Summary
The neurotransmitter dopamine is oxidized to its quinone (DA-Q), which at neutral pH undergoes intramolecular cyclization by 1,4-Michael addition, followed by oxidation to form leukochrome, then aminochrome, and finally neuromelanin. At lower pH, the amino group of DA is partially protonated, allowing the competitive intermolecular 1,4-Michael addition with nucleophiles in DNA to form the depurinating adducts, DA-6-N3Ade and DA-6-N7Gua. Catechol estrogen-3,4-quinones react by 1,4-Michael addition to form the depurinating 4-hydroxyestrone(estradiol)-1-N3Ade [4-OHE1(E2)-1-N3Ade] and 4-OHE1(E2)-1-N7Gua adducts, which are implicated in the initiation of breast and other human cancers. The effect of pH was studied by reacting tyrosinase-activated DA with DNA and measuring the formation of depurinating adducts. The most adducts were formed at pH 4, 5, and 6, and their level was nominal at pH 7 and 8. The N3Ade adduct depurinated instantaneously, but N7Gua had a half-life of 3 H. The slow loss of the N7Gua adduct is analogous to that observed in previous studies of natural and synthetic estrogens. The antioxidants N-acetylcysteine and resveratrol efficiently blocked formation of the DA-DNA adducts. Thus, slightly acidic conditions render competitive the reaction of DA-Q with DNA to form depurinating adducts. We hypothesize that formation of these adducts could lead to mutations that initiate Parkinson’s disease. If so, use of N-acetylcysteine and resveratrol as dietary supplements may prevent initiation of this disease.
Keywords: depurinating dopamine-DNA adducts; dopamine quinone; prevention of dopamine-DNA adduct formation; tyrosinase-activated dopamine; 1,4-Michael addition
INTRODUCTION
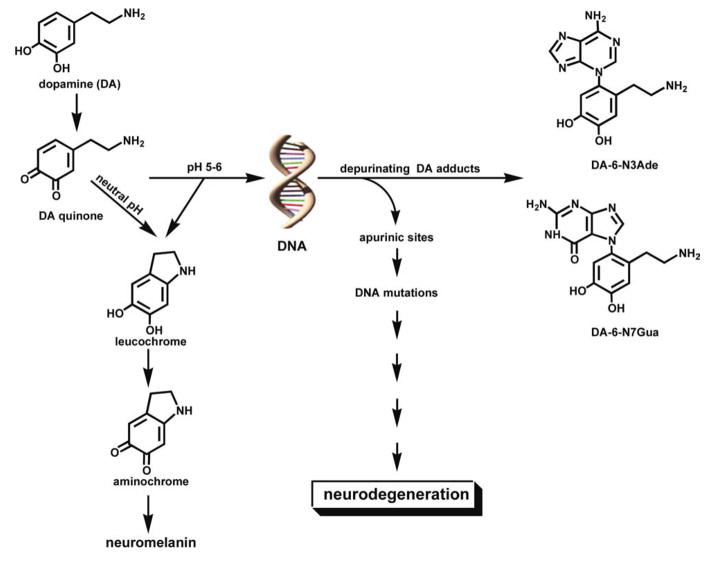
One of the major pathways in the metabolism of the neurotransmitter dopamine (DA) is oxidation to its quinone (DA-Q) (Fig. 1). At neutral pH, this quinone undergoes intramolecular cyclization by 1,4-Michael addition to form leukochrome, which in turn is oxidized to aminochrome. Polymerization of aminochrome leads to neuromelanin (Fig. 1). At lower pH, the amino group of DA is partially protonated, slowing down the intramolecular cyclization and leading to a competitive intermolecular 1,4-Michael addition with nucleophiles, including those of DNA. With DNA this leads to formation of the depurinating DA adducts, DA-6-N3Ade and DA-6-N7Gua (Fig. 1) (1, 2). It was recently reported that an excessive amount of glutamate can result in a lumenal pH of 5.5 in dopaminergic neurons (3), suggesting that DA-Q could form DNA adducts in vivo.
Figure 1.
Intramolecular 1,4-Michael addition of dopamine quinone at neutral pH to form neuromelanin and competitive intermolecular 1,4-Michael addition at pH 5–6, with formation of the depurinating adducts DA-6-N3Ade and DA-6-N7Gua. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Formation of depurinating adducts at the N3 of Ade and N7 of Gua also occurs when the natural estrogens estrone and estradiol are oxidized to their quinones, which react with DNA (4). The resulting apurinic sites in the DNA generate mutations that can initiate breast and other human cancers (5, 6).
