Abstract
Objective:
Groups of nonrefractory patients with schizophrenia, taking antipsychotics other than clozapine, show distinct trajectories of treatment response over time. Whether similar patterns of response occur with clozapine-treated patients remains uncertain.
Method:
We used a cluster analysis approach for longitudinal data (k-means longitudinal) to analyze individual patient data from 2 pivotal studies of clozapine, compared with chlorpromazine. Trajectories and symptom severity were examined in a younger, less chronic, mixed-sample (study 16, n = 100) and in treatment-refractory (study 30, n = 257) patients.
Results:
Early-good and delayed-partial trajectory groups were observed, with the early-good trajectory group comprised of 73/100 (73.0%) from the mixed patient study, and 147/257 (57.2%) refractory patients. In the mixed patient sample, the distribution of clozapine and chlorpromazine treatments did not differ between the early-good and delayed-partial trajectory groups; in refractory patients proportionately more clozapine treatment was present in the early-good (87/147, 59.2%), compared with the delayed-partial (35/110, 31.8%), trajectory group. In the early-good trajectory group, improvement in mean symptom severity was 63% in mixed-study patients. Clozapine resistance appeared to be present in 10/50 (20.0%) mixed-study patients, and in 35/122 (28.9%) refractory patients.
Conclusions:
Early-good and delayed-partial response trajectories are seen in clozapine studies. The advantage of clozapine over chlorpromazine is seen most clearly in previous refractory patients, within the early-good trajectory group. Good and partial or poor responders to clozapine may merit further investigation.
Keywords: antipsychotics, schizophrenia, trajectories, clinical trial, treatment response
Abstract
Objectif :
Des groupes de patients non réfractaires souffrant de schizophrénie et prenant des antipsychotiques autres montrent au fil du temps des trajectoires distinctes de réponses au traitement. Il demeure incertain que des modèles semblables de réponse se produisent avec des patients traités par clozapine.
Méthode :
Nous avons utilisé une méthode d’analyse typologique pour des données longitudinales (longitudinales à K moyennes) afin d’analyser les données des patients individuels de 2 études pivots sur la clozapine, comparée avec la chlorpromazine. Les trajectoires et la gravité des symptômes ont été examinées dans un échantillon plus jeune, moins chronique, mixte (étude 16, n = 100) et chez des patients réfractaires au traitement (étude 30, n = 257).
Résultats :
Des groupes de trajectoires bonnes au début et partiellement tardives ont été observés, le groupe de trajectoires bonnes au début comprenant 73/100 (73,0 %) de l’étude des patients mixtes, et 147/257 (57,2 %) des patients réfractaires. Dans l’échantillon de patients mixtes, la distribution des traitements par clozapine et par chlorpromazine ne différait pas entre les groupes de trajectoires bonnes au début et partiellement tardives; chez les patients réfractaires, le traitement par clozapine était proportionnellement plus présent dans le groupe de trajectoires bonnes au début (87/147, 59,2 %), comparativement au groupe de trajectoires partiellement tardives (35/110, 31,8 %). Dans le groupe de trajectoires bonnes au début, l’amélioration de la gravité moyenne des symptômes était de 63 % chez les patients de l’étude mixte. La résistance à la clozapine semblait être présente chez 10/50 (20,0 %) des patients de l’étude mixte, et chez 35/122 (28,9 %) des patients réfractaires.
Conclusions :
Les trajectoires de la réponse bonnes au début et partiellement tardives sont observées dans les études de la clozapine. L’avantage de la clozapine sur la chlorpromazine est plus évident chez les patients précédemment réfractaires, au sein du groupe des trajectoires bonnes au début. Les répondants bons, partiels ou mauvais à la clozapine doivent faire l’objet de plus de recherche.
Important changes in the understanding of treatment response in schizophrenia have come from meta-analyses of the time course of treatment response.1–3 These studies now define an early response profile for AP treatment. Initial analyses excluded treatment-refractory patients, and patients treated with clozapine. However, the early response profile does appear to apply to refractory patients, and to clozapine.4 Further studies of individual participant data from large trials have helped to understand the heterogeneity in treatment response in schizophrenia, by defining groups with more homogeneous trajectories of response over time.5–9 Similar to the meta-analytic studies of time course, trajectory analyses have largely excluded refractory patients, often use industry-sponsored trials of a limited range of APs, and have excluded clozapine-treated patients.
