Abstract
Objective
The aim of study was to analyze the accuracy of TRUS (transrectal ultrasound) vs. MRI (magnetic resonance imaging) and clinical gynecological examination estimation in the evaluation of tumor dimensions.
Methods
The patients inclusion criterion included primarily pathologically squamous cell carcinoma, but excluded were patients who had not undergone BT (brachytherapy) and treated with palliative intent. We offer two types of treatment for locally advanced cervical cancer: (a) radiochemotherapy followed by surgery and (b) exclusive radiochemotherapy. Imaging tests follow the presence of tumor and tumor size (width and thickness). Each examination was performed by a different physician who had no knowledge of the others’ findings. All patients underwent MRI prior to EBRT (external beam radiation therapy) while 18 of them also at the time of the first brachytherapy application. For the analysis we used the r-Pearson correlation coefficient.
Results
In 2013, 26 patients with cervical cancer were included. A total of 44 gynecological examinations were performed, 44 MRIs and 18 TRUSs. For the comparisons prior to EBRT the correlation coefficient between TRUS vs. MRI was r = 0.79 for AP and r = 0.83 for LL, for GYN vs. MRI was r = 0.6 for AP and r = 0.75 for LL. Prior to BT for GYN vs. MRI, r values were 0.60 and 0.63 for AP and LL, respectively; for GYN vs. TRUS, r values were 0.56 and 0.78 for AP and LL, respectively.
Conclusions
A high correlation between the three examinations was obtained. As such, TRUS can be considered a suitable method in the evaluation of tumor dimensions.
Keywords: Cervical cancer, MRI, Transrectal ultrasound, Gynecological clinical examination
1. Background
Cervical cancer represents a real public health problem in Romania, both in terms of incidence and mortality. Romania is positioned on the first place in Europe and the sixth place in the world for the incidence and mortality of cervical cancer. In Romania, cervical cancer represents 15% of malign tumors ranking first among gynecological tumors (approximately 67%) and being the second cause of female mortality caused by cancer.1
In Romania in 2010, the incidence and mortality were 28.7%000 and 10.8%000, respectively. In the Oncology Institute, “Prof. Dr. Ion Chiricuta” Cluj-Napoca, there were 1085 cervical cancer patients treated in the same year. Of these, 70–75% were locally advanced tumors. For these reasons early diagnosis, pretreatment planning and multidisciplinary approach in the treatment of cervical cancer represent a very important national problem. Therefore a strong emphasis has to be put on the development of efficient tools for cost effective treatment solutions, particularly for advanced disease.
Treatment of locally advanced carcinoma of the cervix includes external beam radiotherapy (EBRT), chemotherapy (CT) and brachytherapy (BT). The addition of concomitant radiochemotherapy by using CT (Cisplatinum) as radio-sensitizer showed significant improvement in local recurrence and survival.2–4 Existing treatments of cervical cancer ensure a 5-year overall survival (OS) of 83% for stage IIB, 69% for stage IIIA, and 63% for stage IIIB.5
Although our institutional local control rates are in accordance with the ones published in literature, we acknowledge the need for improvement especially in stage IIB–IIIB, which represent challenging cases to treat only with 2D standard brachytherapy.
The potential of image-guided adaptive brachytherapy (IGABT) with magnetic resonance imaging (MRI) was demonstrated to have reduced late morbidity and obtained improved local control and overall survival. By applying MRI based IGABT it was reported a local tumor control of 96% for IIB and 86% for IIIB.6 Nevertheless, in order to achieve IGABT, centers must be equipped with MRI, that is how highly equipped centers can benefit from this procedure. Unfortunately, patients in Romania, which is one of the countries with limited financial resources, will barely take advantage from these therapeutic improvements. On the other hand, low-cost imaging modalities, such as ultrasound (US) including transrectal US (TRUS) have already been in use worldwide for some years now.
For our study, the TRUS and MRI were performed to assess local tumor extension (width and thickness) before starting EBRT and 1 day before the brachytherapy application. The aim of this study is to compare the clinical gynecological measurements (width and thickness) with TRUS and MRI, and it is the first of its kind in Romania.
