Abstract
The salience network (SN), a set of brain regions composed of the anterior fronto-insular cortex (aFI) and the anterior cingulate cortex (ACC), is usually involved in interoception, self-regulating, and action selection. Accumulating evidence indicates that dysfunctions in this network are associated with various pathophysiological deficits in both schizophrenia and eating disorders, stemming mainly from dysfunctional information processing of internal or external stimuli. In addition, the metabolic side effects of some antipsychotics (APs), as well as their pharmacological mechanisms of action, also suggest a link between the functional and neurophysiological changes in the brain in both schizophrenia and in eating disorders. Nevertheless, there is still a knowledge gap in explicitly and directly linking the metabolic side effects associated with AP treatment with the dysfunction in SN associated with processing of food-related information in schizophrenia. Here we provide neuroimaging evidence for such a link, by presenting data on a group of schizophrenia patients who followed 16 weeks of olanzapine treatment and undertook a passive viewing task while their brain activity was recorded. In response to food-related dynamic stimuli (video clips), we observed a decreased activity in SN (aFI and ACC) after the treatment, which also correlated with ghrelin plasma concentration and a measure of dietary restraint. Taken together with past findings regarding the role of SN in both schizophrenia and eating disorders, our results suggest that enhancing the reactivity in the SN has the potential to be a treatment strategy in people with anorexia nervosa.
Clinical Trial Registration Number: NCT 00290121
Keywords: anorexia nervosa, antipsychotics, olanzapine, schizophrenia
Abstract
Le réseau de la salience (RS), un ensemble de régions cérébrales formé du cortex fronto-insulaire antérieur (FIa) et du cortex cingulaire antérieur (CCA), est habituellement impliqué dans l’intéroception, l’autorégulation, et la sélection d’actions. Les données probantes qui s’accumulent indiquent que les dysfonctions de ce réseau sont associées avec divers déficits pathophysiologiques dans la schizophrénie et les troubles alimentaires, émanant principalement du traitement dysfonctionnel de l’information des stimuli internes et externes. En outre, les effets secondaires métaboliques de certains antipsychotiques (AP), ainsi que leurs mécanismes d’action pharmacologiques suggèrent aussi un lien entre les changements fonctionnels et neurophysiologiques du cerveau dans la schizophrénie et les troubles alimentaires. Néanmoins, les connaissances ne suffisent pas encore à lier explicitement et directement les effets secondaires métaboliques associés au traitement par AP avec la dysfonction du RS associée au traitement de l’information liée aux aliments dans la schizophrénie. Nous offrons ici des preuves en neuroimagerie de ce lien, en présentent des données d’un groupe de patients souffrant de schizophrénie qui ont suivi un traitement de 16 semaines par olanzapine et qui ont effectué une tâche de visualisation passive pendant que leur activité cérébrale était enregistrée. En réponse aux stimuli dynamiques liés aux aliments (vidéoclips), nous avons observé une activité réduite du RS (FIa et CCA) après le traitement, qui corrélait aussi avec la concentration plasmatique de ghréline et une mesure des restrictions alimentaires. Pris conjointement avec les constatations passées sur le rôle du RS dans la schizophrénie et les troubles alimentaires, nos résultats suggèrent qu’accroître la réactivité du RS pourrait être une stratégie de traitement chez les personnes souffrant d’anorexie mentale.
