Abstract
Aldehydes are generated by oxidized lipids and carbohydrates at increased levels under conditions of metabolic imbalance and oxidative stress during atherosclerosis, myocardial and cerebral ischemia, diabetes, neurodegenerative diseases and trauma. In most tissues, aldehydes are detoxified by oxidoreductases that catalyze the oxidation or the reduction of aldehydes or enzymatic and nonenzymatic conjugation with low molecular weight thiols and amines, such as glutathione and histidine dipeptides. Histidine dipeptides are present in micromolar to millimolar range in the tissues of vertebrates, where they are involved in a variety of physiological functions such as pH buffering, metal chelation, oxidant and aldehyde scavenging. Histidine dipeptides such as carnosine form Michael adducts with lipid-derived unsaturated aldehydes, and react with carbohydrate-derived oxo- and hydroxy- aldehydes forming products of unknown structure. Although these peptides react with electrophilic molecules at lower rate than glutathione, they can protect glutathione from modification by oxidant and they may be important for aldehyde quenching in glutathione-depleted cells or extracellular space where glutathione is scarce. Consistent with in vitro findings, treatment with carnosine has been shown to diminish ischemic injury, improve glucose control, ameliorate the development of complications in animal models of diabetes and obesity, promote wound healing and decrease atherosclerosis. The protective effects of carnosine have been linked to its anti-oxidant properties, it ability to promote glycolysis, detoxify reactive aldehydes and enhance histamine levels. Thus, treatment with carnosine and related histidine dipeptides may be a promising strategy for the prevention and treatment of diseases associated with high carbonyl load.
Keywords: Carnosine, aldehyde, histidine dipeptides, oxidative stress
1. Aldehydes: Occurrence and Association with Disease
Biological aldehydes are ubiquitous products of normal metabolism and are generated during intermediary metabolism and lipid and nucleotide synthesis. In addition, they are present in several types of food and are produced to high levels during the processes of uncontrolled lipid and carbohydrate oxidation [1]. Accumulation of aldehydes due to excessive production has been suggested to contribute to the etiology of several diseases characterized by the presence of oxidative stress such as atherosclerosis, diabetes or Alzheimer’s disease. Because of the presence of an electrophilic carbonyl group, aldehydes are highly reactive and they react readily with nucleophilic functional groups present on the macromolecules such as proteins, lipids and DNA [2]. In addition, recent evidence suggests that aldehydes also act as signaling molecules mediating inflammation and endoplasmic reticulum stress [3].
Although the aldehyde group by itself is only moderately reactive, its reactivity increases dramatically by the presence of other neighboring electron-withdrawing groups. Aldehydes associated with cellular oxidative stress are derived from two major classes of biological reactions: oxidation of polyunsaturated fatty acids and enzymatic or nonenzymatic metabolism of carbohydrates [4]. Aldehydes derived from the oxidation of lipids (lipid-derived aldehydes) are often characterized by the presence of an α,β double bond, or other oxo-or hydroxyl-groups present on the molecule. Major aldehydes derived from lipid peroxidation are: 4-hydroxy-trans-2-nonenal (HNE), 4-hydroxy-trans-2-hexenal (HHE), 4-oxo-trans-2-nonenal (ONE), nonenal, hexenal, etc. These aldehydes are electrophilic in nature and they form Michael adducts with cellular nucleophiles, such as amine and sulfhydryl compounds. The carbohydrate-derived aldehydes, i.e., methylglyoxal, glyoxal, glycolaldehyde, 3-deoxyglucosone, usually contain a keto or hydroxyl group in the α-position relative to the aldehyde group. This structural feature activates the aldehyde group and allows it to attack amine and guanidinium groups in proteins to undergo Schiff base formation, decarboxylation, and α-aminoketone condensation leading to the formation of advanced glycation end-products (AGEs) [5]. Several diseases such as atherosclerosis, diabetes, stroke, myocardial ischemia, Alzheimer disease, schizophrenia, neurodegenerative diseases, and even ageing are associated with increased oxidative stress and aldehyde accumulation. Products of oxidized lipoproteins accumulate in the atherosclerotic lesions of humans and atherosclerosis-prone animals and the circulating levels of lipid oxidation-derived aldehydes correlate strongly with angiographically documented coronary artery disease in humans [6–9]. In support of a causative role of aldehydes in atherogenesis, it has been shown that deletion of enzymes that promote the removal of lipid peroxidation products, such as paraoxonase [10] or aldose reductase [11] increases atherogenesis. Products of glycation of amino acid residues with α-dialdehydes (AGEs) have been shown to accumulate and contribute to the etiology of diabetes [12–14]. Hydroimidazolones, arising from arginine modification with aldehydes, often the major AGEs in proteins of tissues and body fluids, increase in diabetes and associated vascular complications, renal failure, cirrhosis, Alzheimer’s disease, arthritis, Parkinson’s disease and ageing [15]. Because several AGE inhibitors such as pyridoxamine and benfotiamine inhibit the development of retinopathy and neuropathy in streptozotocin (STZ)-induced diabetic rats, it has been suggested that AGEs play a key role in the development of secondary diabetic complications [13, 14].
It has been proposed that aldehydes contribute significantly to the pathological damage in high-metabolic-rate organs, such as liver, heart, and skeletal muscle. This suggestion is based on the observation that injury and dysfunction in several tissues is associated with aldehyde accumulation. For example, HNE adducts are frequently detected in liver samples from patients with non-alcoholic fatty liver disease, but they are absent in control livers [16]. Similarly, urine from subjects with inflammatory cancer-prone liver diseases caused by alcohol abuse or viral infection contains massively higher level of 1, N6-etheno-2-deoxyadenosine, a miscoding etheno-modified DNA adduct formed by reaction of HNE with DNA-bases [17]. Increased levels of HNE or HNE-modified proteins have also been detected in the skeletal muscle of type 2 diabetic patients [18, 19] and adipose tissue of HF fed mice [20].
Like other tissues, the brain is also susceptible to oxidative damage due to its high lipid content and oxygen consumption. Increased levels of lipid peroxidation products malondialdehyde, 4-hydroxynonenal and acrolein have been found in neurodegenerative diseases such as Alzheimer’s, amyotrophic lateral sclerosis, bipolar disorder, epilepsy, Parkinson’s disease, schizophrenia and central nervous system after trauma. Increased levels of specific histidine-HNE and glutathione-HNE Michael adducts have been detected in the brain tissue of Alzheimer’s disease patients [21, 22]. In addition, it has been suggested that increased oxidative stress may be relevant to the pathophysiology of schizophrenia, which is also associated with an increase in lipid-derived aldehydes such as malondialdehyde and HNE [23, 24].
2. Aldehyde metabolism
The toxicity of aldehydes under normal physiological conditions and during disease and injury is likely to depend upon the ability of the biological processes involved in their detoxification. Such detoxification pathways include a combination of enzymatic and nonenzymatic reactions. The major enzymatic pathways of aldehyde detoxification are oxidation by aldehyde dehydrogenases, reduction by aldo-keto reductases and conjugation with glutathione (catalyzed by glutathione-S-transferases) (Fig. 1). Non-enzymatic pathways include conjugation with cellular sulfhydryl and amine-based nucleophiles, such as glutathione and carnosine. As conjugation of unsaturated aldehydes involves the formation of a Michael adduct at the double bond, the aldehyde group remains intact in the conjugate and can be further oxidized or reduced. Indeed, it has been shown that glutathione conjugates of HNE and acrolein are excellent substrates of aldose reductase, AKR1B1, which catalyzes their reduction into chemically inert glutathionyl-dihhydroxynonanol (GS-DHN) and glutathionyl-propanol (GS-propanol) [25, 26]. Glutathione reacts with unsaturated aldehydes much faster than amine-based agents; however, the latter may be important under the condition of that leads to glutathione depletion or in removing aldehydes from extracellular spaces where the concentration of glutathione is two to three orders of magnitude lower than in the cells [27, 28]. For the carbohydrate-derived aldehydes, it has been shown that the interaction with amine-containing compounds plays a major role in their biological disposition [29]. Hydrazine-based antiglycating agents such as aminoguanidine, hydralazine, and pyridoxamine, have been demonstrated to scavenge carbohydrate-derived oxo-aldehydes [30] and lipid-derived α,β-unsaturated aldehydes with high efficiency [31]. Therefore, recent interest has been directed towards the use of endogenous nitrogen-based compounds for aldehyde detoxification. These naturally occurring histidine-containing dipeptides have the potential to reduce the formation of aldehydes, detoxify pre-formed aldehydes, prevent generation of modified proteins and possibly even repair proteins that are covalently adducted to aldehydes and related carbonyls [32].
Fig. 1.
Major pathways of aldehyde detoxification.
3. Histidine Dipeptides
Histidine-containing dipeptides are a family of soluble peptides that consist of a histidine (or a histidine-like amino acid) containing an imidazole ring and an atypical amino acid (e.g. β-alanine or γ-hydroxybutyric acid) at the N-terminus of the peptide [33]. The imidazole ring appears to be important for several of the putative biological roles of this family of dipeptides. Carnosine is a “founding member” of this family and it consists of β-alanine bound to histidine (Fig. 2). Carnosine was first discovered in meat by the Russian scientist Gulewitsch in 1900, hence its name carnosine (Latin carnos=meat) [34]. Variations in the structure of histidine dipeptides include methylation on either π (anserine), or τ (balenine) nitrogen on the imidazole ring of histidine, substitution of γ-aminobutyric acid for β-alanine (homocarnosine), or acetylation of the terminal amino group of β-alanine (N-acetylcarnosine). These modifications are species and tissue-specific and may provide means of fine-tuning the biological function of these peptides according to biological need and their biological roles that vary in a tissue-specific manner. For instance, only carnosine and N-acetylcarnosine are believed to be produced in human heart and skeletal muscle [35], whereas anserine is abundant in the pectoral muscle of birds and the leg muscle of rabbits [36]. Balenine is found mainly in muscles of murine diving mammals such as whales and dolphins [37]. Homocarnosine, which contains γ-hydroxybutyric acid in place of β-alanine, is predominantly present in the central nervous system and olfactory bulb [38, 39]. However, the specific function of each of these dipeptides is still not fully understood.
