Abstract
We report an approach for simple, reproducible and high-yield synthesis of branched GNPs directed by deoxycholate bile acid supramolecular aggregates in Au solution. A growth process involving stepwise trapping of the GNP seeds and Au ions in the deoxycholate bile acid solution yields multiple-branched GNPs. Upon NIR laser irradiation strong NIR absorption for branched GNPs induced photothermal-heating to destroy tumor cells. Subsequently, these branched GNPs were bio-functionalized with cRGD cell penetrating-targeting peptides for photothermal cancer treatment applications. Branched GNPs conjugated with cRGD peptides enhanced internalization of the branched GNPs in BxPC3 human pancreatic adenocarcinoma cells and effectively ablated BxPC3 cells when irradiated with a NIR laser (808 nm). Their potential use as photothermal transducing agents was demonstrated in in vivo settings using a pancreatic cancer xenograft model. The tumors were effectively ablated with cRGD-branched GNPs injection and laser exposure without any observation of tumor recurrence. This firstly reported method for deoxycholate bile acid directed synthesis of branched GNPs opens new possibilities for the production of strong NIR absorbing nanostructures for selective nanophotothermolisys of cancer cells and the further design of novel materials with customized spectral and structural properties for broader applications.
Keywords: gold nanoparticles, bile acid, green chemistry, photothermal treatment, nanomedicine
INTRODUCTION
Plasmonic gold nanoparticles (GNPs) have received enormous interest because of their tunable optical properties[1], catalytic activity[2], surface-enhanced Raman spectroscopic patterns (SERS)[3], and potential for use in biological applications as biosensors[4], diagnostic imaging agents[5] and photothermal transducing agents[6]. To date, among the many different shaped GNPs (rods[7], cages[8], triangle or hexagonal plates[9] and poly-branched[10], hollow[11] and star[12–14] shaped GNPs), branched GNPs including star-, flower-, spiky, urchin-shaped GNPs have exhibited stronger optical and catalysis behavior than spherical GNPs, because of their large surface-to-volume ratio, high surface roughness, and large number of edges and sharp tips. The sharp ends of the tips of the branched GNPs enhance the electromagnetic field without the need for the aggregation of particles. Branched GNPs can exhibit different surface plasmon resonances (SPR) in the visible and near-infrared range, which depend strongly on polarization. In the last decade, the extensive demands of branched GNPs in various fields have led to numerous efforts to optimize the synthesis of such branched GNPs with different sizes and shapes.[15–18] The most common procedure involves the synthesis of small GNPs whose surfaces catalyze growth in a metal ion solution to produce the desired branched GNPs. In brief, gold seeds are prepared by reducing Au ions with a reducing agent such as tri-sodium citrate or NaBH4, and then as-prepared seeds are added to HAuCl4, a reducing agent, and a surfactant to disperse them throughout the growth solution, producing the desired particles. The morphology depends on surfactants or capping molecules that can suppress specific facet growth and induce symmetry breaking. However, many synthetic routes use toxic reagents that make them unsuitable for biological use and also have adverse effects on the environment. Furthermore, these methods are expensive, as these require high energy, long reaction times and sophisticated equipment to carry out the reactions. These latter concerns regarding these chemical and physical synthetic techniques have led to multiple attempts to develop biosynthesis approaches. Recently, green chemistry approaches for biotemplated and biomediated synthesis of GNPs using a combination of biomolecules such as enzymes, proteins, amino acids, vitamins, polysaccharides, cellulose, and organic acids (citrates) has received increasing attention.[19–22] However, these require multi-step protocols and cumbersome experimental procedure and the employed molecules have been utilized only for the synthesis of spherical GNPs with SPR bands in the visible region.
Bile acids are steroid acids synthesized in hepatocytes and found predominantly in the bile. The main function of bile acids is to facilitate the formation of micelles, which promote digestion in the intestine and absorption of dietary fat to remove unwanted cholesterol from the body. The bile acids and their derivatives can be used in supramolecular chemistry, materials chemistry and nanotechnology. Bile acids are facially amphiphilic containing both hydrophobic and hydrophilic faces. Unlike conventional surfactant molecules, bile salts possess a rigid steroid backbone having polar hydroxyl groups on the concave α-face and methyl groups on the convex β-face (Fig. 1a). These chemically different hydroxyl groups (and their varying amounts) and the enantiomeric purity of bile acids combined with their availability and low cost make them ideal building blocks for new synthetic applications. Thus, bile acids have been used as templates to synthesize helical ribbon and nanotubes with their asymmetric and chiral structure in bile acid hydrogels.[23–25] In aqueous media, the unique facial amphiphilicity and arrangement of molecules enable them to aggregate; the formed supramolecular aggregates can be used for a host which complexed with guest molecules (i.e. molecular tweezers).[26] The bile acids and derivatives based supramolecular host open up new avenues to synthesize nanostructures that were previously difficult to synthesize using traditional methods.
Figure 1.
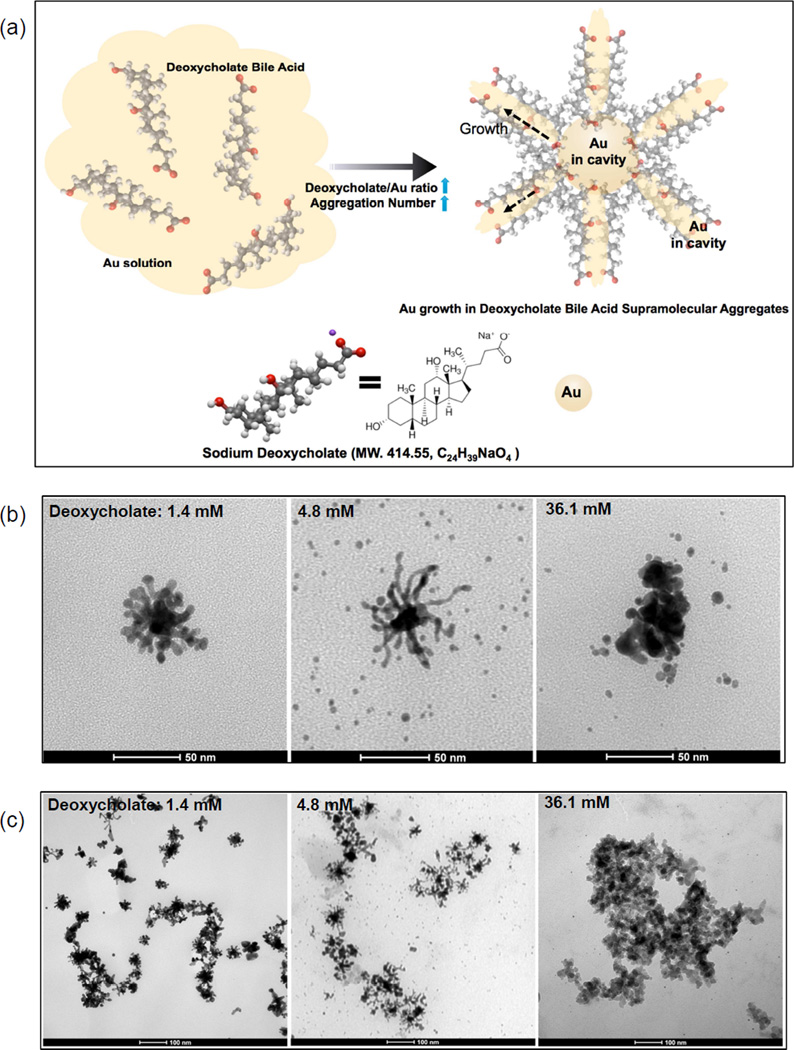
(a) Schematic of the formation of branched GNPs directed by sodium deoxycholate (MW. 414.55, C24H39NaO4) aggregations (increased aggregation number by increasing the concentration of deoxycholate molecules) and (b and c) TEM images of the synthesized branched GNPs (1.4 mM (left)) and GNPs synthesized with different concentrations of sodium deoxycholate solution (4.8 (middle) and 36.1 mM (right)), (d) a photograph and (e) absorption spectra of the synthesized GNP solutions in various concentrations of sodium deoxycholate solution (1.4~36.1 mM).