In this article, we have determined the amount of depurinating DA-DNA adducts formed as a function of pH. We have also investigated the rate of depurination of the DA-6-N3Ade and DA-6-N7Gua from DNA. Finally, we have examined the ability of four specific antioxidants, lipoic acid, melatonin, N-acetylcysteine (NAcCys), and resveratrol, to block the formation of the DA-6-N3Ade and DA-6-N7Gua adducts when DA is oxidized to DA-Q by tyrosinase in the presence of DNA. These antioxidants were selected for a variety of reasons, including their ability to pass through the blood–brain barrier.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
DA hydrochloride, di-tert-butyl-dicarbonate (Boc)-anhydride, Ag2O, trifluoroacetic acid (TFA), formic acid, acetone, CH3OH, CH3CN, DMF, and DMSO-d6 were purchased from Aldrich Chemical Co. (Milwaukee, WI) and used as received. 2′-Deoxyguanosine (dG) and calf thymus DNA were purchased from USB (Cleveland, OH). Mushroom tyrosinase was purchased from Sigma-Aldrich Chemical (St. Louis, MO). The standard depurinating adducts (DA-6-N7Gua and DA-6-N3Ade) were synthesized using a new procedure described below.
Instrumentation
NMR
NMR spectra were recorded on a Varian Unity-Inova 500 instrument operating at a resonance frequency of 500 MHz for 1H and 125 MHz for 13C spectra at 25 °C. Samples were dissolved in 600 μL of DMSO-d6 and referenced to the solvent signals at 2.5 ppm for 1H and 39.7 ppm for 13C. All two-dimensional (2D) experiments were performed by using the standard Varian software (VNMR v6.1c). The structures were further confirmed with the help of 1D and 2D experiments.
UV
The UV spectra were obtained during HPLC by using a Waters 996 or 2996 photodiode array detector (Milford, MA) for all compounds synthesized. HPLC separations were monitored at 290 nm.
HPLC
Analytical HPLC was conducted on a Waters 2690 separations module equipped with a Waters 996 or 2996 photodiode array detector and a reversed phase Phenomenex Luna-2 C-18 column (250 × 4.6 mm, 5 μm; 120 Å, Torrance, CA). The column was eluted starting with 10% CH3CN in H2O (0.1% TFA) at a flow rate of 1 mL/Min to 100% CH3CN in 35 Min using a linear gradient. Retention times: N-Boc-DA: 14.6 Min, N-Boc-DA-Q: 14.1 Min.
Mass Spectrometry
ESI-MS
All experiments were performed on a Waters QuattroMicro triple quadruple mass spectrometer by using electrospray ionization (ESI) in positive ion (PI) mode, with previously published parameters (2).
UPLC-MS/MS
Samples were analyzed on an Acquity UPLC system attached to a Waters QuattroMicro triple quadrupole mass spectrometer as previously described (2). Three-point calibration curves were run for each standard.
Synthesis of Depurinating Adducts of Dopamine
The previously published method for the synthesis of depurinating adducts of DA afforded low yield and required multiple HPLC runs for purification of the final products (1). To overcome this problem, a new method, which increased the yields, was used, and the purification of depurinating adducts became comparatively easy.
To a stirred solution of DA hydrochloride (78.0 mg, 0.41 mmol) in DMF (5 mL) was added a saturated solution of NaHCO3 (1 mL) and Boc2O (107.4 mg, 0.49 mmol). After 30 Min of stirring, the reaction mixture was subjected to preparative HPLC, and the pure peak of N-Boc-DA was collected and lyophilized.
To a stirred solution of N-Boc-DA (2 mg,7.9 μmol) in acetone-d6 (1 mL) was added Ag2O (20 mg, 6 equiv.) at room temperature. The reaction mixture was stirred for 30 Min and then filtered directly into an NMR tube for spectroscopic analysis. A 10-μL aliquot was analyzed by analytical HPLC (described above). 1H NMR (ppm) δ: 7.16 (dd, J = 7.8, 1.5 Hz, 1 H, H-6), 6.32 (d, J = 7.8 Hz, 1 H, H-5), 6.19 (d, J = 1.5 Hz, 1 H, H-2), 3.40 (m, 2 H, CH2), 2.64 (m, 2 H, CH2), 1.35 (s, 9 H, 3 × CH3).
To a stirred solution of N-Boc-DA (50 mg, 0.19 mmol) in acetone (5 mL) was added 6 equiv. of Ag2O (275 mg) and the mixture was refluxed. After 15 Min, the reaction mixture was filtered directly into a solution of dG (338 mg) or Ade (160 mg) in 9.8 mL of acetic acid/H2O (1:1) and 200 μL of DMF. The colored solution was allowed to stir overnight and then analyzed on analytical HPLC, which showed one peak at 9.57 Min for the Gua adduct and 8.50 Min for the Ade adduct (HPLC conditions were those described above). The reaction mixture was then separated by preparative HPLC (Waters Delta Prep 600) by using a SunFire™ Prep C18 OBD™ (10 μm, 30 × 150 mm) column. The column was initially eluted by using 10% CH3CN:H2O (10:90) and then an increasing proportion of CH3CN up to 100% in 20 Min at a flow rate of 30 mL/Min. The major peak eluting at 6.67 Min (Gua adduct) or 3.30 Min (Ade adduct) was collected, lyophilized, and characterized to be the target adduct.
