarcinoma cells [47]. It is speculated that calpain-1 may cleave SERCA2a outside of SR/ER.
5. Conclusions
This study demonstrates that calpain-1 induces ER stress and subsequent JNK1/2 activation, thereby mediating apoptosis in cardiomyocytes. Accordingly, inhibition of calpain prevents ER stress, JNK1/2 activation and apoptosis in cardiomyocytes following H/R and hearts after I/R. Thus, this study provides a new mechanism by which calpain-1 mediates ischemic injury in cardiomyocytes.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (MOP-133657) and the National Natural Science Foundation of China (81470499), and in part by Western Department of Medicine Program of Experimental Medicine (POEM) Research Award. The research in Dr. Guo-Chang Fan’s lab is supported by NIH R01 grant [grant number HL-087861]. T.P. is a recipient of a New Investigator Award from the Canadian Institutes of Health Research.
Abbreviations
- ER
endoplasmic reticulum
- H/R
hypoxia/re-oxygenation
- I/R
ischemia/reperfusion
- JNK1/2
c-Jun N-terminal protein kinase1/2
- MI
myocardial infarction
- MOI
multiplicity of infection
- TAUR
tauroursodeoxycholate
- CI-III
calpain inhibitor-III
- CAST
calpastatin
- PERK
protein kinase-like ER kinase
- IRE1
inositol-requiring kinase 1
- ATF6
activating transcription factor 6
- eIF2α
eukaryotic translation initiation factor 2α
- ATF4
translation of transcription factor ATF4
- XBP1
X-box binding protein1
- CHOP
the transcription factor C/EBP homologous protein
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
Footnotes
Conflict of interest
There is no conflict of interest.
References
- 1.Dorn GW., II Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovasc Res. 2009;81:465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frantz S, Bauersachs J, Ertl G. Post-infarct remodelling: contribution of wound healing and inflammation. Cardiovasc Res. 2009;81:474–481. doi: 10.1093/cvr/cvn292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009;81:482–490. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canadian Cardiovascular Society. CCS Consensus Document on Congestive Heart Failure. 2001. [Google Scholar]
- 5.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Li Y, Shan L, Shen E, Chen R, Peng T. Over-expression of calpastatin inhibits calpain activation and attenuates myocardial dysfunction during endotoxaemia. Cardiovasc Res. 2009;83:72–79. doi: 10.1093/cvr/cvp100. [DOI] [PubMed] [Google Scholar]
- 7.Galvez AS, Diwan A, Odley AM, Hahn HS, Osinska H, Melendez JG, Robbins J, Lynch RA, Marreez Y, Dorn GW., II Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res. 2007;100:1071–1078. doi: 10.1161/01.RES.0000261938.28365.11. [DOI] [PubMed] [Google Scholar]
- 8.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, Baud L. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj G, Sharma RK. TNF-alpha-mediated cardiomyocyte apoptosis involves caspase-12 and calpain. Biochem Biophys Res Commun. 2006;345:1558–1564. doi: 10.1016/j.bbrc.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Arnold JM, Pampillo M, Babwah AV, Peng T. Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med. 2009;46:51–61. doi: 10.1016/j.freeradbiomed.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Feng Q, Arnold M, Peng T. Calpain activation contributes to hyperglycaemia-induced apoptosis in cardiomyocytes. Cardiovasc Res. 2009;84:100–110. doi: 10.1093/cvr/cvp189. [DOI] [PubMed] [Google Scholar]
- 12.Kositprapa C, Zhang B, Berger S, Canty JM, Jr, Lee TC. Calpain-mediated proteolytic cleavage of troponin I induced by hypoxia or metabolic inhibition in cultured neonatal cardiomyocytes. Mol Cell Biochem. 2000;214:47–55. doi: 10.1023/a:1007160702275. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda Y, Young LH, Lefer AM. Attenuation of neutrophil-mediated myocardial ischemia-reperfusion injury by a calpain inhibitor. Am J Physiol Heart Circ Physiol. 2002;282:H1421–H1426. doi: 10.1152/ajpheart.00626.2001. [DOI] [PubMed] [Google Scholar]