Herein, we report a facile synthesis of branched GNPs directed and promoted by sodium deoxycholate bile acid molecules in a reaction system of HAuCl4, silver nitrate and L-ascorbic acid under ambient conditions. Precise control can be exerted when forming highly branched GNPs structures when deoxycholate bile acid molecules are used to control the growth behavior of GNPs. These branched GNPs may serve as ideal efficient near infrared (NIR) transducers with tremendous potential for targeted NIR photothermal cancer therapy. Among many tumor types, pancreatic adenocarcinoma is one of the most difficult tumors to treat due to late initial diagnosis and intrinsic resistance to conventional treatments. Systemic chemotherapy, or combined forms with both chemoradiation and chemotherapy has had little impact; prognosis is particularly poor for this aggressive neoplasm largely due to the characteristics of the surrounding fibrous tissue stroma.[27, 28] A NIR photothermal ablation therapy using highly efficient NIR photothermal nano-transducers with a targeting moiety may be an ideal choice for the treatment of pancreatic cancer. Therefore, the synthesized branched GNPs, with deoxycholate bile acid aggregates, were biofunctionalized with cyclo(Arg-Gly-Asp-D-Phe-Glu) targeting peptides (cRGD) and their potential use as NIR photothermal transducing agents demonstrated in both in vitro using BxPC3 human pancreatic adenocarcinoma cells and in vivo using a pancreatic xenograft tumor model. Our results represent the first report that highly branched GNPs can be formulated by deoxycholate bile acid supramolecular aggregates for potential application as highly efficient NIR photothermal transducers for the treatment of a devastating form of pancreatic cancer.
Results and Discussion
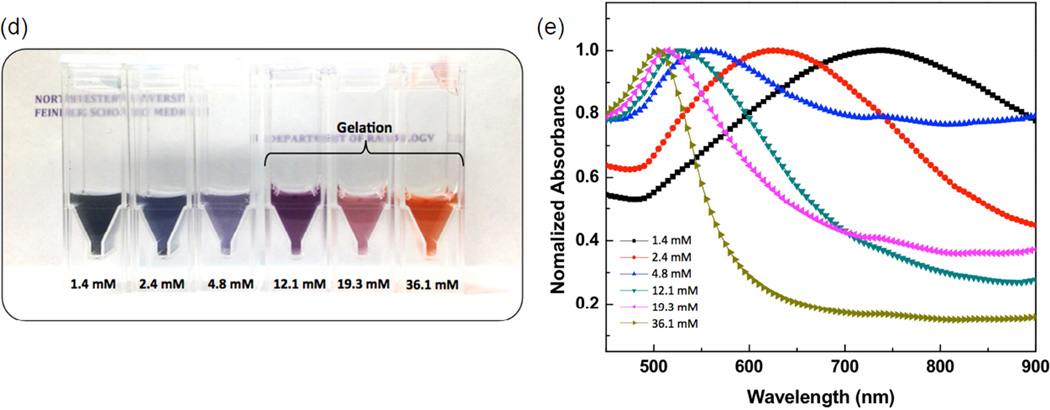
In our study, a sodium deoxycholate molecules-assisted GNPs growth method was first developed for the formation of branched GNPs (Fig. 1a). Briefly, small GNP seeds (sub-10 nm) were formed in sodium deoxycholate solution (24 uM) by adding 10 mM HAuCl4•3H2O and reducing with 10 mM NaBH4, and then branched GNPs were further grown with HAuCl4•3H2O, AgNO3, L-ascorbic acid, the seeds, and sodium deoxycholate solution. Interestingly, multiple-branched GNPs (47 nm; branches: 10~20 nm and core: 20 nm) showing a dendritic morphology along with primary and secondary branches (generally considered difficult to produce) were grown in the deoxycholate solution (1.4 mM deoxycholate in Fig. 1b). XRD patterns of the synthesized branch GNPs confirmed the fcc Au crystalline structure. The peaks at 2θ=38.2, 44.4 and 64.5 were assigned to (111), (200) and (220) reflections of Au (Fig. 2c). In the absence of sodium deoxycholate, only spherical GNPs were observed.
Figure 2.
(a) A photograph image of the branched GNPs solution in 10 ml and 100 ml batches and (inset) their hydrodynamic size, (b) TEM image of the synthesized branched GNPs with a 1.4 mM sodium deoxycholate solution and (inset) their electron diffraction, (c) XRD pattern of the synthesized branched GNPs, (d) schematics for biofunctionalized branched GNPs with carboxyl-PEG-thiol and cRGD peptides to target integrin ανβ3, and (e) IR thermal image of agar phantom including the branched GNPs (0.6 mM) and heating profiles of the branched GNPs at various concentrations (0~0.6 mM) under irradiation with 808 nm laser (0.47 W/cm2).
To further understand the sodium deoxycholate mediated branched GNPs formation, we increased the concentration of sodium deoxycholate in the synthetic protocol. The morphology of the GNPs grown in deoxycholate bile acid molecules was strongly dependent on the concentration of deoxycholate bile acid as identified with TEM (Fig. 1b and c). Increasing the concentration of deoxycholate bile acid from 1.4 mM to 4.8 mM resulted in fiber-like long-branch growth; the core size was ~20 nm, similar to GNPs in 1.4 mM deoxycholate bile acid, but branches were extended to 20–50 nm (4.8 mM in Fig. 1b). However, as the deoxycholate concentration was increased upwards to 36.1 mM, the excessive amounts of deoxycholate bile acid formed irregular massively aggregated GNP structures with excessive amounts of deoxycholate molecules (36.1 mM in Fig. 1b). A rapid reduction of gold occurred right after mixing the seed particles and the growth solution, which led to a mixture of spheres and branched GNPs in both 4.8 mM and 36.1 mM deoxycholate solutions (Fig. 1b). The structural arrangement of deoxycholate creates a unique facial amphiphilicity for this class of molecules, enabling them to form stable supramolecular aggregates with cavities by the hydrophobic association of apolar β-faces of steroid backbones, ion-ion, ion-dipole, and hydrogen bonding interactions (Fig. 1a). The formed bile acid aggregations have been found to be useful for entrapping guest molecules in their core and forming dendritic structures.[29, 30] The formed cholate acid supramolecular aggregations have large empty regions between adjacent cholate anions, such that polar groups can be approached by the guest molecules such as Au ions in the solution toward the lateral surface in addition to the bases of the cholate molecules.[29, 30] These characteristics of the bile acids led to the formation of deoxycholate supramolecular aggregates having empty regions between adjacent deoxycholate anions, such that polar groups can be approached by the guest Au molecules in the solution toward the lateral surface in addition to the bases of the cholate molecules as shown in Fig. 1a. As the concentration of deoxycholate is increased, direct linkages to their hydroxyl groups among the deoxycholate molecules form extended dendritic structures with high aggregation numbers and grow GNPs as shaped dendritic structures.[31–35] These results may suggest that the stable deoxycholate supramolecular aggregates acted as a frame to grow GNPs and the introduced sodium deoxycholate molecules clearly have a critical impact upon the resulting morphology of the GNPs (Fig. 1). Additionally, the silver ions in the seeds, Au ions and deoxycholate bile acid aggregates promoted the growth of branches but excessive amounts of AgNO3 solution (beyond 10 mM) resulted in a decrease in the aspect ratio and decrease in the number of branches.
