Abstract
Survival outcomes in patients with squamous cell carcinoma of the head and neck (HNSCC) vary by extent of disease, behavioral, and socioeconomic factors. We assessed the extent to which pre-treatment pain influences survival in 2340 newly diagnosed patients with HNSCC, adjusting for disease stage, symptoms, pain medications, comorbidities, smoking, alcohol consumption, age, sex, and race/ethnicity. Patients rated their pain at presentation to the cancer center (0= ‘no pain’ and 10= ‘pain as bad as you can imagine’). Survival time was calculated from the date of diagnosis to the date of death of any cause or last follow-up. Five year overall survival was calculated for all the variables assessed in the study. Severe pain (≥7) was most prevalent among those with oral cancer (20.4%; pharynx=18.8%; larynx=16.1%) and significantly varied by tumor stage, fatigue severity, smoking status, comorbid lung disease, and race (all p<0.05) across cancer diagnoses. Overall 5 year survival varied by pain for oral (severe pain=31% versus non-severe=52%; p<0.001) and pharyngeal cancer (severe pain=33%, versus non-severe=53%; p<0.001). Multivariable analyses showed that pain persisted as an independent prognostic factor for survival.
Pain reported prior to treatment should be considered in understanding survival outcomes in HNSCC patients.
Perspective
Pre-treatment pain was an independent predictor of survival in a large sample of HNSCC patients even after accounting for TNM stage, fatigue, age, race/ethnicity, smoking and alcohol intake. Therefore, symptoms at presentation and pre-cancer treatment are important factors to be considered in understanding survival outcomes in HNSCC patients.
Keywords: pain, depression, fatigue, symptoms, survival, head and neck
Introduction
Head and neck cancer is the sixth most common malignancy worldwide. Squamous cell cancer of the head and neck (HNSCC) is the most common head and neck cancer that includes cancers of the oral cavity (including the gums and tongue), pharynx, and larynx. In the US, more than 53,640 men and women are expected to be diagnosed with head and neck cancers in 20131. Relative to certain other cancers, patients with HNSCC have a better prognosis. For all stages combined, the 5-year survival rates for oral and pharyngeal cancers and laryngeal cancers are 56% and 62%, respectively1. However, an estimated two thirds present with advanced stage of disease and with debilitating symptoms that impact their quality of life.
Pain is often one of the first signs of head and neck cancer. Head and neck cancer pain may be due to the disease itself (tumor) or as a consequence of therapy. Nociceptive pain may arise due to the destructive lesions, and direct bone and soft tissue involvement22; and neuropathic pain may arise due to invasion of nerves, the inflammatory milieu adjacent to nerves and as toxicity of treatment6,22. Acute pain due to therapy is extremely common secondary to ablative surgery, chemo- and/or radiotherapy11,24. Up to 80% of patients with head and neck cancer report pain during treatment, and for some 36%, pain persists beyond treatment9. To date, limited data exist on pre-treatment pain and its influence on survival outcomes in head and neck cancer patients.
Tumor (T), nodal (N) and metastasis (M) stage (TNM stage) is the single most important prognostic factor and treatment determinant in HNSCC. Patients diagnosed in early stages have better prognosis and health outcomes. Behavioral factors such as alcohol intake and smoking10,31 have also been shown to influence survival outcomes. While a number of studies also suggest the importance of pain as an independent predictor of survival in patients with HNSCC and other cancers14,19, the limited sample size and a lack of comprehensive assessment of clinical (disease stage, comorbid conditions) behavioral (smoking, alcohol consumption) and epidemiological (age, sex, race/ethnicity) factors known to influence survival in HNSCC, limit the generalizability of study findings.
In the present study, we used a large sample of patients (n=2340) with HNSCC to assess the importance of pain, reported at diagnosis, prior to cancer treatment, in predicting survival outcomes. We assessed the relative importance of pain on survival by including the assessment of clinical (disease stage, comorbid conditions) behavioral (smoking, alcohol consumption) and epidemiological (age, sex, race/ethnicity) factors known to influence survival in HNSCC. Because studies show a high correlation between pain, depression, and fatigue, we also included these symptoms as covariates in our analyses. In the United States, the treatment and management of patients with cancer is based on a multidisciplinary approach, with symptom control as an important aspect in the care of patients with HNSCC. Therefore, understanding the extent to which pre-treatment pain reported at presentation impacts survival outcomes has important clinical significance.
Materials and Methods
Study Population
The study population included newly diagnosed patients with squamous cell carcinoma of the head and neck presenting to The University of Texas M.D. Anderson Cancer Center (MDACC) from January 1, 2000 through December 31, 2009 who received treatment at MDACC for HNSCC. This study was approved by the Institutional Review Board at MDACC.