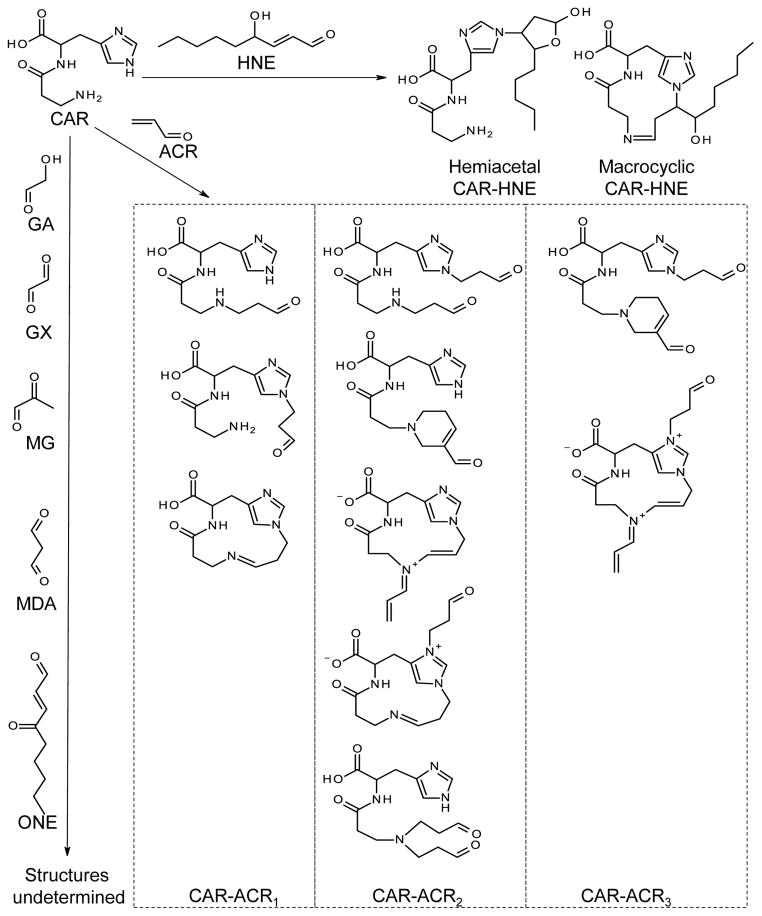
Fig. 2. Structures of carnosine - aldehydes reaction products.
CAR, carnosine; HNE, 4-hydroxy-trans-2-nonenal; ACR, acrolein; GA, glycolaldehyde; GX, glyoxal; MG, methylglyoxal; MDA, malondialdehyde; ONE, 4-oxo-trans-2-nonenal, CAR-ACR1, conjugates and dehydration products with 1:1 carnosine-acrolein ratio; CAR-ACR2, conjugates and dehydration products with 1:2 carnosine-acrolein ratio; CAR-ACR3, conjugates and dehydration products with 1:3 carnosine-acrolein ratio.
Histidine dipeptides are present at the highest level in fast twitch glycolytic muscle fibers, and their levels may depend upon the metabolic needs of the tissue. For instance, higher levels of histidine dipeptides are present in the pectoral muscle of pheasant, chicken and turkey (80–100 mM), species that are involved in explosive flight behaviors where rapid beating of wings for short periods is required, verses endurance flyers such as geese and pigeons [37]. Higher levels of histidine dipeptides have also been found in animals where frequent sprints and prolonged hypoxic dives are important for survival, such as the whale [40, 41]. High levels of these peptides have also been detected in animals bred for athletic competition, such as quarter horses, greyhound dogs, and camels [41]. The highest level of histidine dipeptides (150 mM) has been found in the little piked whale which makes long hypoxic dives [37]. Such wide distribution of these dipeptides among species, their high concentration, and their specific localization attest to the importance of their biological role.
4. Metabolism of carnosine
Carnosine is synthesized in vivo from its constituent amino-acids histidine and β-alanine by the enzyme carnosine synthase (ATPGD1). The availability of β-alanine, which can be derived from panthothenic acid, a ubiquitous grain constituent or synthesized in liver through the degradation of uracil [42], is considered to be a limiting factor in the biosynthesis of carnosine [43–45]. In humans, carnosine is hydrolyzed back into its constituent amino acids by a specific peptidase – carnosinase (CN). Carnosinase is present in two isoforms, CN1 and CN2. While CN1 is secreted into the plasma from the liver and kidneys, CN2 is more ubiquitously expressed and remains in the cytosol [46–48]. The carnosinase gene is subject to polymorphisms in the human population, which determines the efficiency of secretion and plasma activity level. Higher levels of CN1 activity are associated with lower bioavailability of carnosine [49] and progression or susceptibility to diabetic nephropathy.
In addition to synthesis and degradation, carnosine disposition in tissues is determined by its transport in and out of cells. Carnosine is a substrate of the proton-coupled oligopeptide transporter Pept2 [50]. Studies with the Pept2-knockout mice have shown that Pept2 plays a major role in determining the systemic clearance and reabsorption of carnosine in the kidney tubules [51]. In Pept2- knockout mice, higher levels of carnosine are present in skeletal muscle, compared to lower levels in the choroid plexus, olfactory bulb and spleen. However, the uptake of carnosine was severely diminished in the skeletal muscle of these mice. Therefore, it appears an endogenous system exists to maintain carnosine homeostasis in tissues with high and low synthetic capacity.
5. In vivo measurement of carnosine
Carnosine and other histidine dipeptides can be measured using high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE). Two challenges exist for HPLC analysis of histidine-containing dipeptides like carnosine: (1) carnosine is highly hydrophilic and therefore is poorly retained on the reverse-phase C18 HPLC columns, and (2) it lacks of sufficient absorbance in the UV-Visible range. The first issue is addressed by chromatographic separation of carnosine on reverse phase column with addition of surfactant [52] or ion pair agent [53, 54] into the mobile phase. The second issue can be overcome by using detection methods such as amperometric detection [55], or post-column derivatization [56]. Alternatively, pre-column derivatization can be used to improve both chromatographic behavior and detection, but it requires additional steps during sample preparation [57–59].
The combination of mass analysis capabilities of mass spectrometry (MS) or tandem MS (MS/MS) with physical separation capabilities of HPLC offers exceptional sensitivity and specificity, which is especially useful for simultaneous determination of multiple small molecules in a biological sample in “metabolomics” studies [60–64]. Along with HPLC, capillary electrophoresis (CE) offers higher separation efficiency, requires a smaller sample size, and is suitable for separation of peptides [65, 66]. Similar to HPLC-MS, CE-MS has also been used for metabolomics research, and histidine-containing dipeptides were detected in these studies [67–72]
Histidine-containing dipeptides like carnosine can be quantified in a non-invasive manner using nuclear magnetic resonance (NMR) or magnetic resonance spectroscopy (MRS), spectroscopy in human skeletal muscles [73–76], or in intact animals [77]. MRS method has recently been used to show that carnosine is depleted in the gastrocnemius muscle of type 2 diabetic patients [78]. The NMR signal of carnosine has been used to investigate the dynamics of intracellular pH in working muscles [79–81]. Like the MS based methods, NMR is also a method of choice for metabolomics studies, and levels of carnosine measured by this method have been reported [82, 83].
6. Carnosine in normal physiology
Histidine dipeptides are particularly abundant in skeletal muscle (8–50 mM), especially muscles that undergo anaerobic activity and maintain a high level of glycolysis., It has been suggested that in skeletal muscle the physiological function of these dipeptides in skeletal muscle is to maintain pH during strenuous exercise characterized by increased production of lactic acid. It has been estimated that carnosine provides ~ 10% of buffering capacity in vastus lateralis of humans [41]. Carnosine content was found higher in glycolytic type II fast-twitch muscle, than in more oxidative type I fibers, and its benefits in exercise performance have been specifically associated with muscle subjected to anaerobic activity and thus high level of glycolysis [40]. It has also been demonstrated that the levels of carnosine in the muscle are increased through training, and trained athletes in general have higher levels of carnosine in their muscle than untrained individuals [84]. Men have 36 to 82% higher muscle levels of carnosine than women. Vegetarians have lower levels of carnosine, suggesting that diet is an important determinant of carnosine content [85]. It has been shown that carnosine content of the soleus muscle decreases with age both in male and female omnivores [85]. In contrast, polymorphisms in the carnosinase gene, which affect plasma carnosine concentration after ingestion of carnosine, do not affect muscle carnosine content [85]. Supplementation with an isomolar amount of carnosine and its precursor β-alanine have been reported to increase muscle carnosine content by as much as 80% accompanied by improvements in ergometric performance [41, 86, 87].
7. Histidine dipeptides as antioxidants
Histidine dipeptides are capable of binding transition metal ions, including Cu2+ [88], Co2+ [89], Mn2+ [90], Zn2+ [91], Cd2+ [91] and Fe2+ [90]. The chemical character and biological relevance of this chelation capability has been summarized in several reviews [92, 93]. Metal binding affinity of carnosine is utilized in the carnosine-zinc complex, which has membrane-stabilizing [94], antioxidant [95], and wound healing [96] effects, and has been approved in Japan to treat gastric ulcer under the name Polaprezinc [97].
In addition to pH buffering and metal chelation, it has been suggested that carnosine and other histidine dipeptides can also act as antioxidants. A number of early studies indicated that carnosine is a scavenger of hydroxyl, peroxyl, superoxide radicals and singlet oxygen. In several in vitro oxidizing systems, including iron-ascorbate and linoleic acid hydroperoxide – haemoglobin systems, which generate superoxide and lipid peroxyl radicals, respectively, carnosine inhibits oxidation of linoleic acid and phosphatidylcholine liposomes [98]. Carnosine also inhibits iron-dependent hydroxylation of deoxyribose, suggesting that it might be an efficient hydroxyl radical scavenger [98]. Carnosine also inhibits oxidative hydroxylation of deoxyguanosine induced by an ascorbic acid/copper mixture; however, this effect may be due to its ability to chelate copper [99]. In addition to these properties, carnosine has also been shown to enhance ferric-reducing capacity of human plasma and to protect the DNA from damage induced by ferric and copper ascorbate systems to an extent much higher than that of histidine and β-alanine alone or in combination [100].
