Abstract
Objectives:
Weight gain is a major side effect of antipsychotics (APs), which contributes to poor treatment adherence and significant morbidity. The mechanisms involved in AP-induced weight gain are incompletely understood. Recently, it has been proposed that changes in leptin, an cadipocyte-derived hormone exerting anorexigenic effects, may be involved in AP-induced weight gain. Thus far, studies on leptin changes during AP treatment have produced inconsistent results, prompting our group to perform a meta-analysis.
Method:
A search of the literature was performed using PubMed and Embase. Studies were included only if reporting peripheral levels of leptin before and after AP treatment in schizophrenia. Effect size estimates were calculated with Hedges g and were aggregated using a random effects model as results were heterogeneous (P < 0.10). Meta-regression analyses were performed using study length and changes in body mass index (BMI) as moderator variables.
Results:
Twenty-eight studies were retrieved, including 39 comparisons. A moderate and positive effect size was observed across studies. Olanzapine, clozapine, and quetiapine produced moderate leptin elevations, whereas haloperidol and risperidone were associated with small (nonsignificant) leptin changes. Across studies, BMI changes were significantly associated with increases in leptin levels. There was no effect of sex on AP-induced changes in leptin.
Conclusions:
A physiological role of leptin in AP-induced weight gain is supported because the most significant leptin increases were observed with APs inducing the most weight gain and because of the observed association between leptin increases and BMI changes. The overall increase in leptin levels suggests that leptin acts as a negative feedback signal in the event of fat increase.
Keywords: antipsychotic, weight gain, leptin, meta-analysis, metabolic
Abstract
Objectifs :
La prise de poids est un effet secondaire majeur des antipsychotiques (AP), ce qui contribue à une mauvaise observance du traitement et à une morbidité significative. Les mécanismes impliqués dans la prise de poids induite par AP ne sont pas tout à fait compris. Récemment, il a été proposé que les changements de leptine, une hormone dérivée d’un adipocyte exerçant des effets anorexigènes, pourraient participer à la prise de poids induite par AP. Jusqu’ici, les études sur les changements de leptine durant le traitement par AP ont produit des résultats sans cohérence, ce qui a poussé notre groupe à exécuter une méta-analyse.
Méthode :
Une recherche de la littérature a été menée à l’aide de PubMed et Embase. Les études n’ont été incluses que si elles indiquaient les taux périphériques de leptine avant et après le traitement de la schizophrénie par AP. Les estimations de la taille de l’effet ont été calculées avec le g de Hedges et ont été regroupées à l’aide d’un modèle à effets aléatoires puisque les résultats étaient hétérogènes (P < 0,10). Des analyses de méta-régression ont été exécutées à l’aide de la durée de l’étude et des changements de l’indice de masse corporelle (IMC) comme variables modératrices.
Résultats :
Vingt-huit études ont été récupérées, dont 39 comparaisons. Une taille de l’effet modérée et positive a été observée dans toutes les études. L’olanzapine, la clozapine et la quétiapine produisaient des hausses de leptine modérées, alors que l’halopéridol et la rispéridone étaient associés à des changements de leptine mineurs (non significatifs). Dans toutes les études, les changements d’IMC étaient significativement associés à des augmentations des taux de leptine. Il n’y avait pas d’effet du sexe sur les changements de leptine induits par AP.
Conclusions :
Le rôle physiologique de la leptine dans la prise de poids induite par AP est confirmé parce que les hausses de leptine les plus significatives s’observaient quand les AP induisaient le plus de prise de poids et en raison de l’association observée entre les hausses de leptine et les changements d’IMC. L’augmentation globale des taux de leptine suggère que la leptine donne un signal de rétroaction négative dans le cas d’une augmentation des lipides
Second-generation APs have become increasingly popular for the treatment of schizophrenia owing to their low potential to induce extrapyramidal symptoms, relative to FGAs.1 However, some SGAs such as olanzapine, clozapine, and quetiapine (less so) are associated with significant metabolic side effects, including weight gain, and elevations in insulin, triglyceride, glucose, and LDL cholesterol levels.2–5 Metabolic side effects are associated with poor treatment adherence and high rates of diabetes mellitus type 2, cardiovascular disease, and morbidity among schizophrenia patients.6–9
The mechanisms responsible for metabolic side effects associated with SGAs are not completely understood. Leptin—a cytokinelike peptide that is synthesized in adipose tissue—acts to reduce appetite and increase metabolic rate after it reaches the brain through regions outside the blood–brain barrier, including parts of the hypothalamus. Leptin is considered one of the best markers of total body fat in animals and humans.10,11 In mice, there is evidence that ob (obese) and db (diabetes) genes encode leptin and the leptin receptor, respectively—recessive mutations in these genes result in obesity and diabetes.12–15 Subcutaneous leptin infusion to lean mice results in a dose-dependent loss of body weight, whereas chronic infusions of intracerebroventricular leptin results in complete depletion of visible adipose tissue. When subcutaneous leptin is infused into diet-induced obese mice it results in loss of adipose tissue, but less so than in lean mice, suggesting the development of leptin resistance.16
Clinical Implications
Only high-to-moderate risk APs (olanzapine, clozapine, and quetiapine) produced significant leptin elevations.
Hyperleptinemia in schizophrenia is likely to represent a secondary effect related to AP-induced weight gain.
The overall increase in leptin levels suggests that leptin acts as a negative feedback signal in the event of fat increase.
Limitations
We were unable to include some prospective studies that did not report absolute changes in leptin levels and this may have biased our results.
Our analysis contained a low number of studies that treated patients with APs that are known to induce little or no weight gain, and most studies were comprised of a small sample of patients.
We were not able to calculate an effect size for changes in ghrelin.