2. Aim
The aim of this study was to analyze the accuracy of TRUS in comparison to MRI and clinical gynecological examination estimation in the evaluation of tumor dimensions and identification of residual tumor after EBRT and before the first BT insertion in advanced cervical cancer.
3. Materials and methods
3.1. Patients
The inclusion criterion was pathologically confirmed squamous cell carcinoma of the uterine cervix (obtained by tumor biopsy). These patients underwent gynecological examination, chest radiography, MRI and TRUS.
Excluded cases were patients who had not undergone BT, treated with palliative intent, with previous oncological treatments or with hematological, kidney and/or liver disorders.
Only patients included in this study benefited from MRI and TRUS examinations. Imaging tests in our study followed: the presence of tumor and the tumor size (width and thickness). Each examination (gynecological, TRUS and MRI) was performed by different physicians and they had no knowledge of the others’ findings.
At the beginning of the study we had some difficulties in carrying out TRUS and MRI immediately one after another; if at the beginning of the study there was a 3-week break between the two procedures, later on they were performed in the same day or after a few days.
3.2. Treatment protocol
In our institution, due to the high number of patients and limited resources, the standard treatment for locally advanced cervical cancer includes: (a) radiochemotherapy followed by surgery and (b) exclusive radiochemotherapy.7
In both situations, the treatment schedule consists of EBRT to the pelvic region delivered with 15/16 MV X-rays by a linear accelerator according to international protocols, adding Cisplatinum as radiosensitizer. A boost is delivered by HDR or LDR/MDR intracavitary brachytherapy using standard prescription points for the dose. The patients were reevaluated at a EBRT dose of 46 Gy/pelvis and, according to the tumor clinical response and/or patients option, the treatment was continued either with curative intent with radiochemotherapy (RCT) to 60 Gy or with surgery (after 4–6 weeks interval).8,9
As for the BT, we delivered a total dose (TD) of 10 Gy/2 fractions for patients which underwent surgery and a TD of 21 Gy/3 fractions for exclusive curative intent radiochemotherapy patients.10 The BT was performed with weekly endocavitary insertions of one tandem and two ovoid applicators or one tandem and one ring applicator.
The planning system used (Plato BPS v14.2) was based on the Manchester System-2D dosimetry was based on reconstruction from 2-orthogonal planes X-ray radiographs (45° and 315°), with manual or graphical optimization.
A maximum of 70% from the prescribed dose in point A for the rectum and 75% for the bladder were the accepted doses used on the organs at risk.11
3.3. Imaging
The TRUS and MRI were performed outside our institution, according to their standard protocols of our collaborators. All patients underwent MRI prior to EBRT while some of them also at the time of the first brachytherapy application.
3.3.1. Magnetic resonance imaging
Magnetic resonance imaging was performed in a medical imaging center using the standard protocol with a machine with 1.5 Tesla. The axial T1 weighted images were used to evaluate the tumor pre- and post-administration of intravenous contrast. The T2 weighted images were used to analyze the tumor at the level of the uterine cervix (iso/hyper signal); the T2 weighted axial images were used for the evaluation of the pelvic lymph nodes; the T2 weighted oblique axial sections for the depiction of the parametrial invasion (and possibly invasion of the neighboring organs). The T2 sagittal weighted images were used to evaluate the cranio-caudal extension of the tumor, and vaginal invasion, respectively to describe the exo- or endophytic extension.
The thickness of the slices was 6 mm, with 1 mm spacing between them.
The thickness of the oblique axial slices was 5 mm, with 0.5 mm spacing between them.
The maximum latero-lateral dimension of the tumor was measured using the oblique axial sections, and the maximum cranio-caudal dimension was measured with the sagittal sections.
3.3.2. Transrectal ultrasonography
TRUS was performed using the endorectal approach with a machine equipped with broadband transducer frequencies between 4 and 9 MHz. Exploration technique was standardized. Preparations were conducted prior to the exploration, with rectal enema region prepared 2 h earlier. Anal region was lubricated with gel. The position of the patient was gynecological with the basin raised to 30° by applying under the seat a special fabric roll made for this type of exploration. The transducer was inserted into the rectum up to the point where the cervix was visualized. For longitudinal sections, the cervical canal was used as a reference. In the explorations, we used perpendicular sections. The following parameters were evaluated: delimitation, structure, vascularization, mobility and elasticity (by palpation with the transducer). The suggestive criteria for the existence of the cervical tumors were: change of the cervical structure, the presence of a localized vascular exacerbations, existence of distortion or discontinuity of capsulation, increased firmness of the cervix, movement in the bulk of the cervix compared with the neighboring pelvic structures.