The structural evolution of GNPs synthesized with various concentrations of deoxycholate (1.4~36.1 mM) was investigated with extinction spectra. The resulting GNPs solutions were of different color as shown in Fig. 1d and a variety of extinction peaks for these GNPs solutions was obtained (Fig. 1e). The branched GNPs (Fig. 1c, left), synthesized with 1.4 mM deoxycholate molecules were deep blue in color and a significant absorption band was observed at 740 nm near infrared region (Fig. 1e). The formed multiple branches in GNPs are associated with a red-shift in the longitudinal plasmon peak.[36–38] However, as the concentration of deoxycholate was increased (2.4~ 36.1 mM), the absorption spectra of the GNPs shifted from 740 nm toward blue at 510 nm due to the as-shown mixture of spherical GNPs with the highly connected branched GNPs (Fig 1c (middle) and Fig. 1e). At high concentration of deoxycholate solution (12.1~36.1 mM, pH 6.5~6.7), a complete gelation of the GNPs solution was observed that may have been caused by Au3+ ions, unbroken frontage of methylene groups, uninterrupted by methyl or hydroxyl groups within the 1 hour reaction period.[39, 40] This gelation and high aggregations led to an observed spectral shift to blue ~510 nm (Fig. 1e). The resulting highly branched morphology and strong NIR absorption characteristics of the GNPs synthesized when using a 1.4 mM deoxycholate solution should be highly valuable for targeted NIR photothermal cancer treatments. The edge and tips in the multiple branches of the GNPs allow large local electric field enhancements at the plasmon resonance, resulting in efficient transduction of incident light into heat due to electron excitation and relaxation.[41, 42] The strong NIR absorption of branch GNPs also contributes to efficient energy absorption in deep tissues because the NIR light can penetrate tissues with relatively low optical attenuation from water and blood within this spectral range (650~900 nm).[43]
In our study, the branch GNPs synthesized with 1.4 mM deoxycholate solution (Fig. 2a) were tested for potential application in targeted photothermal therapy for the treatment pancreatic cancer. A high yield synthesis approach was used for both our in vitro and in vivo studies. The synthesis of branched GNPs was scaled up to 100 ml in a batch, allowing the production of a large amount of colloidal branched GNPs in a short time period (Fig. 2a and b). Then, the photothermal-heating properties of the branched GNPs were analyzed using various concentrations of the branched GNPs. As we anticipated, the strong NIR absorption at 740 nm for branched GNPs induced fast photothermal-heating (initial heating rate: 0.75 °C/sec at 0.6 mM of branched GNPs) when irradiated with a NIR laser (808 nm; 0.47 W/cm2, branch- GNPs at concentrations from 0 to 0.6 mM in 1% agar phantom) for 2 min (Fig. 2d). Heating rates varied with concentration of the branched GNPs and the photothermal heating was well localized to only agar region containing the branched GNPs (Fig. 2d). To evaluate the photothermal properties of branched GNPs, a photothermal transduction efficiency was calculated according to the thermal balance as described in previous reports.[44] The calculated photothermal transduction efficiency for branched GNPs was determined to be 61%, which is approximately twice that reported for alternative photothermal agents.[44–48] This extraordinarily high photothermal transduction should be particularly advantageous for targeted ablation of tumors. However, with this high photothermal transduction efficiency, there is a strong need to achieve a selective delivery of the branch GNPs to the target tumor cells. An effective method to do so is by coating the surface with cell targeting-penetrating peptides.
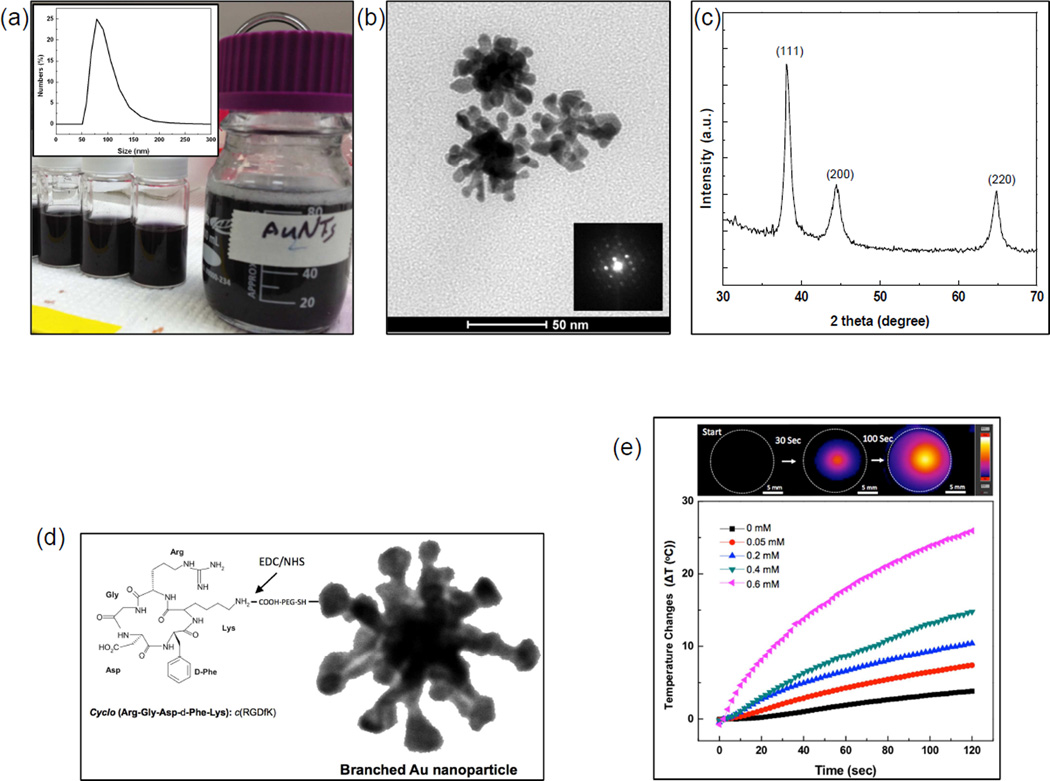
Our synthesized branched GNPs were first modified with carboxy-PEG-thiol and then conjugated with cyclic RGD peptides (cyclo(Arg-Gly-Asp-D-Phe-Glu) recognizing over expressed integrin ανβ3 in pancreatic tumor cells; these were linked to the PEG using NHS/EDC chemistry (Fig. 2c). The resulted cRGD conjugation efficiency with branched GNPs was 89%. The targeting and uptake of the cyclic RGD peptide (cRGD) conjugated branched GNPs (cRGD-branched GNPs) were evaluated in BxPC3 human pancreatic cancer cells. Targeted and uptaken cRGD-branched GNPs after incubating with BxPC3 cells for 24 hours were visualized under confocal fluorescent microscopy and TEM, respectively (Fig. 3a). Cyto780 labeled cRGD-branched GNPs were visualized as red fluorescent on the BxPC3 cells and a TEM image confirmed uptaken and clustering of cRGD-branched GNPs in vesicles within the cytoplasm (Fig. 3a). Next, in vitro efficacy for photothermal treatment of BxPC3 cells was investigated (Fig. 3b). Resulting cytotoxicity was dependent upon the level of NIR photothermal heating induced by the different amounts of branched GNPs (0~0.8 mM) and applied laser powers (0.47 and 0.96 W/cm2). The cell viability of cRGD-branched GNPs mediated photothermal treated groups gradually decreased with increased photothermal temperatures and heating rates induced by increased amounts of cRGDbranched GNPs and increasing laser power. When we added 0.2 mM of cRGD-branched GNPs and irradiated cells with 0.96 W/cm2 laser, an 80% cell killing effect was observed. A dose of 0.8 mM cRGD-branched GNPs demonstrated a stronger ability to absorb NIR laser (0.97 W/cm2) with temperatures rapidly increasing up to 50 °C (Fig. 3b) within 3 min, and higher cell mortality rates with 90% observed cell kill. NIR laser irradiation alone or exposure of cRGD-branched GNPs up to 0.8 mM (without irradiation) resulted in cell mortality rates of less than 20%.