In obese humans, a paradoxical hyperleptinemic state has been observed, which appears to indicate a loss of leptin’s ability to stop overeating behaviour (that is, leptin resistance).17–20 In patients with schizophrenia, hyperleptinemia has also been observed and it is associated with AP-induced weight gain and metabolic side effects.21–25 Numerous studies examined AP-induced changes in leptin among patients with schizophrenia. Thus far, results have been mixed such that some studies report increased leptin levels,26–29 while others report no change.30–32 Taking into account AP-type (high-to-moderate risk, compared with low risk of metabolic side effects) should resolve these issues, but this is not necessarily the case. For example, some studies reveal that only high-to-moderate risk APs (olanzapine, clozapine, and quetiapine) significantly increase leptin levels,33–35 while others show that all APs produce equal changes in leptin (or no changes), independent of their propensity to induce metabolic side effects.36–41 Other confounding factors that may lead to the differential results include age and sex of patients, as well as baseline AP, study duration, and type of blood sample (plasma, compared with serum).
Our meta-analysis of prospective studies aims to resolve some of the discrepant findings by examining the effects of APs on blood leptin levels in patients with schizophrenia. Sufficient data were available for 4 SGAs (olanzapine, clozapine, quetiapine, and risperidone) and 1 FGA (haloperidol). Further, we performed meta-regressions to analyze the relation between changes in leptin, demographic variables, metabolic markers, and other confounding factors.
Methods
Search Strategies
A systematic search was performed in the electronic databases PubMed, clinicaltrials.gov, and Embase using the key words antipsychotic, leptin, and ghrelin. This search identified studies after January 1, 1998, and before January 1, 2014. Additionally, studies were identified by cross-referencing of reviews published on the topic.42,43 Unfortunately, after collecting the ghrelin studies, we observed that they measured heterogeneous ghrelin outcomes (for example, active, compared with inactive, compared with total ghrelin levels). Moreover, 4 studies did not specify which outcome was measured. For these reasons, we did not perform the ghrelin meta-analysis.
Studies were included if they met the following criteria: had involved subjects with DSM or International Classification of Diseases schizophrenia spectrum disorder; had employed a pre–post design, which involved the administration of APs (single or mixed); had measured the level of leptin before and after the treatment in plasma or serum; and, had been published in English. When several articles dealt with the same population, we selected the article with the largest sample. Studies were excluded if any of the following existed: the study design was cross-sectional; the sample of patients comprised patients with DSM Axis I disorders other than schizophrenia spectrum disorders; the sample comprised adolescents with schizophrenia; the study had incomplete data to calculate the effect size estimate; the study was a randomized controlled trial of add-on treatment; and, the study only measured leptin-related gene polymorphisms and did not report blood levels of leptin.
The variables for each article included in the meta-analysis were as follows: sample sizes, sex (proportion of females), participants’ mean age, follow-up length, diagnosis, clinical status (in- or outpatient), and AP at baseline and during treatment, changes in BMI, and changes in metabolic markers. As a gross estimate of study quality, we also performed a subanalysis on the type of study design (randomized or not). Data were extracted by 2 independent reviewers, and conflicts were resolved by consensus. To achieve a high standard of reporting, we have adopted Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.44
Statistical Analysis
Data were analyzed with a quantitative meta-analytical approach using Comprehensive Meta-Analysis software, version 2 (Biostat Inc, Englewood, NJ). Comprehensive Meta-Analysis software employs the same computational algorithms used by the Cochrane collaborators to weight studies by the inverse variance method.45 The primary effect size measure was the difference in blood levels of leptin after AP treatment (end point, compared with baseline). The effect size was estimated by calculating Hedges unbiased g, which corrects for bias from small sample sizes,46 with negative values reflecting decreased leptin levels after AP treatment. Hedges g was chosen instead of Cohen d, because of the low number of patients involved in most of the studies included in the meta-analysis. Effect size estimates were calculated using means, standard deviations, and sample size, or repeated measures statistics (for example, paired t tests or repeated measures analyses of variances). Following the convention of Cohen,47 effect sizes of 0.2, 0.5, and 0.8 were considered small, moderate, and large, respectively. Heterogeneity among study point estimates was assessed with the Q statistics,48 with magnitude of heterogeneity being evaluated with the I2 index.49 As the database was characterized by high heterogeneity (see below), we employed random effects models, which take into account between-study variability and are therefore more conservative than fixed-effect models.50
To determine whether categorical factors modified AP-induced leptin changes, subgroup analyses were performed.48 The influence of continuous moderator variables was tested using meta-regression analyses. To limit risk of false-positive (type I) errors arising from multiple comparisons, we adjusted P < 0.05 by dividing alpha with the number of meta-regressions. The possibility of publication bias was examined with the fail-safe number.51 Finally, to assess the robustness of the results, we performed sensitivity analyses by sequentially removing each study and rerunning the analysis.
Results
Studies
The literature search retrieved 32 potential articles. Among these, the studies from Bromel et al,52 Eder et al,53 and Hinze-Selch et al54 were excluded because their data overlapped with the data of the studies from Theisen et al,55 Ebenbichler et al,56 and Kraus et al,33 respectively. The study from Wang et al57 was excluded because of the use of adjunctive treatment with propranolol. Thus a total of 28 publications were included in the meta-analysis, which comprised 39 study arms and 819 patients (online eTable 1).26–41,55,56,58–67 For leptin, the data set was characterized by high heterogeneity (Q = 167.9; P = 0.001; I2 = 77.4). Among the 28 studies, 10 studies also examined blood levels of ghrelin.27,32,37–39,55,59,62–64
Changes in Blood Levels of Leptin
Main Results
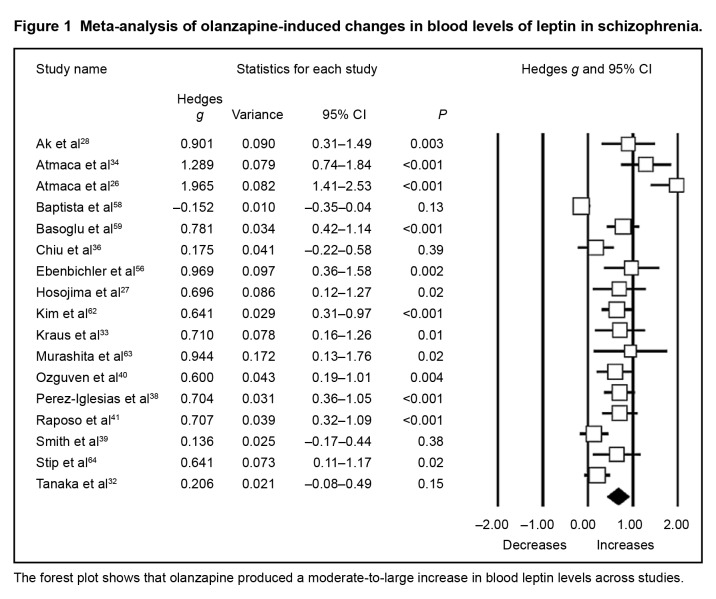
For the whole data set (Table 2), we found that APs induced moderate increases in leptin levels, regardless of the AP. The most prominent increases were observed with clozapine, quetiapine, and olanzapine (Figure 1), while risperidone-induced increases in leptin levels were of small magnitude.