Expanding trajectory analyses of treatment response to cover a wider spectrum of patients, and to include clozapine could provide support for a newly suggested approach to subtyping schizophrenia based on treatment response.10 This subtyping strategy proposes the following groups: schizophrenia-AP responsive (possibly 70% to 80% of patients), schizophrenia-clozapine responsive (possibly 20% to 30% of the remaining patients), and finally, schizophrenia-clozapine resistant. Importantly, treatment response in this strategy refers to improvement of the BPRS or PANSS of 50% or greater, comparable with a CGI improvement of much improved or better.11
Clinical Implications
Treatment response in schizophrenia is highly heterogeneous.
Clozapine responders and nonresponders may represent distinct subtypes of schizophrenia.
Limitations
Our findings are from a secondary analysis of data collected in the 1980s.
Trajectories were in some cases calculated using only 3 time points.
Trajectory analysis is a useful exploratory technique to generate ideas, but findings may be difficult to rigorously replicate.
The 2 pivotal trials for reintroducing clozapine in the 1980s may be informative for trajectory analyses.12,13 A mixed sample of patients with a history of extrapyramidal symptoms but not necessarily poor treatment response was included in study 16, while study 30 was limited to refractory patients. Both trials used chlorpromazine as a comparator. To investigate the treatment-response subtyping model, we hypothesized that there were distinct trajectory groups in these clozapine trials that the distribution of clozapine- and chlorpromazine-treated patients would differ between trajectory groups, and that AP-responsive, clozapine-responsive, and clozapine-resistant patient groups could be described.
Method
Study Descriptions
Data for this secondary analysis study were obtained from 2 trials of clozapine carried out in the United States by Sandoz,12,13 both involving inpatients with schizophrenia. Individual participant data were provided by Novartis. Study 16 was a randomized, double-blind, multi-centre trial comparing clozapine with chlorpromazine.13 Diagnoses of schizophrenia were made according to DSM-II (with a 295 code). Patients had a moderate severity of illness, with a minimum score of 4 on 3 of 6 pre-defined BPRS items. There was no assessment of treatment refractory status in the study description; hospitalization was for less than 6 months. Following a placebo phase of up to 2 weeks, patients were initiated on clozapine or chlorpromazine with increasing doses during the first week. During the next 3 weeks, flexible dosing was allowed (150 to 900 mg per day clozapine or 300 to 1800 mg per day chlorpromazine). Study 30 was a randomized, double-blind, multi-centre trial.12 Diagnoses of schizophrenia were made according to DSM-III. Treatment-refractory illness was defined by 3 periods of treatment in 5 years with APs from at least 2 classes, at doses equivalent to 1000 mg chlorpromazine or more, without significant symptomatic relief, and no period of good functioning within the preceding 5 years. The BPRS total score was required to be at least 45, with a minimum CGI score of 4, as well as a score of 4 on 2 of 4 pre-defined BPRS items. The median length of hospitalization was 2 years, with a mean of 7 prior hospitalizations. A pre-treatment placebo phase of 2 weeks was followed by a trial of haloperidol for 6 weeks. Nonresponders were then administered placebo for 1 week, followed by 6 weeks of study medication. Clozapine up to 900 mg per day was compared with chlorpromazine up to 1800 mg per day administered with benztropine. Both studies used the BPRS for the primary outcome measure of symptom severity.
Data Analysis
Individual participant total BPRS total scores were converted to a per-item score, with a range of 0 to 6. The KmL approach (R version 3.0.3) was used for study 16 and study 30 data separately to examine for clustering of patients into mutually exclusive groups defined by different trajectories of change in BPRS mean item scores over time.14 This iterative algorithm incrementally assigns participants to homogenous groups to minimize the sum of Euclidean distances between each trajectory and the mean (centroid) of the cluster and maximize the distance between clusters.14 The KmL does not assume trajectory shape nor require normality within clusters. The 2- to 6-cluster solutions were examined using multiple quality criteria.15–17 To account for missing values, Euclidean distances with Gower adjustment were used18 and the quality criteria were calculated using bisector linear interpolation.14 The KmL has been used in biomedical studies to define clusters of patients with homogeneous trajectories of change in measures including renal function, and behavioural symptoms related to childhood hyperactivity.19,20 Once patients within each of the 2 trials were assigned to trajectory groups, characteristics including BPRS item scores and type of medication administered were compared between and within trajectory groups using Student t tests and chi-square analyses. Per cent change in BPRS mean item score (0 to 6) from baseline was also examined.