Epidemiology and clinical data collection at presentation to the Cancer Center
Trained M.D. Anderson staff administered questionnaires to patients presenting at the Cancer Center, prior to being seen by clinicians. The questionnaire was developed by an interdisciplinary team of scientists representing the areas of epidemiology, behavioral science, medical oncology, etc. The overarching goal was to understand the epidemiology of the different types of cancers and the underlying factors associated with and risk factors for, cancer, cancer progression, and survival outcomes. Many questionnaire items were considered, but the committee was very cognizant of patient burden, and the final set of questions was decided through consensus. Clinical data including stage of disease were abstracted from patients' charts.
Outcome Variable
Survival time was calculated from the date of diagnosis to the date of death of any cause or last follow-up. Patients who were lost to follow-up or were still alive at the end of the follow-up period were censored. Five year overall survival was calculated for all the variables assessed in the study.
Main Independent Variable
Patients were first asked “have you experienced pain in the last week?” and “circle the number that best describes the pain you are having” on an 11-point numeric scale, (0= ‘no pain’ and 10= ‘pain as bad as you can imagine’), a recommended standard for pain assessment in clinical studies of pain5.
Other Co-factors (potential confounders)
Clinical factors included the extent of disease using AJCC TNM and comorbid conditions. TNM classification, which includes information on the primary tumor (T), lymph node involvement (N), and distant metastasis (M) were abstracted from medical records by trained and certified tumor registrars. Comorbidities reported by the patients included heart disease, stroke, hypertension, diabetes, and lung disease.
Because studies show a high correlation between pain, depression, and fatigue, we also used the following items “during the past 4 weeks, have you felt downhearted and blue?” and “during the past 4 weeks, did you have a lot of energy?” to assess depressed mood and fatigue respectively. These items, with a 6-point Likert response format, were taken from the SF-12. The SF-12 is a validated measure of quality of life and is extensively used in studies of cancer patients34-37.
Behavioral factors included smoking and alcohol intake. Smoking and alcohol intake were assessed at time of presentation and prior to treatment. Smoking was categorized as never smoker; former smoker and current smoker. Alcohol intake was classified as never, social, moderate, and heavy alcohol use. Heavy alcohol use was defined as 4 or more drinks per day for males and females. Alcohol use was classified as moderate if a patient reported alcohol consumption of greater than 14 drinks per week for males, and 7 drinks per week for females18 but less than 4 or more drinks per day.
Epidemiological factors included age (at cancer diagnosis), sex, and race/ethnicity. Race/ethnicity was defined as Non-Hispanic White, Non-Hispanic Black, and Hispanic.
Pain Medications
Charts were reviewed for information on pain medications reported by patients at presentation to the Cancer Center. We used the World Health Organization (WHO) three-step ladder to categorize the medications, as follows: Level 1 includes non-opioid medication such as aspirin, acetaminophen, or non-steroidal anti-inflammatory drugs; Level 2 includes weak opioids such as codeine; Level 3 includes powerful opioids such as morphine. These categorizations were reviewed by a pain specialist (KHT).
Statistical Analyses
Descriptive statistics were used to summarize the patient characteristics. The Kolmogorov-Smirnov Z test was used to assess the normality distribution for pain, fatigue, and depressed mood. Since normality was not met, we used the National Comprehensive Cancer Network cut-off score of ≥ 7 for severe pain.
Based on our previous studies25,26, we also combined responses to the SF-12 questionnaire. For the question, “During the past 4 weeks, have you been feeling downhearted and blue?,” responses of “most of the time” and “all of the time” were combined to indicate severe levels of depressed mood, and “none of the time,” “little of the time,” “some of the time,” and “good bit of the time” were combined to indicate non-severe levels of depressed mood. For the fatigue question (“During the past 4 weeks, have you had a lot of energy?”), we combined the responses “none of the time” and “little of the time” to indicate severe levels of fatigue and the responses “most of the time,” “all of the time,” “some of the time,” and “good bit of the time” to indicate non-severe levels of fatigue.
Pearson's chi-squared tests were used to assess the relationship between pain and clinical, behavioral and sociodemographic factors. Using the Kaplan-Meier method, we generated 5-year overall survival by selected characteristics and assessed statistical significance using log-rank test. Univariate and multivariate Cox proportional hazards regression analyses were used to estimate the strength of association for variables using hazard ratios (HRs) and 95% confidence intervals (CIs). The multivariable model assessed the effect of pain severity on survival, while controlling for pain treatment, clinical (extent of disease, comorbid conditions) and epidemiological (age, sex, race/ethnicity) and behavioral (smoking, alcohol consumption) factors and symptoms (fatigue and depressed mood) found significant in the univariate model (P<0.05). All statistical analyses were performed using SPSS software (SPSS Inc, Chicago, Il). All of the statistical tests were two-sided.
