Abstract
Background
Advancing research on the etiology, prevention, and treatment of psychopathology requires the field to move beyond modular conceptualizations of neural dysfunction toward understanding disturbance in key brain networks. Although some studies of anxiety and depression have begun doing so, they typically suffer from several drawbacks, including: (1) a categorical approach ignoring transdiagnostic processes, (2) failure to account for substantial anxiety and depression comorbidity, (3) examination of networks at rest, which overlooks disruption manifesting only when networks are challenged. Accordingly, the present study examined relationships between transdiagnostic dimensions of anxiety/depression and patterns of functional connectivity while goal maintenance was challenged.
Methods
Participants (n = 179, unselected community members and undergraduates selected to be high/low on anxiety/depression) performed a task in which goal maintenance was challenged (color-word Stroop) while fMRI data were collected. Analyses examined moderation by anxiety/depression of condition-dependent coupling between regions of dorsolateral prefrontal cortex (dlPFC) previously associated with approach and avoidance motivation and amygdala/orbitofrontal cortex (OFC).
Results
Anxious arousal was positively associated with amygdala↔right dlPFC coupling. Depression was positively associated with OFC↔right dlPFC coupling and negatively associated with OFC↔left dlPFC coupling.
Conclusions
Findings advance the field toward an integrative model of the neural instantiation of anxiety/depression by identifying specific, distinct dysfunctions associated with anxiety and depression in networks important for maintaining approach and avoidance goals. Specifically, findings shed light on potential neural mechanisms involved in attentional biases in anxiety and valuation biases in depression and underscore the importance of examining transdiagnostic dimensions of anxiety/depression while networks are challenged.
Keywords: amygdale; anxiety; depression; dorsolateral prefrontal cortex; orbitofrontal cortex, network; transdiagnostic
Given that the human brain is comprised of interconnected and interdependent circuits,[1] advancing research on the etiology, prevention, and treatment of psychiatric symptoms requires the field to move beyond modular conceptualizations of neural dysfunction (i.e., examining function in individual regions)[2, 3] toward characterizing pathology-related disturbance in key brain networks.[4] Doing so fosters a richer representation of the neural instantiation of pathology,[3] increasing diagnostic reliability/validity, and introducing potential novel treatment targets.
Anxiety and depression are extremely prevalent and costly, both for society and for those personally affected.[5-8] Although research has identified some areas of anxiety/depression-related disruption in functional brain circuits,[9-14] these studies have almost uniformly taken a categorical approach (i.e., DSM-IV diagnoses) that ignores processes shared across disorders. This is at odds with recent recognition that dimensional, transdiagnostic approaches will likely have more utility when translating basic neuroscience into clinical treatments.[15-17] Furthermore, relevant studies have rarely examined anxiety and depression simultaneously, despite high comorbidity,[18] leaving unclear whether findings are related to either or both. Finally, the majority of research on functional brain networks in anxiety and depression has employed data collected while participants were at rest, which may overlook disrupted network activity that manifests only when systems are challenged.[19] Accordingly, the present study examined the relationship between dimensions of anxiety/depression and patterns of functional connectivity while goal maintenance was challenged. Given that approach and avoidance goals appear to be differentially disrupted in anxiety and depression (avoidance increased in both, approach decreased only in depression),[20-23] the present study examined one brain network related to approach and another to avoidance,[24,25] allowing for the identification of anxiety/depression-specific dysfunction.
Several regions central to goal pursuit consistently differentiate individuals with and without anxiety/depression, including orbitofrontal cortex (OFC) and amygdala. With regard to anxiety, studies of OFC show consistent hyperactivation to aversive stimuli.[26,27] Both hyper- and hypoactivation have been detected in individuals with Major Depressive Disorder (MDD), depending on stimulus valence. For instance, a meta-analysis of emotional challenge studies and MDD found hyper- and hypoactivation in OFC for sad and happy stimuli, respectively.[28] Given research linking OFC to maintaining the average motivational value of stimuli,[29] hyperactivation in OFC may lead to the cost overestimation seen in anxiety[30] and depression,[31] and hypoactivation may lead to the reward underestimation seen in depression.[32] Both anxiety and depression consistently show hyperactivation in amygdala,[33-38] a subcortical structure central to identifying salient stimuli and enhancing motivationally relevant stimulus features.[39] Consequently, amygdala hyperactivation may play a role in the heightened salience for potential threat observed in anxiety in particular.[40]
Importantly for the focus of the present study on networks, emerging evidence suggests that both OFC and amygdala (anatomically mediated viaOFC) interact with dorsolateral prefrontal cortex (dlPFC) as part of a circuit instantiating the maintenance of approach/avoidance goals.[25] Research consistently implicates dlPFC in top-down biasing of processing (e.g., in OFC/amygdala) to be consistent with goals,[41] and regions of dlPFC have been implicated in the integration of motivational and executive function processes.[24, 42] Specifically, a region of left dlPFC has been selectively related to approach motivation and a region of right dlPFC to avoidance (see Fig. 1).[43,44] Thus, examining interactions between dlPFC and OFC/amygdala as a function of anxiety/depression may be particularly informative, because these connections appear to be important for effectively maintaining approach/avoidance goals.[25]
Figure 1.
