Abstract
Background
Late rectal injury is a common side effect of external beam radiotherapy for prostate cancer.
Aim
The aim of this study was to evaluate what total dose may be safely delivered for prostate patients for 3DCRT and IMRT techniques and the CTV–PTV margin.
Materials and methods
3DCRT and IMRT plans were prepared for 12 patients. For each patient PTV was defined with CTV–PTV margins of 0.4, 0.6, …, 1.0 cm, and total doses of 70, 72, …, 80 Gy, with 2 Gy dose fraction. NTCP values for the rectum were calculated using the Lyman model. Both techniques were compared in terms of population mean DVH.
Results
Significantly smaller NTCPs for IMRT were obtained. For both techniques diminishing the margin CTV–PTV of 2 mm leads to decreasing the NTCP of about 0.03. For total dose of 80 Gy the NTCP was smaller than 10% for the 4 mm margin only. The QUANTEC dose volume constraints were more frequently fulfilled for the IMRT technique than for the 3DCRT technique.
Conclusions
The IMRT technique is safer for prostate patients than the 3DCRT. If very high total doses are applied the CTV–PTV margin of 0.4 cm and the IMRT technique should be used. If the CTV–PTV margin of 0.6 cm is applied, the NTCP is smaller than 10% for the 3DCRT and IMRT techniques for the total doses smaller than 74 Gy and 78 Gy, respectively.
Keywords: Rectum injury, Normal Tissue Complication Probability, 3DCRT and IMRT, CTV–PTV margin
1. Background
Late rectal injury is a common side effect of external beam radiotherapy for prostate cancer, especially if very high dose is prescribed. This observation was confirmed by many retrospective and prospective studies.1–3 The total doses of values larger than 80 Gy delivered with 2 Gy per fraction are recommended for prostate cancer treatment.4 It is quite common to treat patients with larger doses per fraction.5 A shorter course with increased dose per fraction becomes the standard, however this increased the risk of rectum injury. To keep the risk of rectum injury at acceptable level, image guided radiotherapy is used with the Intensity Modulated Radiotherapy (IMRT).6 The larger the equivalent prescribed dose is delivered, the larger is the risk of rectum injury and, therefore, significant efforts are being undertaken to diminish the CTV–PTV margin. To diminish the dose to the rectum, in some clinics the endorectal balloons or hydrogel spacers are used.7,8
Recently, the Quantitative Analysis of Normal Tissue Effects in the Clinic Group (QUANTEC) reviewed the published data on the dose-volume determinants of late rectal injury after external beam therapy. The meta-analysis of QUANTEC revealed that the Lyman–Kutcher–Burman model gave the best estimates of Grade ≥2 late rectal toxicity or rectal bleeding.9 Using this model it is possible to evaluate quantitatively the safety of irradiation of patients with prostate cancer.
2. Aim
The purpose of this study was to evaluate what total dose may be safely delivered for prostate patients depending on the irradiation technique and the CTV–PTV margin.
3. Materials and methods
3.1. Treatment technique
Here is a short description of the technique of irradiation of patients with prostate cancer in our clinic. Twelve patients with localized prostate carcinoma (T2–3 N0 M0) treated in our clinic in 2010 were randomly selected. The median age of treated patients was 71 years and 6 months, PSA median 7.9 ng/ml (range 4.8–22.7 ng/ml), and Gleason score median 4.0 (range 3–7). During the CT scanning, patients were positioned supine with a knee-roll for position's stabilization. According to the protocol, images were taken with empty rectum and full bladder. To achieve this before investigation, patients were asked to empty their bladder and drink half a liter of water 1 h prior to a planning CT scan.10 The same procedure was followed before each treatment session. If the patient was not able to empty the rectum the additional Cone Beam CT was made to correct the patient's position. CT treatment simulation was performed with a CT scanner (Somatom Open, Siemens) with a slice thickness of 3 mm. Images were sent to a contouring station (ROP, CompArt) where the prostate, seminal vesicles, rectum, posterior wall of rectum, bladder and femoral heads were delineated by a physician. The rectum was delineated in all CT slices containing CTV and was extended by 3 cm craniocaudally. The rectum was regarded as the solid organ including the rectal content. The PTV is drawn by adding the margins: cranial–caudal and anterior–posterior margin was 0.7 cm, left-right margin was 0.4 cm. The 15 MV energy photon beams were used. The prescribed dose was 65 Gy delivered in 25 fractions 5 times a week. Dose distributions fulfilled the recommendations of the ICRU Reports 50 and 62: minimum dose to PTV > 95% and maximum dose < 107%. Additionally, according to our internal protocol, minimum dose to CTV > 97%, and maximum dose to CTV < 103% of the prescribed dose were recommended. The rectum dose-limiting constraints were: 25% of the rectum volume could receive the dose of 61 Gy or larger, 2% of the posterior wall of the rectum could receive the dose of 56.2 Gy or larger. The bladder dose-volume limiting constraint was: 50% of the bladder volume could receive the dose of 52 Gy or larger. Treatment plans were prepared with the Eclipse system, version. 10 with an Anisotropic Analytical Algorithm.