Several early studies suggested that carnosine quenches singlet molecular oxygen. In some studies it has been shown that carnosine protects bacteriophage DNA against damage induced by γ-irradiation and inhibits singlet oxygen phosphorescence [101, 102]. In a more biologically relevant setting carnosine has been shown to improve functional recovery of ischemic hearts by singlet oxygen quenching, as established by electron paramagnetic resonance measurements [103]. To determine whether histidine dipeptides have the ability to protect biological lipids from oxidation in metal-free systems, we bubbled oxygen through a suspension of 1-palmitoyl-2-arachidonyl phosphatidylcholine, a naturally occurring phospholipid containing polyunsaturated fatty acid. The formation of an aldehyde product 1-palmitoyl-2-(5′-oxo-valeroyl)-phosphatidylcholine (POVPC) was monitored using electrospray ionization MS. Carnosine reduced the formation of POVPC to the level observed without oxygen bubbling indicating that it protects phospholipids from oxidation (Fig. 3). Hence, a combination of metal chelating and radical scavenging properties makes these dipeptides important and universal antioxidants in vivo.
Fig. 3. Carnosine protects phospholipid from oxidation by molecular oxygen.
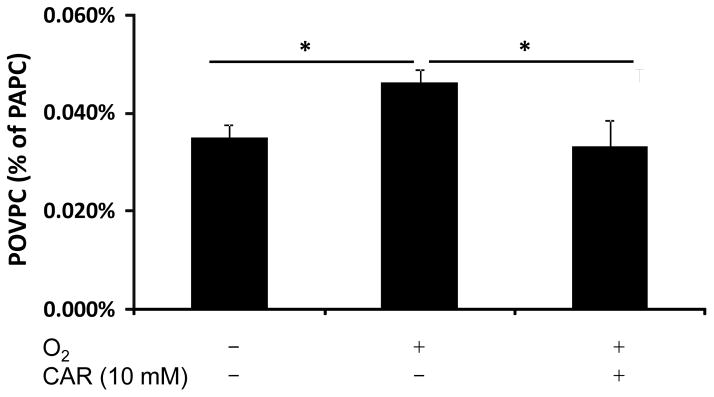
Oxygen was bubbled at a rate of 30 ml/min through a solution containing 1-palmitoyl-2-arachidonyl phosphatidylcholine (PAPC; 25μg/ml) in 3 ml of phosphate-buffered saline (KH2PO4 1.06 mM, NaCl 155.17 mM, Na2HPO4 2.97 mM, pH 7.4) for 1h at 37°C in the presence and absence of 10 mM carnosine. Formation of PAPC oxidation product 1-palmitoyl-2-(5′-oxo-valeroyl)-phosphatidylcholine (POVPC) was determined by ESI mass-spectrometry and POVPC concentration is expressed as the percentage of the parent PAPC ion. *, P<0.05.
8. Reaction of histidine dipeptides with aldehydes
In addition to radical quenching, carnosine has also been found to protect proteins from attack by biologically reactive aldehydes, e.g. aldose sugars [104, 105], malondialdehyde [106], methylglyoxal [107, 108], glycolaldehyde [105], HNE [109], acrolein [110], and ONE [111] in vitro. In the early literature, carnosine was presumed to act as a sacrificial sink due to its structural similarity with liable proteins [104]. This view is supported by the studies of Zhou and Decker, which showed that carnosine quenches α,β-unsaturated aldehydes, such as HNE and trans-2-hexenal [112], and that the quenching ability of carnosine is much higher than its constituent amino acids separately. Products of the reaction of carnosine with aldehydes, such as HNE and acrolein, have been identified (Fig. 2).
The reaction of HNE and carnosine has been investigated using both mass-spectrometry and NMR [109, 113–115] and summarized in [116]. Briefly, the reaction between carnosine and HNE follows two pathways and leads to the formation of two major products (Fig. 2). The first pathway starts with Schiff base formation between the aldehyde functional group and the amino group of the β-alanyl residue, followed by the formation of a 13-member cyclic adduct (macrocyclic CAR-HNE) by ring closure. In the second pathway the reactive C3 of the aldehyde links with Nτ-imidazole nitrogen of the histidine residue by Michael adduction, and then a 5- member ring derivative (hemiacetal CAR-HNE) is formed. The two pathways could occur sequentially, i.e. the second pathway may follow the first one [113]. Due to the lack of a hydroxyl group, the second pathway is not possible for trans-2-nonenal, and therefore this aldehyde reacts with carnosine to form the 13-member cyclic adduct but not the hemi-acetal derivative [113].
In the first pathway (starting with Schiff base formation), the amino group of the β-alanyl residue acts as a catalyst, which explains the higher quenching ability of carnosine than histidine [113]. This catalyzing role is supported by the observation that N-acetyl-carnosine, which lacks free amino group of the β-alanyl residue, has significantly lower quenching ability than carnosine; but quenching ability of anserine, the Nπ-methyl derivative of carnosine, is similar to that of carnosine [113]. Molecular modeling of the Schiff base intermediate shows that the “folded” conformer is the reactive conformation, and aldehyde quenching ability can be optimized by structural modification of carnosine to increase the proportion of this reactive conformation [117].
Among carnosine-HNE reaction products, the hemiacetal CAR-HNE has drawn great interest because it is the only carnosine-aldehyde reaction product that has been detected and quantitatively measured in biological samples. This conjugate has been detected in HNE-spiked rat skeletal muscle [118], urine of Zucker obese rat [119], and apoE-null mice [120] suggesting that hemiacetal CAR-HNE could be used as a specific biomarker of lipid peroxidation.
The reaction between acrolein and carnosine or homocarnosine has been investigated using mass spectrometry [121]. One, two, or three acrolein molecules can be linked to nitrogens of one carnosine molecule (Fig. 2), and different reaction products are generated after different reaction times. In addition to Michael addition and Schiff base formation, aldol condensation and dehydration are also involved in the reaction between carnosine and acrolein. The reaction of homocarnosine with acrolein also follows the same pathway as the carnosine-acrolein reaction [121].
Although reactions between α,β-unsaturated aldehydes and histidine dipeptides have been studied intensively, reactions between histidine dipeptides and carbohydrate-derived aldehydes, such as methylglyoxal, glycolaldehyde, and glyoxal have attracted less attention. However, the reactions between histidine dipeptides and these aldehydes are implied in studies showing that carnosine protects proteins from attack by methylglyoxal [107, 108] and glycolaldehyde [105]. Due to the biological importance of carbohydrate-derived aldehydes, it is important to identify the products and elucidate reaction mechanisms of histidine dipeptides and these aldehydes. To study the chemical reaction between methylglyoxal and carnosine, we monitored the UV spectra of a reaction mixture containing methylglyoxal and carnosine. Incubation of carnosine with methylglyoxal led to a large increase in the absorbance at 280nm. However, incubation of methylglyoxal or carnosine alone did not produce this peak, indicating that the peak at 280 nm is characteristic of the product of the reaction between carnosine and methylglyoxal (Fig. 4). A similar spectroscopic change was observed when carnosine was incubated with glyoxal and glycolaldehyde (not shown), indicating a common structural motif is present in the product formed by these reactions. In a separate experiment, we incubated methylglyoxal with glutathione (GSH) in the absence and presence of carnosine and monitored the consumption of GSH using thiol-specific Ellman’s reagent (5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB) [122]. In the absence of carnosine, GSH was consumed due to reaction with methylglyoxal. However, addition of carnosine slowed down GSH consumption in a dose-dependent manner (Fig. 5). These results indicate that within its physiological concentration range carnosine could prevent cellular GSH from depletion by carbohydrate-derived aldehydes.
Fig. 4. Reaction of carnosine with methylglyoxal.
A, UV spectra of a reaction mixture containing methylglyoxal (1 mM) and carnosine (5 and 40 mM) after 12h of incubation. B, Time course of the reaction between methylglyoxal and carnosine monitored at 280 nm. Carnosine at several concentrations was incubated with 1mM methylglyoxal in 150 mM potassium phosphate buffer, pH 7.4, at 37°C. Change in absorbance was acquired every 60 min.
Fig. 5. Carnosine protects glutathione from methylglyoxal-mediated depletion.
Carnosine at indicated concentrations was incubated with 0.2 mM GSH and 0.2 mM methylglyoxal in 150 mM potassium phosphate buffer, pH 7.4, at 37 °C. Time-dependent consumption of free GSH was monitored spectrophotometrically after the addition of DTNB.
9. Mechanisms of in vivo action of carnosine: pre-clinical and clinical studies
As described above, increased generation of reactive oxygen and carbonyl species has been implicated in the comorbidity of numerous pathological conditions including ischemia, lung injury, diabetes, atherosclerosis, and cardiac dysfunction. A unique nucleophilic structure of carnosine allows this dipeptide to alleviate oxidative stress by scavenging singlet oxygen, hydroxyl and superoxide radicals and bind reactive aldehydes. Given this rationale, carnosine has been investigated in multiple disease states to determine potential benefits due to its antioxidant properties and its function in scavenging free radicals and aldehydes.
Carnosine has been shown to improve functional recovery of rat hearts after ischemia-reperfusion injury, where its positive effect has been attributed to its capacity to avidly intercept singlet oxygen formation generated upon post ischemic reperfusion [103]. Also, studies with the murine models of cerebral ischemia have demonstrated that post and pre-treatment with carnosine reduced infarct size when administered both before and after induction of ischemia. The reduction in the infarct size was accompanied by decreased reactive oxygen species (ROS) levels in the ischemic brain and preservation of normal glutathione levels, suggesting that antioxidant properties of carnosine are at least partly responsible for its neuroprotective effect against cerebral ischemia [123].