Table 2.
Meta-analysis of antipsychotic-induced changes in peripheral leptin levels
| Comparison | Study arms, n | Hedges g | Variance | 95% CI | P |
|---|---|---|---|---|---|
| Clozapine | 4 | 0.570 | 0.049 | 0.137 to 1.003 | 0.01 |
| Haloperidol | 3 | 0.423 | 0.06 | −0.056 to 0.902 | 0.08 |
| Mixed antipsychotics | 8 | 0.431 | 0.022 | 0.142 to 0.721 | 0.004 |
| Olanzapine | 17 | 0.649 | 0.012 | 0.439 to 0.859 | <0.001 |
| Quetiapine | 2 | 0.790 | 0.096 | 0.182 to 1.398 | 0.01 |
| Risperidone | 5 | 0.285 | 0.035 | −0.083 to 0.652 | 0.13 |
| Composite | 39 | 0.533 | 0.005 | 0.397 to 0.668 | <0.001 |
| Sample | |||||
| Plasma | 15 | 0.525 | 0.011 | 0.315 to 0.734 | <0.001 |
| Serum | 24 | 0.534 | 0.007 | 0.367 to 0.701 | <0.001 |
| Baseline drug treatment | |||||
| Antipsychotic | 9 | 0.249 | 0.014 | 0.019 to 0.479 | 0.03 |
| Drug-free | 16 | 0.613 | 0.008 | 0.433 to 0.793 | <0.001 |
| Drug-naive | 8 | 0.707 | 0.014 | 0.472 to 0.942 | <0.001 |
| Not specified | 6 | 0.421 | 0.021 | 0.133 to 0.708 | 0.004 |
| Study quality | |||||
| Single arm | 24 | 0.515 | 0.007 | 0.348 to 0.682 | <0.001 |
| Randomized | 15 | 0.553 | 0.011 | 0.345 to 0.762 | <0.001 |
Figure 1.
Meta-analysis of olanzapine-induced changes in blood levels of leptin in schizophrenia.
The forest plot shows that olanzapine produced a moderate-to-large increase in blood leptin levels across studies.
The blood sample (plasma or serum) used to collect leptin had no influence on results (Table 2). Neither did clinical status (inpatient or outpatient), nor study design (Table 2). Conversely, drug status at baseline significantly influenced results, as AP-induced increases in blood levels of leptin were more prominent in patients who were drug-naive or drug-free at baseline, compared with patients who were already treated with APs (Table 2).
Meta-Regression Analyses
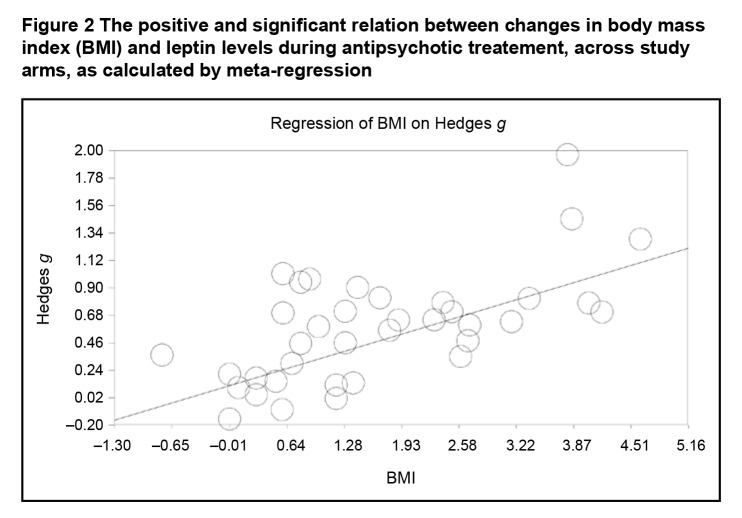
After applying Bonferoni correction, meta-regression analyses revealed significant positive associations between increases in leptin blood levels and changes in BMI during treatment (Figure 2); changes in LDL levels; changes in cholesterol levels; and changes in triglyceride levels (Table 3). Significant negative associations were observed between increases in leptin levels and age and between increases in leptin levels and changes in glucose levels (Table 3). Potential associations with changes in HDL and insulin levels did not emerge as significant (Table 3).
Figure 2.
The positive and significant relation between changes in body mass index (BMI) and leptin levels during antipsychotic treatement, across study arms, as calculated by meta-regression
Table 3.