For this study additional plans were prepared by the authors of this paper. For each patient four PTVs (Planning Target Volume) were drawn consisting of the prostate gland CTV (Clinical Target Volume) and seminal vesicles with uniform margins of 0.4, 0.6, 0.8, and 1.0 cm. For each patient two treatment plans were prepared: (1) 3D conformal plan with a three field arrangement (AP and two lateral opposed fields, with a wedge as required), and (2) IMRT plan (sliding window) consisted of 5 beams at angles of 0°, 45°, 115°, 245° and 315°. For each patient, each margin, and each total dose the optimized plan was prepared. The optimization was performed for total doses of 70, 72, …, 80 Gy and 2 Gy dose per fraction. For Organs at Risk the QUANTEC dose-volume constraints were used.10 The dose was always prescribed to the isocenter which was defined as a center of mass of the CTV.
3.2. Normal Tissue Complication Probability and data analysis
Differential absolute dose volume histograms with dose bins of 1 Gy for each patient, and for each plan were calculated and saved in the computer file. No fractionation correction was made. Using this numerical data in Excel, the Normal Tissue Complication Probability (NTCP) values for the rectum were calculated using the Lyman model with model parameters recommended by QUANTEC for Grade ≥2 late toxicity or rectal bleeding: n = 0.09, m = 0.13, and TD50 = 76.9 Gy.10–12 Population mean Cumulative Dose Volume Histograms were calculated for each margin and both techniques separately. Average population Cumulative Dose Volume Histograms were compared with dose volume constraints proposed by Michalski, i.e. V50 < 50%, V60 < 35%, V65 < 25%, V70 < 20%, and V75 < 15% (VX is the partial volume of an organ which receives dose smaller than X Gy).10
It was assumed that the treatment is safe, if the NTCP of rectum is not larger than 10%.
4. Results
In Fig. 1 the average population DVHs for the 3D-CRT and IMRT techniques is shown (for some points the standard deviation is added). Similar differences in shape of DVHs were observed for all patients. Comparison of the population average dose distribution in the rectum obtained for IMRT and 3DCRT shows that: for the IMRT technique, smaller volume of the rectum was exposed to doses larger than 70% and smaller than 35% of prescribed dose. For doses in the range between 35% and 70% of prescribed dose the DVH is similar for both techniques. Figs. 2 and 3 show the dependence of the NTCP on the total dose for 3D-CRT and IMRT for all CTV–PTV margins. The increase of NTCP with dose is more pronounced for larger CTV–PTV margins, e.g., for 4 mm and 8 mm for dose of 80 Gy the difference between the NTCP for IMRT and 3DCRT are about 5% and 8%, respectively. Fig. 4 shows a comparison of the results obtained for the 3D-CRT and IMRT techniques for 4 mm, and 8 mm margins. For 9 patients the values of NTCP for 3D-CRT for all total doses and all CTV–PTV margins were larger than for IMRT. For one patient better dose distribution was obtained for 3D-CRT. These differences were statistically significant (p < 0.005, paired t-test). In Table 1 the number of dose distribution metrics are presented for which the mean value of VD in the group of 12 patients is smaller than Michalski's dose-volume constraints for a given prescribed dose and CTV–PTV margin for IMRT and 3DCRT.10 The QUANTEC dose volume constraints were more frequently fulfilled for the IMRT technique than for the 3DCRT. For both techniques, the larger the margin was the smaller the number of the dose-volume constraints which were not exceeded.
Fig. 1.

DVHs for IMRT and 3DCRT techniques averaged over all 12 patients.
Fig. 2.
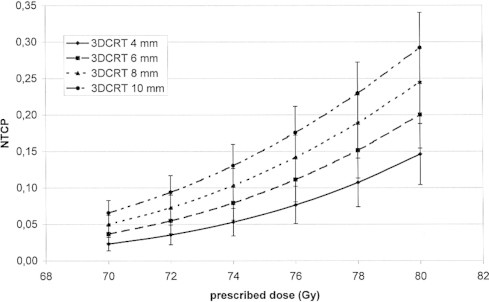
Dependence of the NTCP on the total dose for 3DCRT technique, for different CTV–PTV margins.