Results
Characteristics of the Study Sample
A total of 2340 patients with HNSCC comprised our sample, 1196 with cancer of the oral cavity; 696 with cancer of the pharynx; and 448 with cancer of the larynx. A majority of the sample were men (n=1788; 76%) and Non-Hispanic Whites (1859; 80%). Mean age for the total sample was 59 years (SD=11.7). The most commonly reported comorbid conditions were hypertension (n=970; 41.5%), followed by heart disease (n=433; 18.5%) and diabetes (n=288; 12.3%). One in 4 (24.5%) were current smokers and 22% were heavy drinkers. Patients with laryngeal cancer had the highest proportion of heavy drinkers (31%; versus 19.3% for oral and 28.8% pharyngeal; p<0.001) and smokers (35.6; versus 20.2% for oral and 26.4% pharyngeal; p<0.001).
Pain Severity
Severe pain was reported by as many as 19% of the total sample and was most prevalent among those with oral cancer (20.4%); followed by pharynx (18.8%) and larynx (16.1%).
Table 1 shows that tumor stage, smoking status, lung disease, and race were significant covariates of severe pain (p <0.05) across all cancer diagnoses (oral, pharynx and larynx). Interestingly, alcohol intake was a significant covariate of severe pain for those with oral (p<0.001) and pharyngeal cancer (p<0.001) but not for patients with cancer of the larynx. Patients reporting heavy alcohol intake had the highest proportion reporting severe pain. More female patients (24.2%) with oral cancer also reported severe pain relative to males (19%).
Table 1. Severe Pain by Selected Characteristics*.
| ORAL N= 1196 | PHARYNX N= 696 | LARYNX N= 448 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | No/Yes | P | No/Yes | P | No/Yes | P |
| All individuals | 952/244 | 565/131 | 376/72 | |||
| TNM Classification | ||||||
| Tumor | ||||||
| 0-2 | 557/87 | <0.001 | 303/32 | <0.001 | 189/22 | 0.001 |
| 3-4 | 343/147 | 251/90 | 176/49 | |||
| Node | ||||||
| 0 | 356/78 | NS | 107/28 | NS | 223/39 | NS |
| 1 | 120/44 | 76/13 | 33/12 | |||
| 2 | 176/48 | 126/25 | 58/16 | |||
| 3 | 37/10 | 36/16 | 14/2 | |||
| Metastasis | ||||||
| Non-metastatic | 891/233 | NS | 545/116 | 0.010 | 360/70 | NS |
| Metastatic | 8/2 | 8/7 | 5/0 | |||
| Comorbidities | ||||||
| Heart No | 783/186 | 0.035 | 480/99 | 0.013 | 298/61 | NS |
| Yes | 169/58 | 85/32 | 78/11 | |||
| Lung No | 871/213 | 0.049 | 528/109 | <0.001 | 321/51 | 0.005 |
| Yes | 81/31 | 37/22 | 55/21 | |||
| Hypertension No | 567/145 | NS | 351/78 | NS | 196/33 | NS |
| Yes | 385/99 | 214/53 | 180/39 | |||
| Stroke No | 918/232 | NS | 541/120 | NS | 356/70 | NS |
| Yes | 34/12 | 24/11 | 20/2 | |||
| Diabetes No | 836/208 | 0.282 | 507/109 | 0.047 | 332/60 | NS |
| Yes | 116/36 | 58/22 | 44/12 | |||
| Behavioral Factors | ||||||
| Alcohol Never | 296/79 | <0.001 | 134/21 | <0.001 | 92/23 | NS |
| Social | 323/47 | 188/19 | 103/14 | |||
| Moderate | 119/24 | 81/20 | 48/6 | |||
| Heavy | 142/70 | 131/56 | 106/24 | |||
| Smoking Never | 378/71 | <0.001 | 172/15 | <0.001 | 44/1 | 0.003 |
| Yes, but quit | 412/90 | 263/59 | 208/35 | |||
| Yes, current | 157/83 | 126/57 | 123/36 | |||
| Sociodemographic | ||||||
| Age <50 | 183/60 | NS | 107/28 | NS | 56/15 | NS |
| ≥50 | 769/184 | 458/103 | 320/57 | |||
| Male | 704/165 | 0.053 | 455/111 | NS | 301/52 | NS |
| Female | 248/79 | 110/20 | 75/20 | |||
| Non-Hispanic White | 778/171 | <0.001 | 487/95 | <0.001 | 277/51 | 0.020 |
| Hispanics | 78/34 | 35/14 | 44/3 | |||
| Non-Hispanic Blacks | 33/23 | 21/18 | 42/15 | |||
| Symptoms | ||||||
| Depressed Mood | 838/175 | <0.001 | 498/88 | <0.001 | 310/56 | NS |
| None-moderate | 48/49 | 35/31 | 31/11 | |||
| Severe | ||||||
| Fatigue | ||||||
| None-Moderate | 662/88 | <0.001 | 410/45 | <0.001 | 245/17 | <0.001 |
| Severe | 201/120 | 105/68 | 92/47 | |||
| WHO Ladder | ||||||
| None | 696/139 | <0.001 | 430/76 | <0.001 | 283/40 | <0.001 |
| Level 1 | 110/19 | 47/11 | 38/8 | |||
| Level 2 | 105/53 | 64/27 | 40/9 | |||
| Level 3 | 41/33 | 24/17 | 15/15 | |||
Missing data for some variables
Severe fatigue was a significant covariate of severe pain across cancer diagnoses (oral, pharynx and larynx) and depressed mood was observed to significantly covary with severe pain among those with oral and pharyngeal cancer but not for those with laryngeal cancer. We also found that pain treatment, categorized using the WHO step ladder, significantly varied by pain severity.