3.4. Statistical analysis
Correlation between measurements of a pair of methods was evaluated by the Pearson correlation coefficient (r).12
All coefficients of correlation were significant and majority (7 of 8) had high correlation (over 0.6). We used a simplified linear model y = b*x to see the link between the two methods of evaluation. In this way, if b > 1 the y evaluation is in excess and if b < 1y measure induces an under-evaluation.
4. Results
Between January 2013 and August 2013, 26 patients with stage IB–IIIB cervical cancer (FIGO staging modified by MD Anderson Cancer Centre13) were included in this study. Two patients were in early stage IB–IIA, 15 patients (57.69%) in stage IIB, 6 patients (23.08%) stage IIIA and 3 patients (11.54%) stage IIIB disease (Table 1). The median age was 54.5 years (range 32–69 years) (Table 2).
Table 1.
Patients statistics by FIGO stages.
| Nr. Crt | Stage | Number of patients | % |
|---|---|---|---|
| 1 | IB | 1 | 3.85 |
| 2 | IIA | 1 | 3.85 |
| 3 | IIB | 15 | 57.69 |
| 4 | IIIA | 6 | 23.08 |
| 5 | IIIB | 3 | 11.54 |
| Total | 26 | 100.01 | |
Table 2.
Patients statistics by age.
| Age (years) | |
|---|---|
| Min | 32 |
| Max | 69 |
| Median | 54.5 |
| Media | 52.2 |
Imaging tests in our study followed the presence of the tumor and tumor size (width and thickness). Each examination (gynecological, TRUS and MRI) was performed by a different physician who had no knowledge of the other colleagues’ findings.
Eight patients underwent twice each type of the following examinations: gynecological, TRUS and MRI; 10 patients underwent two gynecological examinations and two MRI; 2 patients underwent once each type of the examinations (gynecological, MRI and TRUS) and 6 patients had only one gynecological examination, namely MRI.
A total of 44 gynecological examinations were performed, 44 MRIs and 18 TRUSs.
The median tumor thickness for the first gynecological examination (performed pre-therapeutically) was 32.5 mm (range 10–70 mm) and for the second examination (performed after the concomitant radiochemotherapy and before the brachytherapy treatment) it was 2.5 mm (range 0–40 mm). The median tumor width for the first gynecological examination was 40 mm (range 10–70 mm) and for the second examination it was 2.5 mm (range 0–35 mm) (Table 3).
Table 3.
Width and thickness statistics for the first and second gynecological, TRUS and MRI examinations.
| 1st GYN thickness (mm) | 1st GYN width (mm) | 1st ECO thickness (mm) | 1st ECO width (mm) | 1st RMN thickness (mm) | 1st RMN width (mm) | |
|---|---|---|---|---|---|---|
| Min | 10 | 10 | 25.5 | 21.5 | 3 | 1.7 |
| Max | 70 | 70 | 55 | 55 | 60 | 70 |
| Median | 32.5 | 40 | 33.5 | 34 | 35 | 37.5 |
| Media |
36.2 |
39.4 |
35.6 |
35.8 |
33.1 |
38.0 |
| 2nd GYN thickness (mm) | 2nd GYN width (mm) | 2nd ECO thickness (mm) | 2nd ECO width (mm) | 2nd RMN thickness (mm) | 2nd RMN width (mm) | |
|---|---|---|---|---|---|---|
| Min | 0 | 0 | 0 | 0 | 0 | 0 |
| Max | 40 | 35 | 18 | 18 | 35 | 35 |
| Median | 2.5 | 2.5 | 5 | 5 | 6 | 6 |
| Media | 6.8 | 6.9 | 7.4 | 8.0 | 8.6 | 10.1 |
The median tumor thickness for the first TRUS was 33.5 mm (range 25.5–55 mm) and for the second examination it was 5 mm (range 0–18 mm). The median tumor width for the first TRUS was 34 mm (range 21.5–55 mm) and for the second examination it was 5 mm (range 0–18 mm) (Table 3).
