Abstract
Hendra virus and Nipah virus are bat-borne paramyxoviruses that are the prototypic members of the genus Henipavirus. The henipaviruses emerged in the 1990s, spilling over from their natural bat hosts and causing serious disease outbreaks in humans and livestock. Hendra virus emerged in Australia and since 1994 there have been 7 human infections with 4 case fatalities. Nipah virus first appeared in Malaysia and subsequent outbreaks have occurred in Bangladesh and India. In total, there have been an estimated 582 human cases of Nipah virus and of these, 54% were fatal. Their broad species tropism and ability to cause fatal respiratory and/or neurologic disease in humans and animals make them important transboundary biological threats. Recent experimental findings in animals have demonstrated that a human monoclonal antibody targeting the viral G glycoprotein is an effective post-exposure treatment against Hendra and Nipah virus infection. In addition, a subunit vaccine based on the G glycoprotein of Hendra virus affords protection against Hendra and Nipah virus challenge. The vaccine has been developed for use in horses in Australia and is the first vaccine against a Biosafety Level-4 (BSL-4) agent to be licensed and commercially deployed. Together, these advances offer viable approaches to address Hendra and Nipah virus infection of livestock and people.
Keywords: Hendra virus, Nipah virus, vaccine, monoclonal antibody, G glycoprotein, horse, nonhuman primate, emerging virus, subunit, immunization
Introduction
Hendra virus and Nipah virus are recently recognized bat-borne paramyxoviruses, each of which have repeatedly emerged causing significant morbidity and mortality in both animal and human populations since the mid to late 1990’s. Hendra virus was isolated in Australia from fatal cases of severe respiratory disease in horses and one person in the Brisbane suburb of Hendra in September, 1994, and was shown to be distantly related to measles virus and other morbilliviruses (Murray et al., 1995). The same virus had also caused fatal infections in horses a month prior in Mackay, Australia, but this emergence was only recognized when one individual who was unknowingly exposed to the infected horses at that time developed a recrudescence of fatal meningoencephalitis 13 months later (O’Sullivan et al., 1997; Wong et al., 2009). Hendra virus’ close relative, Nipah virus, emerged in peninsular Malaysia in 1998–99, in a large outbreak of respiratory disease in pigs along with numerous cases of encephalitis among pig farmers, eventually resulting in more than 100 human fatalities. Genetic and serological studies revealed the relatedness of this new virus to Hendra virus (Chua et al., 2000). Hendra virus and Nipah virus now represent the prototype species of the new genus Henipavirus within the paramyxovirus family (Wang et al., 2013).
Since their discovery, both Hendra virus and Nipah virus have continued to repeatedly cause spillover events into animals and/or people. Hendra virus infection among horses in Australia has occurred annually since 2006 and in total there have now been 7 human cases of which 4 have been fatal (Anonymous, 2009b; Playford et al., 2010). In all 7 human cases, Hendra virus was transmitted from infected horses to humans. Of note, in 2011 from the months of June to October, a significant increase in the number of Hendra virus spillovers occurred with 18 separate episodes of infection in horses in Australia, including the first recognized case of infection in a dog (reviewed in (Broder, 2012)). There were 8 cases of Hendra virus spillovers into horses in 2012 (Anonymous, 2012b) and a further two cases of Hendra virus infection in horses in early 2013 (Anonymous, 2013b). In all, a total of 42 Hendra virus spillover events have occurred since 1994 and 28 of these have occurred in just the past 2 years. Likewise, following the Malaysian outbreak in 1998, nearly annual outbreaks of Nipah virus infection, occurring primarily in Bangladesh but also India have occurred since 2001. The most recent outbreak occurred in early 2013, with apparently 10 fatalities of 12 cases (Anonymous, 2013c). Compared to the original Malaysian outbreak, these Nipah virus spillovers have been smaller in case number, however the fatality rates in people overall have been notably higher, ranging from 75–100%. Importantly, direct transmission of Nipah virus from bats to humans and significant human-to-human transmission have also been documented during outbreaks in India and Bangladesh. The epidemiological details of the spillovers of both Hendra virus and Nipah virus into people since their emergence and recognition have recently been reviewed and summarized in detail (Luby and Gurley, 2012). There have been an estimated 582 cases of Nipah virus infection with 315 human fatalities (Anonymous, 2013c; Luby and Gurley, 2012; Luby et al., 2009; Pallister et al., 2011a).
The henipavirus transboundary threat
The natural reservoir hosts of Hendra virus and Nipah virus are several species of pteropid fruit bats among which they are not known to cause disease (Halpin et al., 2011). However, Hendra and Nipah viruses possess an exceptionally broad species tropism and both natural and experimental infections have demonstrated their capacity to cause disease which can often be fatal in horses, pigs, cats, dogs, ferrets, hamsters, guinea pigs, monkeys, and humans, spanning 6 mammalian Orders (reviewed in (Geisbert et al., 2012)). In disease susceptible animal hosts and people, Nipah virus and Hendra virus cause a systemic infection that is characterized as a wide-spread vasculitis and endothelial cell tropism. Though this pathology is not unique to these henipaviruses, an understanding of Hendra and Nipah virus cellular tropism on the molecular level has provided an explanation to this disease feature which includes the appearance of syncytia, thrombosis, ischemia and necrosis, with parenchymal cell infection and associated pathology in many major organ systems, and prominently in the brain and lung (reviewed in (Weingartl et al., 2009; Wong and Ong, 2011)). The major involvement of the lung and brain in Hendra and Nipah virus infection often manifests as an acute severe respiratory syndrome, encephalitis or a combination of both. Disturbingly however, infection in people can also have longer term consequences, and in addition to an acute symptomatic infection, Hendra and Nipah virus infection can also take a protracted course following recovery from an initial infection. Individuals in these cases can later undergo a recrudescence of virus replication in the central nervous system (CNS) causing a relapse of encephalitis, a process that was first noted in the second fatal case of Hendra virus human infection (O’Sullivan et al., 1997; Wong et al., 2009). Quite remarkably, relapsed-encephalitis caused by Nipah virus has been reported in people from several months to as long as 11 years following infection (Abdullah et al., 2012) (reviewed in (Wong, 2010)). How the henipaviruses survive immune-mediated clearance and can later cause a recrudescence of replication in the CNS is unknown, but this virological feature clearly has important implications for anti-henipavirus therapeutics development.