- 14.Kang MY, Zhang Y, Matkovich SJ, Diwan A, Chishti AH, Dorn GW., II Receptor-independent cardiac protein kinase Calpha activation by calpain-mediated truncation of regulatory domains. Circ Res. 2010;107:903–912. doi: 10.1161/CIRCRESAHA.110.220772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Wei M, Wang Q, Li J, Wang H, Liu W, Lacefield JC, Greer PA, Karmazyn M, Fan GC, Peng T. Deficiency of Capn4 gene inhibits nuclear factor-kappaB (NF-kappaB) protein signaling/inflammation and reduces remodeling after myocardial infarction. J Biol Chem. 2012;287:27480–27489. doi: 10.1074/jbc.M112.358929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mani SK, Balasubramanian S, Zavadzkas JA, Jeffords LB, Rivers WT, Zile MR, Mukherjee R, Spinale FG, Kuppuswamy D. Calpain inhibition preserves myocardial structure and function following myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;297:H1744–H1751. doi: 10.1152/ajpheart.00338.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernando V, Inserte J, Sartorio CL, Parra VM, Poncelas-Nozal M, Garcia-Dorado D. Calpain translocation and activation as pharmacological targets during myocardial ischemia/reperfusion. J Mol Cell Cardiol. 2010;49:271–279. doi: 10.1016/j.yjmcc.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Dorado D, Ruiz-Meana M, Inserte J, Rodriguez-Sinovas A, Piper HM. Calcium-mediated cell death during myocardial reperfusion. Cardiovasc Res. 2012;94:168–180. doi: 10.1093/cvr/cvs116. [DOI] [PubMed] [Google Scholar]
- 19.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickhout JG, Carlisle RE, Austin RC. Interrelationship between cardiac hypertrophy, heart failure, and chronic kidney disease: endoplasmic reticulum stress as a mediator of pathogenesis. Circ Res. 2011;108:629–642. doi: 10.1161/CIRCRESAHA.110.226803. [DOI] [PubMed] [Google Scholar]
- 21.Groenendyk J, Sreenivasaiah PK, Kim do H, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ Res. 2010;107:1185–1197. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- 22.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 23.Jain K, Suryakumar G, Prasad R, Ganju L. Differential activation of myocardial ER stress response: a possible role in hypoxic tolerance. Int J Cardiol. 2013;168:4667–4677. doi: 10.1016/j.ijcard.2013.07.180. [DOI] [PubMed] [Google Scholar]
- 24.French JP, Quindry JC, Falk DJ, Staib JL, Lee Y, Wang KK, Powers SK. Ischemia-reperfusion-induced calpain activation and SERCA2a degradation are attenuated by exercise training and calpain inhibition. Am J Physiol Heart Circ Physiol. 2006;290:H128–H136. doi: 10.1152/ajpheart.00739.2005. [DOI] [PubMed] [Google Scholar]
- 25.Pedrozo Z, Sanchez G, Torrealba N, Valenzuela R, Fernandez C, Hidalgo C, Lavandero S, Donoso P. Calpains and proteasomes mediate degradation of ryanodine receptors in a model of cardiac ischemic reperfusion. Biochim Biophys Acta. 2010;1802:356–362. doi: 10.1016/j.bbadis.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Brostrom MA, Brostrom CO. Calcium dynamics and endoplasmic reticular function in the regulation of protein synthesis: implications for cell growth and adaptability. Cell Calcium. 2003;34:345–363. doi: 10.1016/s0143-4160(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Ma J, Zhu H, Singh M, Hill D, Greer PA, Arnold JM, Abel ED, Peng T. Targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes. 2011;60:2985–2994. doi: 10.2337/db10-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rui T, Feng Q, Lei M, Peng T, Zhang J, Xu M, Abel ED, Xenocostas A, Kvietys PR. Erythropoietin prevents the acute myocardial inflammatory response induced by ischemia/reperfusion via induction of AP-1. Cardiovasc Res. 2005;65:719–727. doi: 10.1016/j.cardiores.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Shan L, Li J, Wei M, Ma J, Wan L, Zhu W, Li Y, Zhu H, Arnold JM, Peng T. Disruption of Rac1 signaling reduces ischemia-reperfusion injury in the diabetic heart by inhibiting calpain. Free Radic Biol Med. 2010;49:1804–1814. doi: 10.1016/j.freeradbiomed.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Peng T, Lu X, Lei M, Feng Q. Endothelial nitric-oxide synthase enhances lipopolysaccharide-stimulated tumor necrosis factor-alpha expression via cAMP-mediated p38 MAPK pathway in cardiomyocytes. J Biol Chem. 2003;278:8099–8105. doi: 10.1074/jbc.M207288200. [DOI] [PubMed] [Google Scholar]