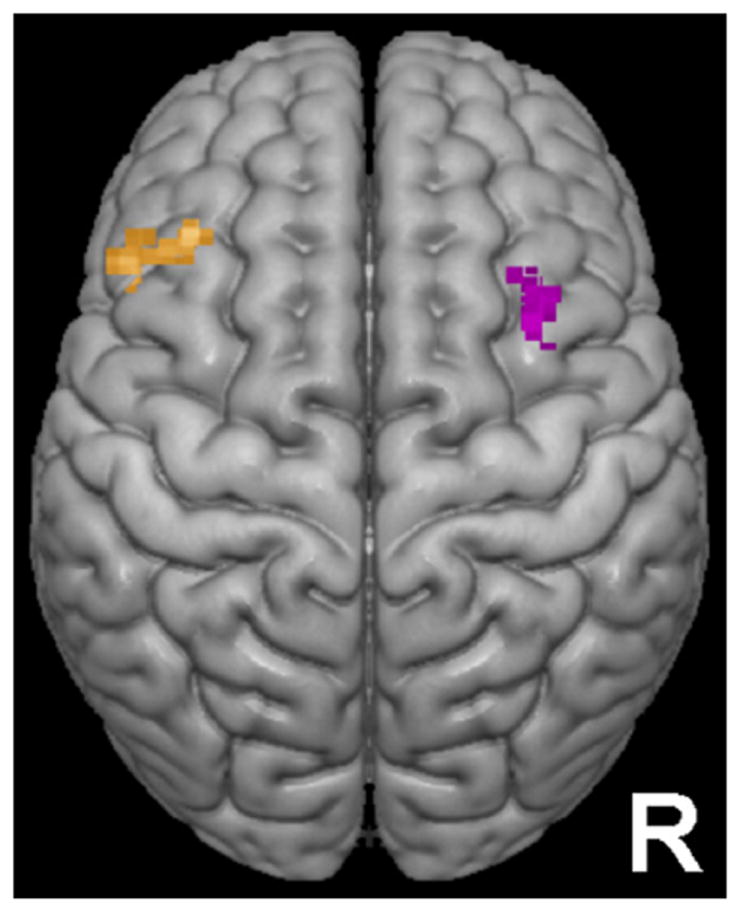
Regions of dorsolateral prefrontal cortex used as seed clusters. The orange cluster above left was previously associated with approach motivation.[43] The purple cluster above right was previously associated with avoidance motivation.[43]
The importance of examining connectivity with dlPFC is underscored by evidence of anxiety/depression-related dysfunction in this region. For example, hyperactivation in right dlPFC has been consistently reported when individuals encounter anxiety-relevant stimuli.[45-48] Several studies have also found an association between depression and rightward dlPFC lateralization.[49, 50] Furthermore, intervention research indicates that rightward dlPFC lateralization is functionally significant. For example, successful psychotherapy normalizes right dlPFC hyperactivation in individuals with Posttraumatic Stress Disorder (PTSD) viewing trauma-related imagery,[45] and disruption of right dlPFC via repetitive transcranial magnetic stimulation leads to reduced anxiety in individuals with Generalized Anxiety Disorder (GAD).[48] Additionally, research in our lab indicates that depression is associated with decreased left dlPFC↔dorsal anterior cingulate coupling[51] (although, a different dlPFC region was examined and coupling was based on source-localized electroencephalography).
Although dlPFC↔amygdala/OFC coupling in anxiety/depression has received little attention, several studies provide initial evidence of dysfunction in this circuit. For example, individuals with GAD[10] and MDD[52] exhibit greater right dlPFC↔amygdala connectivity at rest, and greater right dlPFC↔OFC coupling is evident in individuals with MDD performing a face-matching task.[53] Thus, emerging evidence supports the hypothesis that anxiety and depression involve dysfunction in this circuit, although these studies suffered from the flaws mentioned above.
PRESENT STUDY
The present study tested the hypothesis that anxiety and depression moderate dlPFC↔OFC/amygdala functional connectivity during goal maintenance and evaluated whether they do so differentially. Rather than taking a unitary approach, analyses examined two dimensions of anxiety whose importance appears to cross disorder boundaries. Specifically, the present study examined anxious apprehension (characterized by worry/verbal rumination and particularly evident in GAD[54-56]) and anxious arousal (characterized by somatic tension/sympathetic hyperarousal and particularly evident in Panic Disorder[57]). In addition, the present study examined anhedonic depression, a distinct depression facet that is distinguishable from the general negative affect shared with anxiety.[57,58]
Although anxious apprehension and arousal are separable dimensions, it is not the case that there is no conceptual or empirical overlap between these constructs. For example, both dimensions show strong relationships with avoidance motivation and weaker relationships with approach.[23] Indeed, this overlap is a key reason to examine these dimensions simultaneously (to ascertain unique effects). Despite overlap, there is a growing body of research showing differential effects when unique variance is examined.[51, 59, 60] Thus, it appears conceptually important and methodologically acceptable to examine both dimensions of anxiety.
To elicit goal-maintenance processing, the present task involved a goal that was challenged by irrelevant but distracting information, the color-word Stroop task.[61] Connectivity with two regions of dlPFC was assessed: a left dlPFC cluster associated with approach motivation and a right dlPFC cluster associated with avoidance.[43,44] In a previous study, we showed that connections between these clusters and OFC/amygdala were strengthened when goal maintenance was challenged.[25] Connections between dlPFC and OFC also varied as a function of trait motivation, supporting the relevance of these pathways in particular. Present hypotheses were that (1) both anxiety dimensions and anhedonic depression would be associated with increased right dlPFC↔OFC coupling, (2) anhedonic depression would also be linked to decreased left dlPFC↔OFCcoupling, and (3) both anxiety dimensions and anhedonic depression would exhibit increased right dlPFC↔amygdala coupling.
METHODS
PARTICIPANTS
The sample consisted of undergraduates (n = 81) and community members (n = 98) and was the same as used in.[25] Undergraduates were recruited from a larger pool (n = 2,723) based on their scores on three scales: the Penn State Worry Questionnaire (PSWQ[62]), and the Anxious Arousal (MASQ-AA) and Loss of Interest Anhedonic Depression (MASQ-AD) scales of the Mood and Anxiety Symptom Questionnaire.[57] Participants were contacted if (1) they scored ≥ 80th percentile (PSWQ ≥ 63, MASQ-AA ≥ 33, MASQ-AD ≥ 22) on one dimension and ≤ 50th percentile (PSWQ ≤ 49, MASQ-AA ≤ 25, MASQ-AD ≤ 17) on the others, (2) ≥ 80th percentile on all three, or (3) ≤ 50th percentile on all three. Unselected community members were recruited using advertisements placed in newspapers/electronic list-serves and from a local clinic. Study procedures were identical across undergraduates and community members.