Results
The demographic and clinical characteristics of patients in the 2 studies appear in Table 1. The wide range of illness represented by patients in study 16 differed from the treatment refractory patients in study 30 in several ways. The sex distribution between studies differed (χ2 = 16.2, df = 1, P < 0.001); patients in study 16 were more likely to be women. Patients in study 16 were younger in median age (Wilcoxon z = 3.22, P = 0.001), and had a shorter median duration of illness (Wilcoxon z = 8.26, P < 0.001). Overall severity of clinical symptoms was lower in study 16 patients (Wilcoxon z = 2.68, P = 0.007). Despite study 16 patients being selected for a history of extrapyramidal side effects, the mean scores on the Simpson-Angus Scale were lower, although statistical comparison was not possible as individual data or SDs were not available. Although the median scores for the demographic and clinical measures in study 16 suggested a less severe form of illness in this group, the range of age extended from 18 to 65 years, the disease duration from 0.1 to 47 years, and the BPRS total from 32 to 91. For descriptive purposes and comparisons that follow, we will refer to study 16 as a mixed group, and study 30 as a treatment refractory group.
Table 1.
Demographic and clinical characteristics of patients
| Characteristic | Study 16 | Study 30 |
|---|---|---|
| Sex, na | ||
| Men | 73 | 210 |
| Women | 52 | 58 |
| Age, median (IQR), yearsb | 31.0 (25.0–40.0) | 34.5 (30.0–41.0) |
| Duration of illness, median (IQR), yearsb | 6.0 (2.2–13.0) | 14.0 (10.0–20.0) |
| Brief Psychiatric Rating Scale total, median (IQR)b | 58.0 (48.0–64.5) | 60.0 (53.0–67.0) |
| Simpson-Angus Scale for extrapyramidal side effects score, meanc | 1.27 | 3.25 |
Statistically significant difference in distribution between studies
Statistically significant difference between studies
Only mean values provided in reports
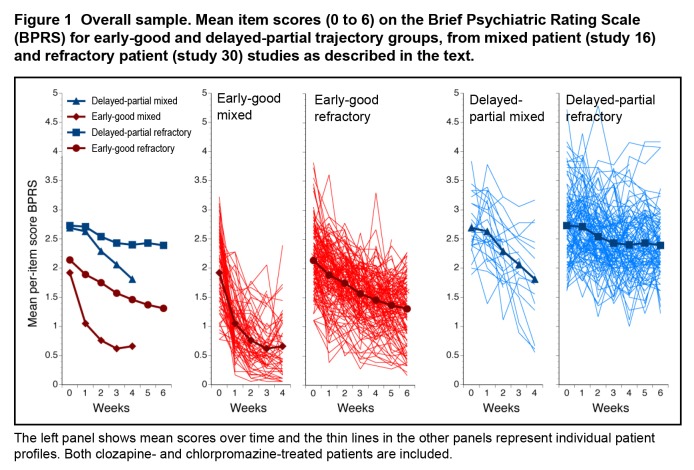
From the overall samples, n = 100 from study 16, and n = 257 from study 30 had at least 3 data points available for trajectory analyses. Separate KmL analyses identified 2 trajectory groups in each study, described here as early-good and delayed-partial trajectory groups (Figure 1). In the mixed-patient study, 73/100 (73.0%) were members of the early-good trajectory group, in contrast to the refractory-patient study, where 147/257 (57.2%) were in the early-good trajectory group. Not unexpectedly, the distribution of patients between the early-good and delayed-partial trajectory groups differed between the studies (χ2 = 7.85, df = 1, P = 0.005).
Figure 1.
Overall sample. Mean item scores (0 to 6) on the Brief Psychiatric Rating Scale (BPRS) for early-good and delayed-partial trajectory groups, from mixed patient (study 16) and refractory patient (study 30) studies as described in the text.
The left panel shows mean scores over time and the thin lines in the other panels represent individual patient profiles. Both clozapine- and chlorpromazine-treated patients are included.
Mean BPRS item scores at baseline differed substantially between the trajectory groups (early-good = 2.07, SD 0.58; delayed-partial = 2.72, SD 0.58; t = 10.38, df = 355, P < 0.001). Within each trajectory group, scores were somewhat lower in the mixed patients (early-good: mixed = 1.91, SD 0.64; refractory = 2.14, SD 0.54; t = 2.72, df = 218, P = 0.007, and delayed-partial: mixed = 2.69, SD 0.47; refractory = 2.73, SD 0.60; t = 0.34, df = 135, P = 0.74). The clearest early response profile was seen in the mixed patients in the early-good trajectory group (Figure 1). The mean change in BPRS item score during 4 weeks in these patients was 63.4%; in contrast, the mean change during 6 weeks for the previously refractory patients in the early-good trajectory group was 39.1%.
