Reactive oxygen species (ROS) are generated either during oxidative metabolism or in defense against pathogens. Their production is increased further by tissue injury or disease. Extensive evidence accumulated during the last 30 years provides compelling evidence that ROS-induced damage is a significant cause of cardiovascular injury and dysfunction. A variety of enzymatic and nonenzymatic anti-oxidants have evolved to protect against the constant onslaught of ROS, and these defenses respond deftly to changes in ROS generation or to the generation of secondary oxidation products. In this issue of Circulation Research, Endo et al1 report that increased mitochondrial accumulation of aldehydes derived from lipid oxidation protects against myocardial ischemia/reperfusion injury. These findings put a new twist on our view of the oxidant–antioxidant balance in the heart and underscore the importance of hormesis in which mild exposure to a stressor elicits an adaptive response that increases resistance to subsequent stress. Understanding how endogenous defense mechanisms are enhanced by stress response signaling could lead to the development of new strategies for combating oxidative stress.
Most biological ROS are highly reactive and, therefore, short-lived. The ones with the greatest reactivity, such as the hydroxyl and alkoxyl radicals, have the shortest life span. As a result, the damage they induce is likely to be restricted to their site of origin and, therefore, of limited significance. Hence, it has long been suspected that ROS generate secondary products that spread injury and amplify damage. Which molecules can amplify and propagate ROS-initiated injury? Although many possibilities exist, one likely class of molecules may be aldehydes generated by the oxidation of unsaturated lipids. With the exception of antioxidants such as vitamin E or glutathione, unsaturated lipids are the most likely targets of ROS. When oxidized, these lipids generate a plethora of bioactive molecules, of which aldehydes are among the most reactive and abundant products. Being engendered by the violent fragmentation of their lipid parents, these aldehydes possess high reactivity. However, they are more stable than ROS, so they can diffuse to sites distant from their site of injury, thereby propagating oxidative injury. Moreover, these aldehydes possess a rich variety of structural features or they acquire additional ones by conjugating with receptive nucleophiles, which allows them to be recognized by cell constituents as signaling molecules. Aldehydes generated from oxidized lipids such as malondialdehyde, 4-hydroxy-trans-2-nonenal (HNE), and 1-palmitoyl-2-oxovaleroyl phosphatidyl choline (POVPC) have been detected in almost all tissues that have experienced oxidative injury. They are found in ischemic, hypertrophic, and failing hearts; in atherosclerotic lesions and restenotic vessels; in apoptotic cells; and in damaged mitochondria.2 Nevertheless, it is unclear whether they are simply markers of oxidative stress (footprints of a disruptive presence long gone) or whether they are active instruments of injury and sounders of alarm signaling.
How does one study the active role of aldehydes in adaptation or injury? One approach is to examine aldehyde metabolism. Like ROS, aldehydes are metabolized and detoxified by several nonenzymatic and enzymatic processes (see the Figure). Previous work has shown that in most cells aldehydes are either reduced (to alcohols), oxidized (to acids), or conjugated with cellular nucleophiles such as glutathione, carnosine (β-alanine-L-histidine), or proteins. Several enzymes involved in metabolizing these aldehydes have been identified.2 In cardiovascular tissue, aldose reductase (AR) catalyzes the reduction of both HNE and POVPC. Oxidation of aldehydes is catalyzed by aldehyde dehydrogenases (ALDHs) and glutathione conjugation is facilitated by glutathione S-transferases. By converting aldehydes to less reactive products, these enzymes prevent the direct toxicity of aldehydes; however, metabolic conversion could also enhance stability of aldehydes and thereby augment their ability to stimulate cell signaling. Hence, investigations into the role of aldehyde-metabolizing enzymes in modulating outcomes of oxidative injury could provide one avenue for understanding the role of aldehydes as mediators of oxidative stress–related signaling.
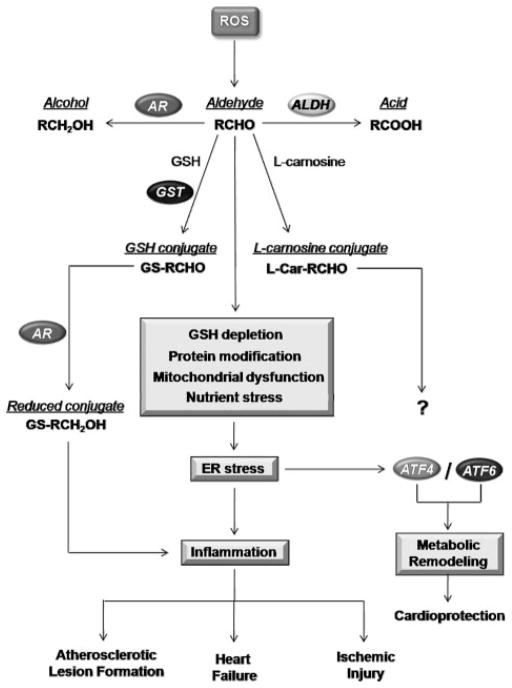
Figure.