Two hundred and twenty-seven participants completed the protocol, and data from 179 (60% female, mean age = 27.32, SD = 10.0) passed data quality screening. Exclusion criteria were (1) moved > 3.3 mm relative to the middle volume or > 2 mm relative to the previous volume, (2) committed errors on > 15% trials, (3) exhibited reaction times (RT) > 3 SD, (4) had susceptibility artifact in relevant areas, or (5) exhibited activation patterns indicative of motion. Questionnaire data were not available for one participant.
PSYCHOPATHOLOGY ASSESSMENT
The 16-item PSWQ assessed anxious apprehension, the 17-item MASQ-AA assessed anxious arousal, and an 8-item subscale of the MASQ-AD assessed anhedonic depression. PSWQ correlated 0.376 with MASQ-AA and 0.421 with MASQ-AD, and MASQ-AA correlated 0.525 with MASQ-AD (Ps < .001).
The Structured Clinical Interview for the DSM-IV (SCID[63]) was also administered. Given our focus on transdiagnostic dimensions of anxiety/depression, SCID data are not reported beyond disorder rates. With regard to depression, 46 participants had a diagnosis of MDD, eight of Dysthymia, and two of Depressive Disorder NOS. With regard to anxiety, 44 participants had at least one diagnosis (GAD = 21, Specific Phobia = 16, Social Phobia = 13, Obsessive Compulsive Disorder = 5, PTSD = 4, Anxiety Disorder NOS = 2, Panic Disorder = 1, Agoraphobia Without Panic = 1).
EXPERIMENTAL DESIGN
Participants completed two tasks during fMRI data collection: a color-word and an emotion-word Stroop (duration of each = 12.33 min). Only data from the color-word task are discussed. In the color-word Stroop, 256 trials were presented in 16 blocks (four congruent, four incongruent, eight neutral), with a variable ITI (2,000 +/−225 ms). Each trial consisted of one word presented in one of four ink colors, each color occurring equally often with each word type. Word meaning was the same as ink color in congruent trials (e.g., ‘RED’ in red ink), whereas word meaning differed from ink color in incongruent trials (e.g., ‘GREEN’ in red ink), and the two were unrelated in neutral trials (e.g., ‘LOT’ in red ink). Task condition alternated by block, and task and condition order were counterbalanced.
BEHAVIORAL DATA PROCESSING
Mean RT and accuracy were calculated per condition and entered into paired t-tests. The impact of psychopathology on behavior was evaluated by entering RT/accuracy into repeated-measures GLMs with psychopathology dimensions as between-participant predictors.
fMRI DATA COLLECTION
fMRI data were 370 EPI images (TR 2,000 ms, TE 25 ms, flip angle 80°, FOV 220 cm), each consisting of 38 axial slices (slice thickness 3 mm, 0.3 mm gap, resolution 3.4375 × 3.4375 mm). A 1 mm3 structural sequence was also acquired.
dlPFC SEED CLUSTERS
dlPFC seed areas were those identified in,[43] which used a subsample of the data used in the present analyses (i.e., undergraduate data). Analyses in[43] identified regions in which activation during incongruent was greater for those individuals who were higher on approach/avoidance temperament. One cluster, located in left dlPFC (BA 9, max z-coordinates = [−36,30,46]), was associated with approach temperament, and one cluster, located in right dlPFC (BA 9/8/6, max z-coordinates = [36,12,44]), was associated with avoidance. These dlPFC seeds were also used in[25], which initially identified the network tested in the present study.
fMRI DATA PROCESSING
Image processing and statistical analysis were implemented primarily via FSL.[64] Data were motion-corrected, intensity-normalized, temporally high-pass filtered, and spatially smoothed (FWHM = 5 mm). Temporal low-pass filtering was carried out using AFNI’s 3dDespike.
Psychophysiological interaction analyses were performed on the functional time series. For each dlPFC ROI, the mean value across voxels was extracted per time point to create a dlPFC predictor. In each analysis, eight predictors were entered: (1) one dlPFC predictor, (2) a predictor modeling incongruent versus congruent (= 1 during incongruent, −1 during congruent, 0 all other times), (3) the interaction of these predictors, (4–6) three nuisance task predictors (incongruent+congruent, neutral, rest), and (7–8) two nuisance predictors modeling mean signal in white matter and cerebral-spinal fluid (to remove brain-wide fluctuations). Task predictors were HRF convolved prior to creating the interaction term. β-maps were nonlinearly warped to the 2009 MNI152 symmetrical 1 mm3 template.[65]
Group-level mixed-effects were computed on the 1st-level β-maps associated with the interaction term. For each dlPFC ROI, PSWQ, MASQ-AA, and MASQ-AD were entered simultaneously as between participant predictors. A nuisance covariate modeled whether participants completed the color-word Stroop first. AlphaSim was used to estimate overall significance level, with an individual-voxel threshold of 2.0537 and an overall corrected P of .05. For a priori directional hypotheses, one-tailed tests were used; two-tailed tests were used otherwise. Masks of OFC and amygdala limited the voxels under consideration to relevant regions.
To examine the relationship between PPI coupling and behavior, mean coupling for each cluster was extracted and entered as a between participant predictor in repeated-measures GLM (one accuracy outlier was excluded). Analyses were rerun with psychopathology scores entered as covariates to ensure that effects were not driven by shared variance. All analyses remained significant.
Task main effects were created by including only task predictors in 1st-level models and averaging across participants (using a gray-matter mask). Given the large sample and task effect size, a voxel threshold of 3.8906 was used.