Administration of carnosine to obese Zucker rats attenuated the development of hypertension, weight gain and dyslipidemia, which were accompanied by reduced indices of oxidative burden, namely, reduced urinary content of oxidative stress markers 8-epi-PGF2α and AGEs, and protein-carbonyl content in kidney [124]. Zucker rats had a 6-fold higher level of carnosine-HNE adduct in urine compared to lean counterparts, suggesting that endogenous carnosine plays a role in elimination of this lipid oxidation-derived aldehyde. Despite the combined level of carnosine and histidine conjugates of HNE was 10-fold lower than the level of its glutathione-derived metabolite, 1,4-dihydroxynonane-mercapturic acid, conjugation with carnosine may play an important role in specific niches, where glutathione is unavailable. The observation prompted the authors to propose that HNE adducts with histidine-containing peptides might be biomarkers of lipid-derived carbonyl stress.
Similarly, the non-hydrolyzable and membrane-permeable analog of carnosine, octyl-D-carnosine, has also been shown to attenuate the development of atherosclerosis in apoE-null mice fed a Western diet. Supplementation with octyl-D-carnosine was associated with reduced protein carbonylation, circulating and tissue ALEs, expression of receptors for these products, and systemic and tissue oxidative stress. Importantly, octyl-D-carnosine feeding led to a 2.5-fold increase of CAR-HNE conjugate in the urine, indicating that the beneficial effect of carnosine could at least in part be attributed to the removal of toxic carbonyl species [120]. These studies raise an interesting possibility that treatment with carnosine or its analogues could prevent or retard the development of atherosclerotic lesions in humans.
On the other hand, in STZ-induced rats, a model of type I diabetes, carnosine prevented podocyte loss and apoptosis of glomerular cells but failed to prevent accumulation of AGEs (Nε-(carboxymethyl)-lysine and methylglyoxal-modified proteins) and nitrotyrosine-containing proteins in kidney cortex [125]. Similarly, oral carnosine treatment prevented retinal vascular damage after 6 months of experimental hyperglycemia; however, the protection was not accompanied ROS- or AGE-inhibition, but associated with significant induction of Hsp27 in activated glial cells and normalization of increased Ang-2 levels in diabetic retinas [126]. These results indicate that the beneficial effects of carnosine may not be limited to its antioxidant and carbonyl-scavenging properties, but may be attributed to gene-induction effects, e.g., induction of protective Hsp27 in activated glial cells and normalization of hyperglycemia-induced Ang-2, or potentially its pro-histaminic properties.
As described in the previous sections, carnosine is hydrolyzed by carnosinase present in plasma and different tissues, and the liberated histidine may be converted to histamine [127]. Because carnosine can act a reservoir of histidine, several investigators suggested that carnosine might control glucose and lipid metabolism through the facilitation of sympathetic nervous system driven by histamine neurons through the H1 receptor activity. Whether this mechanism contributes to the effect of carnosine may be condition and tissue-dependent. Carnosine-induced protection of PC-12 cells from neurotoxicity of N-methyl-D-aspartate was diminished by H1 histamine receptor antagonist pyrilamine and by histidine decarboxylase inhibition [128], implicating histamine generation as part of the mechanism of the neuroprotective effect of carnosine. It has been suggested that histamine generated from endogenous carnosine released from muscle during exercise reduces sympathetic nerves activity, which leads to decrease in blood pressure and blood glucose [129]. Similarly, the protective effect of carnosine against ischemia/reperfusion-induced renal injury in rats could be blocked by histamine H3 receptor antagonist thioperamide and corroborated by agonist R-α-methylhistamine. These observations support the hypothesis that metabolic conversion to histamine plays significant role in the action of carnosine [130].
The data supporting the involvement of histamine as a mediator of carnosine effect are counteracted by experimental evidence showing that the pro-histaminic pathway could not be the only mechanism for the protective effect of carnosine. Anti-obeseogeneic and anti-atherogenic effects of D-carnosine (β-alanine-D-histidine, a non-hydrolyzable enantiomer) or octyl-D-carnosine, which are not precursors of histamine, argue in favor of the carbonyl quenching mechanism of action of carnosine. In addition, the pro-histaminic pathway is tissue-dependent. Indeed, carnosine is an inhibitor of histidine decarboxylase [131], and it has been shown that histamine release from mast cell is decreased after carnosine treatment [132]. Therefore, antioxidant and carbonyl-scavenging activity, as well as pro-histaminic properties may contribute to the effect of carnosine in different tissues under a variety of physiological and pathological conditions. Clearly, additional studies are necessary in order to understand the complexity and multitude of biological effects of a multifaceted peptide such as carnosine.
In a number of human and animal studies, the beneficial effects of carnosine supplementation or genetic association between polymorphisms in the carnosinase gene have been demonstrated; however, the mechanism remains unknown or incompletely understood. These include type II diabetes and its complications, and mental disorders, such as autism [133]. In human diabetic patients, it was found that carnosine levels were decreased in the gastrocnemius muscles of type 2, but not type 1, diabetics compared with healthy subjects [134] providing a rationale for using carnosine in the treatment or prevention of diabetic complications. Administration of local and intraperitoneal carnosine has been shown to enhance wound healing and promote granulation, increase tensile strength, collagen deposition and hyroxyproline content in the wound area in diabetic mice [135]. Supplementation of type 2 diabetic db/db mice with carnosine delayed the development of hyperglycemia, increased fasting insulin levels and preservation of the β-cell mass. Conversely, increased levels of carnosine-degrading enzyme carnosinase correlated with increased fasting plasma glucose, increased HbA1C levels, and lower fasting insulin levels [136].
These findings are consistent with the results of genetic association studies on the role of carnosinase in the development of diabetic complications in humans. Two independent groups have found that polymorphisms in the carnosinase gene are associated with lower serum carnosinase activity as well as the absence of diabetic nephropathy in European Caucasians, Arabs and European Americans [137, 138].
In summary, carnosine supplementation has been found to be beneficial in multiple conditions in human and animal studies including diabetes and its complications, obesity, atherosclerosis, and neurodegenerative and psychological disorders. In addition, polymorphisms in the carnosinase gene (CDNP1), leading to higher enzyme activity in plasma and by inference, higher carnosine turnover, are associated with protection from diabetic nephropathy [139]. Whether these effects are due to antioxidant or aldehyde-scavenging functions of carnosine, its pro-histaminic properties, or other functions, such as regulation of gene expression is not clear in all cases. Further studies are required to connect in vitro observations with in vivo data to establish the mechanistic basis of carnosine action.
10. Conclusion
Histidine dipeptides are present in millimolar concentrations in skeletal muscle and other tissues such as brain, heart, spleen and kidney. These peptides could be involved in pH buffering in the muscle, antioxidant defense, chelation of copper ions, and scavenging of aldehydes. Antioxidant and aldehyde-scavenging properties of these peptides make them efficient agents for combating oxidative stress. Carnosine supplementation has been shown to prevent atherosclerosis, delay the development of diabetic complications and enhance wound healing. Hence, carnosine and other histidine dipeptides promise to be potentially useful therapeutic agents for conditions characterized by carbonyl overload. Several clinical and preclinical trials testing the efficacy of these compounds are under way and additional trials are warranted to assess fully the efficacy of these peptides in prevention or treatment of human diseases associated with high rates of carbohydrate metabolism or oxidative stress.
Acknowledgments
This work was supported in part by the NIH grant GM103492 and HL-89372 (to O.A.B.) and an AHA Beginning-Grant–in-Aid (to S.P.B). We thank Dr. Aruni Bhatnagar for critical reading of the manuscript, and Dr. Jong Min Lee for assistance in implementation of oxygen bubbling.
The abbreviations used are
- HNE
4-hydroxy-trans-2-nonenal
- HHE
4-hydroxy-trans-2-hexenal
- ONE
4-oxo-trans-2-nonenal
- AGEs
advanced glycation end-products
- STZ
streptozotocin
- CN
carnosinase
- MS
mass spectrometry
- MS/MS
tandem MS
- NMR
nuclear magnetic resonance
- MRS
magnetic resonance spectroscopy
- HPLC
high-performance liquid chromatography
- CE
capillary electrophoresis
- POVPC
1-palmitoyl-2-(5′-oxo-valeroyl)-phosphatidylcholine
- GSH
glutathione
- ROS
reactive oxygen species
Footnotes
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zhengzhi Xie, Email: zhengzhi.xie@louisville.edu.
Shahid P. Baba, Email: spbaba01@louisville.edu.
Brooke R. Sweeney, Email: brswee01@louisville.edu.
Oleg A. Barski, Email: o.barski@louisville.edu.