Figure 3.
(a) Cellular targeting and uptake of cRGD-branched GNPs incubated with BxPC-3 cells for 24 h. Fluorescent images (red: cRGD-branched GNPs and blue: nucleus) and a TEM image of the cRGD-branched GNPs uptaken by BxPC3 cells, (b) Viability of BxPC3 cells after incubation with increasing concentrations of branched GNPs and cRGDbranched GNPs for 24 h without laser irradiation, and (c) photothermal destruction of BxPC-3 cells exposed to increasing concentrations of cRGD-branched GNPs (0~0.8 mM) and power densities (0.47 and 0.96 W/cm2) of NIR laser (808 nm) for 3 mins.
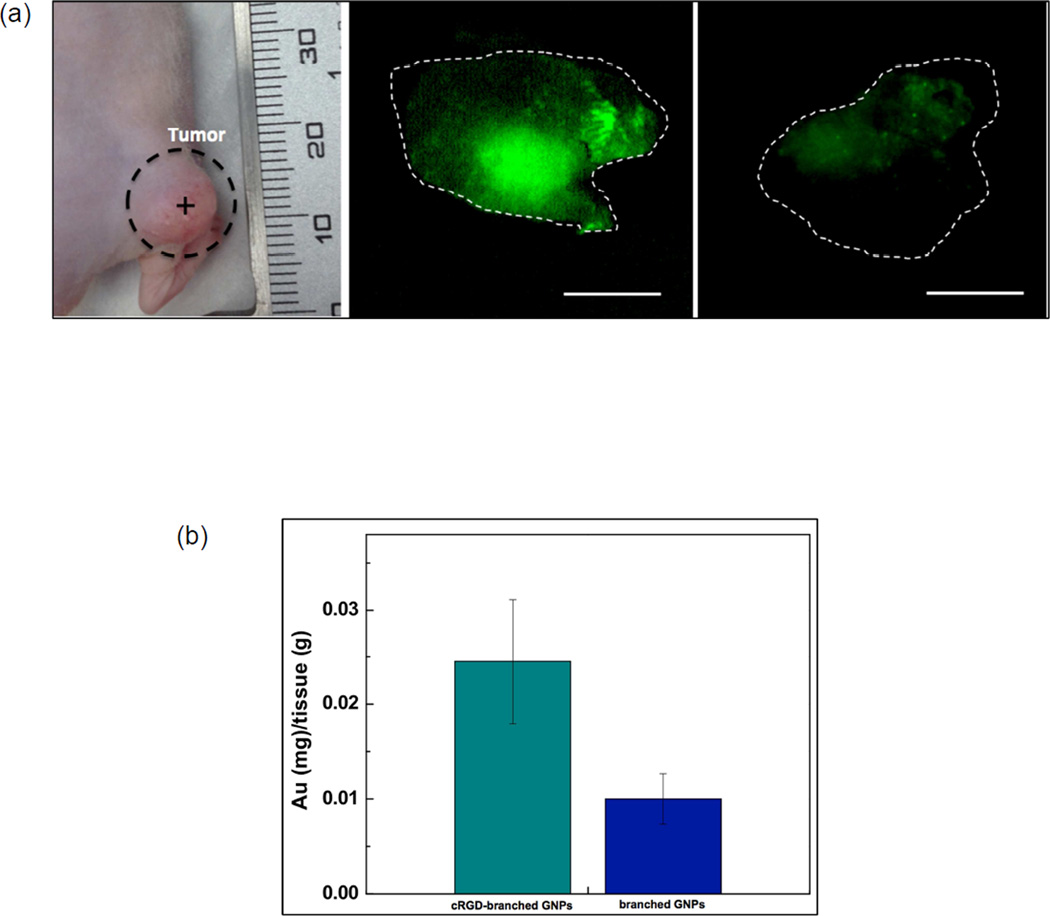
Next, we then investigated the feasibility to perform in vivo cRGD-branched GNPs mediated photothermal cancer treatment with a NIR laser in a pancreatic xenograft tumor model. Targeted photothermal treatment following photothermal transducing cRGD-branched GNPs delivery, as an alternative to open surgery, should reduce risk, pain and recovery time for pancreatic cancer patients. Before in vivo photothermal therapy experiments, improved targeting efficiency for the cRGD-branched GNPs was validated in tumor bearing mice following intratumoral (IT) injection of Cyto780 NIR fluorescent (NIRF) labeled cRGD-branched GNPs and Cyto780 labeled branched GNPs (control without cRGD). Tumors were excised 24 hours after injection and imaged using a fluorescent microscope to establish the presence of Cyto780-branched GNPs. Comparative studies revealed specific targeting and uptakes of the cRGD-branched GNPs in comparison with non-conjugated branched GNPs. A well-distributed and stronger fluorescent signal was consistently observed for cRGD-branched GNPs compared to non-conjugated branched GNPs (without cRGD), as observed in Fig. 4a. The superior targeting efficiency of the cRGD-branched GNPs was further confirmed with quantification of the resulting amounts of branched GNPs in the excised pancreatic tumors. The accumulation amount of cRGD-branched GNPs was 2.2 times higher than the amount observed for non-conjugated branched GNPs.
Figure 4.
(a) A photograph of BxPC3 pancreatic tumor model in nude mouse and fluorescent images of the excised tumors at 24 h after respective intra-tumoral injections of cyto780 labeled cRGD-branched GNPs and non-conjugated branched GNPs (without cRGD). Scale bars= 5 mm (Cyto780 labeled cRGD-branched GNPs (green) and dotted line: border of tumors) and (b) quantified amounts of targeted branched GNPs in the tumor tissues at 24 h post-injection (P < 0.05).