The distribution of medication used for treatment and within trajectory groups appears in Figure 2. In the mixed-patient study, the distribution of clozapine- and chlorpromazine-treated patients did not differ between the early-good and delayed-partial trajectory groups (χ2 = 2.51, df = 1, P = 0.11). In contrast, in the refractory-patient study, the distribution of patients treated with clozapine and with chlorpromazine differed between the trajectory groups (χ2 = 19.21, df = 1, P < 0.001). Clozapine-treated patients represented a greater proportion of the early-good trajectory group (Figure 2). In refractory patients, compared with chlorpromazine, 26.8% more patients treated with clozapine were assigned to the early-good trajectory group.
Figure 2.
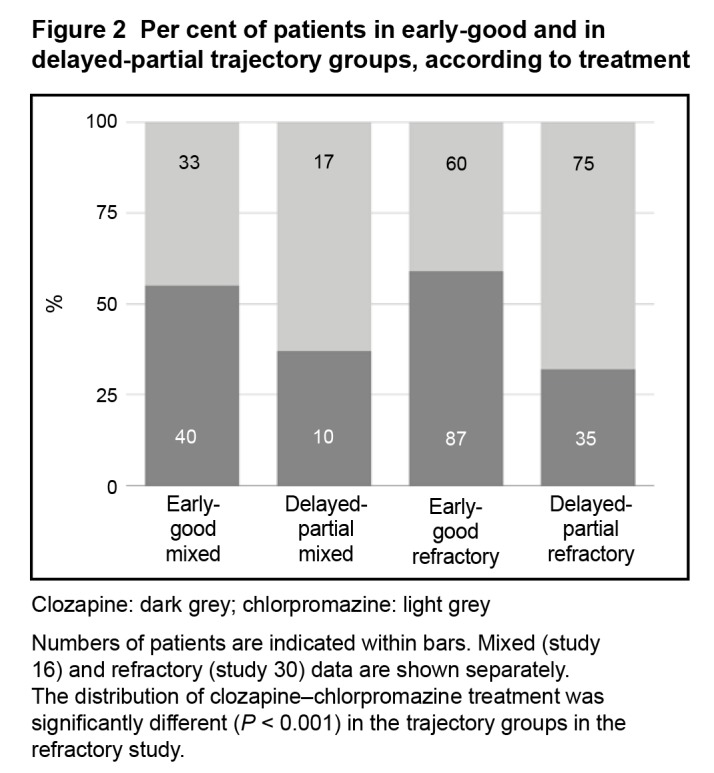
Per cent of patients in early-good and in delayed-partial trajectory groups, according to treatment
Clozapine: dark grey; chlorpromazine: light grey
Numbers of patients are indicated within bars. Mixed (study 16) and refractory (study 30) data are shown separately. The distribution of clozapine–chlorpromazine treatment was significantly different (P < 0.001) in the trajectory groups in the refractory study.
In the mixed patient study, within the early-good and delayed-partial trajectory groups, the profiles of response to clozapine and chlorpromazine appeared similar (Figure 3). The early-good trajectory group showed an initial steep decline, with later attenuation in response over the 4 weeks of the study; while the delayed-partial trajectory group appeared to be slow to respond initially, but was still improving at the end of 4 weeks. In the refractory-patient study, the profiles of response to clozapine and chlorpromazine appeared slightly different. In the early-good trajectory group, clozapine-treated patients appeared to be continuing to improve at the end of 6 weeks (mean BPRS item change 49.2%), while the treatment response in chlorpromazine-treated patients appeared to be attenuating (mean BPRS item change 21.0%). The delayed-partial trajectory group showed a modest initial response, which attenuated for both drugs.
Figure 3.