References
- 1.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Negre-Salvayre A, Coatrieux C, Ingueneau C, Salvayre R. Advanced lipid peroxidation end products in oxidative damage to proteins. Potential role in diseases and therapeutic prospects for the inhibitors. Br J Pharmacol. 2008;153:6–20. doi: 10.1038/sj.bjp.0707395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman HJ. Reactive oxygen species and alpha, beta-unsaturated aldehydes as second messengers in signal transduction. Ann N Y Acad Sci. 2010;1203:35–44. doi: 10.1111/j.1749-6632.2010.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Zwart LL, Meerman JHN, Commandeur JNM, Vermeulen NPE. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic Biol Med. 1999;26:202–226. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Ho CT. Flavour chemistry of methylglyoxal and glyoxal. Chem Soc Rev. 2012;41:4140–4149. doi: 10.1039/c2cs35025d. [DOI] [PubMed] [Google Scholar]
- 6.Tsimikas S, Kiechl S, Willeit J, Mayr M, Miller ER, Kronenberg F, Xu Q, Bergmark C, Weger S, Oberhollenzer F, Witztum JL. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J Am Coll Cardiol. 2006;47:2219–2228. doi: 10.1016/j.jacc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Tsimikas S, Witztum JL. Measuring circulating oxidized low-density lipoprotein to evaluate coronary risk. Circulation. 2001;103:1930–1932. doi: 10.1161/01.cir.103.15.1930. [DOI] [PubMed] [Google Scholar]
- 8.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 9.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50(Suppl):S207–212. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava S, Vladykovskaya E, Barski OA, Spite M, Kaiserova K, Petrash JM, Chung SS, Hunt G, Dawn B, Bhatnagar A. Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein E-null mice. Circ Res. 2009;105:793–802. doi: 10.1161/CIRCRESAHA.109.200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 13.Miyazawa T, Nakagawa K, Shimasaki S, Nagai R. Lipid glycation and protein glycation in diabetes and atherosclerosis. Amino Acids. 2012;42:1163–1170. doi: 10.1007/s00726-010-0772-3. [DOI] [PubMed] [Google Scholar]
- 14.Vander Jagt DL. Methylglyoxal, diabetes mellitus and diabetic complications. Drug Metabol Drug Interact. 2008;23:93–124. doi: 10.1515/dmdi.2008.23.1-2.93. [DOI] [PubMed] [Google Scholar]
- 15.Rabbani N, Thornalley PJ. Methylglyoxal glyoxalase 1 and the dicarbonyl proteome. Amino Acids. 2012;42:1133–1142. doi: 10.1007/s00726-010-0783-0. [DOI] [PubMed] [Google Scholar]
- 16.Seki S, Kitada T, Yamada T, Sakaguchi H, Nakatani K, Wakasa K. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37:56–62. doi: 10.1016/s0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 17.Nair J, Srivatanakul P, Haas C, Jedpiyawongse A, Khuhaprema T, Seitz HK, Bartsch H. High urinary excretion of lipid peroxidation-derived DNA damage in patients with cancer-prone liver diseases. Mutat Res. 2010;683:23–28. doi: 10.1016/j.mrfmmm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Calabrese V, Cornelius C, Leso V, Trovato-Salinaro A, Ventimiglia B, Cavallaro M, Scuto M, Rizza S, Zanoli L, Neri S, Castellino P. Oxidative stress, glutathione status sirtuin and cellular stress response in type 2 diabetes. Biochim Biophys Acta. 2012;1822:729–736. doi: 10.1016/j.bbadis.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Ingram KH, Hill H, Moellering DR, Hill BG, Lara-Castro C, Newcomer B, Brandon LJ, Ingalls CP, Penumetcha M, Rupp JC, Garvey WT. Skeletal muscle lipid peroxidation and insulin resistance in humans. J Clin Endocrinol Metab. 2012;97:E1182–1186. doi: 10.1210/jc.2011-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grimsrud PA, Picklo MJ, Sr, Griffin TJ, Bernlohr DA. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Mol Cell Proteomics. 2007;6:624–637. doi: 10.1074/mcp.M600120-MCP200. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Dang TN, Arseneault M, Ramassamy C. Role of by-products of lipid oxidation in Alzheimer’s disease brain: a focus on acrolein. J Alzheimers Dis. 2010;21:741–756. doi: 10.3233/JAD-2010-100405. [DOI] [PubMed] [Google Scholar]
- 22.LoPachin RM, Gavin T, Petersen DR, Barber DS. Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation. Chem Res Toxicol. 2009;22:1499–1508. doi: 10.1021/tx900147g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciobica A, Padurariu M, Dobrin I, Stefanescu C, Dobrin R. Oxidative stress in schizophrenia - focusing on the main markers. Psychiatr Danub. 2011;23:237–245. [PubMed] [Google Scholar]
- 24.Adibhatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 25.Srivastava S, Dixit BL, Cai J, Sharma S, Hurst HE, Bhatnagar A, Srivastava SK. Metabolism of lipid peroxidation product, 4-hydroxynonenal (HNE) in rat erythrocytes: role of aldose reductase. Free Radic Biol Med. 2000;29:642–651. doi: 10.1016/s0891-5849(00)00351-8. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava S, Watowich SJ, Petrash JM, Srivastava SK, Bhatnagar A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- 27.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 28.Smith CV, Jones DP, Guenthner TM, Lash LH, Lauterburg BH. Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol Appl Pharmacol. 1996;140:1–12. doi: 10.1006/taap.1996.0191. [DOI] [PubMed] [Google Scholar]
- 29.Thornalley PJ. Endogenous alpha-oxoaldehydes and formation of protein and nucleotide advanced glycation endproducts in tissue damage. Novartis Found Symp. 2007;285:229–243. doi: 10.1002/9780470511848.ch17. discussion 243–226. [DOI] [PubMed] [Google Scholar]
- 30.Desai K, Wu L. Methylglyoxal and advanced glycation endproducts: new therapeutic horizons? Recent Pat Cardiovasc Drug Discov. 2007;2:89–99. doi: 10.2174/157489007780832498. [DOI] [PubMed] [Google Scholar]
- 31.Burcham PC, Kaminskas LM, Fontaine FR, Petersen DR, Pyke SM. Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology. 2002;181–182:229–236. doi: 10.1016/s0300-483x(02)00287-1. [DOI] [PubMed] [Google Scholar]
- 32.Hipkiss AR. Carnosine and its possible roles in nutrition and health. Adv Food Nutr Res. 2009;57:87–154. doi: 10.1016/S1043-4526(09)57003-9. [DOI] [PubMed] [Google Scholar]
- 33.Aldini G, Facino RM, Beretta G, Carini M. Carnosine and related dipeptides as quenchers of reactive carbonyl species: from structural studies to therapeutic perspectives. Biofactors. 2005;24:77–87. doi: 10.1002/biof.5520240109. [DOI] [PubMed] [Google Scholar]
- 34.Boldyrev AA. Problems and perspectives in studying the biological role of carnosine. Biochemistry (Mosc) 2000;65:751–756. [PubMed] [Google Scholar]
- 35.Boldyrev AA, Severin SE. The histidine-containing dipeptides, carnosine and anserine: distribution, properties and biological significance. Adv Enzyme Regul. 1990;30:175–194. doi: 10.1016/0065-2571(90)90017-v. [DOI] [PubMed] [Google Scholar]
- 36.Zapp JA, Wilson DW. Quantitative studies of carnosine and anserine in mammalian muscle: II. The distribution of carnosine and anserine in various muscles of different species. J Biol Chem. 1938;126:19–27. [Google Scholar]
- 37.Crush KG. Carnosine and related substances in animal tissues. Comp Biochem Physiol. 1970;34:3–30. doi: 10.1016/0010-406x(70)90049-6. [DOI] [PubMed] [Google Scholar]
- 38.Artero C, Marti E, Biffo S, Mulatero B, Andreone C, Margolis FL, Fasolo A. Carnosine in the brain and olfactory system of amphibia and reptilia: a comparative study using immunocytochemical and biochemical methods. Neurosci Lett. 1991;130:182–186. doi: 10.1016/0304-3940(91)90392-7. [DOI] [PubMed] [Google Scholar]
- 39.Pisano JJ, Wilson JD, Cohen L, Abraham D, Udenfriend S. Isolation of gamma-aminobutyrylhistidine (homocarnosine) from brain. J Biol Chem. 1961;236:499–502. [PubMed] [Google Scholar]
- 40.Culbertson JY, Kreider RB, Greenwood M, Cooke M. Effects of beta-alanine on muscle carnosine and exercise performance: a review of the current literature. Nutrients. 2010;2:75–98. doi: 10.3390/nu2010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill CA, Harris RC, Kim HJ, Harris BD, Sale C, Boobis LH, Kim CK, Wise JA. Influence of beta-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids. 2007;32:225–233. doi: 10.1007/s00726-006-0364-4. [DOI] [PubMed] [Google Scholar]
- 42.Fritzson P. The catabolism of C14-labeled uracil, dihydrouracil, and beta-ureidopropionic acid in rat liver slices. J Biol Chem. 1957;226:223–228. [PubMed] [Google Scholar]
- 43.Horinishi H, Grillo M, Margolis FL. Purification and characterization of carnosine synthetase from mouse olfactory bulbs. J Neurochem. 1978;31:909–919. doi: 10.1111/j.1471-4159.1978.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 44.Dunnett M, Harris RC. Influence of oral beta-alanine and L-histidine supplementation on the carnosine content of the gluteus medius. Equine Vet J Suppl. 1999;30:499–504. doi: 10.1111/j.2042-3306.1999.tb05273.x. [DOI] [PubMed] [Google Scholar]