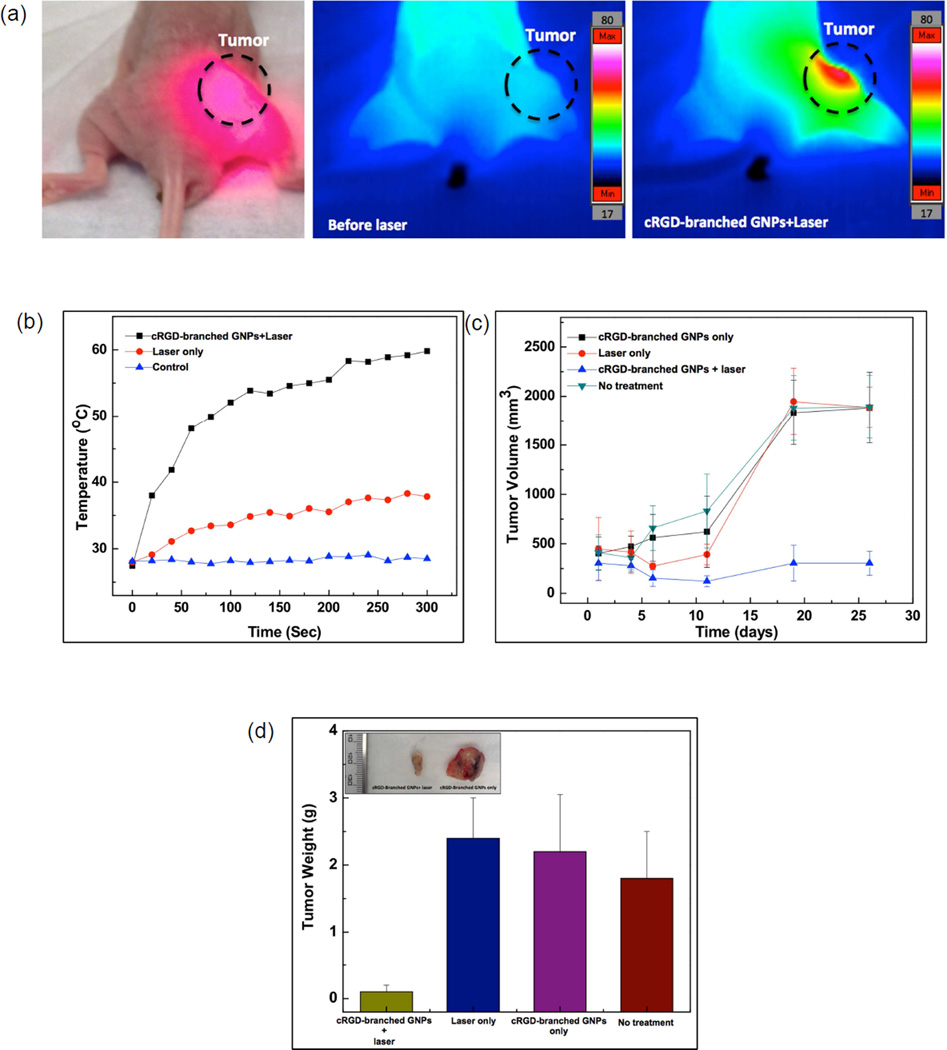
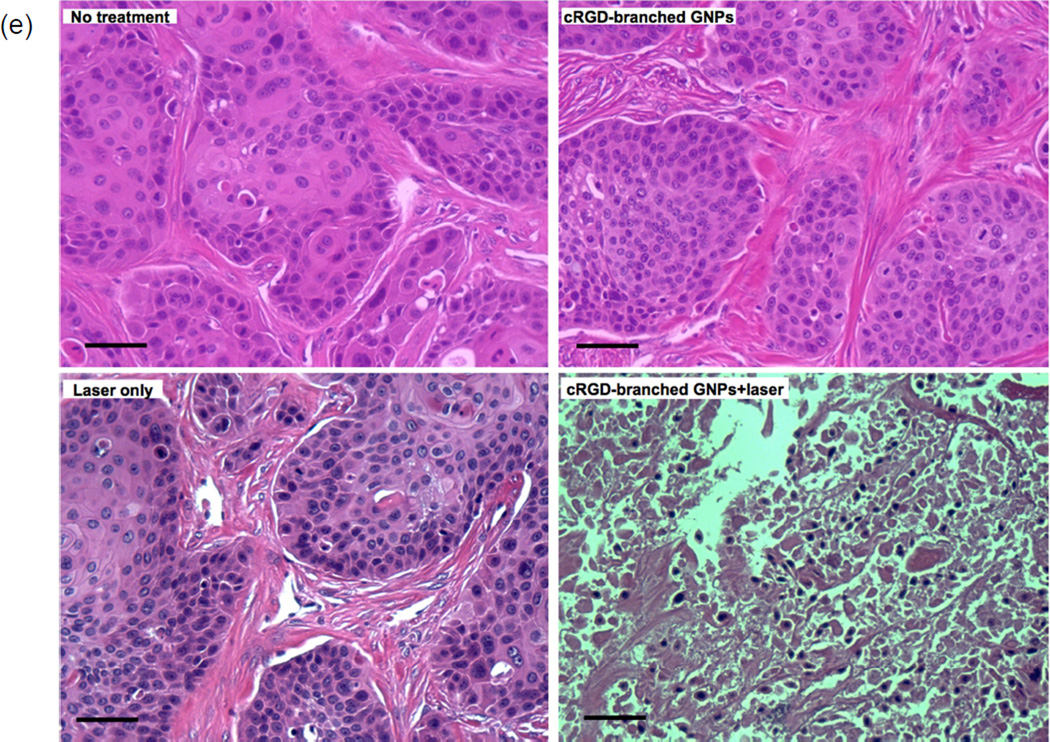
Finally, in vivo cRGD-branched GNPs mediated photothermal cancer therapy was performed in BxPC3 pancreatic tumor-bearing nude mice. cRGD-branched GNPs were administered to mice via IT injection and then, after 24 hours, the mice were exposed to an 808 nm laser (1.4 W/cm2) for 5 mins (Fig. 5a). For comparison purposes, two control groups were included with the first receiving an IT injection of the cRGD-branched GNPs (without irradiation) and the second NIR irradiation (5min) without cRGD-branched GNPs injection. NIR laser irradiation of tumor tissues after accumulation of the cRGD-branched GNPs resulted in significant temperature elevations up to 60°C within 300 seconds (Fig. 5a and b). Highly localized heating with cRGD-branched GNPs during laser irradiation was evidenced by the lack of temperature changes at nearby adjacent positions (within 1.5 cm of the tumor center). The resulting therapeutic efficacy after photothermal heating was evaluated by measuring tumor volume changes for each treatment group. It was found that the tumors were effectively ablated only after cRGD-branched GNPs injection and laser exposure, leaving brown scars at their original sites without any observation of tumor recurrence. Tumors for the laser-only, cRGD-branched GNPs injection-only and no treatment control groups, demonstrated similar growth rates, suggesting that either laser irradiation at this power density (1.4 W/cm2) or cRGD-branched GNPs alone did not impact tumor development (Fig. 5c). These control group tumors grew rapidly with the tumor volumes increasing up to 4 times their original volume within 26 days. The tumors from each mouse were collected to measure weights and perform additional histologic examinations. The mean tumor weight in cRGD-branched GNPs and laser irradiation treated animals on day 26 was only 0.13±0.08g, which was significantly (p<0.05) smaller than that of the laser-only (2.42±0.58g), cRGD-branched GNPs injection-only (2.23±0.85g) and untreated (1.79±0.74g) groups (Fig. 5d). Hematoxylin and eosin (H&E) staining of tumor slices was also carried out. Significant cell damage, such as karyorrhexis and karyolysis of pancreatic cancer cells, was noticed only in the tumors receiving both cRGD-branched GNPs injection and laser irradiation (Fig. 5e), but these findings were not observed in the three control groups, confirming again the therapeutic efficacy resulting from the combination of cRGD-branched GNPs administration and NIR laser irradiation. It should be pointed that a rather low laser power density (1.4 W/cm2) was used to minimize the adverse effects, further suggesting the highly efficient photothermal conversion permitted with these cRGD-branched GNPs.
Figure 5.
(a) A photograph and IR thermal images of a BxPC3 pancreatic tumor bearing mouse after cRGD-branched GNPs injection during NIR irradiation (808 nm; 1.4 W/cm2) for 5 mins, (b) Temperature changes measured in control tumors (without laser irradiation) and during NIR laser irradiation with and without injection of cRGD-branched GNPs, (c) tumor growth curves as a function of time after the different treatment protocols (control, laser irradiation only, cRGDbranched GNPs injection-only and cRGD-branched GNPs injection+laser irradiation) in BxPC3 pancreatic xenograft tumors; (d) average tumor weight and (inset) a digital picture of tumors excised at day 26 after treatment in each group, (e) representative photographs of hematoxylin and eosin-stained slides of tumor tissues from each group (scale bars=50 um).