Given the virulence of Hendra and Nipah virus and the increase in their spillover occurrences over the past decade, strategies to mitigate the risk of Hendra and Nipah virus exposure have become paramount. Both Hendra virus and Nipah virus reside in large wild bat populations, which make controlling virus in the reservoir host or influencing the reservoir host population dynamics difficult to impossible. In extreme instances, bat culling has been proposed to minimize exposure; however, the ecological importance of bats as a whole makes this an unrealistic option. In Malaysia and Australia efforts have been made to reduce livestock interactions with bats; for example, restricting livestock access to areas under fruit trees, covering water and feed containers to prevent contamination and not placing water and feed under fruit trees (Anonymous, 2013a). However, the significant numbers of fruit trees and roosting flying foxes on or near properties containing livestock makes complete separation of the wildlife and livestock populations near impossible. In Bangladesh, measures have been employed to prevent flying foxes access to date palm sap collectors in hopes of preventing contamination with Nipah virus (Luby and Gurley, 2012). Unfortunately, Nipah outbreaks continue to occur every year reflecting the difficulty of implementing a new practice culturally to prevent such a disease that is still considered to be rare. Developing vaccines and antiviral therapies for Hendra and Nipah virus are also viable alternatives for mitigating disease risk. As livestock have been identified as intermediate hosts for both Hendra and Nipah virus, antiviral therapies seem less attractive given the size of horses and pigs and the significant costs associated with producing large quantities of any possible drug. Conversely, vaccination of livestock populations is a highly attractive mitigation strategy since both disease in the target species as well as secondary transmission of virus to humans would be prevented. In areas such as Bangladesh, where no intermediate host has been definitively identified, there is a real need for the development of effective therapies and vaccine strategies to prevent infection. Similarly, for individuals who have potential occupational exposure to Hendra and Nipah virus infection, such as pig farmers and equine veterinarians, therapeutic agents and/or a vaccine to prevent infection would significantly reduce morbidity and mortality associated with Hendra and Nipah viruses.
Hendra and Nipah virus attach to host cell-surface displayed ephrin-B2 or -B3 proteins and infect host cells by the coordinated activity of their attachment (G) and fusion (F) glycoproteins (reviewed in (Aguilar and Iorio, 2012; Lee and Ataman, 2011)). The G glycoprotein monomer consists of a stalk and globular head (Figure 1) and the atomic structures of both the Nipah and Hendra virus G glycoprotein’s globular head domain have been determined alone and in complex with ephrin proteins (reviewed in (Xu et al., 2012a)). The F glycoprotein mediates the membrane fusion process between the viral and host cell membranes by a Class I fusion mechanism that is initiated following the G glycoprotein engagement of ephrin receptor (Lee and Ataman, 2011). The susceptible host species and associated cellular tropism and pathology of Hendra and Nipah virus has in large part been explained by their use of the highly conserved ephrin-B2 and -B3 proteins as entry receptors (reviewed in (Pernet et al., 2012; Wong and Ong, 2011)). In addition and of importance to countermeasure development, the henipavirus G and F envelope glycoprotein spikes are major targets of virus-neutralizing antibodies and as discussed below, the development of potential vaccines have largely focused on these important structural components of the virion (reviewed in (Broder, 2010)).
Figure 1.
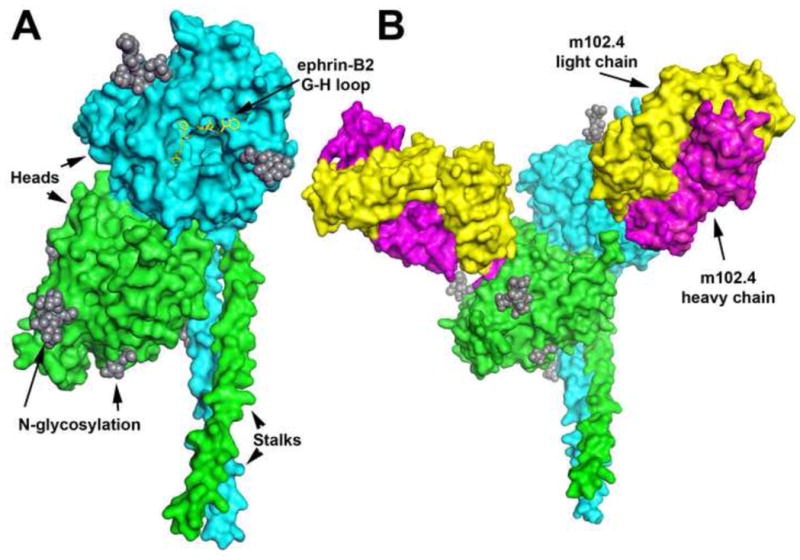
Model of the Hendra virus soluble G glycoprotein subunit vaccine (HeV-sG) and its complex with the henipavirus-neutralizing human monoclonal antibody m102. A: The HeV-sG glycoprotein subunit vaccine is composed of the entire ectodomain (amino acids 76-604) of the HeV G glycoprotein. Here, HeV-sG is shown as dimer with one monomer colored green and the other cyan. The secondary structure elements of the two globular head domains are derived from the crystal structure of the HeV G head domain (Colgrave et al., 2011; Xu et al., 2012b), and the stalk regions of each G monomer (residues 77-136) are modeled (Kelley and Sternberg, 2009). N-linked glycosylation sites shown as gray spheres. The ephrin-binding face of the cyan globular head is facing forward with an overlay of the interacting ephrin-B2 G-H loop residues in yellow. B: The HeV-sG dimer shown in complex with two m102.3 Fab antibody fragments. The two HeV-sG monomers are colored green and cyan as in panel A and rotated slightly to the right. The two Fab m102.3 molecules are shown with their heavy chains colored in magenta and light chain in yellow, each binding one globular head domains of G. (Xu, K. et al., submitted).