Survival Outcomes
There was a total of 828 deaths (oral= 416; pharynx= 251; larynx= 161). Median survival (in days) were as follows: oral= 3143 (95% CI=2790; 3495); pharynx=3307 (95%CI=2628;3985) and larynx=3119 (95%CI=2440;3797). Figure 1 shows Kaplan-Meier estimates of the effect of pain on survival for the entire sample.
Figure 1. Kaplan-Meier estimates of the effect of pain on survival probability.
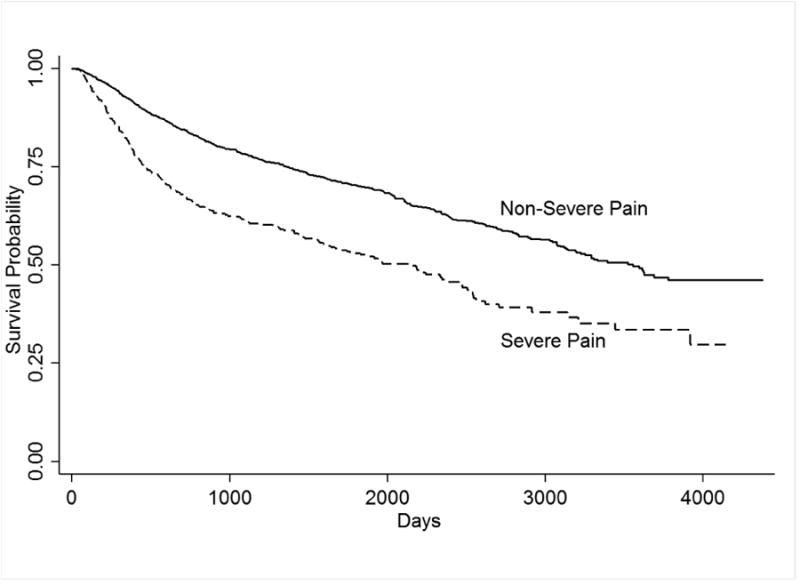
Univariate analyses for 5-year overall survival (data not shown) shows that among patients with oral cancer, overall 5 year survival was 31% among those with severe pain versus 52% of those without severe pain (p<0.001). Similarly, among those with pharyngeal cancer, those reporting severe levels of pain=33% (non-severe=53%; p<0.001) had lower 5-year overall survival.
As expected, extent of disease using TNM as separate variables for tumor size (T), lymph node involvement (N) and metastasis (M) were also significant factors, along with behavioral factors (alcohol intake, smoking status), comorbid conditions (heart disease, lung disease, hypertension, stroke), age and race.
Multivariable Analyses
We conducted multivariable analyses to assess the extent to which symptoms influence survival outcomes for the whole sample by including factors known to influence survival in cancer patients. The variables found significant in the univariate model (P<0.05) were included in the analyses. We observed (data not shown) that compared to patients with laryngeal cancer, patients with oral cancer and pharyngeal cancer are at an increased risk of mortality (oral HR=1.39 and pharyngeal HR=1.52, respectively). Pain (HR=1.30; 95% CI=1.03;1.63; p<0.025) and fatigue (HR= 1.30; 95%CI=1.06;1.59; p<0.011) were significant predictors for overall survival in HNSCC patients. As expected, extent of disease (TNM), smoking and alcohol intake, lung disease and age and race were also significant predictors of survival. When we accounted for pain treatment, using WHO step ladder categories, Table 2 shows that pain and fatigue, disease-related variables (TNM), socio-demographic (age and race/ethnicity) and behavioral factors (smoking and alcohol intake) persisted as important factors for survival.