Pathways of aldehyde-induced stress responses in the cardiovascular system. The peroxidation of membrane lipids by ROS leads to the formation of aldehydes (RCHO). Aldehydes are detoxified by multiple enzymes in the cell; they can be reduced by aldo-keto reductases (AR) to their corresponding alcohols (RCH2OH), oxidized to carboxylates via ALDHs, or conjugated to glutathione (GSH) or L-carnosine to form glutathione conjugates (GS-RCHO) and L-carnosine conjugates (His-RCHO), respectively. The glutathione conjugation reactions are known to be catalyzed by GST. Reduction of the conjugate by AR has been shown to promote inflammation. Aldehydes can deplete glutathione, modify proteins, and promote mitochondrial dysfunction and nutrient stress, which may cause ER stress. ER stress can promote the activation of adaptive arms of the unfolded protein response, leading to induction of proteins, ie, ATF4 and -6, associated with metabolic remodeling and a cardioprotected phenotype. ER stress can also lead to inflammatory responses, which, alone or in combination with mitochondrial dysfunction, could increase atherosclerotic lesion formation or promote myocardial ischemia/reperfusion injury.
In keeping with the view that ROS-mediated injury could be attributed in part to lipid-derived aldehydes, it has been shown that modulation of aldehyde metabolism profoundly affects cardiovascular injury. For example, inhibition of the aldehyde-metabolizing enzyme AR abolishes the late phase of ischemic preconditioning3 and exacerbates atherogenesis.4 Moreover, treatment with small molecule activators of ALDH2 to stimulate the metabolism of cytotoxic aldehydes reduces infarct size by 60%,5 indicating that much of the damage inflicted by ischemia/reperfusion could be attributed to aldehydes generated in the ischemic heart. In contrast, Endo et al1 report that transgenic expression of an Aldh2 gene containing a single nucleotide polymorphism (Aldh2*2), with impaired ALDH activity, diminishes ischemic injury. They reconcile these results by postulating that mitochondrial aldehyde accumulation induces a hormetic response that leads to a cardioprotected phenotype.
The Aldh2*2 allele is present in a large proportion of people of Asian descent and results in a frank inability to tolerate alcohol. In addition to alcohol flushing syndrome, this mutation results in an increase in the levels of serum peroxide and an increase in the risk of late-onset Alzheimer’s disease. Few other physiological effects of this mutation have been reported. Intriguingly, transgenic expression Aldh2*2 in the skeletal muscle and hearts of mice resulted in stunted growth, reduced muscle mass and fat content, osteopenia, and kyphosis. Expression of Aldh2*2 decreased the ability of the heart to metabolize HNE, with extensive metabolic remodeling. Surprisingly, no significant changes in the expression of most of the major antioxidant enzymes were observed, but there was a shift in glucose metabolism from glycolysis to the pentose phosphate pathway. These changes seem to be related to the activation of the eukaryotic initiation factor (eIF)2α–activating transcription factor (ATF)4 pathway, because heterozygous knockout of ATF4 blunted the increase in glutathione in the Aldh2*2 hearts and weakened the ischemia-resistant phenotype.
The eIF2α-ATF4 pathway is a regulator of amino acid metabolism and is activated as a component of the unfolded protein response, which is triggered by the accumulation of unfolded proteins in the endoplasmic reticulum (ER).6 Because transgenic expression of Aldh2*2 was associated with an increase in the accumulation of protein-HNE adducts, it is likely that that HNE-adducted proteins could trigger unfolded protein response pathways. Further studies are required to elucidate the role of ER stress in aldehyde-induced hormesis; however, the findings of Endo et al1 reveal novel pathways of crosstalk between mitochondrial stress, antioxidant defense, and intermediary metabolism. In our present understanding of ischemic preconditioning, brief bouts of ischemia increase cytosolic cardioprotective proteins, which then protect the mitochondria during lethal episodes of ischemia. Here, stress originating primarily from mitochondria established cardioprotection by altering cytosolic metabolism, indicating a new mechanism for sensing mitochondrial stress related to the activation of ATF4.
Although the efficacy of sublethal stress in establishing protection has been demonstrated in a variety of experimental paradigms (eg, ischemic preconditioning, calorie restriction), the true value of such Spartan principles is only now beginning to emerge. For instance, it has been reported recently that decreasing glucose metabolism extends the life span of Caenorhabditis elegans because it increases mitochondrial ROS production.7 Significantly, treatment with antioxidants and vitamins prevented the extension of life span, arguing against many commonly held beliefs in the utility of antioxidant supplements and providing an additional reason why clinical trials with vitamin supplements have been so disappointingly negative.
The observation that ischemic resistance of Aldh2*2 transgenic hearts was related to metabolic remodeling also provides new support to the concept that antioxidant defense could be strengthened, not only by elevating the levels of antioxidants (vitamins C and E) or antioxidant enzymes (superoxide dismutase or catalase) but also by increasing metabolic flux through pathways that generate reducing equivalents (NADPH and glutathione). That this protection was achieved by rechanneling glucose from glycolysis to the shunt and by changing amino acid metabolism points to the presence of ancient stress-activated circuits linking nutrition and energy metabolism to survival and self-defense.