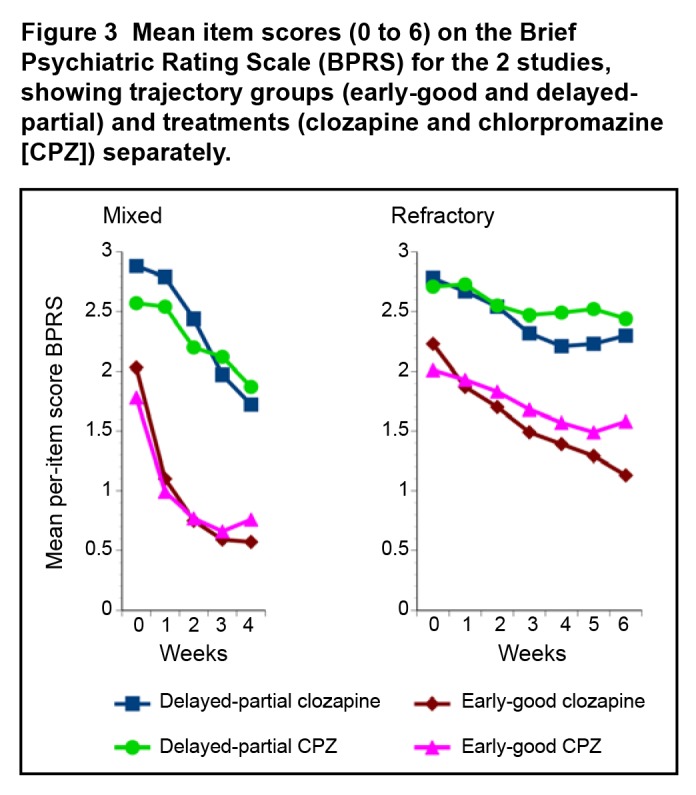
Mean item scores (0 to 6) on the Brief Psychiatric Rating Scale (BPRS) for the 2 studies, showing trajectory groups (early-good and delayed-partial) and treatments (clozapine and chlorpromazine [CPZ]) separately.
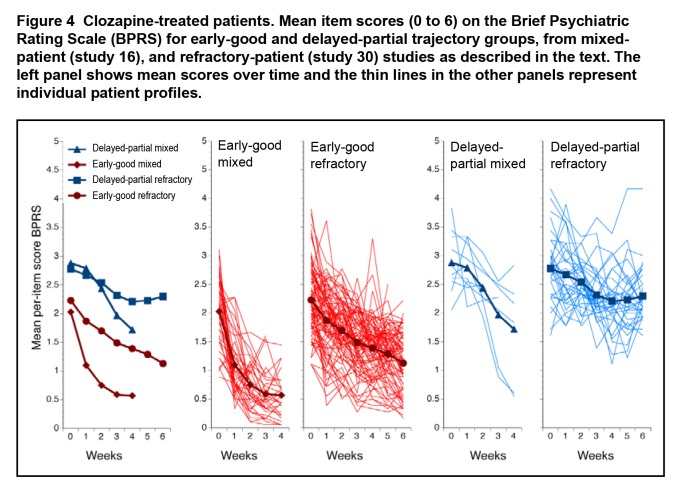
Focusing on the clozapine-treated patients (Figure 4), putative clozapine-resistant patients were observed in both the mixed- (10/50, 20.0%) and the refractory- (35/122, 28.9%) patient studies.
Figure 4.
Clozapine-treated patients. Mean item scores (0 to 6) on the Brief Psychiatric Rating Scale (BPRS) for early-good and delayed-partial trajectory groups, from mixed-patient (study 16), and refractory-patient (study 30) studies as described in the text. The left panel shows mean scores over time and the thin lines in the other panels represent individual patient profiles.
Discussion
In analyses of the early, pivotal studies of clozapine, compared with chlorpromazine, carried out in mixed- and in refractory-patient samples, we observed 2 trajectory groups. Over 70% of the mixed patient sample was assigned to the early-good trajectory group, and the absolute amount of improvement in symptom severity was over 60%. For the refractory patients, about 27% more clozapine-treated, compared with chlorpromazine-treated, patients were observed to have an early-good trajectory. These clozapine-treated patients had mean improvement of symptom severity of more than 45%. Considering clozapine-treated patients in the delayed-partial response trajectory groups to be nonresponders, the rates were 20% in the mixed study, and 29% in the refractory study.