The development of medical countermeasures for use in humans is a time-consuming process, especially for highly pathogenic BSL-4 agents like Hendra and Nipah virus where human efficacy trials are not feasible. Demonstrated efficacy in two animal models of disease is required to support possible licensure. In recent years monoclonal antibodies (mAbs) have attracted considerable attention as viable antiviral and antibacterial therapies, and the Food and Drug Administration (FDA) has approved both humanized and fully human monoclonal antibody (mAb) for use in preventing or treating infectious diseases in humans (Dolgin, 2013; Zhu et al., 2013). The development of human monoclonal antibodies (humAbs) against Hendra and Nipah virus infection has been highly successful and as discussed below, a viable post-exposure mAb therapy is currently in development. In addition, a recombinant subunit vaccine candidate has been successfully trialed in several animal challenge models of Hendra and Nipah virus infection, and has recently been deployed as an effective equine vaccine in Australia; potentially breaking the chain of Hendra virus transmission and a practical cost effective way to mitigate human Hendra virus infection.
Antiviral treatment
There are no approved or licensed therapeutics for treating henipavirus infection or disease in people, and antiviral approaches against the henipaviruses that have been tested in animal models are few (reviewed in (Broder, 2012)). Ribavirin is a well-known first line treatment strategy for suspected viral infections of unknown etiology. Ribavirin exhibits antiviral activity against a wide variety of both RNA and some DNA viruses (Sidwell et al., 1972) and is an accepted or approved treatment for several viral infections including respiratory syncytial virus and arenaviral hemorrhagic-fevers (reviewed in (Snell, 2001)). In vitro studies have shown that ribavirin is effective against both Hendra and Nipah virus replication (Aljofan et al., 2009; Wright et al., 2005). Also, the anti-malarial drug chloroquine was shown early on to block the critical proteolytic processing needed for the maturation and function of the Hendra virus F glycoprotein (Pager et al., 2004), and not surprisingly cholorquine was later shown to inhibit Nipah and Hendra virus infection in cell culture (Porotto et al., 2009).
An open label ribavirin treatment trial was carried out during the outbreak of Nipah virus in Malaysia in 1998 and was reported to reduce mortality by 36% in treated patients when compared to those patients who presented before ribavirin availability or who refused treatment (Chong et al., 2001). Of the recorded human Hendra virus cases, three individuals were treated with ribavirin, and of these, two succumbed to disease and one survived (Playford et al., 2010). Chloroquine was administered along with ribavirin to one HeV-infected individual in 2009 (Anonymous, 2009c) with no apparent clinical benefit. Three additional people received ribavirin treatment in combination with chloroquine after suspected exposure to Hendra virus contaminated secretions from infected horses. While all three individuals survived, infection was not confirmed and therefore it remains unknown whether the treatment had any effect (Anonymous, 2009a). In the absence of other therapies, ribavirin may be an option for treatment of henipavirus infections. However, more recent animal studies have revealed no therapeutic benefit of either drug. Two studies in hamsters and one study in nonhuman primates (African green monkey (AGM)) showed that ribavirin treatment only delayed but did not prevent death after Nipah or Hendra virus infection (Freiberg et al., 2010; Georges-Courbot et al., 2006; Rockx et al., 2010) and AGMs treated with ribavirin following Hendra virus infection had marked increases of neurological symptoms. Similarly, chloroquine was unable to prevent Nipah infection or disease in ferrets (Pallister et al., 2009).
A passive immunotherapy for people
In contrast, passive immunotherapy with polyclonal or monoclonal antibody specific for the viral envelope glycoproteins has proved successful from initial proof-of-concept findings from several studies carried out in hamsters (Guillaume et al., 2004; Guillaume et al., 2006). Presently, the only reported and effective post-exposure therapy against Hendra or Nipah virus infection and one that could likely be approved in the near future for use in people has been a human monoclonal antibody (mAb) known as m102.4 which was isolated from a recombinant nae human phage -displayed Fab library (Zhu et al., 2008).