- 45.Bakardjiev A, Bauer K. Transport of beta-alanine and biosynthesis of carnosine by skeletal muscle cells in primary culture. Eur J Biochem. 1994;225:617–623. doi: 10.1111/j.1432-1033.1994.00617.x. [DOI] [PubMed] [Google Scholar]
- 46.Otani H, Okumura N, Hashida-Okumura A, Nagai K. Identification and characterization of a mouse dipeptidase that hydrolyzes L-carnosine. J Biochem. 2005;137:167–175. doi: 10.1093/jb/mvi016. [DOI] [PubMed] [Google Scholar]
- 47.Lenney JF, Peppers SC, Kucera-Orallo CM, George RP. Characterization of human tissue carnosinase. Biochem J. 1985;228:653–660. doi: 10.1042/bj2280653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teufel M, Saudek V, Ledig JP, Bernhardt A, Boularand S, Carreau A, Cairns NJ, Carter C, Cowley DJ, Duverger D, Ganzhorn AJ, Guenet C, Heintzelmann B, Laucher V, Sauvage C, Smirnova T. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J Biol Chem. 2003;278:6521–6531. doi: 10.1074/jbc.M209764200. [DOI] [PubMed] [Google Scholar]
- 49.Everaert I, Taes Y, De Heer E, Baelde H, Zutinic A, Yard B, Sauerhofer S, Vanhee L, Delanghe J, Aldini G, Derave W. Low plasma carnosinase activity promotes carnosinemia after carnosine ingestion in humans. Am J Physiol Renal Physiol. 2012;302:F1537–1544. doi: 10.1152/ajprenal.00084.2012. [DOI] [PubMed] [Google Scholar]
- 50.Teuscher NS, Shen H, Shu C, Xiang J, Keep RF, Smith DE. Carnosine uptake in rat choroid plexus primary cell cultures and choroid plexus whole tissue from PEPT2 null mice. J Neurochem. 2004;89:375–382. doi: 10.1111/j.1471-4159.2004.02333.x. [DOI] [PubMed] [Google Scholar]
- 51.Kamal MA, Jiang H, Hu Y, Keep RF, Smith DE. Influence of genetic knockout of Pept2 on the in vivo disposition of endogenous and exogenous carnosine in wild-type and Pept2 null mice. Am J Physiol Regul Integr Comp Physiol. 2009;296:R986–991. doi: 10.1152/ajpregu.90744.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gil-Agusti M, Esteve-Romero J, Carda-Broch S. Anserine and carnosine determination in meat samples by pure micellar liquid chromatography. J Chromatogr A. 2008;1189:444–450. doi: 10.1016/j.chroma.2007.11.075. [DOI] [PubMed] [Google Scholar]
- 53.Pappa-Louisi A, Agrafiotou P, Papachristos K. Retention modeling under organic modifier gradient conditions in ion-pair reversed-phase chromatography. Application to the separation of a set of underivatized amino acids. Anal Bioanal Chem. 2010;397:2151–2159. doi: 10.1007/s00216-009-3381-9. [DOI] [PubMed] [Google Scholar]
- 54.Dunnett M, Harris RC. Determination of carnosine and other biogenic imidazoles in equine plasma by isocratic reversed-phase ion-pair high-performance liquid chromatography. J Chromatogr. 1992;579:45–53. doi: 10.1016/0378-4347(92)80361-s. [DOI] [PubMed] [Google Scholar]
- 55.Nardiello D, Cataldi TRI. Determination of carnosine in feed and meat by high-performance anion-exchange chromatography with integrated pulsed amperometric detection. J Chromatogr A. 2004;1035:285–289. doi: 10.1016/j.chroma.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 56.Fekkes D, Voskuilen-Kooyman A, Jankie R, Huijmans J. Precise analysis of primary amino acids in urine by an automated high-performance liquid chromatography method: comparison with ion-exchange chromatography. J Chromatogr B Biomed Sci Appl. 2000;744:183–188. doi: 10.1016/s0378-4347(00)00234-6. [DOI] [PubMed] [Google Scholar]
- 57.Pappa-Louisi A, Nikitas P, Agrafiotou P, Papageorgiou A. Optimization of separation and detection of 6-aminoquinolyl derivatives of amino acids by using reversed-phase liquid chromatography with on line UV, fluorescence and electrochemical detection. Anal Chim Acta. 2007;593:92–97. doi: 10.1016/j.aca.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 58.Tsuruta YM, Kiyoshi, Inoue Hirofumi, Kosha Keiko, Date Yuuko, Okamura Nobuyuki, Eto Seiji, Kojima Eijiro. Sensitive determination of carnosine in urine by high-performance liquid chromatography using 4-(5,6-dimethoxy-2-phthalimidinyl)-2-methoxyphenylsulfonyl chloride as a fluorescent labeling reagent. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:327–332. doi: 10.1016/j.jchromb.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 59.Hu Q-yC, Xiao-hui, Bi Kai-shun, Yao Jin-ping. Content determination of carnosine by pre-column derivatization with reversed phase-high performance liquid chromatography. Shenyang Yaoke Daxue Xuebao. 2009;26:376–378. [Google Scholar]
- 60.Zoppa M, Gallo L, Zacchello F, Giordano G. Method for the quantification of underivatized amino acids on dry blood spots from newborn screening by HPLC-ESI-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:267–273. doi: 10.1016/j.jchromb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 61.Piraud M, Vianey-Saban C, Bourdin C, Acquaviva-Bourdain C, Boyer S, Elfakir C, Bouchu D. A new reversed-phase liquid chromatographic/tandem mass spectrometric method for analysis of underivatised amino acids: evaluation for the diagnosis and the management of inherited disorders of amino acid metabolism. Rapid Commun Mass Spectrom. 2005;19:3287–3297. doi: 10.1002/rcm.2197. [DOI] [PubMed] [Google Scholar]
- 62.Wei R, Li G, Seymour AB. High-throughput and multiplexed LC/MS/MRM method for targeted metabolomics. Anal Chem. 2010;82:5527–5533. doi: 10.1021/ac100331b. [DOI] [PubMed] [Google Scholar]
- 63.Piraud M, Vianey-Saban C, Petritis K, Elfakir C, Steghens JP, Bouchu D. Ion-pairing reversed-phase liquid chromatography/electrospray ionization mass spectrometric analysis of 76 underivatized amino acids of biological interest: a new tool for the diagnosis of inherited disorders of amino acid metabolism. Rapid Commun Mass Spectrom. 2005;19:1587–1602. doi: 10.1002/rcm.1957. [DOI] [PubMed] [Google Scholar]
- 64.Piraud M, Vianey-Saban C, Petritis K, Elfakir C, Steghens JP, Morla A, Bouchu D. ESI-MS/MS analysis of underivatised amino acids: a new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun Mass Spectrom. 2003;17:1297–1311. doi: 10.1002/rcm.1054. [DOI] [PubMed] [Google Scholar]
- 65.Kasicka V. Recent advances in capillary electrophoresis and capillary electrochromatography of peptides. Electrophoresis. 2003;24:4013–4046. doi: 10.1002/elps.200305660. [DOI] [PubMed] [Google Scholar]
- 66.Jiang C-hL, Kuan-Chyun, Tsai Tsun-Chung. Comparison of HPLC and CE to separate and quantify carnosine and anserin in meat product. Taiwan Nongye Huaxue Yu Shipin Kexue. 2003;41:294–299. [Google Scholar]
- 67.Ramautar R, Busnel JM, Deelder AM, Mayboroda OA. Enhancing the Coverage of the Urinary Metabolome by Sheathless Capillary Electrophoresis-Mass Spectrometry. Anal Chem. 2012;84:885–892. doi: 10.1021/ac202407v. [DOI] [PubMed] [Google Scholar]
- 68.Stanova A, Marak J, Rezeli M, Pager C, Kilar F, Kaniansky D. Analysis of therapeutic peptides in human urine by combination of capillary zone electrophoresis-electrospray mass spectrometry with preparative capillary isotachophoresis sample pretreatment. J Chromatogr A. 2011;1218:8701–8707. doi: 10.1016/j.chroma.2011.09.080. [DOI] [PubMed] [Google Scholar]
- 69.Oikawa A, Fujita N, Horie R, Saito K, Tawaraya K. Solid-phase extraction for metabolomic analysis of high-salinity samples by capillary electrophoresis-mass spectrometry. J Sep Sci. 2011;34:1063–1068. doi: 10.1002/jssc.201000890. [DOI] [PubMed] [Google Scholar]
- 70.Wakayama M, Aoki N, Sasaki H, Ohsugi R. Simultaneous Analysis of Amino Acids and Carboxylic Acids by Capillary Electrophoresis-Mass Spectrometry Using an Acidic Electrolyte and Uncoated Fused-Silica Capillary. Anal Chem. 2010;82:9967–9976. doi: 10.1021/ac1019039. [DOI] [PubMed] [Google Scholar]
- 71.Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, Esumi H, Soga T. Quantitative Metabolome Profiling of Colon and Stomach Cancer Microenvironment by Capillary Electrophoresis Time-of-Flight Mass Spectrometry. Cancer Res. 2009;69:4918–4925. doi: 10.1158/0008-5472.CAN-08-4806. [DOI] [PubMed] [Google Scholar]
- 72.Soga T, Kakazu Y, Robert M, Tomita M, Nishioka T. Qualitative and quantitative analysis of amino acids by capillary electrophoresis-electrospray ionization-tandem mass spectrometry. Electrophoresis. 2004;25:1964–1972. doi: 10.1002/elps.200305791. [DOI] [PubMed] [Google Scholar]
- 73.Stvolinskii SL, DD, MV, LT, PN, ZL, BAA Carnosine and anserine in working muscles-- study using proton NMR spectroscopy. Biokhimiia (Moscow, Russia) 1992;57:1317–1323. [PubMed] [Google Scholar]
- 74.Yoshioka Y, Oikawa H, Ehara S, Inoue T, Ogawa A, Kanbara Y, Kubokawa M. Noninvasive measurement of temperature and fractional dissociation of imidazole in human lower leg muscles using 1H-nuclear magnetic resonance spectroscopy. J Appl Physiol. 2005;98:282–287. doi: 10.1152/japplphysiol.00437.2004. [DOI] [PubMed] [Google Scholar]