Only significant effects are reported.
ANALYSES TO RULE-OUT POTENTIAL CONFOUNDS
To ensure that findings were not driven by variance shared between anxiety/depression and approach/avoidance motivation,[25] analyses were rerun with motivation measures as covariates. To ensure that findings were not driven by differential motion-related variance or SNR, analyses were rerun with motion/SNR estimates covaried. Given significant shared variance between psychopathology measures, it is possible that the unique variance associated with each predictor is not measuring the construct it was meant to represent. Therefore, analyses were repeated individually for each predictor. To ensure that performance differences did not drive present findings, analyses were rerun with performance estimates (RT/accuracy interference) covaried. All findings remained highly significant for these analyses.
OVERLAP WITH PREVIOUS STUDIES
Given that previous studies have used datasets that overlap with the present dataset, we provide the degree of sample overlap and a short summary of the analyses used in past studies (Table 1), along with correlations between present measures and those from past studies (Table 2). As evident in these tables, the present study builds on, but is distinct from, the two previous Spielberg et al. studies. Furthermore, the data examined in the present study are distinct from those used in[51, 66] (fMRI vs. EEG connectivity) and[67] (connectivity vs. task effects). In summary, present findings are both conceptually and analytically distinct from previous work in our lab.
TABLE 1.
Overlap with previous studies
| Study | Sample Overlap for fMRI Data | Analysis Strategy |
|---|---|---|
| Silton et al. (2010) | 13.6% | Used CW Stroop fMRI data from sample subset to seed EEG source localization; examined EEG connectivity between left dlPFC and dACC. |
| Silton et al. (2011) | 13.6% | Built on Silton et al. (2010); examined moderation of EEG connectivity between left dlPFC and dACC by depression and anxiety. |
| Spielberg et al. (2011) | 44.6% | Examined relationship between CW Stroop task activation (in bilateral dlPFC) and measures of approach and avoidance temperament; resultant clusters were used as seed clusters for Spielberg et al. (2012) and the present study. |
| Spielberg et al. (2012) | 97.2% | Built on Spielberg et al. (2011); examined fMRI psychophysiological connectivity between dlPFC seed clusters and OFC, amygdala, basal ganglia, and cingulate; examined moderation of connectivity by approach and avoidance temperament; resultant connectivity model used as the basis for the present study. |
| Warren et al. (2013) | 40.8% | Examined relationship between CW Stroop task activation and two measures of inhibition (Stroop RT interference, self-report measure); examined whether depression and anxiety moderated activation in resultant clusters. |
Note: fMRI = function magnetic resonance imaging; CW = color-word (Stroop); EEG = electroencephalography; dlPFC = dorsolateral prefrontal cortex; dACC = dorsal anterior cingulate; OFC = orbitofrontal cortex; RT = reaction time.
TABLE 2.
Correlation between psychopathology scores and measures used in previous studies with some overlapping data
| Measure | Approach temperament (n = 174) | Avoidance temperament (n = 174) | BRIEF inhibition (n = 73) | Color-word Stroop Incongruent – Neutral RT (n = 73) |
|---|---|---|---|---|
| Anxious Apprehension (PSWQ) | −0.44** | 0.80** | 0.09 | −0.13 |
| Anxious Arousal (MASQ-AA) | −0.19** | 0.41** | 0.36** | −0.17 |
| Anhedonic Depression (MASQ-AD) | −0.37** | 0.60** | 0.28* | −0.11 |
Note:
P < .05;
P < .01;
fMRI = function magnetic resonance imaging; CW= color-word (Stroop); EEG = electroencephalography; dlPFC = dorsolateral prefrontal cortex; dACC = dorsal anterior cingulate; OFC = orbitofrontal cortex; RT = reaction time.
RESULTS
TASK MAIN EFFECTS
Behavioral Data
Participants had slower RT (t(168) = 22.9, P < .001) and made more errors (RT; t(168) = 11.0, P < .001) during incongruent trials.
fMRI Data
Twelve clusters emerged in which activation for incongruent was greater than for congruent trials, whereas seven clusters had the converse pattern (Table 3).
TABLE 3.
Task main effects
| Region | Cluster size (mm3) | Mean z-value | Location
|
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| B IFG/MFG/SFG/OFC/insula/precentral/parahippocampus/GP/putamen/thalamus/SN/caudate/dACC/PCC /paracingulate (BA 6/8/9/10/13/23/24/29/30/32/44/45/46/47) | 168,250 | 5.45 | −4 | 9 | 20 |
| L ITG/MTG /fusiform (BA 20/21/37) | 15,010 | 4.83 | −45 | −62 | −17 |
| L MTG/STG/MOG/SOG/IPL/SPL/cuneus/precuneus/supramarginal (BA 7/19/31/39 /40) | 47,999 | 5.70 | −26 | −60 | 46 |
| R MTG (BA 21) | 129 | 4.06 | 59 | −37 | −6 |
| R IOG/fusiform/B lingual (BA 17/18/37) | 12,538 | 4.51 | 20 | −83 | −17 |
| R ITG/fusiform (BA 20/37) | 446 | 4.18 | 63 | −53 | −14 |
| R STG/IPL/angular/supramarginal/precuneus (BA 7/31/39/40) | 12,110 | 4.61 | 36 | −60 | 42 |
| L MFG/SFG (BA 9/10) | 1,602 | 4.35 | −27 | 49 | 21 |
| R MFG (BA 10) | 318 | 4.26 | 38 | 52 | 2 |
| L cuneus/lingual (BA 18/30) | 2,053 | 4.30 | −6 | −70 | 6 |
| R cuneus/lingual (BA 18/30) | 974 | 4.32 | 12 | −72 | 8 |
| M precuneus (BA 7) | 197 | 4.20 | −1 | −50 | 70 |
| M OFC (BA 11) | 4,364 | −4.59 | 0 | 41 | −20 |
| M sgACC (BA 25) | 254 | −4.19 | 0 | 18 | −10 |
| L insula/precentral (BA 13) | 1,513 | −4.49 | −41 | −18 | 15 |
| R insula/precentral (BA 13) | 666 | −4.32 | 45 | −14 | 13 |
| R insula/precentral (BA 6/13) | 482 | −4.40 | 52 | −6 | 3 |
| R precentral/postcentral (BA 4/6/43) | 129 | −4.12 | 60 | −5 | 21 |
| L precentral/postcentral (BA 3/4) | 302 | −4.08 | −63 | −11 | 37 |
Note: L = left; R = right; B = bilateral; M = medial; BA = Brodmann’s area; IFG = inferior frontal gyrus; MFG = middle frontal gyrus; SFG = superior frontal gyrus; OFC = orbitofrontal cortex; ITG = inferior temporal gyrus; MTG = middle temporal gyrus; STG = superior temporal gyrus; IPL = inferior parietal lobule; SPL = superior parietal lobule; IOG = inferior occipital gyrus; MOG = middle occipital gyrus; SOG = superior occipital gyrus; dACC = dorsal anterior cingulate cortex; sgACC = subgenual anterior cingulate cortex; PCC = posterior cingulate cortex; GP = globus pallidus; SN = substantia nigra; Location = coordinates are for the center of mass.