The m102.4 mAb has exceptionally potent neutralizing activity against both Nipah and Hendra viruses and its epitope maps to the ephrin receptor binding site (Figure 1). Testing of m102.4 has confirmed its neutralization activity against several isolates; NiV-Malaysia, HeV-1994, HeV-Redlands, NiV-Bangladesh (Bossart et al., 2009). Effective post-exposure efficacy with m102.4 has now been demonstrated in both ferrets and nonhuman primates (African green monkey (AGM)) infected with either Hendra virus or Nipah virus (Table 1). The successful m102.4 passive immunotherapy in the AGM was recently reported in a study designed to reflect a possible real life scenario requiring mAb as a post-exposure treatment, and was a follow-up from the initial successful m102.4 post-exposure therapy carried out in ferrets (Bossart et al., 2009). Fourteen monkeys were challenged intratracheally with Hendra virus and 12 animals were infused twice with a 100 mg dose (~20 mg/kg) of m102.4 beginning at 10 hr, 24 hr or 72 hr p.i. with the second infusion ~48 hrs later. All 12 animals that received m102.4 survived infection; whereas the untreated control subjects succumbed to severe systemic disease by day 8 (Bossart et al., 2011). There was no evidence of Hendra virus mediated pathology in any of the m102.4-treated animals and no infectious Hendra virus could be recovered from any tissues from any m102.4-treated subjects.
Table 1.
Evaluation of post-exposure therapy with the henipavirus G glycoprotein-specific human monoclonal antibody m102.4 against lethal henipavirus challenge in laboratory animals.
| Virus | Animal model | Experimental design and resultsa | Reference |
|---|---|---|---|
| Hendra | AGM | A 10, 24, or 72 hr post-exposure use of m102.4 could protect against a 10-fold lethal intratracheal virus challenge | (Bossart et al., 2011) |
| ferret | A 10 or 24 hr post-exposure dose of m102.4 could protect against a 10-fold lethal oronasal virus challenge | (Pallister J., unpublished) | |
| Nipah | ferret | A 10 hr post-exposure dose of m102.4 could protect against a 10-fold lethal oronasal virus challenge | (Bossart et al., 2009) |
| AGM | A 24, 72, or 120 hr post-exposure use of m102.4 could protect against a 10-fold lethal intratracheal virus challenge | (Geisbert T., unpublished) |
The administration of m102.4 mAb was performed by infusion in all studies with the exception of one ferret in one study were it had to be administered by intraperitoneal injection (Bossart et al., 2009). All studies to date have reported that all animals receiving m102.4 post-exposure therapy have survived virus challenge, some with varying levels of mild to moderate illness.
In May of 2010, an instance of possible Hendra virus infection in two individuals was reported on the Sunshine Coast, north of Brisbane, Australia. Both individuals had extensive close contact with a horse just prior to and during the development of clinical illness in the animal. Following a diagnosis of Hendra virus infection in the horse, both individuals were considered to have had high-risk exposure to Hendra virus (Anonymous, 2010). A request was made by Australian health authorities to obtain m102.4 as a possible compassionate use therapeutic option even though clinical trials in human had not been undertaken and safety data of the mAb in humans was lacking. These two individuals were administered the m102.4 mAb (Miles, 2010). Both individuals ultimately did not develop detectable Hendra virus infection but whether this was due to the mAb therapy could not be determined. In 2010, the cell line expressing the human m102.4 mAb was provided to the Queensland Government, Queensland Health, to allow health authorities to manufacture m102.4 for its potential use on a compassionate basis in future cases of high-risk human exposure. In 2012, a third asymptomatic individual who experienced high-risk Hendra virus exposure was also given m102.4 mAb therapy (Anonymous, 2012a; Guest, 2012). There have been no adverse effects observed or reported in these cases.
A one-health solution - vaccination
Initial immunization strategies using the henipavirus G or F viral glycoproteins were first evaluated using recombinant vaccinia viruses providing evidence that complete protection from disease was achievable by eliciting an immune response to the Nipah virus envelope glycoproteins (Guillaume et al., 2004). Other studies using recombinant canarypox-based vaccine candidates for potential use in pigs have also been carried out (Weingartl et al., 2006). To date, the most widely evaluated henipavirus vaccine antigen has been a subunit, consisting of a recombinant soluble and oligomeric form of the G glycoprotein (sG) of Hendra virus (HeV-sG) (Bossart et al., 2005). The HeV-sG subunit vaccine (Figure 1) is a secreted version of the molecule in which the transmembrane and cytoplasmic tail domains have been deleted from the coding sequence. HeV-sG is produced in mammalian cell culture expression systems and is properly N-linked glycosylated and retains many native characteristics including its oligomerization into dimers and tetramers, ability to bind ephrin receptors and elicit potent cross-reactive (Hendra and Nipah virus) neutralizing antibody responses (reviewed in (Broder et al., 2012)).