The median tumor thickness for the first MRI was 35 mm (range 3–60 mm) and for the second examination it was 6 mm (range 0–35 mm). The median tumor width for the first MRI was 37.5 mm (range 1.7–70 mm) and for the second examination it was 6 mm (range 0–35 mm) (Table 3).
The linear regression analysis for the first (at diagnosis) (Fig. 1a and b) and second (before the brachytherapy application) (Fig. 2a and b) gynecological and MRI examinations for width and thickness demonstrated a high correlation of r = 0.75 and r = 0.60, respectively, and r = 0.63 and r = 0.60, respectively.
Fig. 1.
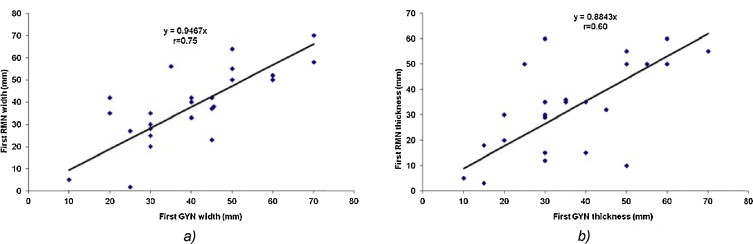
The linear regression analysis for (a) the tumor width and (b) thickness between the gynecological and MRI examination at the first examination (at diagnosis).
Fig. 2.
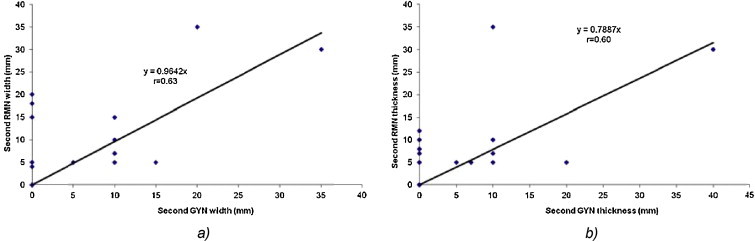
The linear regression analysis for the (a) tumor width and (b) tumor thickness between the gynecological and MRI examination at the second examination (before brachytherapy application).
At the first gynecological and TRUS examinations, the linear regression analysis for width and thickness demonstrated a reasonable correlation of r = 0.59 (Fig. 3a) and r = 0.45, respectively; for the second examination for width, we obtained a high correlation of r = 0.78 (Fig. 3b) and a reasonable correlation of r = 0.56 for thickness (Fig. 3c).
Fig. 3.
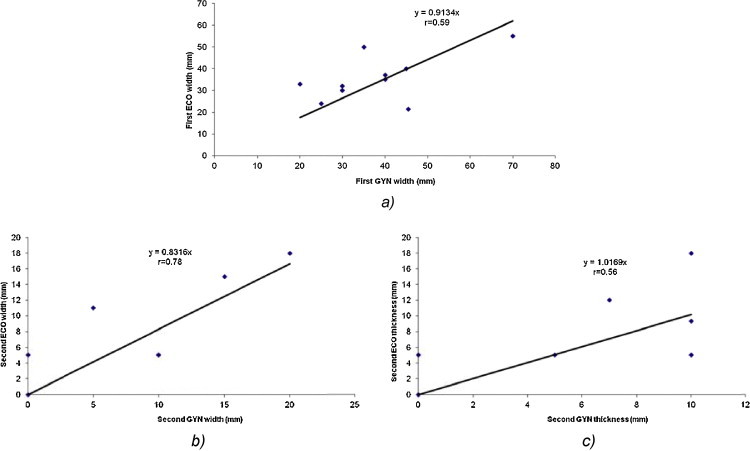
(a) The linear regression analysis for the tumor width between the gynecological and TRUS examination at the first examination (at diagnosis). The linear regression analysis for (b) the tumor width and (c) tumor thickness between the gynecological and TRUS examination at the second examination (before brachytherapy application).
The linear regression analysis between TRUS and MRI at the first examination (Fig. 4a and b) for width and thickness demonstrated a very high and high correlation of r = 0.83 and r = 0.79, respectively. At the second examination, the linear regression analysis demonstrated a high correlation of r = 0.64 for width (Fig. 5) and a very weak correlation for thickness.