Table 2. Predictors of Survival in Patients with HNSCC (Multivariable Analyses).
| Variable | Odds Ratio | 95% CI | p-value | |
|---|---|---|---|---|
|
| ||||
| Lower | Upper | |||
| *Cancer | 0.004 | |||
| Larynx | 1.0 | REF | ||
| Oral | 1.111 | .896 | 1.379 | .337 |
| Pharynx | .719 | .567 | .912 | .007 |
| Symptoms | ||||
| Pain Severity (0-10) | 1.038 | 1.005 | 1.073 | .023 |
| WHO Ladder | ||||
| 0 | 1.0 | REF | 0.286 | |
| 1 | 1.033 | .759 | 1.406 | .835 |
| 2 | .818 | .614 | 1.089 | .169 |
| 3 | 1.215 | .860 | 1.716 | .269 |
| Fatigue severity | ||||
| Non-Severe | 1.0 | REF | ||
| Severe | 1.302 | 1.06 | 1.59 | 0.011 |
| TNM Classification | ||||
| T | ||||
| 0-2 | 1.0 | REF | ||
| 3-4 | 1.646 | 1.355 | 1.999 | <0.001 |
| Node | <0.001 | |||
| 0 | 1.0 | REF | ||
| 1 | 1.117 | .868 | 1.437 | .391 |
| 2 | 1.141 | .905 | 1.437 | .264 |
| 3 | 2.229 | 1.628 | 3.052 | .000 |
| Metastasis | ||||
| Non-metastatic | 1.0 | REF | ||
| Metastatic | 4.220 | 2.525 | 7.052 | .000 |
| Comorbidites | ||||
| Lung diseases | ||||
| No | 1.0 | REF | ||
| Yes | 1.527 | 1.194 | 1.954 | .001 |
| Behavioral Factors | ||||
| Alcohol Intake | 0.077 | |||
| Non-drinker | 1.0 | REF | ||
| Social | .989 | .765 | 1.279 | .933 |
| Moderate | 1.175 | .869 | 1.588 | .294 |
| Heavy | 1.314 | 1.019 | 1.695 | .035 |
| Smoking | <0.001 | |||
| Never | 1.0 | REF | ||
| Yes, but quit | 1.557 | 1.188 | 2.040 | .001 |
| Yes, current | 1.882 | 1.406 | 2.518 | .000 |
| Socio-demographics | ||||
| Race | 0.025 | |||
| NH White | 1.0 | REF | ||
| Hispanics | .950 | .690 | 1.308 | .753 |
| NH Blacks | 1.514 | 1.110 | 2.064 | .009 |
| Age | ||||
| Age <50 | 1.0 | REF | ||
| Age ≥50 | 2.256 | 1.686 | 3.018 | .001 |
Discussion
Our study is one of the first to examine pretreatment pain severity as a predictor of survival in a large sample of HNSCC patients. The results indicate that pretreatment pain is an independent predictor of 5-year overall survival in patients with HNSCC. Previous studies have found that pain severity at post treatment30 and 2 years after treatment32 were significant predictors of survival in patients with head and neck cancer, however, these studies had limited sample size and lacked a comprehensive assessment of the influence of clinical (disease stage, comorbid conditions) behavioral (smoking, alcohol consumption) and epidemiological (age, sex, race/ethnicity) factors known to influence survival in HNSCC, thus, limiting the generalizability of study findings.
Studies have hypothesized the potential link between symptoms and survival as reflecting inflammatory processes that also underlie cancer progression, e.g., increased preoperative concentration of C-reactive protein was found to be associated with poorer survival in patients with oral cancer7. Pain molecules including endothelin, prostaglandin, bradykinin and nerve growth factor--molecules which have been shown to evoke pain in animal models, also influence growth and neovascularization of tumors 17. Another study15 demonstrated a direct role for protease-activated receptor 2 (PAR2) in acute cancer pain. PAR2 is known to uniquely trigger tumor cell migration. The authors suggested that PAR2 upregulation may favor the development and maintenance of chronic cancer pain and that targeting the PAR2–serine protease interaction is a promising approach to the treatment of acute cancer pain and prevention of chronic cancer pain. Additional research is needed to explore the biological mechanisms that might explain the association between pain and survival in HNSCC.
Severe pain was reported prior to cancer treatment by 19% of the total sample and was most prevalent (20.4%) among patients with oral cancer. These results are somewhat higher than those found in a previous study of patients with HNSCC. Scharpf and colleagues30 found a prevalence rate for severe pretreatment pain of 10.9% in a sample of 339 patients with head and neck cancer. However, a study of Sato and colleagues 29 showed 37% of patients with untreated primary oral cancer reported spontaneous pain. The differing prevalence rates may be due to characteristics of these samples, including sample size and the distribution of oral, pharynx, laryngeal cancers. In addition, pain in cancer patients may arise from several factors, including tumor growth, treatment or other causes unrelated to cancer. In our sample, pain was assessed prior to treatment, thus excluding treatment-related pain, but we cannot exclude pain unrelated to cancer (back pain, etc.).