Fig. 3.
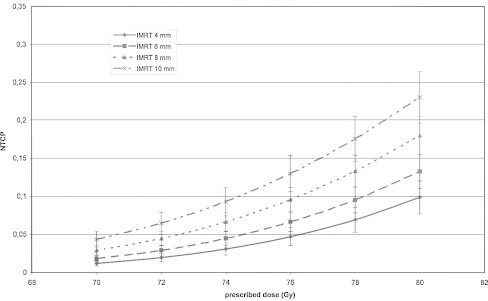
Dependence of the NTCP on the total dose for IMRT technique, for different CTV–PTV margins.
Fig. 4.
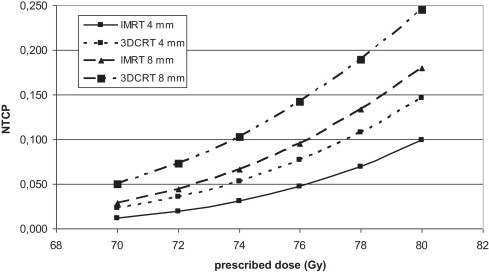
Comparison of NTCP for 3DCRT and IMRT for two CTV–PTV margins, 4 and 8 mm.
Table 1.
Number of indices for which the mean value in the group of patients is smaller than dose-volume constraints proposed by QUANTEC for a given prescribed dose and CTV–PTV margin for IMRT and 3DCRT.
| IMRT/3DCRT | 70 Gy | 72 Gy | 74 Gy | 76 Gy | 78 Gy | 80 Gy |
|---|---|---|---|---|---|---|
| 4 mm | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 | 5/5 |
| 6 mm | 5/5 | 5/4 | 5/3 | 5/2 | 5/2 | 3/1 |
| 8 mm | 5/3 | 5/3 | 5/2 | 3/2 | 3/2 | 2/1 |
| 10 mm | 5/3 | 4/3 | 3/2 | 3/2 | 1/1 | 1/1 |
4/3 means 4 for IMRT and 3 for 3DCRT.
5. Discussion
In the case of external beam therapy of prostate patients, very high total doses are delivered, even exceeding 80 Gy in 2 Gy dose per fraction with modern treatment techniques and modern technologies.3,4 The proximity of sensitive normal structures, the rectum and the bladder, makes this treatment very challenging. Decreasing the dose delivered to the rectum and bladder may be obtained by preparing a very conformal dose distribution, and by diminishing the CTV–PTV margin size. In this study we investigated quantitatively the dependence of the risk of rectum injury on the CTV–PTV margin size for two treatment techniques, 3DCRT and IMRT, and for several total doses delivered in 2 Gy per fraction. The risk was quantitatively expressed in terms of the Normal Tissue Complication Probability. The NTCP was calculated using the Lyman–Kutcher–Burman model with parameters recommended by QUANTEC for Grade ≥2 late toxicities or rectal bleeding.
The results showed that the NTCP is an increasing function of the CTV–PTV margin size, and that the IMRT technique enabled to reduce the risk of rectum injury. For each total dose and each patient, except one, the NTCP for IMRT is smaller than for the 3DCRT (differences of up to 0.08). These differences are statistically significant (p < 0.005). Similar results were obtained by Luxton.13 In his study, for 19 of 22 patients smaller complication probability for the rectum was obtained for IMRT than for 3DCRT technique. For some patients this difference was very small, for others quite large. The smaller the total dose was the smaller the difference between 3DCRT and IMRT. For the largest total dose investigated in this study, i.e. 80 Gy, the mean values of NTCP for 3DCRT and IMRT for the smallest margin of 4 mm were 0.15 and 0.10, respectively. For the same dose but for the margin of 8 mm the mean values of NTCPs increased to 0.25 and 0.17 for 3DCRT and IMRT, respectively. For a total dose of 76 Gy, and for the margin of 8 mm, for 3DCRT and IMRT the mean values of NTCPs were 0.15 and 0.07, respectively. In general, we may conclude that for the same risk of injury for the IMRT technique a dose larger by about 2–4 Gy may be delivered than with the 3DCRT technique. In Fig. 1 typical DVH for IMRT and 3DCRT are compared. Smaller NTCPs for the IMRT technique resulted from a smaller volume of the rectum exposed to doses larger than 60% of prescribed dose. Better dose distribution in the high dose region makes the IMRT technique less risky than the 3DCRT technique. For both techniques, diminishing the margin CTV–PTV of 2 mm leads to decreasing the NTCP by about 0.03.