Conclusion
In summary, we have presented a simple approach for synthesis of branch GNPs directed by deoxycholate bile acid supramolecular aggregates at room temperature. We showed that upon mixing Au solution with deoxycholate bile acid solution, the deoxycholate molecules sequestered Au molecules entrapping them. The reduction ability of deoxycholate supramolecular aggregates and progressive reduction of the entrapped Au ions due to the addition of L-ascorbic acid with AgNO3 led to the formation of branch GNPs. The resulting branched GNPs permitted strong NIR absorption for highly efficient photothermal heating as demonstrated with both phantom and in vivo studies. Branched GNPs conjugated with cell penetrating cRGD peptides were effective for targeted tumor ablation. These branched GNPs synthesized with deoxycholate bile acids should be an ideal candidate to serve as effective photothermal agents for clinical photothermal ablation procedures. Furthermore, this deoxycholate bile acid supramolecular aggregates directed branched GNPs synthesis approach may open up new avenues for the design of novel materials with customized spectral and structural properties for broad applications in nanoelectronics, medicine, ultrasensitive chemical sensing (SERS), life science, and optical devices.
Experimental Section
Chemicals and Materials
Hydrogen tetrachloroaurate(III) trihydrate (HAuCl4•3H20, 99.9%), silver nitrate (AgNO3, 99%), L-ascorbic acid (AA; C6H8O6, ≥98%), sodium borohydrate (NaBH4, 98%) and sodium deoxycholate (C24H39NaO4, ≥97%) were used as purchased from Sigma-Aldrich (St. Louis, USA). Carboxy-PEG-thiol (MW. 5K) was purchased from Laysan Bio (Arab, Al, USA). 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-Hydroxysuccinimide (NHS) were purchased from Pierce (Thermo Scientific Inc., Rockford, IL, USA). c(RGDfE) (cyclo(Arg-Gly-Asp-D-Phe-Glu)) peptide was purchased from Peptides International Inc. (Louisville, KY,USA). Cyto780 near infrared fluorescent dye was purchased from Cytodiagnostics (Ontario, Canada). For MTT assay, 3-[4,5–dimethylthiazol-2-yl]-3,5-diphenyltetrazolium bromide salt and dimethylsulfoxide (DMSO) were purchased from Sigma Chemicals Co. (St. Louis, USA). All chemicals were used without further purification. Milli-Q water (Millipore Corp. Billerica, MA, USA) was used throughout the experiments.
Deoxycholate Bile Acid Directed Synthesis of Branched GNPs
Branched GNPs were synthesized in sodium deoxycholate solutions. A seed solution was prepared in a sodium deoxycholate solution. 250 uL of 10 mM aqueous hydrogen tetrachloroaurate(III) trihydrate in Milli-Q water was added to 10 ml of 24 uM aqueous sodium deoxycholate solution. Then, a freshly prepared and ice-cold 300 ul of 10 mM sodium borohydrate was added to the solution, followed by rapid inversion mixing for 2 min. To grow GNPs, 10 ml sodium deoxycholate solution (concentrations: 1.4~36.1 mM), 1 ml of 10 mM hydrogen tetrachloroaurate(III) trihydrate, and 150 uL of 10 mM silver nitrate were added in that order, one by one, to a test tube, followed by gentle mixing by inversion. The solution at this stage appeared yellow in color. Then 160 uL of 10 mM L-ascorbic acid was added. The solution became colorless upon addition and mixing of AA. Finally, 150 uL of the prepared seed solution was added, and the reaction mixture was gently shaken for 20 s and left undisturbed for at least 2 h. It changed from colorless to dark blue or red, indicating the formation of GNPs.
Characterization
The crystal structure, morphology and size of the synthesized samples were characterized using an Xray diffractometer (XRD; Scintag XDS-2000), transmission electronic microscope (TEM; FEI Tecnai Spirit G2) operating at 120 kV and scanning transmission electronic microscope (STEM; Hitachi HD-2300 Dual EDS Cryo STEM). The absorption spectra of the samples were recorded on a Lambda 1050 spectrophotometer (Perkin-Elmer, USA). The hydrodynamic size and size-distribution of the samples were investigated with dynamic light scattering (DLS) using a Zetasizer Nano-S (Malvern, Herrenberg, Germany) equipped with a 4 mW HeNe laser.
NIR laser induced photothermal properties
A fiber-coupled near infrared (NIR) (808 nm) diode-laser (BWF5, B&W Tek, Inc., DE, USA) was used to investigate photothermal characteristics in vitro. Each sample was illuminated at laser powers from 478 mW/cm2 to 1.5 W/cm2. The sample solution was suspended in 1% agar phantom and pipetted into a cuvette (Malvern, Herrenberg, Germany). An infrared (IR) thermal camera (ICI7320P, Beaumont, TX, USA) was used to measure the temperature of the samples. The photothermal transduction efficiency, which is the conversion efficiency of absorbed light radiation into thermal energy, was determined as described in a previous report.[44]
Preparation of fluorescent cRGD peptide conjugated branched GNPs
Cyclic RGD peptide (c(RGDfE) (cyclo(Arg- Gly-Asp-D-Phe-Glu)) and Cyto 780 NIR fluorescent dye were conjugated onto branched GNPs by using coupling agents (EDC and NHS).[49, 50] To prepare PEG-modified branched GNPs, 100 uL of aqueous carboxy-PEG-thiol (2 mM) was added to 10 mL of branched GNPs (0.02 M). The mixture was stirred and incubated at room temperature for 3 h to allow complete PEG modification of the GNPs with thiol of PEG. The mixture was then purified by centrifugation at 8,500 rpm for 10 min. The supernatant was decanted, and the pellet was re-suspended in 5 mL PBS buffer. Then, the prepared PEG modified branched GNPs were conjugated with c(RGDfE) and Cyto780 NIR fluorescent dye. EDC (10 mg) and NHS (8 mg) were added into the branched GNPs solution (10 ml) prepared above and the mixture was stirred at room temperature for 4 h. After the reaction, c(RGDfE) (5 mg) and 30 uL Cyto 780 NIR-fluorescent dye were added to the activated branched GNPs solution and the new mixture was incubated over-night. Subsequently, the solution was centrifuged at 8,500 rpm for 10 min. The residual small molecules including EDC, NHS, c(RGDfE) and Cyto780 dye in the supernatant were discarded. 5 mL PBS was utilized to dissolve the precipitate.
Cell Lines and Cell Culture
A human pancreatic cancer cell line (BxPC-3) was obtained from American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured in RPMI-1640 medium (Gibco, Invitrogen Co., Grand Island, NY, US) supplemented with 10% FBS, according to ATCC guidelines. Cells were incubated at 37°C with 5% CO2 and 95% humidity.
Cell Uptake of cRGD-branched GNPs
BxPC-3 human pancreatic cancer cells were transferred and cultured onto 20-mm glass coverslips in a 24-well plate and allowed to grow for 2 days. Then the medium was replaced with 1 mL of fresh culture medium containing Cyto780 labeled cRGD-branched GNPs. Cell nuclei were stained with DAPI. After incubation for 24 h, the cell monolayer on the coverslip was removed, repeatedly rinsed with PBS, and then mounted for microscopic examination. The cellular fluorescence images were examined under a Zeiss Axio Observer Z1 fluorescence microscope (Carl Zeiss MicroImaging GmbH, USA).
Photothermal ablation in vitro
In a 96-well plate, 3×104 BxPC3 human pancreatic cancer cells were placed in each well and exposed to a 0~0.8 mM dose of cRGD-branched GNPs. We investigated the efficacy of this photothermal approach when using different power densities during irradiation. After allowing 24 hours for cellular uptake, the samples exposed to these five different GNPs concentrations were divided into two groups, exposed to laser irradiation with power densities of 0.47 and 0.96 W/cm2, respectively, for 3 minutes, the other group was left untreated thus serving as control. After exposure, the cells were returned to a 37°C incubator for another 24 hours before cell viability tests. Then, a standard cell viability test using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was carried out to determine the relative viabilities in each treated group. The cell viability was calculated based on the total viable BxPC3 cells after treatment compared with the control group. Each sample was counted in triplicate.