Numéro d’enregistrement d’essai clinique : NCT 00290121
In recent years, there has been an increased interest in studying the role of SN in schizophrenia. The SN, a set of brain regions composed of aFI and ACC, is involved in assessing the relevance of, and orienting to, internal and external stimuli in support of behavioural choices.1 Several review articles2,3 synthesized the available clinical and neuroimaging evidence and suggested that various pathophysiological deficits in schizophrenia may be linked to dysfunctions in the SN. The basis for this link between schizophrenia symptoms and the SN functional deficit is the mismatch between prior expectations and incoming sensory information leading to a prediction error. For instance, both the anterior insula and the ACC have been repeatedly shown as important cortical regions involved in prediction error coding, and many neuroimaging studies revealed functional and structural deficits in the SN in people with schizophrenia, compared with their healthy counterpart.2,3
Accumulating evidence indicates that dysfunctions in the SN seem to also be related to pathophysiological deficits seen in eating disorders. For example, in a recent study, McFadden et al4 found that neural activity in the SN was significantly decreased (particularly in the ACC) in women with, or recovered from, AN as compared with healthy control subjects. Similarly, a recent fMRI study,5 which used a conditioning paradigm in women with AN or BN and healthy control subjects, found that activity in ACC was diminished both when sucralose or sucrose was delivered in women with AN, compared with control subjects, but increased in the BN group. Finally, a recent anatomical neuroimaging study6 has found a reduced volume in the right anterior insula grey matter in people with AN and recovered AN individuals and an increased volume in the left anterior insula in the BN group, compared with the healthy control subjects. These results suggest that the brain circuitry that has been associated with taste pleasantness and reward value of food is changed at the structural level in people affected by eating disorders.
Taken together, these sets of findings in schizophrenia and eating disorder domains seem to indicate 2 things. First, they suggest that different symptoms, seen in different pathologies, may have a common substrate: the dysfunction in the information processing system that is responsible for evaluating the relevance of, and orienting the person toward, internal and external stimuli. Second, they offer new avenues for intervention, either nonpharmacological (for example, through psychotherapy, by helping people interpret and reframe their evaluations and reactions to various stimuli) or pharmacological (for example, by using drugs that target specifically the SN or repurposing drugs used successfully in one domain to be employed into the other).
Clinical Implications
Dysfunctions within the SN in the brain seem to be associated with symptoms in both schizophrenia and eating disorders.
There may be support for the clinical practice to sometimes use the appetite-linked or metabolic side effects of APs to help patients with eating disorders.
Limitations
In the presented fMRI study, the subjects were not required to perform any task during scanning, it is impossible to know the degree to which they were cognitively engaged while stimuli were presented.
Another particularly interesting link between schizophrenia and eating disorders is the changes of food intake and body weight gains usually associated with AP treatment. In fact, the increasing evidence for metabolic side effects produced by some atypical APs, including olanzapine, seems to be consistent with the overlap between both the neuropharmacological mechanism of action of AP and the neural substrate (that is, SN) underlying both the schizophrenia and eating disorders, as outlined above. To date, there are studies suggesting that AP treatment is associated with structural and functional changes in SN.3,7 Nevertheless, these studies have not considered the association between AP treatment, SN, and metabolic side effects in schizophrenia. Similarly, recent neuroimaging findings have indicated that, in healthy subjects, olanzapine enhanced the response to food in brain reward circuitry,8 whereas in schizophrenia,9 olanzapine treatment led to an increased sensitivity to food-related stimuli in insular cortices and amygdala. Again, these studies did not explicitly consider the role of SN in linking the metabolic side effects of AP treatment with the processing of food-related information. As such, despite a considerable volume of conjectural evidence, there is still a knowledge gap in explicitly and directly linking the metabolic side effects associated with olanzapine treatment and the dysfunction in the SN associated with evaluation of, or reaction to, food-related information in schizophrenia.
In a recent study,9 we reported increased insular activation in a group of patients with schizophrenia during viewing of static food-related cues (images) following 16 weeks of olanzapine treatment. Moreover, these functional changes in insula were correlated with the changes in triglyceride and low-density lipoprotein cholesterol concentrations. However, given that we observed functional changes in a wider network than insula and a lack of activation in ACC,9 the link between SN, olanzapine metabolic side effects, and processing of food-related information could not be explicitly made. One possible explanation for the lack of specificity of our past findings in relation to SN may be the fact that we employed static visual stimuli (images) in a passive viewing paradigm. As such, it is possible that these were not salient enough or did not capture the full attention of our participants. However, in the same experiment, we have also exposed our participants to a second task consisting of passively viewing dynamic food-related stimuli, in a form of restaurants or gastronomy video clips. This data was not analyzed and presented in the previous study, which focused only on the task with the static visual stimuli. In our study, we present the data pertaining to the dynamic visual stimuli task. We hypothesized that the dynamic aspect of the food-related stimuli will elicit specific changes in activation in the SN as a function of olanzapine treatment, thus providing a direct link between SN, olanzapine metabolic side effects, and processing of food-related information.