Fig. 4.
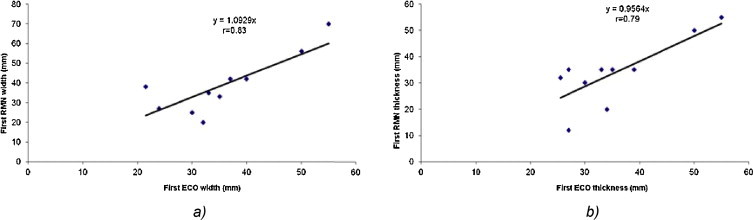
The linear regression analysis for (a) the tumor width and (b) tumor thickness between TRUS and MRI at the first examination (at diagnosis).
Fig. 5.
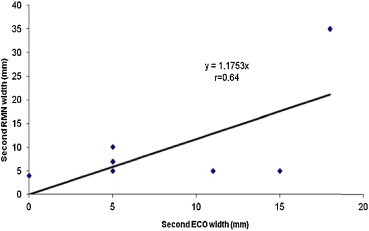
The linear regression analysis for the tumor width between TRUS and MRI at the second examination (before brachytherapy application).
5. Discussion
Imaging techniques (TRUS, computed tomography-CT, MRI) are involved in all the steps of patient management: disease detection, staging, type of therapeutic treatment, defining the target volume, selection of appropriate radiotherapy treatment and treatment planning, early estimation of therapeutic response results, and follow-up.14,15 What is more, literature provides a vast range of evidence that, in comparison with CT, MRI is significantly more accurate in the evaluation of uterine cervix tumor stage and volume.16–18 Consequently, the role of MRI in the management of cervical cancer has evolved dramatically in the last decades. Today, there is considerable evidence in the literature that MRI is not only useful in evaluating the primary tumor condition, but it is also recommended for tumor target definition prior to BT as well as at the time of BT.19,6,20 Furthermore, the positive impact of GEC-ESTRO Recommendations of image-guided BT (IGBT) with repetitive MRI in clinical practice, which gained a worldwide impact,21,22 with MRI superiority to CT and clinical examination in the appreciation of the local tumor extension and in the evaluation of the parametrial involvement, the use of MRI is considered the “Gold Standard” for IGBT.23–29
Another technique that can be used for the evaluation of the tumor dimensions in the cervical cancer is TRUS. Zaritzky et al. were the first to apply TRUS in gynecology.30 Innocenti et al. reported in 1992 that the accuracy of staging with TRUS in comparison to clinical examination was 83% vs. 79%.16,31 In early-stage cervical cancer patients, TRUS may be more precise in evaluating the primary tumors and assessing parametrial infiltration than MRI.32 Also, TRUS is more affordable than MRI, having a lower cost and being a relatively quick procedure, as Testa et al. conclude in their prospective study.33 TRUS eliminates the risk of bleeding from the tumor and it is considered a suitable method in the work-up of cervical cancer. Also, it does not require any special bowel preparation. Thus, by using TRUS we can make a maximum therapeutic impact with minimal patient morbidity.34 Moreover, TRUS provides real time images of the primary tumor, and is also very useful in verifying the position of the applicator for BT and helps in avoiding injury to OARs.25,35–37 However, TRUS has its limitations due to artifacts related to air in the bowel, presence of the applicator and interstitial needles in place.38 According to Fischerova, the accuracy of TRUS is dependent on three variables: the operator, that requires adequate practical expertise based on exposure to abnormal findings and complementary imaging methods, the equipment such as a high-end ultrasound machine equipped with sensitive Doppler and endocavitary probe, and the patient.34 Schmid et al. in their recent study regarding the cervical cancer patients compared TRUS and MRI at the time of diagnosis and at the time of brachytherapy, showing a high correlation for the target width and thickness.23
In our study we compared the tumor dimensions (width and thickness) measured by three different examinations (gynecological, TRUS and MRI). These examinations were performed at the diagnosis, prior to the treatment, and a day before the brachytherapy application, by three different physicians that had independent findings. As such, the highest correlation (r = 0.83) between TRUS and MRI was obtained for the width tumor dimension at the first examination. For all the three examinations we observed that the correlation regarding the tumor width was higher than the thickness. Furthermore, for the second examination, we obtained a high correlation (r = 0.78) between gynecological and TRUS examination; we observed that the correlations between gynecological vs. MRI examination and TRUS vs. MRI were reasonable, yet lower than the correlations at the first examinations. We consider that the fibrosis that appeared after EBRT could be an explanation for this, and not the period of time between the examinations, since at the second examinations there were only maximum 4 days between them. A good correlation (60%) between gynecological exam and MRI was also obtained by Valduvienci in his assessment of the local response after treatment.39 Last but not least, at the second examination we considered the tumor dimension to be zero if the macroscopic tumor was not visualized at the cervical level.