Trajectory analysis may be a useful strategy to reduce heterogeneity in schizophrenia, and provide insight into clinically meaningful subgroups of patients. Previous trajectory analyses have used parametric techniques, with sample sizes at least as large as that available in the present analysis,5–9,21–23 and some with 10 times more patients. These studies often discern 4 or more trajectory classes, but with some as small as 2% or less of the sample. The KmL technique for trajectory analysis used in the present analysis has the advantage of being nonparametric, is independent from time scaling, and does not require assumptions of normality of distribution within clusters, or about the shape of the trajectory over time.14
Trajectory analysis of the mixed study 16 indicates that as many as 73% of patients were in the early-good trajectory group. Others suggest that only a minority of patients with schizophrenia have an adequate response to treatment.7 The small size of the mixed study examined here may result in an overestimate of the proportion of patients expected to have an early-good trajectory. Additionally, how the delayed-partial response trajectory in study 16 compares with the trajectories in study 30 is not entirely clear. However, another consideration is that there are relatively few studies that include clozapine as a treatment option for nonrefractory patients.4 A limitation of using study 16 for this purpose is that the proportion of putatively refractory patients that may have been included is uncertain. However, the overall lower age, shorter duration of illness, less severe symptoms, and milder extrapyramidal side effects suggest that this heterogeneous sample was not biased toward more refractory patients. While clozapine does not appear to have an advantage over other APs in the treatment of first-episode schizophrenia,24–26 this remains a topic of research and debate.27,28 Clozapine is less well studied in multi-episode, nonrefractory patients, and could have a response profile and effectiveness as a maintenance treatment more analogous to that seen in refractory schizophrenia.29 The overall high proportion of patients assigned to the early-good trajectory group here is consistent with the high proportion of patients with first-episode schizophrenia having complete resolution of psychosis with AP treatment.30 In our analysis of patients in the mixed study—early-good trajectory group—the outcome of 63.4% reduction in mean BPRS item score would be consistent with classification as much improved. Notably, first-episode patients with delayed-partial response to other APs may respond very well to clozapine.31
Treatment refractory patients also showed early-good and delayed-partial trajectories, with a smaller proportion but still a majority (57%) being assigned to the early-good trajectory group. This supports the goal of optimizing treatment response, and specifically using clozapine for this purpose. While the overall outcome for the refractory early-good trajectory group was not as good as in the mixed group, the reduction of mean BPRS item score by 49% likely represents a clinically meaningful improvement.11,32 As well, long-term outcomes for seriously ill patients prescribed clozapine may be encouraging.33 Interestingly, the percentage of clozapine-treated (previously) refractory patients in the early-good trajectory group (71%) exceeds the percentage of chlorpromazine patients in the early-good trajectory group (44%) by almost exactly the same difference (27%) as described for clozapine, compared with chlorpromazine responders (30%, compared with 4%, respectively; 26% difference) in the initial report of this study.12 A schizophrenia-clozapine responsive type appears to be a real entity, perhaps in as many as 15% to 20% of patients overall.
Clozapine-treated patients also appeared in the delayed-partial response trajectory groups in both the mixed- and the refractory-patient studies. In the mixed study, the absence of attenuation in the response profile suggests that a duration of treatment longer than 4 weeks may be necessary before the full extent of treatment response can be assessed in this trajectory group. The refractory patients, treated with clozapine, and members of the delayed-partial trajectory group are among the most challenging cases in psychiatry. Symptom severity was very high, and the small initial improvement appeared to attenuate. However, the study was limited to 6 weeks, and improvement related to clozapine treatment may sometimes require many months.34 Conversely, not all AP polypharmacy with clozapine can be attributed to delayed-partial optimization of clozapine monotherapy. Clozapine-resistant patients are a reality,35 and perhaps the most compelling of the treatment response subtypes of schizophrenia for the application of translational research strategies.36,37
Conclusions
Our series of analyses indicate early-good and delayed-partial trajectories of treatment response are seen in clozapine studies. The main caveat is that the trajectory analysis approach is exploratory rather than definitive, and requires replication. The distribution of clozapine responders and nonresponders among the trajectory groups supports the proposal of subtyping schizophrenia according to treatment response.
Acknowledgments
Novartis provided data for our analyses, but had no other role. Dr Honer has received consulting fees or has sat on paid advisory boards for MultiDimensional Healthcare Consulting, In Silico, Eli Lilly, Lundbeck, and Roche. Dr Barr has received grants from Bristol-Myers Squibb. Dr Procyshyn has received consulting fees or has sat on paid advisory boards for AstraZeneca, Bristol-Myers Squibb, Janssen, Otsuka, Pfizer, and Sunovion. Andrea A Jones, Dr Thornton, and Dr Vila-Rodriguez report no conflicts of interest.