Methods and Procedures
To test this hypothesis, the neural response to dynamic food stimuli was assessed in a group of 15 schizophrenia patients through fMRI before and after 16 weeks of olanzapine treatment, together with laboratory assessment of metabolic indices and responses to a questionnaire about eating behaviours (TFEQ). Details about the demographic characteristics of the patients, the type of metabolic indices collected, as well as specific information about medication have been reported elsewhere.9 In fact, the whole experimental procedure is identical with the one reported in our 2012 paper,9 with the exception of the type of stimuli presented during the task: video clips in the current, compared with static images in the previous report. Specifically, in each fMRI session (before and after treatment), a subjects’ brain activity was measured while they participated in a passive viewing task depicting 2 film excerpts (placed in 2 blocks of 180 seconds each and separated by 15 seconds break), in 2 experimental conditions: appetizing and neutral. The first part consisted of presentation of various gardening clips (neutral condition), whereas the second part contained clips which presented various food items in restaurants or gastronomy scenes (appetizing condition). These conditions were always presented in this order given that appetite is a state that may last for some time after food stimuli are presented. The preprocessing and analysis of neuroimaging data followed the same procedure as previously reported.9
Results
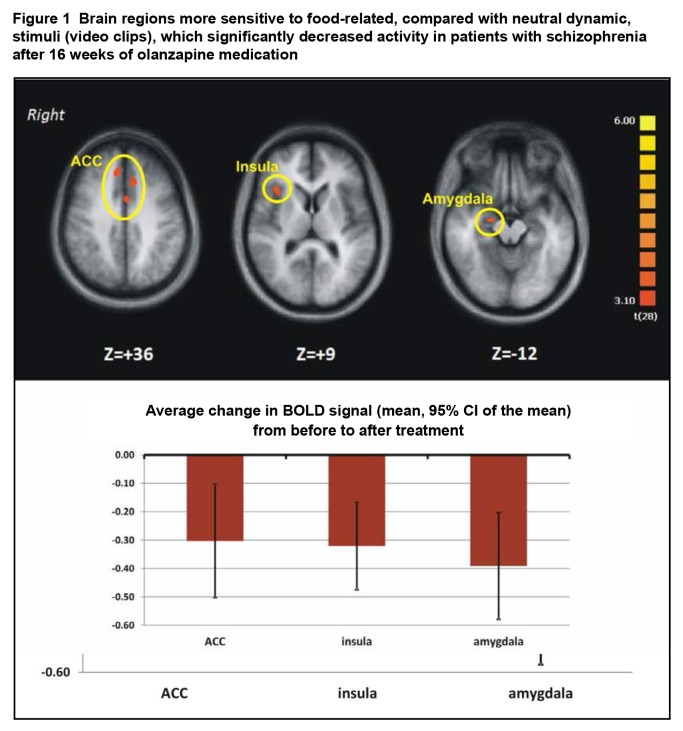
We found that, relative to pretreatment, patients’ activation in the right aFI cortex was reduced in the posttreatment scan, as well as that in amygdala, thalamus, and ACC (Figure 1) only when dynamic food-related stimuli were presented. These regions include more nodes of the SN than those reported by McFadden et al4 and are similar to the SN found by Oberndorfer et al.5 Thus, the fMRI results support our hypothesis that dynamic food-related visual stimuli engage more specifically the regions in the SN, in conjunction with the olanzapine treatment in schizophrenia (that is, difference pre- to posttreatment).
Figure 1.