Studies showing the HeV-sG subunit immunogen as a successful vaccine against lethal Hendra virus or Nipah virus challenge have been carried out in the cat (McEachern et al., 2008; Mungall et al., 2006), ferret (Pallister et al., 2011b) and nonhuman primates (Bossart et al., 2012) (Table 1), and details of the results from these studies have been reviewed elsewhere (Broder et al., 2012). The success of the HeV-sG vaccine-mediated protection observed in multiple animal challenge models led to the consideration of the HeV-sG as a safe and effective vaccine for horses against Hendra virus infection in Australia following a human fatality in 2009 and the human exposure cases in 2010 discussed above. The adopted equine vaccination strategy was to both prevent infection in horses and thus ameliorate the risk of Hendra virus transmission to people. A series of horse HeV-sG vaccination and Hendra virus challenge studies have been carried out in Australia; at the high containment biological safety level 4 (BSL-4) facilities of the Animal Health Laboratories (AAHL), Commonwealth Scientific and Industrial Research Organisation (CSIRO), in Geelong. The development of HeV-sG as an equine vaccine against Hendra virus was a collaborative research program between the Uniformed Services University of the Health Sciences and the Henry M. Jackson Foundation, the AAHL and Pfizer Animal Health (now Zoetis, Inc.). Findings from these initial studies were reported at Australian Veterinary Association, Annual Conference in Adelaide, in May 2011 (Balzer, 2011). The HeV-sG subunit glycoprotein was used to vaccinate horses (a 2 dose regime with a 3 week interval) and both a high and a low dose of HeV-sG antigen was examined. Following a high dose oronasal challenge with Hendra virus, all vaccinated horses remained clinically disease-free, and there was no evidence of virus replication or virus shedding in any of the immunized horses. On November 1, 2012, the vaccine called Equivac HeV® was released for use in Australia, and it is the first vaccine licensed and commercially deployed against a BSL-4 agent and currently is the only licensed prophylactic treatment for henipaviruses.
Concluding remarks
The Nipah virus and Hendra virus are zoonotic paramyxoviruses that can infect and cause lethal disease across a broad range of vertebrate species including humans. They are present in a variety of bat reservoirs, can be isolated and propagated and because of their associated high morbidity and mortality they pose a risk from natural outbreaks, laboratory accidents or deliberate misuse. For all of these reasons, the development of effective prevention and treatment strategies has been pursued. Over the past decade a considerable amount of research has focused on the henipavirus envelope glycoproteins and their roles in the virus attachment and infection process. These efforts have now led to the development and testing of both passive and active immunization strategies applicable to both human and animal use. Presently, a cross-reactive human mAb (m102.4) has been demonstrated as an exceptionally efficacious post-exposure therapy in protecting both ferrets and nonhuman primates from lethal henipavirus disease, and its effectiveness led to its application in people as a compassionate use post-exposure prophylaxis in Australia. Also, as an active vaccination strategy for preventing Hendra virus infection and disease in horses in Australia and thus blocking its potential transmission to people, a recombinant subunit vaccine, HeV-sG, which has been shown to provide protection against henipavirus challenge in cats, ferrets, monkeys and now horses, has been licensed and deployed for use in Australia.
To date, henipavirus antivirals have only been deployed in Australia in the fight against Hendra virus. As Nipah virus causes significantly more instances of human disease, increased efforts are needed to advance Nipah-targeted countermeasures in endemic regions. Animal models have demonstrated that both the HeV-sG vaccine and the m102.4 human antibody can prevent both Nipah virus infection and/or disease. Efforts are currently under way to develop HeV-sG for human use as well as for use in pigs. However, the cost of the vaccine per animal and uptake of the vaccine in the absence of repeated outbreaks or disease will be critical factors influencing the feasibility of its application in Southeast Asia. The pre-clinical development of recombinant HeV-sG for use in people is only a first step and the acquisition of further support for manufacture and clinical trials will certainly be challenging. Clinical trials will also be needed for the m102.4 human antibody therapy, and both the United States and Australia are developing the m102.4 antibody for human use as a Nipah and Hendra virus countermeasure. Nipah virus has not occurred in Malaysia since 1998 and requests for compassionate use of the m102.4 antibody in India or Bangladesh following high-risk Nipah virus exposure or cases of infection have not occurred and may be difficult to orchestrate. Whether the antibody could be pre-positioned in Nipah virus endemic areas will largely depend on international cooperation and financial support.
Table 2.
Evaluation of the protective efficacy of henipavirus sG vaccines against lethal henipavirus challenge in laboratory animals.
| Virus | Animal model | Experimental design and resultsa | Reference |
|---|---|---|---|
| Hendra | ferret | Hendra-sG used to immunize followed by 10-fold lethal oronasal virus challenge | (Pallister et al., 2011b) |
| AGM | Hendra-sG used to immunize followed by 10-fold lethal intratracheal virus challenge | (Bossart et al., 2012) | |
| horse | Hendra-sG is used to immunize horses followed by lethal oronasal virus challenge | (Balzer, 2011) (Middleton D., et al., submitted) | |
| Nipah | cat | Hendra-sG or Nipah-sG used to immunize followed by 10-fold lethal subcutaneous virus challenge; Hendra-sG used to immunize followed by 100-fold lethal oronasal virus challenge | (McEachern et al., 2008; Mungall et al., 2006) |
| ferret | Hendra-sG used to immunize followed by 10-fold lethal oronasal virus challenge | (Pallister J., unpublished) | |
| AGM | Hendra-sG used to immunize followed by 10-fold lethal intratracheal virus challenge | (Geisbert T., unpublished) |
All studies to date have reported that all Hendra-sG immunized animals can be completely protected from infection and disease following either a Hendra or Nipah virus challenge. No evidence of virus replication or shedding has been reported in the majority of challenged subjects.
Advanced antivirals against Nipah and Hendra are a Hendra-sG subunit vaccine and a human monoclonal antibody, m102.4.
The Hendra-sG vaccine was launched in Australia for equine use and is the first BSL-4 agent vaccine for public use.
m102.4 has been used in people on a compassionate use basis in Australia and is presently in pre-clinical development.
A structural model of the Hendra-sG vaccine and its complex with the neutralizing human monoclonal antibody is shown.