- 75.Ozdemir MS, Reyngoudt H, De Deene Y, Sazak HS, Fieremans E, Delputte S, D’Asseler Y, Derave W, Lemahieu I, Achten E. Absolute quantification of carnosine in human calf muscle by proton magnetic resonance spectroscopy. Phys Med Biol. 2007;52:6781–6794. doi: 10.1088/0031-9155/52/23/001. [DOI] [PubMed] [Google Scholar]
- 76.Tallon MJ, Harris RC, Maffulli N, Tarnopolsky MA. Carnosine, taurine and enzyme activities of human skeletal muscle fibres from elderly subjects with osteoarthritis and young moderately active subjects. Biogerontology. 2007;8:129–137. doi: 10.1007/s10522-006-9038-6. [DOI] [PubMed] [Google Scholar]
- 77.Hitzig BM, Perng WC, Burt T, Okunieff P, Johnson DC. 1H-NMR measurement of fractional dissociation of imidazole in intact animals. Am J Physiol. 1994;266:R1008–1015. doi: 10.1152/ajpregu.1994.266.3.R1008. [DOI] [PubMed] [Google Scholar]
- 78.Gualano B, Everaert I, Stegen S, Artioli GG, Taes Y, Roschel H, Achten E, Otaduy MC, Lancha AH, Harris R, Derave W. Reduced muscle carnosine content in type 2, but not in type 1 diabetic patients. Amino Acids. 2012;43:21–24. doi: 10.1007/s00726-011-1165-y. [DOI] [PubMed] [Google Scholar]
- 79.Damon BM, Hsu AC, Stark HJ, Dawson MJ. The carnosine C-2 proton’s chemical shift reports intracellular pH in oxidative and glycolytic muscle fibers. Magn Reson Med. 2003;49:233–240. doi: 10.1002/mrm.10384. [DOI] [PubMed] [Google Scholar]
- 80.Bruhn H, Frahm J, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized Proton Nmr-Spectroscopy Using Stimulated Echoes - Applications to Human Skeletal-Muscle Invivo. Magn Reson Med. 1991;17:82–94. doi: 10.1002/mrm.1910170113. [DOI] [PubMed] [Google Scholar]
- 81.Baguet A, Everaert I, De Naeyer H, Reyngoudt H, Stegen S, Beeckman S, Achten E, Vanhee L, Volkaert A, Petrovic M, Taes Y, Derave W. Effects of sprint training combined with vegetarian or mixed diet on muscle carnosine content and buffering capacity. Eur J Appl Physiol. 2011;111:2571–2580. doi: 10.1007/s00421-011-1877-4. [DOI] [PubMed] [Google Scholar]
- 82.Jung Y, Lee J, Kwon J, Lee K-S, Ryu DH, Hwang G-S. Discrimination of the Geographical Origin of Beef by 1H NMR-Based Metabolomics. J Agric Food Chem. 2010;58:10458–10466. doi: 10.1021/jf102194t. [DOI] [PubMed] [Google Scholar]
- 83.Chikayama E, Sekiyama Y, Okamoto M, Nakanishi Y, Tsuboi Y, Akiyama K, Saito K, Shinozaki K, Kikuchi J. Statistical Indices for Simultaneous Large-Scale Metabolite Detections for a Single NMR Spectrum. Anal Chem. 2010;82:1653–1658. doi: 10.1021/ac9022023. [DOI] [PubMed] [Google Scholar]
- 84.Parkhouse WS, McKenzie DC, Hochachka PW, Ovalle WK. Buffering capacity of deproteinized human vastus lateralis muscle. J Appl Physiol. 1985;58:14–17. doi: 10.1152/jappl.1985.58.1.14. [DOI] [PubMed] [Google Scholar]
- 85.Everaert I, Mooyaart A, Baguet A, Zutinic A, Baelde H, Achten E, Taes Y, De Heer E, Derave W. Vegetarianism, female gender and increasing age, but not CNDP1 genotype, are associated with reduced muscle carnosine levels in humans. Amino Acids. 2011;40:1221–1229. doi: 10.1007/s00726-010-0749-2. [DOI] [PubMed] [Google Scholar]
- 86.Harris RC, Wise JA, Price KA, Kim HJ, Kim CK, Sale C. Determinants of muscle carnosine content. Amino Acids. 2012;43:5–12. doi: 10.1007/s00726-012-1233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Derave W, Ozdemir MS, Harris RC, Pottier A, Reyngoudt H, Koppo K, Wise JA, Achten E. beta-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J Appl Physiol. 2007;103:1736–1743. doi: 10.1152/japplphysiol.00397.2007. [DOI] [PubMed] [Google Scholar]
- 88.Freeman HC, Szymanski JJ. Model compounds for metal–protein interaction: the crystal structure of the copper(II) complex of β-alanyl-L-histidine (carnosine) Chem Commun (London) 1965:598–599. [Google Scholar]
- 89.Brown CE, Antholine WE. Multiple Forms of the Cobalt(Ii)-Carnosine Complex. Biochem Biophys Res Commun. 1979;88:529–536. doi: 10.1016/0006-291x(79)92080-1. [DOI] [PubMed] [Google Scholar]
- 90.Brown CE, Margolis FL, Williams TH, Pitcher RG, Elgar G. Carnosine in Olfaction - Proton Magnetic-Resonance Spectral Evidence for Tissue-Specific Carnosine Binding-Sites. Neurochem Res. 1977;2:555–579. doi: 10.1007/BF00966015. [DOI] [PubMed] [Google Scholar]
- 91.Brown CE, Antholine WE. Chelation Chemistry of Carnosine - Evidence That Mixed Complexes May Occur Invivo. J Phys Chem. 1979;83:3314–3319. [Google Scholar]
- 92.Torreggiani A, Trinchero A, Tamba M, Fini G. Vibrational characterisation and biological activity of carnosine and its metal complexes. Ital J Biochem. 2003;52:87–97. [PubMed] [Google Scholar]
- 93.Baran EJ. Metal complexes of carnosine. Biochemistry (Mosc) 2000;65:789–797. [PubMed] [Google Scholar]
- 94.Cho CH, Luk CT, Ogle CW. The membrane-stabilizing action of zinc carnosine (Z-103) in stress-induced gastric ulceration in rats. Life Sci. 1991;49:PL189–194. doi: 10.1016/0024-3205(91)90321-2. [DOI] [PubMed] [Google Scholar]
- 95.Yoshikawa T, Naito Y, Tanigawa T, Yoneta T, Kondo M. The antioxidant properties of a novel zinc-carnosine chelate compound, N-(3-aminopropionyl)-L-histidinato zinc. Biochim Biophys Acta. 1991;1115:15–22. doi: 10.1016/0304-4165(91)90005-2. [DOI] [PubMed] [Google Scholar]
- 96.Seiki M, Aita H, Ueki S, Yoneta T, Takemasa T, Hori Y, Morita H, Chaki K, Tagashira E. Effect of Z-103 on Wound-Healing by Dermal Incision in Guinea-Pigs. Folia Pharmacologica Japonica. 1992;100:165–172. doi: 10.1254/fpj.100.165. [DOI] [PubMed] [Google Scholar]
- 97.Matsukura T, Tanaka H. Applicability of zinc complex of L-carnosine for medical use. Biochemistry (Mosc) 2000;65:817–823. [PubMed] [Google Scholar]
- 98.Babizhayev MA, Seguin MC, Gueyne J, Evstigneeva RP, Ageyeva EA, Zheltukhina GA. L-Carnosine (Beta-Alanyl-L-Histidine) and Carcinine (Beta-Alanylhistamine) Act as Natural Antioxidants with Hydroxyl-Radical-Scavenging and Lipid-Peroxidase Activities. Biochem J. 1994;304:509–516. doi: 10.1042/bj3040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kohen R, Yamamoto Y, Cundy KC, Ames BN. Antioxidant Activity of Carnosine, Homocarnosine, and Anserine Present in Muscle and Brain. Proc Natl Acad Sci U S A. 1988;85:3175–3179. doi: 10.1073/pnas.85.9.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mozdzan M, Szemraj J, Rysz J, Nowak D. Antioxidant properties of carnosine re-evaluated with oxidizing systems involving iron and copper ions. Basic Clin Pharmacol Toxicol. 2005;96:352–360. doi: 10.1111/j.1742-7843.2005.pto_03.x. [DOI] [PubMed] [Google Scholar]
- 101.Egorov S, Kurella EG, Boldyrev AA, Krasnovsky AA., Jr Quenching of singlet molecular oxygen by carnosine and related antioxidants. Monitoring 1270-nm phosphorescence in aqueous media. Biochem Mol Biol Int. 1997;41:687–694. doi: 10.1080/15216549700201731. [DOI] [PubMed] [Google Scholar]
- 102.Hartman PE, Hartman Z, Ault KT. Scavenging of singlet molecular oxygen by imidazole compounds: high and sustained activities of carboxy terminal histidine dipeptides and exceptional activity of imidazole-4-acetic acid. Photochem Photobiol. 1990;51:59–66. doi: 10.1111/j.1751-1097.1990.tb01684.x. [DOI] [PubMed] [Google Scholar]
- 103.Lee JW, Miyawaki H, Bobst EV, Hester JD, Ashraf M, Bobst AM. Improved functional recovery of ischemic rat hearts due to singlet oxygen scavengers histidine and carnosine. J Mol Cell Cardiol. 1999;31:113–121. doi: 10.1006/jmcc.1998.0850. [DOI] [PubMed] [Google Scholar]
- 104.Hipkiss AR, Michaelis J, Syrris P, Kumar S, Lam Y. Carnosine Protects Proteins against in-Vitro Glycation and Cross-Linking. Biochem Soc Trans. 1994;22:S399–S399. doi: 10.1042/bst022399s. [DOI] [PubMed] [Google Scholar]
- 105.Ukeda H, Hasegawa Y, Harada Y, Sawamura M. Effect of carnosine, related compounds on the inactivation of human Cu, Zn-superoxide dismutase by modification of fructose and glycolaldehyde. Biosci Biotechnol Biochem. 2002;66:36–43. doi: 10.1271/bbb.66.36. [DOI] [PubMed] [Google Scholar]
- 106.Hipkiss AR, Preston JE, Himswoth DTM, Worthington VC, Abbot NJ. Protective effects of carnosine against malondialdehyde-induced toxicity towards cultured rat brain endothelial cells. Neurosci Lett. 1997;238:135–138. doi: 10.1016/s0304-3940(97)00873-2. [DOI] [PubMed] [Google Scholar]
- 107.Hipkiss AR, Chana H. Carnosine protects proteins against methylglyoxal-mediated modifications. Biochem Biophys Res Commun. 1998;248:28–32. doi: 10.1006/bbrc.1998.8806. [DOI] [PubMed] [Google Scholar]
- 108.Pietkiewicz J, Bronowicka-Szydelko A, Dzierzba K, Danielewicz R, Gamian A. Glycation of the Muscle-Specific Enolase by Reactive Carbonyls: Effect of Temperature and the Protection Role of Carnosine. Pirydoxamine and Phosphatidylserine, Protein J. 2011;30:149–158. doi: 10.1007/s10930-011-9307-3. [DOI] [PubMed] [Google Scholar]