Meta-regression analyses of the relations between changes in leptin levels during treatment and other clinical variables
| Clinical variable | Study arms, n | Slope (β) | P |
|---|---|---|---|
| Study length, weeks | 39 | 0.00422 | 0.04a |
| Age of patients, years | 37 | −0.04028 | <0.001 |
| Sex, % of male patients | 36 | −0.00022 | 0.85 |
| Changes in body mass index | 37 | 0.21324 | <0.001 |
| Changes in glucose levelsb | 13 | −0.03050 | <0.001 |
| Changes in HDL levels | 14 | −0.03886 | 0.03a |
| Changes in LDL levels | 14 | 0.02816 | <0.001 |
| Changes in insulin levels | 13 | −0.01586 | 0.04a |
| Changes in total cholesterol levels | 13 | 0.03537 | <0.001 |
| Changes in triglycerides levels | 18 | 0.00948 | <0.001 |
HDL = high-density lipoprotein; LDL = low-density lipoprotein
Nonsignificant after applying Bonferoni correction
For metabolic markers, standard international units were used
Publication Bias
The fail-safe number of additional negative studies (that is, studies reporting no changes in leptin levels during treatment) required to nullify the significance of our composite analysis was high. Indeed, 2479 studies with negative results would need to be published to render nonsignificant the moderate AP-induced increase in peripheral leptin levels observed across the 39 study arms included in the meta-analysis.
Sensitivity Analysis
Sensitivity analysis confirmed the results were robust as no study affected the composite meta-analytic estimate by more than 6.8%. That is, the removal of the most outlier study (Atmaca et al26) would only lower the composite effect size estimate to Hedges g = 0.494 (instead of Hedges g = 0.533).
Discussion
Our meta-analysis of prospective studies aimed at examining the differential effect of APs on leptin levels in schizophrenia. We also performed meta-regression analyses to analyze the relation between changes in leptin, demographic variables, metabolic markers, and other confounding factors. Our analyses revealed a moderate and positive effect size across studies, indicating that APs as a whole produced elevations in leptin among schizophrenia patients. A subanalysis by AP type revealed that only high-to-moderate risk APs (olanzapine, clozapine, and quetiapine) produced significant leptin elevations. Across studies, BMI changes were significantly associated with increases in leptin levels. Moreover, changes in leptin were positively associated with changes in triglycerides, LDL, and total cholesterol, whereas they were negatively associated with changes in glucose. Finally, there was an effect of age (but not sex) on AP-induced changes in leptin.
Many mechanisms have been suggested to be responsible for weight gain and metabolic side effects observed following treatment with APs, including antagonism of H1 histamine receptor, 5-HT2C serotonergic receptors, and M3 muscarinic receptors.68–70 Although all 3 mechanisms may be involved, only affinity for M3 receptors (which regulate insulin secretion, glucose homeostasis, and body weight) seems to explain the large differences in weight gain and metabolic side effects observed between high-to-moderate risk APs and low-risk APs.71,72 Indeed, studies have shown that only olanzapine, clozapine, and quetiapine (through its metabolite nor-quetiapine) have substantial affinity for M3 receptors among SGAs.73,74 In animals, there is evidence that mice deficient in the M3 receptor display a significant decrease in food intake, reduced body weight, and peripheral fat deposits, and very low levels of serum leptin and insulin.75 In patients with schizophrenia, there is evidence that affinity for M3 is among the best predictors for the propensity of APs to induce type 2 diabetes and weight gain.76,77 This interpretation is consistent with our findings as risperidone produced small (nonsignificant) leptin elevations and it has no affinity for M3 receptors.78
Our results show a significant positive association between BMI and leptin levels across studies. Similar associations were reported among subjects undergoing diet-induced weight gain. An early study by Considine et al17 found elevated serum leptin levels among obese subjects, relative to healthy control subjects, and there was a strong positive correlation between serum leptin concentrations and percentage of body fat. Another study by the same authors revealed that serum leptin was 318% higher in obese than lean subjects.18 However, the leptin concentration in the CSF in obese subjects was only 30% higher than in lean subjects, suggesting that leptin enters the brain by a saturable transport system. Similar differences in CSF to blood ratio of leptin between obese and normal subjects were found by Banks et al19 and Schwartz et al.20 Overall, these data suggest that the capacity of leptin transport into the central nervous system is lower in obese subjects, and may provide a mechanism for leptin resistance. These data may also explain why many patients receiving APs that are associated with high risk for metabolic side effects report increased appetite despite the presence of hyperleptinemia63,64—a state that should normally be associated with anorexigenic effects.15 Future studies should elucidate whether there are equal amounts of leptin resistance in AP-treated patients with schizophrenia.
Our analysis of the relation between leptin levels and metabolic markers showed that changes in leptin were positively associated with changes in triglycerides, LDL, and total cholesterol. These results correspond to the known link between AP treatment, increased adiposity, hyperlipidemia, hyperleptinemia, and more serious health problems, such as diabetes and cardiovascular disease.8,21,22 Conversely, we found a negative relation between changes in leptin and changes in fasting blood glucose levels. As leptin normally inhibits insulin secretion, the development of leptin resistance may lead to hyperinsulinemia and reduced glucose elevations.11 However, a continued state of hyperinsulinemia is expected to eventually lead to insulin resistance, glucose elevations, and development of diabetes type 2. However, it must be considered that the relation between changes in leptin and changes in glucose levels was rather small, and that most studies examining this association have not produced significant results.32,58
Although we found significant associations between leptin changes and changes in some metabolic markers (for example, triglycerides, LDL, and total cholesterol), these associations were weaker than the association between leptin changes and changes in BMI, meaning that mechanisms, other than changes in metabolic markers, may be involved in AP-induced weight gain. The overall increase in leptin levels and its association with BMI suggests that leptin acts as a negative feedback signal in the event of fat increase. This may explain the small (but nonsignificant) leptin elevations observed following treatment with haloperidol and risperidone. Indeed, recent meta-analyses suggest that almost all APs (both SGAs and FGAs) produce some weight gain, compared with placebo, on average (even if it is minor), regardless of their propensity to induce other metabolic side effects.1,4 Alternatively, the small leptin elevations associated with haloperidol and risperidone may be a type I error owing to the small number of patients per study and the low number of studies that measured leptin during therapy with these agents.