ANXIETY AND DEPRESSION MODERATING RIGHT DLPFC↔OFC/AMYGDALA COUPLING
Consistent with hypothesis 1, MASQ-AD was uniquely associated with greater right dlPFC↔medial OFC coupling (Table 4, Fig. 2) when maintenance of the current goal was challenged. This OFC cluster overlapped the region that evidenced decreased activation for task main effects. Greater coupling in this circuit during the incongruent condition was related to more errors (F(1,166) = 4.42, P = .037). Consistent with hypothesis 3, MASQ-AA was uniquely associated with greater right dlPFC↔left amygdala coupling.
TABLE 4.
Moderation by psychopathology of coupling with dorsolateral prefrontal cortex
| Region | Cluster Size (mm3) | Mean z-value | Location
|
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Anxious apprehension | |||||
| – | – | – | – | – | – |
| Anxious arousal | |||||
| Coupling with Right dlPFC (Related to Avoidance Motivation) | |||||
| Left amygdala | 587 | 2.42 | −21 | 2 | −21 |
| Anhedonic depression | |||||
| Coupling with Left dlPFC (Related to Approach Motivation) | |||||
| Right lateral OFC (BA 47) | 484 | −2.38 | 51 | 29 | −11 |
| Coupling with Right dlPFC (Related to Avoidance Motivation) | |||||
| Medial OFC (BA 11) | 578 | 2.46 | −2 | 39 | −16 |
Note: OFC = orbitofrontal cortex; dlPFC = dorsolateral prefrontal cortex; Location = coordinates are for the center of mass.
Figure 2.
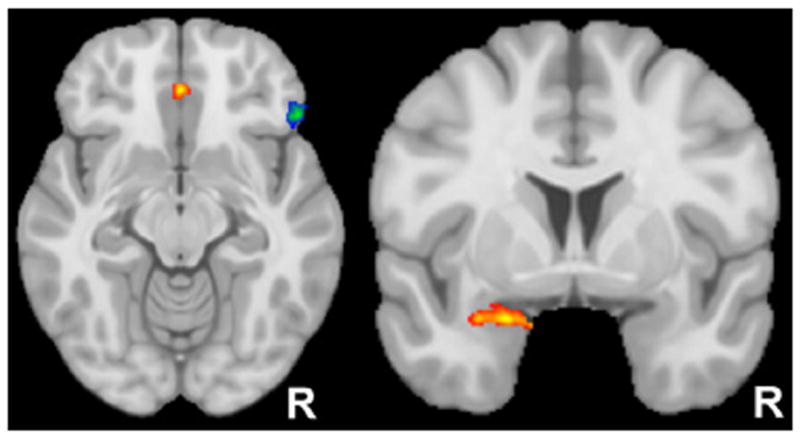
Regions where anhedonic depression or anxious arousal moderated coupling with dorsolateral prefrontal cortex. The axial slice above left (z = −12) displays the two clusters in orbitofrontal cortex (OFC) exhibiting condition-dependent connectivity that was moderated by anhedonic depression. Specifically, depression was associated with decreased coupling between left dorsolateral prefrontal cortex (associated with approach motivation) and the blue/green OFC cluster. In contrast, depression was associated with increased coupling between right dorsolateral prefrontal cortex (associated with avoidance motivation) and the red/yellow cluster. The coronal slice above right (y = 3) displays the amygdala cluster exhibiting condition-dependent connectivity that was moderated by anxious arousal. Specifically, anxious arousal was associated with increased coupling between right dorsolateral prefrontal cortex (associated with avoidance motivation) and the red/yellow amygdala cluster.
ANXIETY AND DEPRESSION MODERATING LEFT DLPFC↔OFC/AMYGDALA COUPLING
Consistent with hypothesis 2, higher MASQ-AD was uniquely associated with reduced left dlPFC↔rightlateral OFC coupling (Table 4, Fig. 2).
DISCUSSION
The present study tested the hypothesis that transdiagnostic dimensions of anxiety and depression are associated with processing differences in brain networks related to approach/avoidance motivation. Consistent with predictions, anxious arousal was uniquely associated with greater right dlPFC↔amygdala coupling (Fig. 2). This right dlPFC region has been previously linked with avoidance motivation,[43,44] both individually and as a central node in a network thought to implement avoidance goal pursuit.[24,25]
Anhedonic depression was uniquely associated with both increased and reduced dlPFC↔OFC coupling (Fig. 2). Specifically, increased coupling was observed for right dlPFC and reduced coupling was evident with left dlPFC. As mentioned above, this right dlPFC region appears to play an important role in avoidance goal pursuit, and evidence suggests that the left dlPFC region examined is similarly central for approach goal pursuit.[24,25,43,44] Importantly, differences in coupling were specific to times when goal maintenance was challenged, rather than at rest as examined in most previous studies in this area.