Acknowledgments
The views expressed in the manuscript are solely those of the authors, and they do not represent official views or opinions of the Department of Defense or The Uniformed Services University of the Health Sciences. CCB, KNB and TWG are supported in part by grants from the United States, Department of Health and Human Services, National Institutes of Health (NIH). ZZ and DSD are supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Conflict of interest
CCB, ZZ and DSD are United States federal employees. CCB, DSD and ZZ are coinventors on patents pertaining to human monoclonal antibodies against Hendra and Nipah viruses, and CCB and KNB are coinventors on patents pertaining to soluble forms of Hendra and Nipah G glycoproteins; assignees are The United States of America as represented by the Department of Health and Human Services (Washington, DC), Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (Bethesda, MD).
All other authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah S, Chang LY, Rahmat K, Goh KT, Tan CT. Late-onset Nipah virus encephalitis 11 years after the initial outbreak: A case report. Neurology Asia. 2012;17:71–74. [Google Scholar]
- Aguilar HC, Iorio RM. Henipavirus membrane fusion and viral entry. Curr Top Microbiol Immunol. 2012;359:79–94. doi: 10.1007/82_2012_200. [DOI] [PubMed] [Google Scholar]
- Aljofan M, Saubern S, Meyer AG, Marsh G, Meers J, Mungall BA. Characteristics of Nipah virus and Hendra virus replication in different cell lines and their suitability for antiviral screening. Virus Res. 2009;142:92–99. doi: 10.1016/j.virusres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous. Hendra virus, human, equine - Australia (02): Queensland, Pro-med. International Society for Infectious Diseases; 2009a. Aug 21, archive no. 20090826.2998. Available at www.promedmail.org. [Google Scholar]
- Anonymous. Hendra virus, human, equine - Australia (04): Queensland, fatal, Pro-med. International Society for Infectious Diseases; 2009b. Sep 3, p. archive no. 20090903.3098. Available at www.promedmail.org. [Google Scholar]
- Anonymous. International Society for Infectious Diseases; 2009c. Sep 10, Hendra virus, human, equine - Australia (05): Queensland, Pro-med; p. archive no. 20090910.3189. Available at www.promedmail.org. [Google Scholar]
- Anonymous. Hendra virus, equine - Australia (05): Queensland: human exposure. Pro-MED. International Society for Infectious Diseases; 2010. May 27, p. archive no. 20100527.1761. Available at www.promedmail.org. [Google Scholar]
- Anonymous. Hendra virus, equine - Australia (08) Queensland: possible human case. Pro-med. International Society for Infectious Diseases; 2012a. Jul 19, p. archive no. 20120720.1208397. Available at www.promedmail.org. [Google Scholar]
- Anonymous. Hendra virus, equine - Australia (12): Queensland: vaccine. Pro-med. International Society for Infectious Diseases; 2012b. Nov 3, p. archive no. 20121104.1390394. Available at www.promedmail.org. [Google Scholar]
- Anonymous. Hendra virus. The State of Queensland, Department of Agriculture, Fisheries and Forestry, Queensland Government; 2013a. Available at www.daff.qld.gov.au/4790_20801.htm. [Google Scholar]
- Anonymous. Hendra virus, equine - Australia: (02) Queensland. Pro-med. International Society for Infectious Diseases; 2013b. Feb 22, p. archive no. 20130224.1557005. Available at www.promedmail.org. [Google Scholar]
- Anonymous. Nipah encephalitis, human - Bangladesh (03), Pro-med. International Society for Infectious Diseases; 2013c. Feb 5, p. archive no. 20130205.1530748. Available at www.promedmail.org. [Google Scholar]
- Balzer M. Hendra vaccine success announced. Aust Vet J. 2011;89:N2–3. doi: 10.1111/j.1751-0813.2011.news_v89_i7.x. [DOI] [PubMed] [Google Scholar]
- Bossart KN, Crameri G, Dimitrov AS, Mungall BA, Feng YR, Patch JR, Choudhary A, Wang LF, Eaton BT, Broder CC. Receptor binding, fusion inhibition, and induction of cross-reactive neutralizing antibodies by a soluble g glycoprotein of hendra virus. J Virol. 2005;79:6690–6702. doi: 10.1128/JVI.79.11.6690-6702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart KN, Geisbert TW, Feldmann H, Zhu Z, Feldmann F, Geisbert JB, Yan L, Feng YR, Brining D, Scott D, Wang Y, Dimitrov AS, Callison J, Chan YP, Hickey AC, Dimitrov DS, Broder CC, Rockx B. A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge. Sci Transl Med. 2011;3:105ra103. doi: 10.1126/scitranslmed.3002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart KN, Rockx B, Feldmann F, Brining D, Scott D, Lacasse R, Geisbert JB, Feng YR, Chan YP, Hickey AC, Broder CC, Feldmann H, Geisbert TW. A hendra virus g glycoprotein subunit vaccine protects african green monkeys from nipah virus challenge. Sci Transl Med. 2012;4:146ra107. doi: 10.1126/scitranslmed.3004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart KN, Zhu Z, Middleton D, Klippel J, Crameri G, Bingham J, McEachern JA, Green D, Hancock TJ, Chan YP, Hickey AC, Dimitrov DS, Wang LF, Broder CC. A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog. 2009;5:e1000642. doi: 10.1371/journal.ppat.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder CC. Therapeutics and Vaccines against Hendra and Nipah Viruses. In: Levine MM, Dougan G, Good MF, Liu MA, Nabel GJ, Nataro JP, Rappuoli R, editors. New Generation Vaccines. 4. Informa Healthcare; USA, New York: 2010. pp. 885–894. [Google Scholar]