Brain regions more sensitive to food-related, compared with neutral dynamic, stimuli (video clips), which significantly decreased activity in patients with schizophrenia after 16 weeks of olanzapine medication
In addition, we found significant correlation between changes in brain activation and physiological measurements of metabolites. For instance, the treatment-induced changes in insular and ACC activation in response to dynamic food-related visual stimuli correlated negatively and significantly with the level of ghrelin posttreatment (r = −0.548; P < 0.05, n = 15 for insula and r = −0.538; P < 0.05, n = 15 for ACC). This indicates that the more brain activity in each of these regions diminished more post- than pretreatment in response to food-related visual stimuli, the higher the plasma concentration of ghrelin measured posttreatment. Also, the difference pre- to posttreatment measured for factor 1 of the TFEQ (evaluating the amount of dietary restraint) correlated positive with the changes in brain activity in aFI and ACC (r = 0.546; P < 0.05, n = 15 for insula and r = 0.491; P < 0.05, n = 15 for ACC). This indicates that training-induced decreases in aFI and ACC were associated with decreased dietary restraint in patients with schizophrenia following the olanzapine treatment.
Discussion
We presented additional and previously unreported data from a published neuroimaging experiment9 with the aim of testing the hypothesis that dynamic food-related visual stimuli will engage more specifically the SN in schizophrenia patients after 16 weeks of olanzapine treatment. The results supported this hypothesis. Specifically, we found significant decreases in brain activity in important SN nodes (for example, aFI and ACC) in response to food-related visual stimuli 16 weeks after the initiation of the AP treatment with olanzapine. In addition, these changes correlated negatively with posttreatment ghrelin plasma concentration and with changes in dietary restraint. Usually, peptides that influence feeding exert an inhibitory effect (anorexigenic peptides). In contrast, only a few exert a stimulating effect (orexigenic), such as ghrelin which is involved in short-term regulation of food intake as its plasma levels increase before meals and decrease strongly post-prandially, leading to adiposity.
Thus, this data explicitly links indicators of metabolic side effects due to AP treatment with changes in the SN in response to processing of food-related information in schizophrenia.
Our results are consistent with previous naturalistic and controlled clinical trials10–14 which have suggested that olanzapine can lead to changes in the patterns of food intake by increasing appetite15–19 and preference for certain diets, including increasing caloric intake.20,21 While these studies have focused on appetite changes and dietary preferences, there is evidence that social and hedonic value of food-related visual cues could also play an important role. For example, a recent study8 showed that olanzapine enhanced the response to food in brain reward circuitry in healthy people. Similarly, there is increasing evidence21–23 suggesting that there are links between obesity, perception of appetitive cues, food intake, and reward that are mediated by specific changes in brain activity. While our study showed that dynamic food-related visual cues engaged the SN, there is a need for more neuroimaging studies with schizophrenia patients to investigate the extent to which this effect is specifically related to the social or reward value associated with this type of stimuli.
Our study added to the cumulating evidence that indicates not only that neural networks contributing to interoception, self-regulating, and action selection may play a role in pathological eating in people with AN, but also that they may account, at least in part, for the individual variability in changes of food intake and body weight associated with AP treatment in schizophrenia. Thus, despite variable findings24 APs with known metabolic side effects, such as olanzapine, should deserve a closer look and may eventually become at least a prototype of the first pharmacologic treatment for AN.
To date, one of the most compelling neuroimaging pieces of evidence about the involvement of the SN in AN and its association with hedonic value of food comes from the study conducted by Oberndorfer et al.5 Using a conditioning paradigm in women with AN or BN and healthy control subjects, the authors found that activity in ACC was diminished both when sucralose or sucrose was delivered in women with AN, compared with control subjects, but increased in the BN group. However, the group by condition analysis revealed that activity in aFI, and not ACC, was diminished in the AN group, not only in general, but more so in response to sucrose than sucralose, with the opposite pattern in the BN group, when compared with their healthy counterparts. This indicates that different nodes of the SN may respond differently to various aspects of the food-related stimuli, such as their reward or caloric properties. In this context, it is important to mention that in the same experiment done with static images,9 we observed increases in insula, rather than decreases, as reported in our study, with dynamic stimuli. However, the insular activation previously reported, albeit seen on the same side of the brain (right), was observed in a cluster located in the posterior insular cortex. In contrast, the cluster reported in our article is located anterior to this structure, which is consistent with the part of the insula that constitutes a node in the SN.1 This gives more support to our hypothesis that dynamic stimuli will more likely engage the SN.