ANXIOUS AROUSAL AND RIGHT DLPFC↔AMYGDALA COUPLING
In light of extensive evidence that dlPFC engages in top-down biasing,[41] one interpretation of present findings is that right dlPFC biases amygdala to overidentify goal-irrelevant stimuli as salient/threatening in high anxious arousal individuals. Given the correlational nature of present analyses, it is also possible that amygdala influences dlPFC, and this hypothesis has some support.[68] Specifically, amygdala may overidentify irrelevant stimuli as salient/threatening in anxious arousal, in turn increasing the maintenance of avoidance goals in right dlPFC via bottom-up influence.
Regardless of whether present findings reflect dlPFC↑amygdala influence or vice versa, findings delineate a potential neural mechanism supporting the attentional biases toward goal-irrelevant stimuli found in anxiety.[69] Given that this bias is observed across anxiety disorders[69] and that a transdiagnostic anxiety dimension (anxious arousal) was used in the present study, dysfunction in this circuit could serve as a biomarker for anxiety-related attentional biases. Considered in the context of proposals that biased attention is an endophenotype for anxiety,[17] present findings further define this potential transdiagnostic endophenotype.
ANHEDONIC DEPRESSION AND DLPFC↔OFC COUPLING
Given evidence that OFC is key to maintaining average stimulus value, one interpretation of present findings is that the biases in valuation observed in depression[31,32] are due to differences in top-down dlPFC regulation of OFC. Specifically, when healthy individuals maintain approach goals, left dlPFC may bias OFC to represent the value of goal-relevant stimuli as more rewarding than that of irrelevant stimuli, whereas this adaptive process may not occur in depression. Similarly, present findings suggest that, when individuals with depression maintain avoidance goals, right dlPFC biases OFC to overvalue the aversive aspects of stimuli. If so, present findings indicate that changing biased valuation in depression may be accomplished by targeting this circuit (i.e., upregulating left dlPFC↔OFC coupling/downregulating right dlPFC↔OFC coupling).
Interestingly, the medial OFC region found to have increased coupling with right dlPFC largely overlapped the region evidencing decreased activation for task main effects (incongruent–congruent). Thus, potential upregulation of this region by dlPFC may actually impair performance, which is supported by the finding that increased coupling in this circuit was related to more errors.
As mentioned earlier, present analyses cannot determine direction of effects. Thus, it is possible that, in depression, biased valuation in OFC influences goal-maintenance processes in dlPFC. For example, when healthy individuals encounter potentially rewarding stimuli, OFC may increase the likelihood of pursuing approach goals via upregulation of relevant left dlPFC processing. Thus, decreased coupling in this circuit is a potential mechanism by which individuals high in anhedonic depression fail to pursue approach goals.
Regardless of the direction of effect, present findings provide insight into the manner in which biases in stimulus valuation interact with the pursuit of goals in depression. Importantly, anhedonic depression was associated with differences in coupling with both approach- and avoidance-related dlPFC regions, in contrast to anxious arousal, which evidenced only differential coupling of the avoidance-related area. This is consistent with previous research indicating that dysfunction in approach motivation is depression-specific.[23] Present findings provide insight into one potential source of this difference, namely coupling with left dlPFC. Thus, it is possible that hypocoupling with left dlPFC serves as a predisposing/maintaining factor for depression uniquely, whereas hypercoupling with right dlPFC is relevant for both anxiety and depression.
Contrary to prediction, no findings emerged for anxious apprehension. It is possible that dlPFC↔amygdala/OFC coupling is not central to this anxiety dimension. For example, recent research indicates that anxious apprehension involves dysfunctional coupling between Broca’s area and attention-related regions.[60] Furthermore, the task used in the present study may not have engaged processes relevant to the core pathology of anxious apprehension. For example, given the future orientation of anxious apprehension,[70] future-oriented tasks (e.g., intertemporal choice) may be needed to elicit biased coupling. Given evidence of strong biases in punishment-related valuation[71] and a strong tendency to pursue avoidance goals[23] in anxious apprehension, future research should examine right dlPFC↔OFC coupling during a future-oriented task.
STRENGTHS AND LIMITATIONS
The present study benefited from an unusually large fMRI sample and examination of transdiagnostic anxiety/depression dimensions rather than DSM diagnoses. Examining anxiety and depression simultaneously allowed for appropriate parsing of variance, despite considerable comorbidity. As well, the present study examined network activity while goal maintenance was challenged rather than at rest, ensuring that dysfunctional activation evident only when the system is challenged would not be missed[19]. Finally, the present study examined brain circuits rather than only activation in specific regions, increasing the level of complexity in representing and understanding the neuropathology of anxiety/depression.
Limitations of the present study include the correlational nature of analyses, leaving direction of influence unresolved. Also, the present design is cross-sectional, leaving unclear whether observed differences are predisposing or maintaining factors or consequences of having anxiety/depression. Future research with a prospective design is needed to discern the degree to which dysfunctional coupling is part of the etiological chain causing or maintaining anxiety/depression. Finally, the present study administered just one of many relevant challenges that may reveal dysfunctional coupling. On balance, present findings suggest specific elements of a model of the neural instantiation of anxiety and depression, including both shared and distinct mechanisms.