- Broder CC. Henipavirus outbreaks to antivirals: the current status of potential therapeutics. Curr Opin Virol. 2012;2:176–187. doi: 10.1016/j.coviro.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder CC, Geisbert TW, Xu K, Nikolov DB, Wang LF, Middleton D, Pallister J, Bossart KN. Immunization Strategies Against Henipaviruses. Curr Top Microbiol Immunol. 2012;359:197–223. doi: 10.1007/82_2012_213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong HT, Kamarulzaman A, Tan CT, Goh KJ, Thayaparan T, Kunjapan SR, Chew NK, Chua KB, Lam SK. Treatment of acute Nipah encephalitis with ribavirin. Ann Neurol. 2001;49:810–813. doi: 10.1002/ana.1062. [DOI] [PubMed] [Google Scholar]
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Ksiazek TG, Rollin PE, Zaki SR, Shieh W, Goldsmith CS, Gubler DJ, Roehrig JT, Eaton B, Gould AR, Olson J, Field H, Daniels P, Ling AE, Peters CJ, Anderson LJ, Mahy BW. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- Colgrave ML, Snelling HJ, Shiell BJ, Feng YR, Chan YP, Bossart KN, Xu K, Nikolov DB, Broder CC, Michalski WP. Site occupancy and glycan compositional analysis of two soluble recombinant forms of the attachment glycoprotein of Hendra virus. Glycobiology. 2011;22:572–584. doi: 10.1093/glycob/cwr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgin E. Animal rule for drug approval creates a jungle of confusion. Nat Med. 2013;19:118–119. doi: 10.1038/nm0213-118. [DOI] [PubMed] [Google Scholar]
- Freiberg AN, Worthy MN, Lee B, Holbrook MR. Combined chloroquine and ribavirin treatment does not prevent death in a hamster model of Nipah and Hendra virus infection. J Gen Virol. 2010;91:765–772. doi: 10.1099/vir.0.017269-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Feldmann H, Broder CC. Animal Challenge Models of Henipavirus Infection and Pathogenesis. Curr Top Microbiol Immunol. 2012;359:153–177. doi: 10.1007/82_2012_208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Courbot MC, Contamin H, Faure C, Loth P, Baize S, Leyssen P, Neyts J, Deubel V. Poly(I)-poly(C12U) but not ribavirin prevents death in a hamster model of Nipah virus infection. Antimicrob Agents Chemother. 2006;50:1768–1772. doi: 10.1128/AAC.50.5.1768-1772.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guest A. The World Today. ABC News (Australian Broadcasting Corporation); 2012. Experimental therapy for Hendra virus patient. [Google Scholar]
- Guillaume V, Contamin H, Loth P, Georges-Courbot MC, Lefeuvre A, Marianneau P, Chua KB, Lam SK, Buckland R, Deubel V, Wild TF. Nipah virus: vaccination and passive protection studies in a hamster model. J Virol. 2004;78:834–840. doi: 10.1128/JVI.78.2.834-840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume V, Contamin H, Loth P, Grosjean I, Courbot MC, Deubel V, Buckland R, Wild TF. Antibody Prophylaxis and Therapy against Nipah Virus Infection in Hamsters. J Virol. 2006;80:1972–1978. doi: 10.1128/JVI.80.4.1972-1978.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin K, Hyatt AD, Fogarty R, Middleton D, Bingham J, Epstein JH, Rahman SA, Hughes T, Smith C, Field HE, Daszak P, The H. Pteropid Bats are Confirmed as the Reservoir Hosts of Henipaviruses: A Comprehensive Experimental Study of Virus Transmission. Am J Trop Med Hyg. 2011;85:946–951. doi: 10.4269/ajtmh.2011.10-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Lee B, Ataman ZA. Modes of paramyxovirus fusion: a Henipavirus perspective. Trends Microbiol. 2011;19:389–399. doi: 10.1016/j.tim.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby SP, Gurley ES. Epidemiology of henipavirus disease in humans. Curr Top Microbiol Immunol. 2012;359:25–40. doi: 10.1007/82_2012_207. [DOI] [PubMed] [Google Scholar]
- Luby SP, Hossain MJ, Gurley ES, Ahmed BN, Banu S, Khan SU, Homaira N, Rota PA, Rollin PE, Comer JA, Kenah E, Ksiazek TG, Rahman M. Recurrent zoonotic transmission of Nipah virus into humans, Bangladesh, 2001–2007. Emerg Infect Dis. 2009;15:1229–1235. doi: 10.3201/eid1508.081237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern JA, Bingham J, Crameri G, Green DJ, Hancock TJ, Middleton D, Feng YR, Broder CC, Wang LF, Bossart KN. A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine. 2008;26:3842–3852. doi: 10.1016/j.vaccine.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles J. The Courier-Mail.com.au. Queensland Newspapers Pty Ltd, Brisbane; Queensland 4001, Australia: 2010. Hendra therapy for mum, daughter. [Google Scholar]
- Mungall BA, Middleton D, Crameri G, Bingham J, Halpin K, Russell G, Green D, McEachern J, Pritchard LI, Eaton BT, Wang LF, Bossart KN, Broder CC. Feline model of acute nipah virus infection and protection with a soluble glycoprotein-based subunit vaccine. J Virol. 2006;80:12293–12302. doi: 10.1128/JVI.01619-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, Westbury H, Hiley L, Selvey L, Rodwell B, et al. A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–97. doi: 10.1126/science.7701348. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JD, Allworth AM, Paterson DL, Snow TM, Boots R, Gleeson LJ, Gould AR, Hyatt AD, Bradfield J. Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet. 1997;349:93–95. doi: 10.1016/s0140-6736(96)06162-4. [DOI] [PubMed] [Google Scholar]