6. Conclusions
Based on our study, a high correlation between the gynecological and TRUS examinations was obtained. TRUS is less expensive than MRI, is a relatively quick procedure, it has a widespread availability and it is recommended to be the imagistic technique in the countries with low-income facilities. TRUS carried out by an experienced sonographer specialized in gyneological oncology is to be considered a suitable method in the evaluation of tumor dimensions (width and thickness) for patients with cervical cancer, and can be an alternative imaging method to MRI.
In Romania, the implementation of TRUS would have a major impact given that the cost of an MRI examination is five times higher than TRUS.
Based on promising results published in the literature, we can affirm that TRUS has a high potential, not only for detecting and characterizing the primary tumor, but also for offering efficient information regarding the parametrial tissue and invasion to adjacent organs.
Therefore, the next step in our further studies will be to appreciate the high-risk clinical target volume (HR-CTV); this information will help us implement IGBT in our institution.
Conflict of interest
None declared.
Financial disclosure
The authors acknowledge the financial support provided by the UEFISCDI Program – PN II – Capacities Module III – Bilateral Cooperation – Romania-Austria.
Acknowledgements
The authors thank all the patients who agreed to participate in this study.
References
- 1.Suteu O., Ghilezan N., Todor N., Scortan E. Uterine cervix cancer epidemiology in Romania 1959–1999. Radiother Oncol Med. 2001;7:40–51. [Google Scholar]
- 2.Nagy V., Ordeanu C., Coza O. Radiotherapy versus concurrent 5-day cisplatin and radiotherapy in locally advanced cervical carcinoma. Strahlenther Onkol. 2009;3:177–182. doi: 10.1007/s00066-009-1893-z. [DOI] [PubMed] [Google Scholar]
- 3.Haie-Meder C., Fervers B., Fondrinier E. SOR guidelines for concomitant chemoradiotherapy for patients with uterine cervical cancers; evidence update bulletin 2004. Ann Oncol. 2005;16:1100–1108. doi: 10.1093/annonc/mdi220. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricins and Gynecologists ACOG practice bulletin number 25, May 2002. Diagnosis and treatment of cervical carcinomas. Int J Gynaecol Obstet. 2002;78:79–91. doi: 10.1016/s0020-7292(02)90092-5. [DOI] [PubMed] [Google Scholar]
- 5.Nagy V., Ordeanu C., Coza O., Rancea A., Traila A., Todor N. Randomized phase 3 trial comparing 2 cisplatin dose schedules in 326 patients with locally advanced squamous cell cervical carcinoma: long-term follow-up. Int J Gynecol Cancer. 2012;22(9):1538–1544. doi: 10.1097/IGC.0b013e318270590a. [DOI] [PubMed] [Google Scholar]
- 6.Pötter R., Georg P., Dimopoulos J.C.A. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol. 2011;100(July (1)):116–123. doi: 10.1016/j.radonc.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marita A., Sturzu D., Ordeanu C. Neo-adjuvant chemotherapy associated to concurrent radio-chemotherapy in locally advanced cervical cancer: a feasibility study of the Institute of Oncology Prof. Dr. Ion Chiricuta. J. Radiother. Med. Oncol. 2013;19(1):17–22. [Google Scholar]
- 8.Roszak A., Warenczak-Florczak Z., Bratos K., Milecki P. Incidence of radiation toxicity in cervical cancer and endometrial cancer patients treated with radiotherapy alone versus adjuvant radiotherapy. Rep Pract Oncol Radiother. 2012;17(6):332–338. doi: 10.1016/j.rpor.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tossi M.T.B., Ghorbani M., Makhdoumi Y. A retrospective analysis of rectal and bladder dose for gynecological brachytherapy treatments with GZP6 HDR afterloading system. Rep Pract Oncol Radiother. 2012;17(6):352–357. doi: 10.1016/j.rpor.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy V.M. Univ Med Ed Cluj-Napoca; 2008. Oncology propedeutics; pp. 89–102. [Google Scholar]