References
- 1.Sporns O. The human connectome: a complex network. Ann NY Acad Sci. 2011;1224:109–125. doi: 10.1111/j.1749-6632.2010.05888.x. [DOI] [PubMed] [Google Scholar]
- 2.Meehan TP, Bressler SL. Neurocognitive networks: findings, models, and theory. Neurosci Biobehav Rev. 2012;36(10):2232–2247. doi: 10.1016/j.neubiorev.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Morris SE, Cuthbert BN. Research Domain Criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin Neurosci. 2012;14(1):29–37. doi: 10.31887/DCNS.2012.14.1/smorris. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC, DuPont RL, Berglund P, Wittchen HU. Impairment in pure and comorbid Generalized Anxiety Disorder and major depression at 12 months in two national surveys. Am J Psychiatry. 1999;156(12):1915–1923. doi: 10.1176/ajp.156.12.1915. [DOI] [PubMed] [Google Scholar]
- 7.Wittchen HU, Carter RM, Pfister H, et al. Disabilities and quality of life in pure and comorbid Generalized Anxiety Disorder and major depression in a national survey. Int Clin Psychopharmacol. 2000;15(6):319–328. doi: 10.1097/00004850-200015060-00002. [DOI] [PubMed] [Google Scholar]
- 8.Simon G, Ormel J, VonKorff M, Barlow W. Health care costs associated with depressive and anxiety disorders in primary care. Am J Psychiatry. 1995;152(3):352–357. doi: 10.1176/ajp.152.3.352. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, et al. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139(1):56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etkin A, Prater KE, Schatzberg AF, et al. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66(12):1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- 11.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56(3):881–889. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 12.Lanius RA, Bluhm RL, Coupland NJ, et al. Default mode network connectivity as a predictor of post-traumatic stress disorder symptom severity in acutely traumatized subjects. Acta Psychiatr Scand. 2010;121(1):33–40. doi: 10.1111/j.1600-0447.2009.01391.x. [DOI] [PubMed] [Google Scholar]
- 13.Sheline YI, Barch DM, Price JL, et al. The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veer IM, Beckmann CF, van Tol MJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4 doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casey BJ, Craddock N, Cuthbert BN, et al. DSM-5 and RDoC: progress in psychiatry research? Nat Rev Neurosci. 2013;14(11):810–814. doi: 10.1038/nrn3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126s. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller GA, Rockstroh B. Endophenotypes in psychopathology research: where do we stand? Annu Rev Clin Psychol. 2013;9:177–213. doi: 10.1146/annurev-clinpsy-050212-185540. [DOI] [PubMed] [Google Scholar]
- 18.Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- 19.Coan JA, Allen JJ, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biol Psychol. 2006;72(2):198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickson JM, MacLeod AK. Approach and avoidance goals and plans: their relationship to anxiety and depression. Cogn Ther Res. 2004;28(3):415–432. [Google Scholar]
- 21.Stein MB, Paulus MP. Imbalance of approach and avoidance: the yin and yang of anxiety disorders. Biol Psychiatry. 2009;66(12):1072–1074. doi: 10.1016/j.biopsych.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trew JL. Exploring the roles of approach and avoidance in depression: an integrative model. Clin Psychol Rev. 2011;31(7):1156–1168. doi: 10.1016/j.cpr.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Spielberg JM, Heller W, Silton RL, et al. Approach and avoidance profiles distinguish dimensions of anxiety and depression. Cogn Ther Res. 2011;35(4):359–371. [Google Scholar]
- 24.Spielberg JM, Heller W, Miller GA. Hierarchical brain networks active in approach and avoidance goal pursuit. Front Hum Neurosci. 2013;7:284. doi: 10.3389/fnhum.2013.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spielberg JM, Miller GA, Warren SL, et al. A brain network instantiating approach and avoidance motivation. Psychophysiology. 2012;49(9):1200–1214. doi: 10.1111/j.1469-8986.2012.01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bystritsky A, Pontillo D, Powers M, et al. Functional MRI changes during panic anticipation and imagery exposure. Neuroreport. 2001;12(18):3953–3957. doi: 10.1097/00001756-200112210-00020. [DOI] [PubMed] [Google Scholar]
- 27.Veit R, Flor H, Erb M, et al. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci Lett. 2002;328(3):233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29(6):683–695. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Doherty JP. Lights, camembert, action! The role of human orbitofrontal cortex in encoding stimuli, rewards, and choices. Ann N Y Acad Sci. 2007;1121:254–272. doi: 10.1196/annals.1401.036. [DOI] [PubMed] [Google Scholar]
- 30.Foa EB, Franklin ME, Perry KJ, Herbert JD. Cognitive biases in generalized social phobia. J Abnorm Psychol. 1996;105(3):433–439. [PubMed] [Google Scholar]
- 31.Voncken MJ, Bogels SM, Peeters F. Specificity of interpretation and judgemental biases in social phobia versus depression. Psychol Psychother. 2007;80(Pt 3):443–453. doi: 10.1348/147608306X161890. [DOI] [PubMed] [Google Scholar]
- 32.Chentsova-Dutton Y, Hanley K. The effects of anhedonia and depression on hedonic responses. Psychiatry Res. 2010;179(2):176–180. doi: 10.1016/j.psychres.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biol Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Stein MB, Goldin PR, Sareen J, et al. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59(11):1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 35.Rauch AV, Reker M, Ohrmann P, et al. Increased amygdala activation during automatic processing of facial emotion in schizophrenia. Psychiatry Res. 2010;182(3):200–206. doi: 10.1016/j.pscychresns.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 37.Siegle GJ, Thompson W, Carter CS, et al. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61(2):198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 38.Abler B, Erk S, Herwig U, Walter H. Anticipation of aversive stimuli activates extended amygdala in unipolar depression. J Psychiatr Res. 2007;41(6):511–522. doi: 10.1016/j.jpsychires.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neurosci. 2010;11(11):773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bar-Haim Y, Lamy D, Pergamin L, et al. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 41.Banich MT. Executive function the search for an integrated account. Curr Direc Psychol Sci. 2009;18(2):89–94. [Google Scholar]