- 109.Liu YH, Xu GZ, Sayre LM. Carnosine inhibits (E)-4-hydroxy-2-nonenal-induced protein cross-linking: Structural characterization of carnosine-HNE adducts. Chem Res Toxicol. 2003;16:1589–1597. doi: 10.1021/tx034160a. [DOI] [PubMed] [Google Scholar]
- 110.Burcham PC, Kerr PG, Fontaine F. The antihypertensive hydralazine is an efficient scavenger of acrolein. Redox Rep. 2000;5:47–49. doi: 10.1179/rer.2000.5.1.47. [DOI] [PubMed] [Google Scholar]
- 111.Zhu X, Gallogly MM, Mieyal JJ, Anderson VE, Sayre LM. Covalent cross-linking of glutathione and carnosine to proteins by 4-oxo-2-nonenal. Chem Res Toxicol. 2009;22:1050–1059. doi: 10.1021/tx9000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou S, Decker EA. Ability of carnosine and other skeletal muscle components to quench unsaturated aldehydic lipid oxidation products. J Agric Food Chem. 1999;47:51–55. doi: 10.1021/jf980780j. [DOI] [PubMed] [Google Scholar]
- 113.Aldini G, Carini M, Beretta G, Bradamante S, Facino RM. Carnosine is a quencher of 4- hydroxy-nonenal: through what mechanism of reaction? Biochem Biophys Res Commun. 2002;298:699–706. doi: 10.1016/s0006-291x(02)02545-7. [DOI] [PubMed] [Google Scholar]
- 114.Aldini G, Granata P, Carini M. Detoxification of cytotoxic alpha, beta-unsaturated aldehydes by carnosine: characterization of conjugated adducts by electrospray ionization tandem mass spectrometry and detection by liquid chromatography/mass spectrometry in rat skeletal muscle. J Mass Spectrom. 2002;37:1219–1228. doi: 10.1002/jms.381. [DOI] [PubMed] [Google Scholar]
- 115.Liu YH, Xu GZ, Sayre LM. Carnosine inhibits (E)-4-hydroxy-2-nonenal-induced protein cross-linking: Structural characterization of carnosine-HNE adducts. Abstracts of Papers of the American Chemical Society. 2001;222:U291–U291. doi: 10.1021/tx034160a. [DOI] [PubMed] [Google Scholar]
- 116.Guiotto A, Calderan A, Ruzza P, Borin G. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12:2293–2315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- 117.Vistoli G, Orioli M, Pedretti A, Regazzoni L, Canevotti R, Negrisoli G, Carini M, Aldini G. Design, synthesis, and evaluation of carnosine derivatives as selective and efficient sequestering agents of cytotoxic reactive carbonyl species. Chem Med Chem. 2009;4:967–975. doi: 10.1002/cmdc.200800433. [DOI] [PubMed] [Google Scholar]
- 118.Orioli M, Aldini G, Beretta G, Facino RM, Carini M. LC-ESI-MS/MS determination of 4- hydroxy-trans-2-nonenal Michael adducts with cysteine and histidine-containing peptides as early markers of oxidative stress in excitable tissues. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:109–118. doi: 10.1016/j.jchromb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 119.Orioli M, Aldini G, Benfatto MC, Facino RM, Carini M. HNE Michael adducts to histidine and histidine-containing peptides as biomarkers of lipid-derived carbonyl stress in urines: LC-MS/MS profiling in Zucker obese rats. Anal Chem. 2007;79:9174–9184. doi: 10.1021/ac7016184. [DOI] [PubMed] [Google Scholar]
- 120.Menini S, Iacobini C, Ricci C, Scipioni A, Blasetti Fantauzzi C, Giaccari A, Salomone E, Canevotti R, Lapolla A, Orioli M, Aldini G, Pugliese G. D-carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation. Br J Pharmacol. 2012;166:1344–1356. doi: 10.1111/j.1476-5381.2012.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Carini M, Aldini G, Beretta G, Arlandini E, Facino RM. Acrolein-sequestering ability of endogenous dipeptides: characterization of carnosine and homocarnosine/acrolein adducts by electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2003;38:996–1006. doi: 10.1002/jms.517. [DOI] [PubMed] [Google Scholar]
- 122.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 123.Rajanikant GK, Zemke D, Senut MC, Frenkel MB, Chen AF, Gupta R, Majid A. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke. 2007;38:3023–3031. doi: 10.1161/STROKEAHA.107.488502. [DOI] [PubMed] [Google Scholar]
- 124.Aldini G, Orioli M, Rossoni G, Savi F, Braidotti P, Vistoli G, Yeum KJ, Negrisoli G, Carini M. The carbonyl scavenger carnosine ameliorates dyslipidaemia and renal function in Zucker obese rats. J Cell Mol Med. 2011;15:1339–1354. doi: 10.1111/j.1582-4934.2010.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Riedl E, Pfister F, Braunagel M, Brinkkotter P, Sternik P, Deinzer M, Bakker SJL, Henning RH, van den Born J, Kramer BK, Navis G, Hammes HP, Yard B, Koeppel H. Carnosine Prevents Apoptosis of Glomerular Cells and Podocyte Loss in STZ Diabetic Rats. Cell Physiol Biochem. 2011;28:279–288. doi: 10.1159/000331740. [DOI] [PubMed] [Google Scholar]
- 126.Pfister F, Riedl E, Wang Q, vom Hagen F, Deinzer M, Harmsen MC, Molema G, Yard B, Feng YX, Hammes HP. Oral Carnosine Supplementation Prevents Vascular Damage in Experimental Diabetic Retinopathy. Cell Physiol Biochem. 2011;28:125–136. doi: 10.1159/000331721. [DOI] [PubMed] [Google Scholar]
- 127.Flancbaum L, Fitzpatrick JC, Brotman DN, Marcoux AM, Kasziba E, Fisher H. The presence and significance of carnosine in histamine-containing tissues of several mammalian species. Agents Actions. 1990;31:190–196. doi: 10.1007/BF01997607. [DOI] [PubMed] [Google Scholar]
- 128.Shen Y, Fan Y, Dai H, Fu Q, Hu W, Chen Z. Neuroprotective effect of carnosine on necrotic cell death in PC12 cells. Neurosci Lett. 2007;414:145–149. doi: 10.1016/j.neulet.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 129.Nagai K, Tanida M, Niijima A, Tsuruoka N, Kiso Y, Horii Y, Shen J, Okumura N. Role of L-carnosine in the control of blood glucose, blood pressure, thermogenesis, and lipolysis by autonomic nerves in rats: involvement of the circadian clock and histamine. Amino Acids. 2012;43:97–109. doi: 10.1007/s00726-012-1251-9. [DOI] [PubMed] [Google Scholar]
- 130.Kurata H, Fujii T, Tsutsui H, Katayama T, Ohkita M, Takaoka M, Tsuruoka N, Kiso Y, Ohno Y, Fujisawa Y, Shokoji T, Nishiyama A, Abe Y, Matsumura Y. Renoprotective effects of l-carnosine on ischemia/reperfusion-induced renal injury in rats. J Pharmacol Exp Ther. 2006;319:640–647. doi: 10.1124/jpet.106.110122. [DOI] [PubMed] [Google Scholar]
- 131.Sakamoto Y, Watanabe T, Hayashi H, Taguchi Y, Wada H. Effects of Various Compounds on Histidine-Decarboxylase Activity - Active-Site Mapping. Agents and Actions. 1985;17:32–37. doi: 10.1007/BF01966677. [DOI] [PubMed] [Google Scholar]
- 132.Shen Y, Zhang SH, Fu L, Hu WW, Chen Z. Carnosine attenuates mast cell degranulation and histamine release induced by oxygen-glucose deprivation. Cell Biochem Funct. 2008;26:334–338. doi: 10.1002/cbf.1447. [DOI] [PubMed] [Google Scholar]
- 133.Chez MG, Buchanan CP, Aimonovitch MC, Becker M, Schaefer K, Black C, Komen J. Double-blind, placebo-controlled study of L-carnosine supplementation in children with autistic spectrum disorders. J Child Neurol. 2002;17:833–837. doi: 10.1177/08830738020170111501. [DOI] [PubMed] [Google Scholar]
- 134.Gualano B, Everaert I, Stegen S, Artioli GG, Taes Y, Roschel H, Achten E, Otaduy MC, Junior AH, Harris R, Derave W. Reduced muscle carnosine content in type 2, but not in type 1 diabetic patients. Amino Acids. 2012;43:21–24. doi: 10.1007/s00726-011-1165-y. [DOI] [PubMed] [Google Scholar]
- 135.Ansurudeen I, Sunkari VG, Grunler J, Peters V, Schmitt CP, Catrina SB, Brismar K, Forsberg EA. Carnosine enhances diabetic wound healing in the db/db mouse model of type 2 diabetes. Amino Acids. 2012;43:127–134. doi: 10.1007/s00726-012-1269-z. [DOI] [PubMed] [Google Scholar]
- 136.Sauerhofer S, Yuan G, Braun GS, Deinzer M, Neumaier M, Gretz N, Floege J, Kriz W, van der Woude F, Moeller MJ. L-carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes. 2007;56:2425–2432. doi: 10.2337/db07-0177. [DOI] [PubMed] [Google Scholar]
- 137.Freedman BI, Hicks PJ, Sale MM, Pierson ED, Langefeld CD, Rich SS, Xu J, McDonough C, Janssen B, Yard BA, van der Woude FJ, Bowden DW. A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol Dial Transplant. 2007;22:1131–1135. doi: 10.1093/ndt/gfl717. [DOI] [PubMed] [Google Scholar]
- 138.Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, Rychlik I, Cerna M, Romzova M, de Heer E, Baelde H, Bakker SJ, Zirie M, Rondeau E, Mathieson P, Saleem MA, Meyer J, Koppel H, Sauerhoefer S, Bartram CR, Nawroth P, Hammes HP, Yard BA, Zschocke J, van der Woude FJ. Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes. 2005;54:2320–2327. doi: 10.2337/diabetes.54.8.2320. [DOI] [PubMed] [Google Scholar]
- 139.Janssen B, Hohenadel D, Brinkkoetter P, Peters V, Rind N, Fischer C, Rychlik I, Cerna M, Romzova M, de Heer E, Baelde H, Bakker SJL, Zirie M, Rondeau E, Mathieson P, Saleem MA, Meyer J, Koppel H, Sauerhoefer S, Bartram CR, Nawroth P, Hammes HP, Yard BA, Zschocke J, van der Woude FJ. Carnosine as a protective factor in diabetic nephropathy - Association with a leucine repeat of the carnosinase gene CNDP1. Diabetes. 2005;54:2320–2327. doi: 10.2337/diabetes.54.8.2320. [DOI] [PubMed] [Google Scholar]