It is not surprising that we observed severe pain as more common among cancer patients with advanced disease. Pain in advanced cancer may result from the primary activation of visceral or somatic nociceptors by a metastatic tumor (nociceptive pain), the impingement of the tumor on adjacent tissues22, the obstruction of blood vessels or the inflammation caused by the tumor-induced mediators, such as cytokines28. The finding that severe pain was more prevalent among non-Hispanic Black and Hispanic patients is consistent with other studies demonstrating a higher prevalence of severe pain among racial and ethnic minority patients with cancer2. The racial and ethnic differences in pain persisted even when we stratified by stage of disease or TNM. Additional research is needed to identify the causes of the higher pain prevalence among minority patients with head and neck cancer.
It should be noted that the significant covariates of severe pain differed somewhat across cancer diagnoses. Metastatic disease was a significant covariate among patients with cancer of the pharynx but not among patients with oral or laryngeal cancer. Alcohol intake was a covariate of severe pain for patients with oral and pharyngeal cancers. Associations between pain and alcohol consumption have been shown in population-based studies4,20. However, there have been limited investigations of these associations in cancer patients. Among patients with oral cancer, we observed that more women than men with oral cancer report severe pain. This is consistent with previous studies of orofacial pain. Women have a greater risk of pain and report more severe pain, more frequent pain, and longer pain durations than men 8. These gender differences are partially attributed to the action of sex hormones, which may influence central and peripheral mechanisms of nociceptive pain transmission, pain sensitivity, and pain perception 38.
We also observed that compared to patients with laryngeal cancer, patients with oral cancer and pharyngeal cancer are at an increased risk of mortality (oral HR=1.39 and pharyngeal HR=1.52, respectively). While these results can be partially explained by the fact that there are more effective surgical treatment options for salvage of recurrent laryngeal cancer compared to oral and pharyngeal cancers, it should be noted that when we accounted for pain medications, the increased risk for mortality among patients with oral cancer was no longer statistically significant.
We observed that patients who smoke had a higher prevalence of severe pain compared to nonsmokers 21,33. It has been hypothesized that smoking has a bidirectional relationship with pain. Smoking leads to physiologic changes, ie, down regulation in the hypothalamic pituitary axis that may increase pain sensitivity and pain perception. Alternatively, smoking is a way of coping for patients with pain. Additional studies are needed to further explore this relationship in patients with cancer.
Severe fatigue and depression were significantly associated with severe pain in our patient sample. These findings are consistent with other studies27 demonstrating that the three symptoms are often associated in samples of patients with cancer. For many patients with cancer, receiving a diagnosis of a potentially fatal disease and the prospect of aggressive disease management generate significant emotional turmoil. Most individuals with cancer report some feelings of distress, depression or anxiety during the course of their disease and its treatment23. Scharpf and colleagues 30 found that post treatment depression was significantly associated with severe pain in their sample of patients with head and neck cancer. Fatigue has been correlated with severe pain and depression in other samples of patients with cancer. It has been suggested that these symptoms share common biological pathways and may be related to inflammatory changes associated with cancer and cancer treatment 16,28.
In addition to pain, fatigue was a significant predictor of survival across cancer diagnoses. Fatigue is one of the most common and distressing symptoms associated with cancer3. To our knowledge, our study is one of the first to evaluate pretreatment fatigue as a predictor of survival in head and neck cancer patients. A previous study of breast cancer patients found that fatigue was a significant predictor of recurrence-free survival, after controlling for clinical variables13. Indeed, both pain and fatigue may be important markers for survival due to their association with inflammatory changes. Among cancer patients, chronic inflammation acts as a tumor promoter, resulting in aggressive tumor growth and spread.
Aside from pain and fatigue, significant predictors of overall survival across cancer diagnoses (oral, pharynx, larynx) in multivariate analyses included extent of disease (TNM). TNM stage is the single most important risk factor for recurrence and survival and treatment determinant for HNSCC. It also is not surprising that the co-morbidity of chronic lung disease influences overall survival. Patients who have comorbid conditions in addition to HNSCC are at risk for early mortality from multiple causes.
Our results also support the importance of assessing alcohol intake and smoking status, behaviors that were significant predictors of survival in our total sample. Patients who report smoking and/or significant alcohol intake can be referred for smoking cessation programs and/or further assessment and treatment of possible alcohol abuse. Thompson and colleagues 32 found that patients with pain or poor overall quality of life 2 years after diagnosis were more likely to die from all causes, whereas those still smoking were more likely to die from their cancer. They concluded that in addition to older age and advanced stage; pain, poor quality of life, and tobacco use 2 years after diagnosis characterize patients who might need longer and more intense follow-up care32.
There are limitations to our study. We did not include type of cancer treatment as a covariate since treatment is driven by extent of disease, thus, inextricably associated with tumor stage (hence high multicollinearity). Information on human papillomavirus (HPV) status was missing. Studies have found that patients with HPV-positive tumors tend to have better survival rates than patients with HPV-negative tumors12. Another limitation is our measure of depression, which relied on patient self-report. A structured psychiatric interview would provide a more reliable assessment of clinical depression. Anxiety, a known covariate of pain, was also not assessed.