- 42.Sakagami M, Watanabe M. Integration of cognitive and motivational information in the primate lateral prefrontal cortex. Ann N Y Acad Sci. 2007;1104:89–107. doi: 10.1196/annals.1390.010. [DOI] [PubMed] [Google Scholar]
- 43.Spielberg JM, Miller GA, Engels AS, et al. Trait approach and avoidance motivation: lateralized neural activity associated with executive function. NeuroImage. 2011;54(1):661–670. doi: 10.1016/j.neuroimage.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spielberg JM, Miller GA, Warren SL, et al. Trait motivation moderates neural activation associated with goal pursuit. Cogn Affect Behav Neurosci. 2012;12(2):308–322. doi: 10.3758/s13415-012-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lindauer RJ, Booij J, Habraken JB, et al. Effects of psychotherapy on regional cerebral blood flow during trauma imagery in patients with Post-traumatic Stress Disorder: a randomized clinical trial. Psychol Med. 2008;38(4):543–554. doi: 10.1017/S0033291707001432. [DOI] [PubMed] [Google Scholar]
- 46.Morey RA, Petty CM, Cooper DA, et al. Neural systems for executive and emotional processing are modulated by symptoms of Posttraumatic Stress Disorder in Iraq War veterans. Psychiatry Res. 2008;162(1):59–72. doi: 10.1016/j.pscychresns.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Heuvel OA, Veltman DJ, Groenewegen HJ, et al. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry. 2005;62(8):922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- 48.Bystritsky A, Kerwin L, Feusner J. A pilot study of cranial electrotherapy stimulation for generalized anxiety disorder. J Clin Psychiatry. 2008;69(3):412–417. doi: 10.4088/jcp.v69n0311. [DOI] [PubMed] [Google Scholar]
- 49.Herrington JD, Heller W, Mohanty A, et al. Localization of asymmetric brain function in emotion and depression. Psychophysiology. 2010;47(3):442–454. doi: 10.1111/j.1469-8986.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller GA, Crocker LD, Spielberg JM, et al. Issues in localization of brain function: the case of lateralized frontal cortex in cognition, emotion, and psychopathology. Front Integr Neurosci. 2013;7:2. doi: 10.3389/fnint.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silton RL, Heller W, Engels AS, et al. Depression and anxious apprehension distinguish frontocingulate cortical activity during top-down attentional control. J Abnorm Psychol. 2011;120(2):272–285. doi: 10.1037/a0023204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yue Y, Yuan Y, Hou Z, et al. Abnormal functional connectivity of amygdala in late-onset depression was associated with cognitive deficits. PLoS One. 2013;8(9):e75058. doi: 10.1371/journal.pone.0075058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frodl T, Bokde AL, Scheuerecker J, et al. Functional connectivity bias of the orbitofrontal cortex in drug-free patients with major depression. Biol Psychiatry. 2010;67(2):161–167. doi: 10.1016/j.biopsych.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 54.Andrews V, Borkovec TD. The differential effects of inductions of worry, somatic anxiety, and depression on emotional experience. J Behav Ther Exp Psychiatry. 1988;19(1):21–26. doi: 10.1016/0005-7916(88)90006-7. [DOI] [PubMed] [Google Scholar]
- 55.Barlow DH, Blanchard EB, Vermilyea JA, et al. Generalized anxiety and generalized anxiety disorder: description and reconceptualization. Am J Psychiatry. 1986;143(1):40–44. doi: 10.1176/ajp.143.1.40. [DOI] [PubMed] [Google Scholar]
- 56.Barlow DH. Disorders of emotion. Psychological Inquiry. 1991;2(1):58–71. [Google Scholar]
- 57.Watson D, Clark LA, Weber K, et al. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. J Abnorm Psychol. 1995;104(1):15–25. doi: 10.1037//0021-843x.104.1.15. [DOI] [PubMed] [Google Scholar]
- 58.Nitschke JB, Heller W, Imig JC, et al. Distinguishing dimensions of anxiety and depression. Cogn Ther Res. 2001;25(1):1–22. [Google Scholar]
- 59.Engels AS, Heller W, Spielberg JM, et al. Co-occurring anxiety influences patterns of brain activity in depression. Cogn Affect Behav Neurosci. 2010;10(1):141–156. doi: 10.3758/CABN.10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spielberg JM, Leon AA, Bredemeier K, et al. Anxiety type modulates immediate versus delayed engagement of attention-related brain regions. Brain Behav. 2013;3(5):532–551. doi: 10.1002/brb3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643. [Google Scholar]
- 62.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28(6):487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 63.First M, Spitzer R, Gibbon M, Williams J. Structural Clinical Interview for DSM-IV-TR, Research Version, Non-patient Edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 64.Jenkinson M, Beckmann CF, Behrens TE, et al. Fsl. NeuroImage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 65.Fonov V, Evans A, McKinstry R, et al. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47:S102. [Google Scholar]
- 66.Silton RL, Heller W, Towers DN, et al. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. NeuroImage. 2010;50(3):1292–1302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Warren SL, Crocker LD, Spielberg JM, et al. Cortical organization of inhibition-related functions and modulation by psychopathology. Front Hum Neurosci. 2013;7:271. doi: 10.3389/fnhum.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402(6759):294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- 69.Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: an integrative review. Clin Psychol Rev. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Watkins E, Moulds M, Mackintosh B. Comparisons between rumination and worry in a non-clinical population. Behav Res Ther. 2005;43(12):1577–1585. doi: 10.1016/j.brat.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 71.Berenbaum H, Thompson RJ, Pomerantz EM. The relation between worrying and concerns: the importance of perceived probability and cost. Behav Res Ther. 2007;45(2):301–311. doi: 10.1016/j.brat.2006.03.009. [DOI] [PubMed] [Google Scholar]