Michalski proposed, for the rectum, following dose volume constraints: V50 < 50%, V60 < 35%, V65 < 25%, V70 < 20%, and V75 < 15% as a conservative starting point for 3D planning.10 We compared our results with the constraints proposed by Michalski. The comparison was made in terms of the population mean values of all VD listed by Michalski. For each dose and each CTV–PTV margin, we calculated the mean value of each VD for IMRT and 3DCRT techniques separately. Next, we calculated for how many dose volume constraints the average population results were not larger than VD proposed by Michalski. The results are presented in Table 1. For 4 mm CTV–PTV margin for both techniques, all population mean values of VD were smaller than the dose volume constraints proposed by Michalski. For 8 and 10 mm margins and for 3DCRT, the population average values of VD were always larger than proposed by Michalski. For the IMRT technique and for a 6 mm margin, all constraints were not fulfilled only for 80 Gy. For the IMRT technique for an 8 mm margin for doses of 70, 72, 74 and 76 Gy, all population average values of VD were smaller than the proposed constraints. Perhaps the QUATEC model overestimated the NTCP values. Liu et al. tested the QUANTEC model. They showed that the model consistently predicted higher NTCP values than it was clinically observed.14 The QUANTEC group recommends to contour the rectum from above the anal verge up to the turn into the sigmoid colon, including the rectal contents. In our work the rectum was delineated as a solid organ 3 cm above and below the CTV. The influence of the rectum contouring on rectal dose-volume histograms, and rectal toxicity was investigated by several authors. Liu emphasized that DVHs varied drastically with different techniques of contouring, which may lead to different interpretations.15 The method of rectum delineation influences the dose volume histogram in the low dose region, where only scattered dose is delivered. Consequently, it may change the VD values due to enlarging the total volume of the rectum. In our case, it may influence the comparison of the calculated DVHs and dose volume constraints proposed by Michalski. Contouring the rectum according to the QUANTEC recommendation would lead to enlargement of the total volume of the rectum and, consequently, move all the points on the DVH graph downwards. It would lead to dose distributions which will more often be acceptable according to the QUANTEC recommendations. However, it would have almost no influence on the results of the NTCP calculations.
The smaller is the CTV–PTV margin, the smaller is the risk of rectum injury but the larger is the risk of a geographical miss. In modern radiotherapy several treatment methods are applied to make the treatment geometrically precise.16 In the case of the prostate, this is especially important because the prostate is a moving target. Depending on the method of positioning control implemented in a radiotherapy center, different CTV–PTV margin sizes ensure delivering the full dose to the CTV. If only conventional portal control on bony structures is performed, a margin of about 1 cm must be used.17,18 Using the more sophisticated techniques of prostate positioning control, the margin may be diminished to about 7 mm. In our center the adaptive protocol is used which enables the use of a 7 mm CTV–PTV margin.10 On-line daily verification with permanent implants (gold markers) enables a decrease of the margin to about 5 mm.19 The smallest margin of 4 mm may be used if the system based on a transducer would be used.20 Further decrease of the margin size, in the authors’ opinion, is not safe due to possible doctor's delineation error and difficulties in visualization of extracapsular invasion of the tumor.21 Therefore, with the most sophisticated positioning control system for a prescribed dose of 80 Gy the risk of rectum injury of Grade ≥2 is about 10%. Additional improvement is expected from planning the treatment with a rotational IMRT techniques.22
6. Conclusions
The results indicate that the IMRT technique is safer for prostate patients than the 3D conformal radiotherapy. For both techniques diminishing the CTV–PTV margin by 2 mm leads to decreasing the NTCP by about 3%. If the CTV–PTV margin of 0.6 cm is applied, the NTCP is smaller than 10% for the 3DCRT and IMRT techniques for the total doses smaller than 74 Gy and 78 Gy, respectively. For the IMRT technique, if the CTV–PTV margin of 0.4 cm is applied for the total dose 80 Gy for some patients the NTCP is still larger than 10%.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Jung H., Beck-Bornholdt H.P., Svoboda V. Late complications after radiotherapy for prostate cancer. Strahlenther Onkol. 2012;188:965–974. doi: 10.1007/s00066-012-0142-z. [DOI] [PubMed] [Google Scholar]