We also acknowledge that other limitations include the lack information on the location, type of pain (back pain, etc.) and etiology of pain. Further, pain was only assessed at presentation and follow-up pain assessments were not conducted. The study was also limited to head and neck cancer patients at one tertiary care cancer center. Thus, additional prospective studies are needed to validate our findings.
In sum, our study provide empirical evidence that pain at presentation is a prognostic marker for survival, even after accounting for disease, socio-demographic and other clinical factors associated with survival outcomes. Among the important implications of our findings is that patients who present with severe pain at diagnosis need to be closely monitored, with prompt treatment and management of symptoms incorporated in treatment planning. Additional prospective studies are needed to validate our findings.
Acknowledgments
Disclosures: The research is supported by NIH R01 DE 022891.
Footnotes
Conflict of Interest: Nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer Facts and Figures 2011. Atlanta, GA: American Cancer Society; 2012. 2011. Retrieved October 3, 2011. [Google Scholar]
- 2.Anderson KO. Racial and ethnic disparities in pain: causes and consequences of unequal care. 2009 doi: 10.1016/j.jpain.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: mechanisms, contributing factors, and treatment implications. Brain Behav Immun. 2013;30(Suppl):S48–S57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan PL, Soohoo S. Pain and use of alcohol in later life: prospective evidence from the health and retirement study. J Aging Health. 2013;25:656–677. doi: 10.1177/0898264313484058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caraceni A, Cherny N, Fainsinger R, Kaasa S, Poulain P, Radbruch L, De Conno F. Pain measurement tools and methods in clinical research in palliative care: recommendations of an Expert Working Group of the European Association of Palliative Care. J Pain Symptom Manage. 2002;23:239–255. doi: 10.1016/s0885-3924(01)00409-2. [DOI] [PubMed] [Google Scholar]
- 6.Caraceni A, Portenoy RK. An international survey of cancer pain characteristics and syndromes. IASP Task Force on Cancer Pain International Association for the Study of Pain. Pain. 1999;82:263–274. doi: 10.1016/S0304-3959(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen HH, Chen IH, Liao CT, Wei FC, Lee LY, Huang SF. Preoperative circulating C-reactive protein levels predict pathological aggressiveness in oral squamous cell carcinoma: a retrospective clinical study. Clin Otolaryngol. 2011;36:147–153. doi: 10.1111/j.1749-4486.2011.02274.x. [DOI] [PubMed] [Google Scholar]
- 8.Dao TT, Leresche L. Gender differences in pain. J Orofac Pain. 2000;14:169–184. [PubMed] [Google Scholar]
- 9.Epstein JB, Hong C, Logan RM, Barasch A, Gordon SM, Oberle-Edwards L, McGuire D, Napenas JJ, Elting LS, Spijkervet FK, Brennan MT. A systematic review of orofacial pain in patients receiving cancer therapy. Support Care Cancer. 2010;18:1023–1031. doi: 10.1007/s00520-010-0897-7. [DOI] [PubMed] [Google Scholar]
- 10.Farshadpour F, Kranenborg H, Calkoen EV, Hordijk GJ, Koole R, Slootweg PJ, Terhaard CH. Survival analysis of head and neck squamous cell carcinoma: influence of smoking and drinking. Head Neck. 2011;33:817–823. doi: 10.1002/hed.21549. [DOI] [PubMed] [Google Scholar]
- 11.Fischer DJ, Klasser GD, Epstein JB. Cancer and orofacial pain. Oral Maxillofac Surg Clin North Am. 2008;20:287–301. vii. doi: 10.1016/j.coms.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 13.Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 14.Inman BA, Tran VT, Fradet Y, Lacombe L. Carcinoma of the upper urinary tract: predictors of survival and competing causes of mortality. Cancer. 2009;115:2853–2862. doi: 10.1002/cncr.24339. [DOI] [PubMed] [Google Scholar]
- 15.Lam DK, Dang D, Zhang J, Dolan JC, Schmidt BL. Novel animal models of acute and chronic cancer pain: a pivotal role for PAR2. J Neurosci. 2012;32:14178–14183. doi: 10.1523/JNEUROSCI.2399-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain Behav Immun. 2003;17(Suppl 1):S125–S131. doi: 10.1016/s0889-1591(02)00079-x. [DOI] [PubMed] [Google Scholar]
- 17.Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7:797–809. doi: 10.1038/nrn1914. [DOI] [PubMed] [Google Scholar]
- 18.http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderatebinge-drinking. 2013.