Taken together with past findings from the neuroimaging literature on the SN in both schizophrenia and eating disorders, our results suggest that enhancing the reactivity in the SN when people with AN engage in eating behaviour has the potential to be a treatment strategy. An important mechanism of action by which olanzapine and other second-generation APs may induce increases in appetite is the blockade of receptors, such as 5-hydroxytryptamine2A, 5-hydroxytryptamine2C serotoninergic; D1, D2, D3, and D4 dopaminergic; and H1 histaminergic, α1 and α2 adrenergic and cholinergic25–33—which are known to be involved in appetite regulation. Thus pharmacological modulation of insular reactivity through APs may increase sensitivity to food in patients with AN.
Acknowledgments
Dr Stip is the holder of the Eli Lilly Chair of Schizophrenia from the University of Montreal. His research has received funding from Lundbeck Canada Inc. and Otsuka Canada Pharmaceutical Inc. He has served on the advisory boards and been a lecturer for Lundbeck Canada Inc, Otsuka Canada Pharmaceutical Inc, and Janssen. Dr Potvin received no funding for this editorial.
Abbreviations
- ACC
anterior cingulate cortex
- aFI
anterior fronto-insular cortex
- AN
anorexia nervosa
- AP
antipsychotic
- BN
bulimia nervosa
- fMRI
functional magnetic resonance imaging
- SN
salience network
- TFEQ
Three-Factor Eating Questionnaire
References
- 1.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012;37:17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palaniyappan L, White TP, Liddle PF. The concept of salience network dysfunction in schizophrenia: from neuroimaging observations to therapeutic opportunities. Curr Top Med Chem. 2012;12:2324–2338. doi: 10.2174/156802612805289881. [DOI] [PubMed] [Google Scholar]
- 4.McFadden KL, Tregellas JR, Shott ME, et al. Reduced salience and default mode network activity in women with anorexia nervosa. J Psychiatry Neurosci. 2014;39(3):178–188. doi: 10.1503/jpn.130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oberndorfer TA, Frank GK, Simmons AN, et al. Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am J Psychiatry. 2013;170:1143–1151. doi: 10.1176/appi.ajp.2013.11111745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank GK, Shott ME, Hagman JO, et al. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170:1152–1160. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung M, Cheung C, Yu K, et al. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophr Bull. 2011;37:199–211. doi: 10.1093/schbul/sbp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathews J, Newcomer JW, Mathews JR, et al. Neural correlates of weight gain with olanzapine. Arch Gen Psychiatry. 2012;69:1226–1237. doi: 10.1001/archgenpsychiatry.2012.934. [DOI] [PubMed] [Google Scholar]
- 9.Stip E, Lungu OV, Anselmo K, et al. Neural changes associated with appetite information processing in schizophrenic patients after 16 weeks of olanzapine treatment. Transl Psychiatry. 2012;2:e128. doi: 10.1038/tp.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arjona AA, Zhang SX, Adamson B, et al. An animal model of antipsychotic-induced weight gain. Behav Brain Res. 2004;152:121–127. doi: 10.1016/j.bbr.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 11.Cope MB, Nagy TR, Fernandez JR, et al. Antipsychotic drug-induced weight gain: development of an animal model. Int J Obes (Lond) 2005;29:607–614. doi: 10.1038/sj.ijo.0802928. [DOI] [PubMed] [Google Scholar]
- 12.Hartfield AW, Moore NA, Clifton PG. Effects of clozapine, olanzapine and haloperidol on the microstructure of ingestive behaviour in the rat. Psychopharmacology (Berl) 2003;167:115–122. doi: 10.1007/s00213-002-1368-8. [DOI] [PubMed] [Google Scholar]
- 13.Ota M, Mori K, Nakashima A, et al. Peripheral injection of risperidone, an atypical antipsychotic, alters the bodyweight gain of rats. Clin Exp Pharmacol Physiol. 2002;29:980–989. doi: 10.1046/j.1440-1681.2002.t01-1-03755.x. [DOI] [PubMed] [Google Scholar]