Biodistribution of cRGD-branched GNPs in tumor bearing mice
Female athymic nude mice (5–7 weeks old) were purchased from the Charles River Laboratories. All procedures involving experimental animals were performed in accordance with protocols approved by the Northwestern University Institutional Animal Care and Use Committee. BxPC3 pancreatic tumor models were established by subcutaneous injection of BxPC3 (high αvβ3 receptor expression) tumor cells (~5×106 in 100 µL of PBS) into the femoral tissue of female athymic nude mice (n=6). To investigate the targeting ability of the cRGD-branched GNPs, as the tumors grew to a diameter of up to about 0.5 cm, Cyto780 fluorescent labeled cRGD-branched GNPs (150 uL, 50 ug) and nonconjugated branched GNPs were injected into BxPC3 tumor-bearing mice through intratumoral injection (n=3 in each group). After 24 hours, the distribution of the GNPs in tumor regions of each group were imaged with OV100 small animal imaging system (Olympus, Tokyo, Japan). Then, mice were euthanized and tumors excised to determine gold content using Inductively Coupled Plasma Mass Spectrometer (ICP-MS) (Perkin Elmer, Waltham, MA, USA).
Photothermal treatment with targeted cRGD-branched GNPs and NIR Laser in vivo
BxPC3 pancreatic tumor mice models (n=18) were used to investigate the feasibility and effectiveness of in vivo cRGD-branched GNPs mediated photothermal cancer treatment. When the tumor volumes reached roughly 300–400 mm3, the mice were randomly divided into four different experimental groups (n = 12): intratumoral injection of cRGD-branched GNPs (150 uL, 50 ug) and 808 nm NIR laser (1.4 W/cm2 for 5 min) irradiation (n=6); only intratumoral injection of cRGD-branched GNPs (150 uL, 50 ug) (n=6); as control groups (n=6): 808 nm NIR laser (1.4 W/cm2 for 5 min) irradiation only (n=3); no treatment (n=3). During photothermal treatment, real-time thermal imaging of the tumor was performed using an IR thermal camera (ICI7320P, Beaumont, TX, USA). Body weight and tumor growth or regression for each group was monitored for 26 days continuously. Tumor dimensions were determined using a digital caliper. Tumor volume (V) (mm3) was calculated using the following formula: V=(tumor length)×(tumor width)2/2. The treated tumors of mice were collected and weight measured at end of the study.
Histopathology assessments
Tumors were harvested and fixed in 10% neutral buffered formalin for 24 hours, and the tissue specimens were sliced at 1mm intervals; these slices were sectioned into 5 µm thick sections for H&E staining to identify regions of necrotic tumor tissues. H&E slides were viewed and digitized (x200 magnification) using a TissueFAXS microscope (TissueGnostics GmbH, Vienna, Austria).
Statistics
Significant differences were determined using the Student’s t-test where differences were considered significant (p < 0.05).
ACKNOWLEDGMENT
This work was supported by Basic Research Grant from ACS (American Cancer Society, ACS 279148) and by four grants R01CA159178, R01CA141047, R21CA173491 and R21EB017986 from the National Cancer Institute and National Institute of Biomedical Imaging and Bioengineering. This work was supported by the Center for Translational Imaging at Northwestern University. We thank J. Chen, Dr. Y. Guo, Dr. Z. Zhang, Dr. I.W. Jung, and A. Gordon for discussion and assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lv WP, Wang Y, Feng WQ, Qi JJ, Zhang GL, Zhang FB, et al. Robust and smart gold nanoparticles: one-step synthesis, tunable optical property, and switchable catalytic activity. J Mater Chem. 2011;21:6173–6178. [Google Scholar]
- 2.Turner M, Golovko VB, Vaughan OPH, Abdulkin P, Berenguer-Murcia A, Tikhov MS, et al. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters. Nature. 2008;454:981-U31. doi: 10.1038/nature07194. [DOI] [PubMed] [Google Scholar]
- 3.Thacker VV, Herrmann LO, Sigle DO, Zhang T, Liedl T, Baumberg JJ, et al. DNA origami based assembly of gold nanoparticle dimers for surface-enhanced Raman scattering. Nat Commun. 2014;5 doi: 10.1038/ncomms4448. [DOI] [PubMed] [Google Scholar]
- 4.Hutter E, Maysinger D. Gold-nanoparticle-based biosensors for detection of enzyme activity. Trends Pharmacol Sci. 2013;34:497–507. doi: 10.1016/j.tips.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Peng G, Tisch U, Adams O, Hakim M, Shehada N, Broza YY, et al. Diagnosing lung cancer in exhaled breath using gold nanoparticles. Nat Nanotechnol. 2009;4:669–673. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 6.Park JH, von Maltzahn G, Xu MJ, Fogal V, Kotamraju VR, Ruoslahti E, et al. Cooperative nanomaterial system to sensitize, target, and treat tumors. P Natl Acad Sci USA. 2010;107:981–986. doi: 10.1073/pnas.0909565107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Juste J, Pastoriza-Santos I, Liz-Marzan LM, Mulvaney P. Gold nanorods: Synthesis, characterization and applications. Coordin Chem Rev. 2005;249:1870–1901. [Google Scholar]
- 8.Wang YC, Black KCL, Luehmann H, Li WY, Zhang Y, Cai X, et al. Comparison Study of Gold Nanohexapods, Nanorods, and Nanocages for Photothermal Cancer Treatment. Acs Nano. 2013;7:2068–2077. doi: 10.1021/nn304332s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong S, Shuford KL, Park S. Shape Transformation of Gold Nanoplates and their Surface Plasmon Characterization: Triangular to Hexagonal Nanoplates. Chem Mater. 2011;23:2011–2013. [Google Scholar]
- 10.Zhu MS, Lei B, Ren FF, Chen PL, Shen YF, Guan B, et al. Branched Au Nanostructures Enriched with a Uniform Facet: Facile Synthesis and Catalytic Performances. Sci Rep-Uk. 2014;4 doi: 10.1038/srep05259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.You J, Zhang GD, Li C. Exceptionally High Payload of Doxorubicin in Hollow Gold Nanospheres for Near-Infrared Light-Triggered Drug Release. Acs Nano. 2010;4:1033–1041. doi: 10.1021/nn901181c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song HM, Wei QS, Ong QK, Wei A. Plasmon-Resonant Nanoparticles and Nanostars with Magnetic Cores: Synthesis and Magnetomotive Imaging. Acs Nano. 2010;4:5163–5173. doi: 10.1021/nn101202h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HY, Zhang X, Dai SH, Ma YX, Cui SS, Achilefu S, et al. Multifunctional Gold Nanostar Conjugates for Tumor Imaging and Combined Photothermal and Chemo-therapy. Theranostics. 2013;3:633–649. doi: 10.7150/thno.6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Hu Y, Yang J, Wei P, Sun W, Shen M, et al. Hyaluronic acid-modified Fe3O4@Au core/shell nanostars for multimodal imaging and photothermal therapy of tumors. Biomaterials. 2015;38:10–21. doi: 10.1016/j.biomaterials.2014.10.065. [DOI] [PubMed] [Google Scholar]