- 31.Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol. 2006;291:H1411–H1420. doi: 10.1152/ajpheart.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parameswaran S, Sharma RK. Altered expression of calcineurin, calpain, calpastatin and HMWCaMBP in cardiac cells following ischemia and reperfusion. Biochem Biophys Res Commun. 2014;443:604–609. doi: 10.1016/j.bbrc.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Inserte J, Garcia-Dorado D, Hernando V, Soler-Soler J. Calpain-mediated impairment of Na+/K+-ATPase activity during early reperfusion contributes to cell death after myocardial ischemia. Circ Res. 2005;97:465–473. doi: 10.1161/01.RES.0000181170.87738.f3. [DOI] [PubMed] [Google Scholar]
- 34.Lu XY, Chen L, Cai XL, Yang HT. Overexpression of heat shock protein 27 protects against ischaemia/reperfusion-induced cardiac dysfunction via stabilization of troponin I and T. Cardiovasc Res. 2008;79:500–508. doi: 10.1093/cvr/cvn091. [DOI] [PubMed] [Google Scholar]
- 35.Chen Q, Paillard M, Gomez L, Ross T, Hu Y, Xu A, Lesnefsky EJ. Activation of mitochondrial mu-calpain increases AIF cleavage in cardiac mitochondria during ischemia-reperfusion. Biochem Biophys Res Commun. 2011;415:533–538. doi: 10.1016/j.bbrc.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkard N, Becher J, Heindl C, Neyses L, Schuh K, Ritter O. Targeted proteolysis sustains calcineurin activation. Circulation. 2005;111:1045–1053. doi: 10.1161/01.CIR.0000156458.80515.F7. [DOI] [PubMed] [Google Scholar]
- 37.Panther F, Williams T, Ritter O. Inhibition of the calcineurin-NFAT signalling cascade in the treatment of heart failure. Recent Pat Cardiovasc Drug Discov. 2009;4:180–186. doi: 10.2174/157489009789152276. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Matkovich SJ, Duan X, Diwan A, Kang MY, Dorn GW., II Receptor-independent protein kinase C alpha (PKCalpha) signaling by calpain-generated free catalytic domains induces HDAC5 nuclear export and regulates cardiac transcription. J Biol Chem. 2011;286:26943–26951. doi: 10.1074/jbc.M111.234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang CY, Xie JW, Wang T, Xu Y, Cai JH, Wang X, Zhao BL, An L, Wang ZY. Hypoxia-triggered m-calpain activation evokes endoplasmic reticulum stress and neuropathogenesis in a transgenic mouse model of Alzheimer’s disease. CNS Neurosci Ther. 2013;19:820–833. doi: 10.1111/cns.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muruganandan S, Cribb AE. Calpain-induced endoplasmic reticulum stress and cell death following cytotoxic damage to renal cells. Toxicol Sci. 2006;94:118–128. doi: 10.1093/toxsci/kfl084. [DOI] [PubMed] [Google Scholar]
- 41.Lim YJ, Choi HH, Choi JA, Jeong JA, Cho SN, Lee JH, Park JB, Kim HJ, Song CH. Mycobacterium kansasii-induced death of murine macrophages involves endoplasmic reticulum stress responses mediated by reactive oxygen species generation or calpain activation. Apoptosis. 2013;18:150–159. doi: 10.1007/s10495-012-0792-4. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, Kain V, Sitasawad SL. High glucose-induced Ca2+ overload and oxidative stress contribute to apoptosis of cardiac cells through mitochondrial dependent and independent pathways. Biochim Biophys Acta. 2012;1820:907–920. doi: 10.1016/j.bbagen.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol. 2011;3:a004317. doi: 10.1101/cshperspect.a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank KF, Bolck B, Erdmann E, Schwinger RH. Sarcoplasmic reticulum Ca2+-ATPase modulates cardiac contraction and relaxation. Cardiovasc Res. 2003;57:20–27. doi: 10.1016/s0008-6363(02)00694-6. [DOI] [PubMed] [Google Scholar]
- 45.Shimoke K, Kishi S, Utsumi T, Shimamura Y, Sasaya H, Oikawa T, Uesato S, Ikeuchi T. NGF-induced phosphatidylinositol 3-kinase signaling pathway prevents thapsigargin-triggered ER stress-mediated apoptosis in PC12 cells. Neurosci Lett. 2005;389:124–128. doi: 10.1016/j.neulet.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 46.Shareef MA, Anwer LA, Poizat C. Cardiac SERCA2A/B: therapeutic targets for heart failure. Eur J Pharmacol. 2014;724:1–8. doi: 10.1016/j.ejphar.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 47.Hood JL, Brooks WH, Roszman TL. Differential compartmentalization of the calpain/calpastatin network with the endoplasmic reticulum and Golgi apparatus. J Biol Chem. 2004;279:43126–43135. doi: 10.1074/jbc.M408100200. [DOI] [PubMed] [Google Scholar]