- 11.Gerbaulet A., Pötter R., Mazeron J.J., Meertens H., Van Limbergen E. ACCO; 2002. The GEC ESTRO handbook of brachytherapy; pp. 331–332. [Google Scholar]
- 12.Rosner B. Cengage Learning; 2010. Fundamentals of biostatistics; pp. 427–515. [Google Scholar]
- 13.Eifel P.J. Problems with the clinical staging of carcinoma of the cervix. Semin Radiat Oncol. 1994;4:1–8. doi: 10.1053/SRAO00400001. [DOI] [PubMed] [Google Scholar]
- 14.Valduvieco I., Biete A., Rios I. Correlation between clinical findings and magnetic resonance imaging for the assessment of local response after standard treatment in cervical cancer. Rep Pract Oncol Radiother. 2013;18(4):214–219. doi: 10.1016/j.rpor.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez C.A., Mutic S. Advances and future of Radiation Oncology. Rep Pract Oncol Radiother. 2013;18(6):329–332. doi: 10.1016/j.rpor.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischerova D., Cibula D., Stenhova H. Transrectal ultrasound and magnetic resonance imaging and staging of early cervical cancer. Int J Gynaecol Cancer. 2008;18:766–772. doi: 10.1111/j.1525-1438.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim S.H., Choi B.I., Han J.C. Preoperative staging of uterine cervical carcinoma: comparison of CT and MRI in 99 patients. J Comput Assist Tomogr. 1993;17:633–640. doi: 10.1097/00004728-199307000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Choi S.H., Kim S.H., Choi H.J. Preoperative magnetic resonance imaging staging of uterine cervical carcinoma: results of prospective study. J Comput Assist Tomogr. 2004;28:620–627. doi: 10.1097/01.rct.0000138007.77725.0a. [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos J.C.A., Petrow P., Tanderup K. Recommendations from Gynecological (GYN) GEC-ESTRO Working Group (IV): basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiother Oncol. 2012;103(April (1)):113–122. doi: 10.1016/j.radonc.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brocker K.A., Alt C.D., Eichbaum M. Imaging of female pelvic malignancies regarding MRI, CT, and PET/CT: Part 1. Strahlenther Onkol. 2011;187(October (10)):611–618. doi: 10.1007/s00066-011-4001-0. [DOI] [PubMed] [Google Scholar]
- 21.Haie-Meder C., Potter R., van Limbergen E. Recommendations from the Gynaecologycal (GYN) GEC ESTRO Working Group: concepts and therms in 3D-image based 3D-treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2004;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Potter R., Haie-Meder C., van Limbergen E. Recommendations from the Gynaecologycal (GYN) GEC ESTRO Working Group (II): concepts and terms in 3D-image based 3D-treatment planning in cervix cancer brachytherapy-3D dose-volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Schmid M.P., Potter R., Brader P. Feasibility of transrectal ultrasonography for assessment of cervical cancer. Strahlenther Onkol. 2013;189:123–128. doi: 10.1007/s00066-012-0258-1. [DOI] [PubMed] [Google Scholar]
- 24.Schmid M.P., Mansmann B., Federico M. Residual tumour volumes and grey zones after external beam radiotherapy (±chemotherapy) in cervix cancer patients: a low field MRI study. Strahlenther Onkol. 2013;189:238–245. doi: 10.1007/s00066-012-0260-7. [DOI] [PubMed] [Google Scholar]
- 25.Brocker K.A., Alt C.D., Eichbaum M. Imaging of female pelvic malignancies regarding MRI, CT, and PET/CT: Part 1. Strahlenther Onkol. 2011;187:611–618. doi: 10.1007/s00066-011-4001-0. [DOI] [PubMed] [Google Scholar]