- Pager CT, Wurth MA, Dutch RE. Subcellular localization and calcium and pH requirements for proteolytic processing of the Hendra virus fusion protein. J Virol. 2004;78:9154–9163. doi: 10.1128/JVI.78.17.9154-9163.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallister J, Middleton D, Broder CC, Wang LF. Henipavirus Vaccine Development. J Bioterr Biodef. 2011a:S1. [Google Scholar]
- Pallister J, Middleton D, Crameri G, Yamada M, Klein R, Hancock TJ, Foord A, Shiell B, Michalski W, Broder CC, Wang LF. Chloroquine administration does not prevent Nipah virus infection and disease in ferrets. J Virol. 2009;83:11979–11982. doi: 10.1128/JVI.01847-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallister J, Middleton D, Wang LF, Klein R, Haining J, Robinson R, Yamada M, White J, Payne J, Feng YR, Chan YP, Broder CC. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine. 2011b;29:5623–5630. doi: 10.1016/j.vaccine.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet O, Wang YE, Lee B. Henipavirus receptor usage and tropism. Curr Top Microbiol Immunol. 2012;359:59–78. doi: 10.1007/82_2012_222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford EG, McCall B, Smith G, Slinko V, Allen G, Smith I, Moore F, Taylor C, Kung YH, Field H. Human Hendra virus encephalitis associated with equine outbreak, Australia, 2008. Emerg Infect Dis. 2010;16:219–223. doi: 10.3201/eid1602.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porotto M, Orefice G, Yokoyama CC, Mungall BA, Realubit R, Sganga ML, Aljofan M, Whitt M, Glickman F, Moscona A. Simulating henipavirus multicycle replication in a screening assay leads to identification of a promising candidate for therapy. J Virol. 2009;83:5148–5155. doi: 10.1128/JVI.00164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B, Bossart KN, Feldmann F, Geisbert JB, Hickey AC, Brining D, Callison J, Safronetz D, Marzi A, Kercher L, Long D, Broder CC, Feldmann H, Geisbert TW. A novel model of lethal Hendra virus infection in African green monkeys and the effectiveness of ribavirin treatment. J Virol. 2010;84:9831–9839. doi: 10.1128/JVI.01163-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Huffman JH, Khare GP, Allen LB, Witkowski JT, Robins RK. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972;177:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- Snell NJ. Ribavirin--current status of a broad spectrum antiviral agent. Expert Opin Pharmacother. 2001;2:1317–1324. doi: 10.1517/14656566.2.8.1317. [DOI] [PubMed] [Google Scholar]
- Wang L-F, Mackenzie JS, Broder CC. Henipaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia: 2013. pp. 1070–1085. [Google Scholar]
- Weingartl HM, Berhane Y, Caswell JL, Loosmore S, Audonnet JC, Roth JA, Czub M. Recombinant nipah virus vaccines protect pigs against challenge. J Virol. 2006;80:7929–7938. doi: 10.1128/JVI.00263-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weingartl HM, Berhane Y, Czub M. Animal models of henipavirus infection: a review. Vet J. 2009;181:211–220. doi: 10.1016/j.tvjl.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Wong KT. Emerging epidemic viral encephalitides with a special focus on henipaviruses. Acta Neuropathol. 2010;120:317–325. doi: 10.1007/s00401-010-0720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KT, Ong KC. Pathology of acute henipavirus infection in humans and animals. Patholog Res Int. 2011;2011:567248. doi: 10.4061/2011/567248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KT, Robertson T, Ong BB, Chong JW, Yaiw KC, Wang LF, Ansford AJ, Tannenberg A. Human Hendra virus infection causes acute and relapsing encephalitis. Neuropathol Appl Neurobiol. 2009;35:296–305. doi: 10.1111/j.1365-2990.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- Wright PJ, Crameri G, Eaton BT. RNA synthesis during infection by Hendra virus: an examination by quantitative real-time PCR of RNA accumulation, the effect of ribavirin and the attenuation of transcription. Arch Virol. 2005;150:521–532. doi: 10.1007/s00705-004-0417-5. [DOI] [PubMed] [Google Scholar]
- Xu K, Broder CC, Nikolov DB. Ephrin-B2 and ephrin-B3 as functional henipavirus receptors. Semin Cell Dev Biol. 2012a;23:116–123. doi: 10.1016/j.semcdb.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Chan YP, Rajashankar KR, Khetawat D, Yan L, Kolev MV, Broder CC, Nikolov DB. New insights into the hendra virus attachment and entry process from structures of the virus g glycoprotein and its complex with ephrin-b2. PLoS One. 2012b;7:e48742. doi: 10.1371/journal.pone.0048742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Bossart KN, Bishop KA, Crameri G, Dimitrov AS, McEachern JA, Feng Y, Middleton D, Wang LF, Broder CC, Dimitrov DS. Exceptionally potent cross-reactive neutralization of Nipah and Hendra viruses by a human monoclonal antibody. J Infect Dis. 2008;197:846–853. doi: 10.1086/528801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Prabakaran P, Chen W, Broder CC, Gong R, Dimitrov DS. Human monoclonal antibodies as candidate therapeutics against emerging viruses and HIV-1. Virologica Sinica. 2013;28:71–80. doi: 10.1007/s12250-013-3313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