- 15.Jena BK, Raj CR. Synthesis of flower-like gold nanoparticles and their electrocatalytic activity towards the oxidation of methanol and the reduction of oxygen. Langmuir. 2007;23:4064–4070. doi: 10.1021/la063243z. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Wu J, Zhang X, Liu Y, Zhou D, Sun HZ, et al. Controllable Synthesis of Stable Urchin-like Gold Nanoparticles Using Hydroquinone to Tune the Reactivity of Gold Chloride. J Phys Chem C. 2011;115:3630–3637. [Google Scholar]
- 17.Sanchez-Gaytan BL, Swanglap P, Lamkin TJ, Hickey RJ, Fakhraai Z, Link S, et al. Spiky Gold Nanoshells: Synthesis and Enhanced Scattering Properties. J Phys Chem C. 2012;116:10318–10324. [Google Scholar]
- 18.Li Z, Li W, Camargo PH, Xia Y. Facile synthesis of branched au nanostructures by templating against a self-destructive lattice of magnetic fe nanoparticles. Angewandte Chemie. 2008;47:9653–9656. doi: 10.1002/anie.200804634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilchis-Nestor AR, Sanchez-Mendieta V, Carnacho-Lopez MA, Gomez-Espinosa RM, Camacho-Lopez MA, Arenas-Alatorre JA. Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater Lett. 2008;62:3103–3105. [Google Scholar]
- 20.Daniel MC, Astruc D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 21.Hall SR. Biotemplated syntheses of anisotropic nanoparticles. P R Soc A. 2009;465:335–366. [Google Scholar]
- 22.Zhang TJ, Wang W, Zhang DY, Zhang XX, Ma YR, Zhou YL, et al. Biotemplated Synthesis of Gold Nanoparticle- Bacteria Cellulose Nanofiber Nanocomposites and Their Application in Biosensing. Adv Funct Mater. 2010;20:1152–1160. [Google Scholar]
- 23.Ono Y, Nakashima K, Sano M, Hojo J, Shinkai S. Template effect of cholesterol-based organogels on sol-gel polymerization creates novel silica with a helical structure. Chem Lett. 1999:1119–1120. [Google Scholar]
- 24.Jung JH, Ono Y, Shinkai S. Sol-gel polycondensation of tetraethoxysilane in a cholesterol-based organogel system results in chiral spiral silica. Angew Chem Int Edit. 2000;39:1862-+. doi: 10.1002/(sici)1521-3773(20000515)39:10<1862::aid-anie1862>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Kalyanikutty KP, Nikhila M, Maitra U, Rao CNR. Hydrogel-assisted synthesis of nanotubes and nanorods of US, ZnS and Cus, showing some evidence for oriented attachment. Chem Phys Lett. 2006;432:190–194. [Google Scholar]
- 26.DSouza LJ, Maitra U. Design, synthesis, and evaluation of bile acid-based molecular tweezers. J Org Chem. 1996;61:9494–9502. [Google Scholar]
- 27.Kleeff J, Michalski C, Friess H, Buchler MW. Pancreatic cancer: from bench to 5-year survival. Pancreas. 2006;33:111–118. doi: 10.1097/01.mpa.0000229010.62538.f2. [DOI] [PubMed] [Google Scholar]
- 28.Guo Y, Zhang Z, Kim DH, Li W, Nicolai J, Procissi D, et al. Photothermal ablation of pancreatic cancer cells with hybrid iron-oxide core gold-shell nanoparticles. International journal of nanomedicine. 2013;8:3437–3446. doi: 10.2147/IJN.S47585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giglio E, Loreti S, Pavel NV. Exafs - a New Approach to the Structure of Micellar Aggregates. J Phys Chem-Us. 1988;92:2858–2862. [Google Scholar]
- 30.Zhang JW, Luo JT, Zhu XX, Junk MJN, Hinderberger D. Molecular Pockets Derived from Cholic Acid as Chemosensors for Metal Ions. Langmuir. 2010;26:2958–2962. doi: 10.1021/la9028996. [DOI] [PubMed] [Google Scholar]
- 31.Nonappa, Maitra U. Unlocking the potential of bile acids in synthesis, supramolecular/materials chemistry and nanoscience. Org Biomol Chem. 2008;6:657–669. doi: 10.1039/b714475j. [DOI] [PubMed] [Google Scholar]
- 32.Balasubramanian R, Maitra U. Design and synthesis of novel chiral dendritic species derived from bile acids. J Org Chem. 2001;66:3035–3040. doi: 10.1021/jo0013305. [DOI] [PubMed] [Google Scholar]
- 33.Ropponen J, Tamminen J, Lahtinen M, Linnanto J, Rissanen K, Kolehmainen E. Synthesis, characterization, and thermal behavior of steroidal dendrons. Eur J Org Chem. 2005:73–84. [Google Scholar]
- 34.Posa M, Sebenji A. Determination of number-average aggregation numbers of bile salts micelles with a special emphasis on their oxo derivatives-the effect of the steroid skeleton. Biochimica et biophysica acta. 2014;1840:1072–1082. doi: 10.1016/j.bbagen.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Warren DB, Chalmers DK, Hutchison K, Dang WB, Pouton CW. Molecular dynamics simulations of spontaneous bile salt aggregation. Colloid Surface A. 2006;280:182–193. [Google Scholar]
- 36.Hao E, Bailey RC, Schatz GC, Hupp JT, Li SY. Synthesis and optical properties of "branched" gold nanocrystals. Nano Lett. 2004;4:327–330. [Google Scholar]
- 37.Hao F, Nehl CL, Hafner JH, Nordlander P. Plasmon resonances of a gold nanostar. Nano letters. 2007;7:729–732. doi: 10.1021/nl062969c. [DOI] [PubMed] [Google Scholar]
- 38.Nehl CL, Liao HW, Hafner JH. Optical properties of star-shaped gold nanoparticles. Nano Lett. 2006;6:683–688. doi: 10.1021/nl052409y. [DOI] [PubMed] [Google Scholar]
- 39.Harry Sobotka NC. The gelation of bile salt solutions. Journal of Colloid Science. 1958;13:188–191. [Google Scholar]
- 40.Rich A, Blow DM. Formation of a helical steroid complex. Nature. 1958;182:423–426. doi: 10.1038/182423a0. [DOI] [PubMed] [Google Scholar]
- 41.Kennedy LC, Bickford LR, Lewinski NA, Coughlin AJ, Hu Y, Day ES, et al. A New Era for Cancer Treatment: Gold-Nanoparticle-Mediated Thermal Therapies. Small. 2011;7:169–183. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- 42.Huang XH, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 43.Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 44.Pattani VP, Tunnell JW. Nanoparticle-mediated photothermal therapy: a comparative study of heating for different particle types. Lasers in surgery and medicine. 2012;44:675–684. doi: 10.1002/lsm.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baffou G, Quidant R, Girard C. Heat generation in plasmonic nanostructures: Influence of morphology. Appl Phys Lett. 2009;94 [Google Scholar]
- 46.Baffou G, Quidant R, de Abajo FJG. Nanoscale Control of Optical Heating in Complex Plasmonic Systems. Acs Nano. 2010;4:709–716. doi: 10.1021/nn901144d. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Oliveros R, Sanchez-Gil JA. Gold nanostars as thermoplasmonic nanoparticles for optical heating. Opt Express. 2012;20:621–626. doi: 10.1364/OE.20.000621. [DOI] [PubMed] [Google Scholar]
- 48.Pattani VP, Tunnell JW. Nanoparticle-mediated photothermal therapy: A comparative study of heating for different particle types. Laser Surg Med. 2012;44:675–684. doi: 10.1002/lsm.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim DH, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, et al. Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction. Nature materials. 2010 doi: 10.1038/nmat2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DH, Guo Y, Zhang Z, Procissi D, Nicolai J, Omary RA, et al. Temperature-sensitive magnetic drug carriers for concurrent gemcitabine chemohyperthermia. Advanced healthcare materials. 2014;3:714–724. doi: 10.1002/adhm.201300209. [DOI] [PMC free article] [PubMed] [Google Scholar]