Abbreviations
- AP
antipsychotic
- BPRS
Brief Psychiatric Rating Scale
- CGI
Clinical Global Impression
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- KmL
k-means for longitudinal data
- PANSS
Positive and Negative Syndromes Scale
References
- 1.Agid O, Kapur S, Arenovich T, et al. Delayed-onset hypothesis of antipsychotic action: a hypothesis tested and rejected. Arch Gen Psychiatry. 2003;60:1228–1235. doi: 10.1001/archpsyc.60.12.1228. [DOI] [PubMed] [Google Scholar]
- 2.Leucht S, Busch R, Hamann J, et al. Early-onset hypothesis of antipsychotic drug action: a hypothesis tested, confirmed and extended. Biol Psychiatry. 2005;57:1543–1549. doi: 10.1016/j.biopsych.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Sherwood M, Thornton AE, Honer WG. A meta-analysis of profile and time-course of symptom change in acute schizophrenia treated with atypical antipsychotics. Int J Neuropsychopharmacol. 2006;9:357–366. doi: 10.1017/S1461145705005961. [DOI] [PubMed] [Google Scholar]
- 4.Sherwood M, Thornton AE, Honer WG. A quantitative review of the profile and time course of symptom change in schizophrenia treated with clozapine. J Psychopharmacol. 2012;26:1175–1184. doi: 10.1177/0269881112440513. [DOI] [PubMed] [Google Scholar]
- 5.Case M, Stauffer VL, Ascher-Svanum H, et al. The heterogeneity of antipsychotic response in the treatment of schizophrenia. Psychol Med. 2010;41:1291–1300. doi: 10.1017/S0033291710001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marques TR, Arenovich T, Agid O, et al. The different trajectories of antipsychotic response: antipsychotics versus placebo. Psychol Med. 2010;41:1481–1488. doi: 10.1017/S0033291710002035. [DOI] [PubMed] [Google Scholar]
- 7.Stauffer V, Case M, Kollack-Walker S, et al. Trajectories of response to treatment with atypical antipsychotic medication in patients with schizophrenia pooled from 6 double-blind, randomized clinical trials. Schizophr Res. 2011;130:11–19. doi: 10.1016/j.schres.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Levine SZ, Leucht S. Elaboration on the early-onset hypothesis of antipsychotic drug action: treatment response trajectories. Biol Psychiatry. 2010;68:86–92. doi: 10.1016/j.biopsych.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Levine SZ, Rabinowitz J, Faries D, et al. Treatment response trajectories and antipsychotic medications: examination of up to 18 months treatment in the CATIE chronic schizophrenia trial. Schizophr Res. 2012;137:141–146. doi: 10.1016/j.schres.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Farooq S, Agid O, Foussias G, et al. Using treatment response to subtype schizophrenia: proposal for a new paradigm in classification. Schizophr Bull. 2013;39:1169–1172. doi: 10.1093/schbul/sbt137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leucht S, Davis JM, Engel RR, et al. Defining “response” in antipsychotic drug trials: recommendations for the use of scale-derived cutoffs. Neuropsychopharmacol. 2007;32:1903–1910. doi: 10.1038/sj.npp.1301325. [DOI] [PubMed] [Google Scholar]
- 12.Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 13.Claghorn J, Honigfeld G, Abuzzahab FS, et al. The risks and benefits of clozapine versus chlorpromazine. J Clin Psychopharmacol. 1987;7:377–384. [PubMed] [Google Scholar]
- 14.Genolini C, Falissard B. Kml: A package to cluster longitudinal data. Comp Meth Prog Biomed. 2011;104:e112–e121. doi: 10.1016/j.cmpb.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Caliński T, Harabasz J. A dendrite method for cluster analysis. Comm Statistics. 1974;3:1–27. [Google Scholar]
- 16.Ray S, Turi RH. Determination of number of clusters in k-means clustering and application in colour image segmentation. Proceedings of the 4th International Conference on Advances in Pattern Recognition and Digital Techniques (ICAPRDT’99); Calcutta India. New Delhi (IN): Narosa Publishing House; 2000. pp. 137–143. [Google Scholar]
- 17.Davies DL, Bouldin DW. A cluster separation measure. IEEE Trans Pattern Anal Mach Intell. 1979;1:224–227. [PubMed] [Google Scholar]
- 18.Gower JC. A general coefficient of similarity and some of its properties. Biometrics. 1971;27:857–871. [Google Scholar]