It has long been suspected that glucose metabolism has a primary role in antioxidant defense. However, glucose could have opposing effects: it could increase ROS production (by increasing metabolism) or prevent oxidative injury (by providing reducing equivalents). Endo et al1 were able to uncover an unambiguous role of glucose in cardioprotection because they were looking at the effects of secondary products rather than ROS themselves. Direct exposure to ROS alters many things simultaneously and as a result it is difficult to distinguish cause from effect or primary from secondary changes. Surprisingly, they found that instead of an increase in the levels of the usual suspects (catalase, glutathione peroxidases, reductases, superoxide dismutases), there was an upregulation of glycine and serine metabolism and an ATF4-mediated increase in glutathione synthesis. These observations provide fresh support to the idea that to protect cells from oxidative stress-related injury it may be necessary to increase the metabolism of lipid-derived aldehydes rather than to simply increase ROS quenching.
That increased generation of aldehydes could trigger extensive and complex remodeling in the heart attests to the high biological activity of lipid-derived aldehydes and their unique ability to trigger stress signaling. Nevertheless, these conclusions are based on strategies that alter aldehyde metabolism. Elucidation of the in vivo role of aldehydes by altering their metabolism is, however, not straightforward. For example, AR which metabolizes aldehydes also reduces glucose to sorbitol. Hence, some of the effects observed with changes in AR levels may relate to changes in glucose metabolism. Similarly, ALDH2 not only metabolizes HNE but several other aldehydes as well, and it is significant that in the study by Endo et al1 the Aldh2*2 transgenic hearts were smaller in size suggesting that the enzyme may be necessary for growth-regulating metabolism or that changes in the metabolism of aldehydes in the skeletal muscle (where the Aldh2*2 transgene was also expressed) could indirectly affect myocardial sensitivity to ischemia. Moreover, complete deletion of the Aldh2 gene did not affect aldehyde metabolism, indicating that this enzyme does not metabolize aldehydes in vivo. Aldehyde metabolism was, however, affected in Aldh2*2 hearts, which the authors speculate may be attributable to its ability to bind to other ALDHs. If ALDH2 is not the main enzyme catalyzing aldehyde oxidation, then why does the activation of this enzyme by small molecule activators prevent ischemic injury5? Clearly, like all studies of merit, the study by Endo et al raises more questions than it answers. Nonetheless, the present findings are intriguing and strengthen the rationale for studying whether the presence of the Aldh2*2 allele in humans is associated with an increase in resistance to ischemic heart disease or other cardiovascular diseases associated with an increase in ROS production.
Acknowledgments
Sources of Funding
Supported by National Heart, Lung, and Blood Institute (NIH) grants HL55477, HL59378, and HL078825 and National Center for Research Researches (NIH) grant RR24489.
Non-standard Abbreviations and Acronyms
- ALDH
aldehyde dehydrogenase
- AR
aldose reductase
- ATF
activating transcription factor
- eIF
eukaryotic initiation factor
- ER
endoplasmic reticulum
- HNE
4-hydroxy-trans-2-nonenal
- POVPC
1-palmitoyl-2-oxovaleroyl phosphatidyl choline
- ROS
reactive oxygen species
Footnotes
The opinions expressed in this editorial are not necessarily those of the editors or of the American Heart Association.
Disclosures
None.
References
- 1.Endo J, Sano M, Katayama T, Hishiki T, Shinmura K, Morizane S, Matsuhashi T, Katsumata Y, Zhang Y, Ito H, Nagahata Y, Marchitti S, Nishimaki K, Wolf AM, Nakanishi H, Hattori F, Vasiliou V, Adachi T, Ohsawa I, Taguchi R, Hirabayashi Y, Ohta S, Suematsu M, Ogawa S, Fukuda K. Metabolic remodeling induced by mitochondrial aldehyde stress stimulates tolerance to oxidative stress in the heart. Circ Res. 2009;105:1118–1127. doi: 10.1161/CIRCRESAHA.109.206607. [DOI] [PubMed] [Google Scholar]
- 2.Conklin D, Prough R, Bhatanagar A. Aldehyde metabolism in the cardiovascular system. Mol Biosyst. 2007;3:136–150. doi: 10.1039/b612702a. [DOI] [PubMed] [Google Scholar]
- 3.Shinmura K, Bolli R, Liu SQ, Tang XL, Kodani E, Xuan YT, Srivastava S, Bhatnagar A. Aldose reductase is an obligatory mediator of the late phase of ischemic preconditioning. Circ Res. 2002;91:240–246. doi: 10.1161/01.res.0000029970.97247.57. [DOI] [PubMed] [Google Scholar]
- 4.Srivastava S, Vladykovskaya E, Barski OA, Spite M, Kaiserova K, Petrash JM, Chung SS, Hunt G, Dawn B, Bhatnagar A. Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein E-null mice. Circ Res. 2009;105:793–802. doi: 10.1161/CIRCRESAHA.109.200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]