- 26.Alt C.D., Brocker K.A., Eichbaum M. Imaging of female pelvic malignancies regarding MRI, CT, and PET/CT: Part 2. Strahlenther Onkol. 2011;187:705–714. doi: 10.1007/s00066-011-4002-z. [DOI] [PubMed] [Google Scholar]
- 27.Dimopoulos J.C.A., Schirl G., Baldinger A. MRI assessment of cervical cancer for adaptive radiotherapy. Strahlenther Onkol. 2009;185:282–287. doi: 10.1007/s00066-009-1918-7. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell D.G., Snyder B., Koakley F. Early invasive cervical cancer: tumour delineation by magnetic resonance imaging, computed tomography and clinical examination, verified by pathologic results in the ACRIN 6651/GOG 183 intergroup study. J Clin Oncol. 2006;24:5687–5694. doi: 10.1200/JCO.2006.07.4799. [DOI] [PubMed] [Google Scholar]
- 29.Dimopoulos J.C.A., Schard G., Berger D. Systematic evaluation of MRI findings in different stages of treatment of cervical cancer: potential of MRI of delineation of target, pathoanatomic structures, and organs at risk. Int J Rad Oncol Biol Phys. 2006;64:1380–1388. doi: 10.1016/j.ijrobp.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Zaritzky D., Blake D., Willard J. Transrectal ultrasonography in the evaluation of cervical carcinoma. Obstet Gynecol. 1979;53:105–108. [PubMed] [Google Scholar]
- 31.Innocenti P., Pulli F., Savino L. Staging of cervical cancer: reliability of transrectal US. Radiology. 1992;185:201–205. doi: 10.1148/radiology.185.1.1523308. [DOI] [PubMed] [Google Scholar]
- 32.Epstein E., Testa A., Gaurilcikas A. Early-stage cervical cancer: tumour delineation by magnetic resonance imaging and ultrasound – a European multicenter trial. Gynecol Oncol. 2013;128(March (3)):449–453. doi: 10.1016/j.ygyno.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 33.Testa A.C., Ludovisi M., Manfredi R. Transvaginal ultrasonography and magnetic resonance imaging for assessment of presence, size and extent of invasive cervical cancer. Ultrasound Obstet Gynecol. 2009;34:335–344. doi: 10.1002/uog.7325. [DOI] [PubMed] [Google Scholar]
- 34.Fischerova D. Ultrasound scanning of the pelvis and abdomen for staging of gynecological tumours: a review. Ultrasound Obstet Gynecol. 2011;38:246–266. doi: 10.1002/uog.10054. [DOI] [PubMed] [Google Scholar]
- 35.Mahantshetty U., Khanna N., Swamidas J. Trans-abdominal ultrasound (US) and magnetic resonance imaging (MRI) correlation for conformal intracavitary brachytherapy in carcinoma of the uterine cervix. Radiother Oncol. 2012;102(January (1)):130–134. doi: 10.1016/j.radonc.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Small W., Jr., Strauss J.B., Hwang C.S., Cohen L., Lurain J. Should uterine tandem applicators ever be placed without ultrasound guidance? No: a brief report and review of the literature. Int J Gynecol Cancer. 2011;21(July (5)):941–944. doi: 10.1097/IGC.0b013e31821bca53. [DOI] [PubMed] [Google Scholar]
- 37.Weitmann H.D., Knocke T.H., Waldhäusl C., Pötter R. Ultrasound-guided interstitial brachytherapy in the treatment of advanced vaginal recurrences from cervical and endometrial carcinoma. Strahlenther Onkol. 2006;182(February (2)):86–95. doi: 10.1007/s00066-006-1420-4. [DOI] [PubMed] [Google Scholar]
- 38.Van Dyk S., Narayan K., Fisher R., Bernshaw D. Conformal brachytherapy planning for cervical cancer using transabdominal ultrasound. Int J Rad Oncol Biol Phys. 2009;75:64–70. doi: 10.1016/j.ijrobp.2008.10.057. [DOI] [PubMed] [Google Scholar]
- 39.Valduvienci I., Biete A., Rios I. Correlation between clinical findings and magnetic resonance imaging for the assessment of local response after standard treatment in cervical cancer. Rep Pract Oncol Radiother. 2013;18(4):214–219. doi: 10.1016/j.rpor.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]