- 19.Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009;7:102. doi: 10.1186/1477-7525-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moos RH, Brennan PL, Schutte KK, Moos BS. Older adults' health and late-life drinking patterns: a 20-year perspective. Aging Ment Health. 2010;14:33–43. doi: 10.1080/13607860902918264. [DOI] [PubMed] [Google Scholar]
- 21.Novy DM, Lam C, Gritz ER, Hernandez M, Driver LC, Koyyalagunta D. Distinguishing features of cancer patients who smoke: pain, symptom burden, and risk for opioid misuse. J Pain. 2012;13:1058–1067. doi: 10.1016/j.jpain.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portenoy RK. Cancer pain. Epidemiology and syndromes. Cancer. 1989;63:2298–2307. doi: 10.1002/1097-0142(19890601)63:11<2298::aid-cncr2820631140>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Raison CL, Miller AH. Depression in cancer: new developments regarding diagnosis and treatment. Biol Psychiatry. 2003;54:283–294. doi: 10.1016/s0006-3223(03)00413-x. [DOI] [PubMed] [Google Scholar]
- 24.Redding SW. Cancer therapy-related oral mucositis. J Dent Educ. 2005;69:919–929. [PubMed] [Google Scholar]
- 25.Reyes-Gibby CC, El OB, Spitz MR, Parsons H, Kurzrock R, Wu X, Shete S, Bruera E. The influence of tumor necrosis factor-alpha -308 G/A and IL-6 -174 G/C on pain and analgesia response in lung cancer patients receiving supportive care. Cancer Epidemiol Biomarkers Prev. 2008;17:3262–3267. doi: 10.1158/1055-9965.EPI-08-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes-Gibby CC, Shete S, Yennurajalingam S, Frazier M, Bruera E, Kurzrock R, Crane CH, Abbruzzese J, Evans D, Spitz MR. Genetic and nongenetic covariates of pain severity in patients with adenocarcinoma of the pancreas: assessing the influence of cytokine genes. J Pain Symptom Manage. 2009;38:894–902. doi: 10.1016/j.jpainsymman.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reyes-Gibby CC, Swartz MD, Yu X, Wu X, Yennurajalingam S, Anderson KO, Spitz MR, Shete S. Symptom clusters of pain, depressed mood, and fatigue in lung cancer: assessing the role of cytokine genes. Support Care Cancer. 2013 doi: 10.1007/s00520-013-1885-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reyes-Gibby CC, Wu X, Spitz M, Kurzrock R, Fisch M, Bruera E, Shete S. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncol. 2008;9:777–785. doi: 10.1016/S1470-2045(08)70197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato M, Ohashi J, Tsuchiya N, Kashiwase K, Ishikawa Y, Arita H, Hanaoka K, Tokunaga K, Yabe T. Association of HLA-A*3303-B*4403-DRB1*1302 haplotype, but not of TNFA promoter and NKp30 polymorphism, with postherpetic neuralgia (PHN) in the Japanese population. Genes Immun. 2002;3:477–481. doi: 10.1038/sj.gene.6363890. [DOI] [PubMed] [Google Scholar]
- 30.Scharpf J, Karnell LH, Christensen AJ, Funk GF. The role of pain in head and neck cancer recurrence and survivorship. Arch Otolaryngol Head Neck Surg. 2009;135:789–794. doi: 10.1001/archoto.2009.107. [DOI] [PubMed] [Google Scholar]
- 31.Shen GP, Xu FH, He F, Ruan HL, Cui C, Chen LZ, Zeng YX, Jia WH. Pretreatment lifestyle behaviors as survival predictors for patients with nasopharyngeal carcinoma. PLoS One. 2012;7:e36515. doi: 10.1371/journal.pone.0036515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson TL, Pagedar NA, Karnell LH, Funk GF. Factors associated with mortality in 2-year survivors of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2011;137:1100–1105. doi: 10.1001/archoto.2011.179. [DOI] [PubMed] [Google Scholar]
- 33.Vandenkerkhof EG, Macdonald HM, Jones GT, Power C, Macfarlane GJ. Diet, lifestyle and chronic widespread pain: results from the 1958 British Birth Cohort Study. Pain Res Manag. 2011;16:87–92. doi: 10.1155/2011/727094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vilaseca I, Chen AY, Backscheider AG. Long-term quality of life after total laryngectomy. Head Neck. 2006;28:313–320. doi: 10.1002/hed.20268. [DOI] [PubMed] [Google Scholar]
- 35.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey:construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 37.Wettergren L, Bjorkholm M, Axdorph U, Langius-Eklof A. Determinants of health-related quality of life in long-term survivors of Hodgkin's lymphoma. Qual Life Res. 2004;13:1369–1379. doi: 10.1023/B:QURE.0000040790.43372.69. [DOI] [PubMed] [Google Scholar]
- 38.Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–145. doi: 10.1016/s1550-8579(05)80042-7. [DOI] [PubMed] [Google Scholar]