Our analysis of the relation between leptin levels and demographic variables showed that age was associated with leptin elevations, but there was no association with sex. Previous studies in nonschizophrenia subjects revealed that aging is associated with increased leptin levels, although these differences tend to disappear (or even result in leptin decreases) when adjusted for increased adiposity.79–82 Similarly, we found a negative association between age and leptin levels, which may be related to leptin elevations being more pronounced among drug-naive patients, who are also likely to be younger than those treated with APs. In addition, our analysis of leptin changes between males and females revealed no effect of sex. Cross-sectional studies among patients with schizophrenia and nonschizophrenia patients show that females have higher blood leptin levels at baseline, relative to males.23,79–81,83,84 Among prospective studies of patients with schizophrenia, some studies show no effect of sex on AP-induced leptin elevations30,34,58; however, other studies report larger increases in leptin among males, compared with females.24,25,31,67 We may have failed to find an effect of sex on leptin changes because only a few studies performed separate analyses for each sex. Thus we analyzed the impact of sex according to the percentage of male patients in our study.
Our meta-analysis has both strengths and limitations. Importantly, this is the first meta-analysis of AP-induced leptin changes. Here, we analyzed only prospective studies, thus eliminating the potential bias of cross-sectional studies that measure outcomes at a single time point and cannot answer whether changes were due to baseline differences or APs themselves. Additionally, we analyzed numerous potential confounding factors, such as blood sample (plasma, compared with serum), study length, and baseline drug treatment (AP, compared with drug-free, compared with drug-naive, compared with not specified). We found no effect of blood sample and study length, suggesting that they did not bias our results. By contrast, our meta-analysis showed that AP-induced leptin increases are more prominent when patients are drug-free or drug-naive, relative to studies where patients are already treated with APs at baseline. This is noteworthy as it may explain the variability in results among studies, suggesting that future studies should avoid measuring AP-induced changes in leptin levels in patients who are already receiving stable treatment with APs.
One limitation of our meta-analysis is that we were not able to include some prospective studies23–25 that did not report absolute changes in leptin levels, and this may have biased our results. However, the fail-safe number of studies that are required to make our results insignificant was calculated, and the calculated number of studies emerged as being high (n = 2479). Further, our analysis contained a low number of studies that treated patients with APs that are known to induce little or no weight gain, and most studies were comprised of a small sample of patients. This may have led to the larger effect size that we observed for leptin changes with haloperidol, even though this AP is typically associated with less weight gain than risperidone.1,4 Finally, our meta-analysis is limited because we were not able to calculate an effect size for changes in ghrelin. Examination of AP-induced changes in ghrelin is important because this hormone is associated with increased appetite and body fat mass, and inhibition of the anorexigenic effects of leptin.85 However, studies measured heterogeneous ghrelin outcomes (for example, active, compared with inactive, compared with total ghrelin, levels) or did not specify which outcome was measured.
Conclusion
We conducted a meta-analysis of prospective studies that examined AP-induced changes in leptin levels. Our results suggest that hyperleptinemia in schizophrenia is likely to represent a secondary effect related to AP-induced weight gain. This is supported by the most significant leptin increases being observed with APs inducing the most weight gain and by the association between leptin increases and BMI changes. The overall increase in leptin levels and its association with BMI suggests that leptin acts as a negative feedback signal in the event of fat increase. Future studies should examine whether there is evidence of leptin resistance in AP-treated patients, as has been shown in nonpsychotic obese subjects.17–20 Further research is also required on AP-induced leptin changes in outpatients, as 25 out of 28 studies included herein examined inpatients. In addition, there is a need for studies to measure changes in AP-induced changes in other hormones involved in appetite regulation, such as ghrelin, adiponectin, agouti-related protein, neuropeptide Y, and cholecystokinin. Finally, AP-induced changes in leptin levels will also need to be studied in greater detail in psychiatric disorders other than schizophrenia. This is because APs are often used to treat multiple psychiatric conditions, and the incidence of side effects (including metabolic changes) may differ between disorders.2,3
Acknowledgments
Dr Stip is the holder of the Eli Lilly Chair of Schizophrenia from the University of Montreal. His research has received funding from Lundbeck Canada Inc. and Otsuka Canada Pharmaceutical Inc. He has served on the advisory boards and been a lecturer for Lundbeck Canada Inc, Otsuka Canada Pharmaceutical Inc, and Janssen. Dr Zhornitsky is a recipient of a post-doctoral fellowship from the MS Society of Canada. The authors thank Joée Lafontaine for her assistance in data entry. Among the 28 trials included in our meta-analysis, 5 were disclosed as funded by the pharmaceutical company, namely, the study from Ozguven et al40 (AstraZeneca and Eli Lilly), Smith et al39 (Eli Lilly), Stip et al64 (Eli Lilly), Tschoner et al35 (BMS, Janssen and Servier), and Zhang et al67 (Pfizer).