- 19.Bojan M, Lopez-Lopez V, Pouard P, et al. Limitations of early serum creatinine variations for the assessment of kidney injury in neonates and infants with cardiac surgery. PLoS ONE. 2013;8:e79308. doi: 10.1371/journal.pone.0079308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pingault J-B, Côté SM, Lacourse E, et al. Childhood hyperactivity, physical aggression and criminality: a 19-year prospective population-based study. PLoS ONE. 2013;8:e62594. doi: 10.1371/journal.pone.0062594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine SZ, Rabinowitz J, Case M, et al. Treatment response trajectories and their antecedents in recent-onset psychosis: a 2-year prospective study. J Clin Psychopharmacol. 2010;30:446–449. doi: 10.1097/JCP.0b013e3181e68e80. [DOI] [PubMed] [Google Scholar]
- 22.Levine SZ, Lurie I, Kohn R, et al. Trajectories of the course of schizophrenia: from progressive deterioration to amelioration over three decades. Schizophr Res. 2011;126:184–191. doi: 10.1016/j.schres.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Levine SZ, Rabinowitz J. Trajectories and antecedents of treatment response over time in early-episode psychosis. Schizophr Bull. 2010;36:624–632. doi: 10.1093/schbul/sbn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woerner MG, Robinson DG, Alvir JMJ, et al. Clozapine as a first treatment for schizophrenia. Am J Psychiatry. 2003;160:1514–1516. doi: 10.1176/appi.ajp.160.8.1514. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacol. 2003;28:995–1003. doi: 10.1038/sj.npp.1300157. [DOI] [PubMed] [Google Scholar]
- 26.Girgis RR, Phillips MR, Li X, et al. Clozapine v chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199:281–288. doi: 10.1192/bjp.bp.110.081471. [DOI] [PubMed] [Google Scholar]
- 27.Sanz-Fuentenebro J, Taboada D, Palomo T, et al. Randomized trial of clozapine vs. risperidone in treatment-naïve first-episode schizophrenia: results after one year. Schizophr Res. 2013;149:156–161. doi: 10.1016/j.schres.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Remington G, Agid O, Foussias G, et al. Clozapine’s role in the treatment of first-episode schizophrenia. Am J Psychiatry. 2013;170:146–151. doi: 10.1176/appi.ajp.2012.12060778. [DOI] [PubMed] [Google Scholar]
- 29.Meltzer HY, Bobo WV, Lee MA, et al. A randomized trial comparing clozapine and typical neuroleptic drugs in non-treatment-resistant schizophrenia. Psychiatry Res. 2010;177:286–293. doi: 10.1016/j.psychres.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Agid O, Arenovich T, Sajeev G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72:1439–1444. doi: 10.4088/JCP.09m05785yel. [DOI] [PubMed] [Google Scholar]
- 31.Agid O, Remington G, Kapur S, et al. Early use of clozapine for poorly responding first-episode psychosis. J Clin Psychopharmacol. 2007;27:369–373. doi: 10.1097/jcp.0b013e3180d0a6d4. [DOI] [PubMed] [Google Scholar]
- 32.Leucht S, Kane JM, Etschel E, et al. Linking the PANSS, BPRS, and CGI: clinical implications. Neuropsychopharmacol. 2006;10:2318–2325. doi: 10.1038/sj.npp.1301147. [DOI] [PubMed] [Google Scholar]
- 33.Wheeler A, Humberstone V, Robinson G. Outcomes for schizophrenia patients with clozapine treatment: how good does it get? J Psychopharmacol. 2009;23:957–965. doi: 10.1177/0269881108093588. [DOI] [PubMed] [Google Scholar]
- 34.Meltzer HY, Bastani B, Kwon KY, et al. A prospective study of clozapine in treatment-resistant schizophrenic patients. I. Preliminary report. Psychopharmacol. 1989;99(Suppl):S68–S72. doi: 10.1007/BF00442563. [DOI] [PubMed] [Google Scholar]
- 35.Mouaffak F, Tranulis C, Gourevitch R, et al. Augmentation strategies of clozapine with antipsychotics in the treatment of ultraresistant schizophrenia. Clin Neuropharmacol. 2006;29:28–33. doi: 10.1097/00002826-200601000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Mouaffak F, Kebir O, Chayet M, et al. Association of Disrupted in Schizophrenia 1 (DISC1) missense variants with ultra-resistant schizophrenia. Pharmacogenomics J. 2011;11:267–273. doi: 10.1038/tpj.2010.40. [DOI] [PubMed] [Google Scholar]
- 37.Honer WG, Procyshyn RM, Chen EYH, et al. A translational research approach to poor treatment response in patients with schizophrenia: clozapine-antipsychotic polypharmacy. J Psychiatry Neurosci. 2009;34:433–442. [PMC free article] [PubMed] [Google Scholar]