- 14.Thornton-Jones Z, Neill JC, Reynolds GP. The atypical antipsychotic olanzapine enhances ingestive behaviour in the rat: a preliminary study. J Psychopharmacol. 2002;16:35–37. doi: 10.1177/026988110201600111. [DOI] [PubMed] [Google Scholar]
- 15.Briffa D, Meehan T. Weight changes during clozapine treatment. Aust N Z J Psychiatry. 1998;32:718–721. doi: 10.3109/00048679809113128. [DOI] [PubMed] [Google Scholar]
- 16.Bromel T, Blum WF, Ziegler A, et al. Serum leptin levels increase rapidly after initiation of clozapine therapy. Mol Psychiatry. 1998;3:76–80. doi: 10.1038/sj.mp.4000352. [DOI] [PubMed] [Google Scholar]
- 17.Eder U, Mangweth B, Ebenbichler C, et al. Association of olanzapine-induced weight gain with an increase in body fat. Am J Psychiatry. 2001;158:1719–1722. doi: 10.1176/appi.ajp.158.10.1719. [DOI] [PubMed] [Google Scholar]
- 18.Kinon BJ, Kaiser CJ, Ahmed S, et al. Association between early and rapid weight gain and change in weight over one year of olanzapine therapy in patients with schizophrenia and related disorders. J Clin Psychopharmacol. 2005;25:255–258. doi: 10.1097/01.jcp.0000161501.65890.22. [DOI] [PubMed] [Google Scholar]
- 19.Gothelf D, Falk B, Singer P, et al. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry. 2002;159:1055–1057. doi: 10.1176/appi.ajp.159.6.1055. [DOI] [PubMed] [Google Scholar]
- 20.Kane JM, Barrett EJ, Casey DE, et al. Metabolic effects of treatment with atypical antipsychotics. J Clin Psychiatry. 2004;65:1447–1455. doi: 10.4088/jcp.v65n1102. [DOI] [PubMed] [Google Scholar]
- 21.Burger KS, Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage. 2011;55:233–239. doi: 10.1016/j.neuroimage.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng J, Stice E, Yokum S, et al. An fMRI study of obesity, food reward, and perceived caloric density. Does a low-fat label make food less appealing? Appetite. 2011;57:65–72. doi: 10.1016/j.appet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stice E, Spoor S, Bohon C, et al. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117:924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klenotich SJ, Seiglie MP, McMurray MS, et al. Olanzapine, but not fluoxetine, treatment increases survival in activity-based anorexia in mice. Neuropsychopharmacology. 2012;37:1620–1631. doi: 10.1038/npp.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey DE, Zorn SH. The pharmacology of weight gain with antipsychotics. J Clin Psychiatry. 2001;62(Suppl 7):4–10. [PubMed] [Google Scholar]
- 26.Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S–115S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds GP, Templeman LA, Zhang ZJ. The role of 5-HT2C receptor polymorphisms in the pharmacogenetics of antipsychotic drug treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1021–1028. doi: 10.1016/j.pnpbp.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Richelson E. Receptor pharmacology of neuroleptics: relation to clinical effects. J Clin Psychiatry. 1999;60(Suppl 10):5–14. [PubMed] [Google Scholar]
- 29.Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- 30.Seeman P, Tallerico T, Corbett R, et al. Role of dopamine D2, D4 and serotonin (2A) receptors in antipsychotic and anticataleptic action. J Psychopharmacol. 1997;11:15–17. doi: 10.1177/026988119701100104. [DOI] [PubMed] [Google Scholar]
- 31.Van Tol HH, Bunzow JR, Guan HC, et al. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- 32.Wirshing DA, Marshall BD, Jr, Green MF, et al. Risperidone in treatment-refractory schizophrenia. Am J Psychiatry. 1999;156:1374–1379. doi: 10.1176/ajp.156.9.1374. [DOI] [PubMed] [Google Scholar]
- 33.Zeng XP, Le F, Richelson E. Muscarinic m4 receptor activation by some atypical antipsychotic drugs. Eur J Pharmacol. 1997;321:349–354. doi: 10.1016/s0014-2999(96)00956-9. [DOI] [PubMed] [Google Scholar]