- 2.Cahlon O., Zelefsky M.J., Shippy A. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71:330–337. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky M.J., Xin P., Chou J.F. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60:1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polkinghorn W.R., Zelefsky M.J. Improving outcomes in high-risk prostate cancer with radiotherapy. Rep Pract Oncol Radiother. 2013;18:333–337. doi: 10.1016/j.rpor.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogelius I.R., Bentzen S.M. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: bad news, good news, or no news? Int J Radiat Oncol Biol Phys. 2013;85:89–94. doi: 10.1016/j.ijrobp.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piotrowski T., Yartsevd S., Rodrigue G., Bajon T. Comparative analysis of image guidance in two institutions for prostate cancer patients. Rep Pract Oncol Radiother. 2014;19:206–213. doi: 10.1016/j.rpor.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smeenk R.J., Teh B.S., Butler E.B., van Lin E.N., Kaanders J.H. Is there a role for endorectal balloons in prostate radiotherapy? A systematic review. Radiother Oncol. 2010;95:277–282. doi: 10.1016/j.radonc.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Pinkawa M., Piroth M.D., Holy R. Quality of life after intensity-modulated radiotherapy for prostate cancer with a hydrogel spacer. Strahlenther Onkol. 2012;188:917–925. doi: 10.1007/s00066-012-0172-6. [DOI] [PubMed] [Google Scholar]
- 9.Michalski J.M., Gay H., Jackson A. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76:S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piziorska M., Kukołowicz P., Zawadzka A. Adaptive off-line protocol for prostate external radiotherapy with cone beam computer tomography. Strahlenther Onkol. 2012;188:1003–1009. doi: 10.1007/s00066-012-0226-9. [DOI] [PubMed] [Google Scholar]
- 11.Lyman J.T. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8:S13–S19. [PubMed] [Google Scholar]
- 12.Kutcher G.J., Burman C., Brewster L., Goitein M., Mohan R. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys. 1991;21:137–146. doi: 10.1016/0360-3016(91)90173-2. [DOI] [PubMed] [Google Scholar]
- 13.Luxton G., Hancock S.L., Boyer A.L. Dosimetry and radiobiologic model comparison of IMRT and 3D conformal radiotherapy in treatment of carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2004;59:267–284. doi: 10.1016/j.ijrobp.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Liu M., Moiseenko V., Agranovich A. Normal Tissue Complication Probability (NTCP) modeling of late rectal bleeding following external beam radiotherapy for prostate cancer: a test of the QUANTEC-recommended NTCP model. Acta Oncol. 2010;49:1040–1044. doi: 10.3109/0284186X.2010.509736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu M., Berthelet E., Patterson K. Various techniques of contouring the rectum and their impact on rectal dose-volume histograms. Med Dos. 2003;28:189–192. doi: 10.1016/S0958-3947(03)00071-2. [DOI] [PubMed] [Google Scholar]
- 16.Pugh T.J., Amos R.A., Baptiste J.S. Multifield optimization intensity-modulated proton therapy (MFO-IMPT) for prostate cancer: robustness analysis through simulation of rotational and translational alignment errors. Med Dos. 2013;38:344–350. doi: 10.1016/j.meddos.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bel A., van Herk M., Bartelink H., Lebesque J.V. A verification procedure to improve patient set-up accuracy using portal imaging. Radiother Oncol. 1993;29:253–260. doi: 10.1016/0167-8140(93)90255-7. [DOI] [PubMed] [Google Scholar]
- 18.Boer H.C., Heijmen B.J. A protocol for the reduction of systematic patient setup errors with minimal portal imaging workload. Int J Radiat Oncol Biol Phys. 2001;50:1350–1365. doi: 10.1016/s0360-3016(01)01624-8. [DOI] [PubMed] [Google Scholar]
- 19.Graf R., Bohmer D., Budach V., Wust P. Residual translational and rotational errors after kV X-ray image-guided corrections of prostate location using implanted fiducials. Strahlenther Onkol. 2010;186:544–550. doi: 10.1007/s00066-010-2030-8. [DOI] [PubMed] [Google Scholar]
- 20.Langen K.M., Willoughby T.R., Meeks S.L. Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys. 2008;71:1084–1090. doi: 10.1016/j.ijrobp.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 21.Bonin S.R., Hanlon A.L., Lee W.L. Evidence of increased failure in the treatment of prostate carcinoma patients who have perineural invasion treated with three-dimensional conformal radiation therapy. Cancer. 1997;79:75–80. doi: 10.1002/(sici)1097-0142(19970101)79:1<75::aid-cncr11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 22.Robar J.L., Thomas C. HybridArc: a novel radiation therapy technique combining optimized dynamic arcs and intensity modulation. Med Dos. 2012;37:358–368. doi: 10.1016/j.meddos.2012.02.001. [DOI] [PubMed] [Google Scholar]