Abbreviations
- AP
antipsychotic
- BMI
body mass index
- CSF
cerebrospinal fluid
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- FGA
first-generation AP
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- SGA
second-generation AP
References
- 1.Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 2.Moteshafi H, Zhornitsky S, Brunelle S, et al. Comparing tolerability of olanzapine in schizophrenia and affective disorders: a meta-analysis. Drug Saf. 2012;35(10):819–836. doi: 10.1007/BF03261978. [DOI] [PubMed] [Google Scholar]
- 3.Moteshafi H, Stip E. Comparing tolerability profile of quetiapine, risperidone, aripiprazole and ziprasidone in schizophrenia and affective disorders: a meta-analysis. Expert Opin Drug Saf. 2012;11(5):713–732. doi: 10.1517/14740338.2012.712682. [DOI] [PubMed] [Google Scholar]
- 4.Parsons B, Allison DB, Loebel A, et al. Weight effects associated with antipsychotics: a comprehensive database analysis. Schizophr Res. 2009;110(1–3):103–110. doi: 10.1016/j.schres.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2–3):225–233. doi: 10.1016/j.schres.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischhacker WW, Meise U, Günther V, et al. Compliance with antipsychotic drug treatment: influence of side effects. Acta Psychiatr Scand Suppl. 1994;382:11–15. [PubMed] [Google Scholar]
- 7.Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277–288. doi: 10.1016/s0165-1781(01)00234-7. [DOI] [PubMed] [Google Scholar]
- 8.Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334–1349. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
- 9.Mundet-Tudurí X, Iglesias-Rodal M, Olmos-Domínguez C, et al. Cardiovascular risk factors in chronic treatment with antipsychotic agents used in primary care. Rev Neurol. 2013;57(11):495–503. [PubMed] [Google Scholar]
- 10.Prolo P, Wong ML, Licinio J. Leptin. Int J Biochem Cell Biol. 1998;30(12):1285–1290. doi: 10.1016/s1357-2725(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 11.Van Gaal LF, Wauters MA, Mertens IL, et al. Clinical endocrinology of human leptin. Int J Obes Relat Metab Disord. 1999;23(Suppl 1):29–36. doi: 10.1038/sj.ijo.0800792. [DOI] [PubMed] [Google Scholar]
- 12.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 13.Coleman DL, Hummel KP. The influence of genetic background on the expression of the obese (ob) gene in the mouse. Diabetologia. 1973;9(4):287–293. doi: 10.1007/BF01221856. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 15.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 16.Halaas JL, Boozer C, Blair-West J, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci U S A. 1997;94(16):8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 18.Caro JF, Kolaczynski JW, Nyce MR, et al. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348(9021):159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 19.Banks WA, Kastin AJ, Huang W, et al. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17(2):305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz MW, Peskind E, Raskind M, et al. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med. 1996;2(5):589–593. doi: 10.1038/nm0596-589. [DOI] [PubMed] [Google Scholar]
- 21.Jin H, Meyer J, Mudaliar S, et al. Use of clinical markers to identify metabolic syndrome in antipsychotic-treated patients. J Clin Psychiatry. 2010;71(10):1273–1278. doi: 10.4088/JCP.09m05414yel. [DOI] [PubMed] [Google Scholar]
- 22.Melkersson KI, Hulting AL, Brismar KE. Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related psychoses. J Clin Psychiatry. 2000;61(10):742–749. doi: 10.4088/jcp.v61n1006. [DOI] [PubMed] [Google Scholar]
- 23.Hägg S, Söderberg S, Ahrén B, et al. Leptin concentrations are increased in subjects treated with clozapine or conventional antipsychotics. J Clin Psychiatry. 2001;62(11):843–848. [PubMed] [Google Scholar]
- 24.Ehrlich S, Leopold K, Merle JV, et al. Trajectories of agouti-related protein and leptin levels during antipsychotic-associated weight gain in patients with schizophrenia. J Clin Psychopharmacol. 2012;32(6):767–772. doi: 10.1097/JCP.0b013e318270e5c5. [DOI] [PubMed] [Google Scholar]
- 25.Kluge M, Schuld A, Schacht A, et al. Effects of clozapine and olanzapine on cytokine systems are closely linked to weight gain and drug-induced fever. Psychoneuroendocrinology. 2009;34(1):118–128. doi: 10.1016/j.psyneuen.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Atmaca M, Tezcan E, Ustundag B. Plasma nitric oxide and leptin values in patients with olanzapine-induced weight gain. J Psychiatr Res. 2007;41(1–2):74–79. doi: 10.1016/j.jpsychires.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Hosojima H, Togo T, Odawara T, et al. Early effects of olanzapine on serum levels of ghrelin, adiponectin and leptin in patients with schizophrenia. J Psychopharmacol. 2006;20(1):75–79. doi: 10.1177/0269881105056647. [DOI] [PubMed] [Google Scholar]
- 28.Ak M, Sezlev D, Sutcigil L, et al. The investigation of leptin and hypothalamic neuropeptides role in first attack psychotic male patients: olanzapine monotherapy. Psychoneuroendocrinology. 2013;38(3):341–347. doi: 10.1016/j.psyneuen.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Templeman LA, Reynolds GP, Arranz B, et al. Polymorphisms of the 5-HT2C receptor and leptin genes are associated with antipsychotic drug-induced weight gain in Caucasian subjects with a first-episode psychosis. Pharmacogenet Genomics. 2005;15(4):195–200. doi: 10.1097/01213011-200504000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Kivircik BB, Alptekin K, Çaliskan S, et al. Effect of clozapine on serum leptin, insulin levels, and body weight and composition in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(5):795–799. doi: 10.1016/S0278-5846(03)00111-8. [DOI] [PubMed] [Google Scholar]
- 31.Sarandol A, Kirli S, Akkaya C, et al. Coronary artery disease risk factors in patients with schizophrenia: effects of short term antipsychotic treatment. J Psychopharmacol. 2007;21(8):857–863. doi: 10.1177/0269881107077609. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K, Morinobu S, Ichimura M, et al. Decreased levels of ghrelin, cortisol, and fasting blood sugar, but not n-octanoylated ghrelin, in Japanese schizophrenic inpatients treated with olanzapine. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1527–1532. doi: 10.1016/j.pnpbp.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Kraus T, Haack M, Schuld A, et al. Body weight and leptin plasma levels during treatment with antipsychotic drugs. Am J Psychiatry. 1999;156(2):312–314. doi: 10.1176/ajp.156.2.312. [DOI] [PubMed] [Google Scholar]
- 34.Atmaca M, Kuloglu M, Tezcan E, et al. Serum leptin and triglyceride levels in patients on treatment with atypical antipsychotics. J Clin Psychiatry. 2003;64(5):598–604. doi: 10.4088/jcp.v64n0516. [DOI] [PubMed] [Google Scholar]
- 35.Tschoner A, Engl J, Rettenbacher M, et al. Effects of six second generation antipsychotics on body weight and metabolism: risk assessment and results from a prospective study. Pharmacopsychiatry. 2009;42(1):29–34. doi: 10.1055/s-0028-1100425. [DOI] [PubMed] [Google Scholar]
- 36.Chiu CC, Chen KP, Liu HC, et al. The early effect of olanzapine and risperidone on insulin secretion in atypical-naïve schizophrenic patients. J Clin Psychopharmacol. 2006;26(5):504–507. doi: 10.1097/01.jcp.0000237947.80764.d9. [DOI] [PubMed] [Google Scholar]
- 37.Popovic V, Doknic M, Maric N, et al. Changes in neuroendocrine and metabolic hormones induced by atypical antipsychotics in normal-weight patients with schizophrenia. Neuroendocrinology. 2007;85(4):249–256. doi: 10.1159/000103868. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Iglesias R, Vazquez-Barquero JL, Amado JA, et al. Effect of antipsychotics on peptides involved in energy balance in drug-naive psychotic patients after 1 year of treatment. J Clin Psychopharmacol. 2008;28(3):289–295. doi: 10.1097/JCP.0b013e318172b8e6. [DOI] [PubMed] [Google Scholar]
- 39.Smith RC, Lindenmayer JP, Hu Q, et al. Effects of olanzapine and risperidone on lipid metabolism in chronic schizophrenic patients with long-term antipsychotic treatment: a randomized five month study. Schizophr Res. 2010;120(1–3):204–209. doi: 10.1016/j.schres.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Ozguven HD, Baskak B, Oner O, et al. Metabolic effects of olanzapine and quetiapine: a six-week randomized, single blind, controlled study. Open Neuropsychopharmacol J. 2011;4:10–17. [Google Scholar]
- 41.Raposo NRB, Ferreira AS, Gattaz WF. Body mass index increase, serum leptin, adiponectin, neuropeptide Y and lipid levels during treatment with olanzapine and haloperidol. Pharmacopsychiatry. 2011;44(5):169–172. doi: 10.1055/s-0031-1280793. [DOI] [PubMed] [Google Scholar]
- 42.Jin H, Meyer JM, Mudaliar S, et al. Impact of atypical antipsychotic therapy on leptin, ghrelin and adiponectin. Schizophr Res. 2008;100(1–3):70–85. doi: 10.1016/j.schres.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sentissi O, Epelbaum J, Olié JP, et al. Leptin and ghrelin levels in patients with schizophrenia during different antipsychotics treatment: a review. Schizophr Bull. 2008;34(6):1189–1199. doi: 10.1093/schbul/sbm141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borenstein M, Hedges L, Higgins J, et al. Comprehensive meta-analysis version 2. Englewood (NJ): Biostat; 2005. [Google Scholar]
- 46.Hedges L, Holkin I. Statistical methods for meta-analysis. New York (NY): Academic Press; 1985. [Google Scholar]
- 47.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Erlbaum; 1988. [Google Scholar]
- 48.Paulson JF, Bazemore SD. Prenatal and postpartum depression in fathers and its association with maternal depression: a meta-analysis. JAMA. 2010;303(19):1961–1969. doi: 10.1001/jama.2010.605. [DOI] [PubMed] [Google Scholar]
- 49.Lipsey M, Wilson D. Practical meta-analysis. Thousand Oaks (CA): Sage Publications; 2000. [Google Scholar]
- 50.Cooper H, Hedges LV, Valentine J. Handbook of research synthesis and meta-analysis. 2nd ed. New York (NY): Russell Sage Foundation; 2009. [Google Scholar]
- 51.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638–641. [Google Scholar]
- 52.Bromel T, Blum WF, Ziegler A, et al. Serum leptin levels increase rapidly after initiation of clozapine therapy. Mol Psychiatry. 1998;3(1):76–80. doi: 10.1038/sj.mp.4000352. [DOI] [PubMed] [Google Scholar]
- 53.Eder U, Mangweth B, Ebenbichler C, et al. Association of olanzapine-induced weight gain with an increase in body fat. Am J Psychiatry. 2001;158(10):1719–1722. doi: 10.1176/appi.ajp.158.10.1719. [DOI] [PubMed] [Google Scholar]
- 54.Hinze-Selch D, Deuschle M, Weber B, et al. Effect of coadministration of clozapine and fluvoxamine versus clozapine monotherapy on blood cell counts, plasma levels of cytokines and body weight. Psychopharmacology (Berl) 2000;149(2):163–169. doi: 10.1007/s002139900351. [DOI] [PubMed] [Google Scholar]
- 55.Theisen FM, Gebhardt S, Brömel T, et al. A prospective study of serum ghrelin levels in patients treated with clozapine. J Neural Transm. 2005;112(10):1411–1416. doi: 10.1007/s00702-005-0284-6. [DOI] [PubMed] [Google Scholar]
- 56.Ebenbichler C, Laimer M, Kranebitter M, et al. The soluble leptin receptor in olanzapine-induced weight gain: results from a prospective study. Schizophr Res. 2005;75(1):143–146. doi: 10.1016/j.schres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 57.Wang HC, Chen PS, Lee IH, et al. Rapid leptin elevation after initiation of olanzapine? Neuropsychobiology. 2006;54(3):182–185. doi: 10.1159/000099945. [DOI] [PubMed] [Google Scholar]
- 58.Baptista T, Davila A, El Fakih Y, et al. Similar frequency of abnormal correlation between serum leptin levels and BMI before and after olanzapine treatment in schizophrenia. Int Clin Psychopharmacol. 2007;22(4):205–211. doi: 10.1097/YIC.0b013e328080ca44. [DOI] [PubMed] [Google Scholar]
- 59.Basoglu C, Oner O, Gunes C, et al. Plasma orexin A, ghrelin, cholecystokinin, visfatin, leptin and agouti-related protein levels during 6-week olanzapine treatment in first-episode male patients with psychosis. Int Clin Psychopharmacol. 2010;25(3):165–171. doi: 10.1097/YIC.0b013e3283377850. [DOI] [PubMed] [Google Scholar]
- 60.Brandl EJ, Frydrychowicz C, Tiwari AK, et al. Association study of polymorphisms in leptin and leptin receptor genes with antipsychotic-induced body weight gain. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):134–141. doi: 10.1016/j.pnpbp.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Fitzgerald PB, Scaffidi A, Morris MJ, et al. The relationship of changes in leptin, neuropeptide Y and reproductive hormones to antipsychotic induced weight gain. Hum Psychopharmacol Clin Exp. 2003;18(7):551–557. doi: 10.1002/hup.519. [DOI] [PubMed] [Google Scholar]
- 62.Kim BJ, Sohn JW, Park CS, et al. Body weight and plasma levels of ghrelin and leptin during treatment with olanzapine. J Korean Med Sci. 2008;23(4):685–690. doi: 10.3346/jkms.2008.23.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murashita M, Kusumi I, Inoue T, et al. Olanzapine increases plasma ghrelin level in patients with schizophrenia. Psychoneuroendocrinology. 2005;30(1):106–110. doi: 10.1016/j.psyneuen.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Stip E, Lungu OV, Anselmo K, et al. Neural changes associated with appetite information processing in schizophrenic patients after 16 weeks of olanzapine treatment. Transl Psychiatry. 2012;2:e128. doi: 10.1038/tp.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Venkatasubramanian G, Chittiprol S, Neelakantachar N, et al. A longitudinal study on the impact of antipsychotic treatment on serum leptin in schizophrenia. Clin Neuropharm. 2010;33(6):288–292. doi: 10.1097/WNF.0b013e3181fa2a6f. [DOI] [PubMed] [Google Scholar]
- 66.Yanik T, Kursungoz C, Sutcigil L, et al. Weight gain in risperidone therapy: investigation of peripheral hypothalamic neurohormone levels in psychotic patients. J Clin Psychopharmacol. 2013;33(5):608–613. doi: 10.1097/JCP.0b013e318297980e. [DOI] [PubMed] [Google Scholar]
- 67.Zhang ZJ, Yao ZJ, Liu W, et al. Effects of antipsychotics on fat deposition and changes in leptin and insulin levels: magnetic resonance imaging study of previously untreated people with schizophrenia. Br J Psychiatry. 2004;184:58–62. doi: 10.1192/bjp.184.1.58. [DOI] [PubMed] [Google Scholar]
- 68.Deng C, Weston-Green K, Huang XF. The role of histaminergic H1 and H3 receptors in food intake: a mechanism for atypical antipsychotic-induced weight gain? Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(1):1–4. doi: 10.1016/j.pnpbp.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Roth BL, Sheffler DJ, Kroeze WK. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov. 2004;3(4):353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 70.Roerig JL, Steffen KJ, Mitchell JE. Atypical antipsychotic-induced weight gain: insights into mechanisms of action. CNS Drugs. 2011;25(12):1035–1059. doi: 10.2165/11596300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 71.Ruiz de Azua I, Gautam D, Jain S, et al. Critical metabolic roles of β-cell M3 muscarinic acetylcholine receptors. Life Sci. 2012;91(21–22):986–991. doi: 10.1016/j.lfs.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weston-Green K, Huang XF, Deng C. Second generation antipsychotic-induced type 2 diabetes: a role for the muscarinic M3 receptor. CNS Drugs. 2013;27(12):1069–1080. doi: 10.1007/s40263-013-0115-5. [DOI] [PubMed] [Google Scholar]
- 73.Bymaster FP, Felder CC, Tzavara E, et al. Muscarinic mechanisms of antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(7):1125–1143. doi: 10.1016/j.pnpbp.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Jensen NH, Rodriguiz RM, Caron MG, et al. N-desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine’s antidepressant activity. Neuropsychopharmacology. 2008;33(10):2303–2312. doi: 10.1038/sj.npp.1301646. [DOI] [PubMed] [Google Scholar]
- 75.Yamada M, Miyakawa T, Duttaroy A, et al. Mice lacking the M3 muscarinic acetylcholine receptor are hypophagic and lean. Nature. 2001;410(6825):207–212. doi: 10.1038/35065604. [DOI] [PubMed] [Google Scholar]
- 76.Silvestre JS, Prous J. Research on adverse drug events. I. Muscarinic M3 receptor binding affinity could predict the risk of antipsychotics to induce type 2 diabetes. Methods Find Exp Clin Pharmacol. 2005;27(5):289–304. doi: 10.1358/mf.2005.27.5.908643. [DOI] [PubMed] [Google Scholar]
- 77.Matsui-Sakata A, Ohtani H, Sawada Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet. 2005;20(5):368–378. doi: 10.2133/dmpk.20.368. [DOI] [PubMed] [Google Scholar]
- 78.Bolden C, Cusack B, Richelson E. Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther. 1992;260(2):576–580. [PubMed] [Google Scholar]
- 79.Isidori AM, Strollo F, Morè M, et al. Leptin and aging: correlation with endocrine changes in male and female healthy adult populations of different body weights. J Clin Endocrinol Metab. 2000;85(5):1954–1962. doi: 10.1210/jcem.85.5.6572. [DOI] [PubMed] [Google Scholar]
- 80.Ryan AS, Berman DM, Nicklas BJ, et al. Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26(8):2383–2388. doi: 10.2337/diacare.26.8.2383. [DOI] [PubMed] [Google Scholar]
- 81.Marques-Vidal P, Bochud M, Paccaud F, et al. Distribution of plasma levels of adiponectin and leptin in an adult Caucasian population. Clin Endocrinol (Oxf) 2010;72(1):38–46. doi: 10.1111/j.1365-2265.2009.03628.x. [DOI] [PubMed] [Google Scholar]
- 82.Schautz B, Later W, Heller M, et al. Impact of age on leptin and adiponectin independent of adiposity. Br J Nutr. 2012;108(2):363–370. doi: 10.1017/S0007114511005605. [DOI] [PubMed] [Google Scholar]
- 83.Haupt DW, Luber A, Maeda J, et al. Plasma leptin and adiposity during antipsychotic treatment of schizophrenia. Neuropsychopharmacology. 2005;30(1):184–191. doi: 10.1038/sj.npp.1300563. [DOI] [PubMed] [Google Scholar]
- 84.Herrán A, García-Unzueta MT, Amado JA, et al. Effects of long-term treatment with antipsychotics on serum leptin levels. Br J Psychiatry. 2001;179:59–62. doi: 10.1192/bjp.179.1.59. [DOI] [PubMed] [Google Scholar]
- 85.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86(12):5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.