N-Boc-DA-6-N7Gua, yield 32%: 1H NMR (ppm) δ: 10.7 (s, 1 H, NH-Gua, exchangeable with D2O), 9.15 (s, 2 H, Ar-OH, exchangeable with D2O), 7.83 (s, 1 H, H-8-Gua), 6.67 (s, 1 H, H-2), 6.59 (s, 1 H, H-5), 6.11 (s, 2 H, NH2-Gua, exchangeable with D2O), 2.87 (m, 2 H, CH2), 2.30 (m, 2 H, CH2), 1.32 (s, 9 H, 3 × CH3). A 10-mg sample of adduct was re-dissolved in 3 mL of CH3OH and the resulting suspension was treated with TFA (1.5 mL) until the suspension dissolved. The successful deprotection with Boc was confirmed by analytical HPLC; the disappearance of the peak at 9.57 Min and appearance of a new peak at 3.86 Min indicated 100% deprotection of the N-Boc group. Finally, the solvent was evaporated and the residue was triturated with ether to obtain a brownish crystal of the product.
N-Boc-DA-6-N3Ade, yield 38%: 1H NMR (ppm) δ: 9.48 (br. s, 2 H, Ar-OH, exchangeable with D2O), 8.14 (s, 2 H, NH2-Ade, exchangeable with D2O), 8.23 (s, 1 H, H-2-Ade), 7.69 (s, 1 H, H-8-Ade), 6.71 (s, 1 H, H-2), 6.77 (s, 1 H, H-5), 2.87 (m, 2 H, CH2), 2.30 (m, 2 H, CH2), 1.32 (s, 9 H, 3 × CH3). The final adduct was obtained by deprotection, as described above.
Covalent Binding of Tyrosinase-Activated Dopamine to DNA at Different pH
From a stock solution of DA (1.33 mg in 50 mL acetone), 5 μL was mixed with DNA in a 10-mL reaction mixture containing 3 mM calf thymus DNA in 0.1 M sodium–potassium phosphate (pH 6, 7 or 8) or 0.1 M sodium acetate (pH 4 or 5) and 1 mg of mushroom tyrosinase (3,660 units). The reaction mixture was incubated at 37 °C for 1 or 10 H. At the indicated times, two volumes of ethanol was used to precipitate the DNA, and the supernatant containing depurinating DA adducts was evaporated and redissolved with 0.5 mL of CH3OH:H2O (1:1) and passed through a 3,000 MW cut-off filter (Ultrafree-MC, Millipore Corporation, Billerica, MA). Control reactions were carried out under identical conditions with either no enzyme or no DA.
Kinetics of Depurinating Adduct Formation After Reaction of DNA With Tyrosinase-Activated Dopamine
DA (5 μL of 1.33 mg in 50 mL acetone) was incubated with DNA and tyrosinase in a 10-mL reaction mixture at pH 5. At different time points (1, 3, 5, 7, 9, and 10 H), aliquots of the mixtures were treated quickly with two volumes of ethanol to remove DNA, and the supernatant was analyzed for the formation of depurinating adducts, as described above (2). The reactions were performed in triplicate and control experiments were performed with 5 μL of acetone in place of the solution of DA.
Preventive Effect of Natural Antioxidants on Covalent Binding of Tyrosinase-Activated Dopamine With DNA
Tyrosinase-catalyzed reactions were carried out in 10-mL reaction mixtures containing 3 mM calf thymus DNA in 0.1 M sodium acetate, pH 5, 87 μM DA (0.132 mg in 5 μL of acetone) and 1 mg of mushroom tyrosinase (3,660 units), incubated at 37 °C for 10 H. In addition, incubations were carried out in the presence of different ratios (1:0, 1:0.1, 1:0.3, 1:1, and 1:3) of DA to an antioxidant [lipoic acid (reduced form), melatonin, NAcCys or resveratrol]. After precipitation of DNA with ethanol, the supernatant was used for analysis of the depurinating adducts, as previously described (2). Under similar conditions, control reactions were carried out with either no substrate or no enzyme.
RESULTS
Synthesis of Depurinating Dopamine Adducts
A new and easy approach for the synthesis of depurinating adducts of DA was used. These adducts served as the standards to identify the depurinating adducts formed when DA was oxidized in vitro by the enzyme tyrosinase in the presence of DNA.
To overcome the previously encountered problems (1), we protected the amino group of DA with N-Boc, which blocks the intramolecular cyclization and dimerization of DA ortho-quinone. The DA ortho-quinone synthesized by this method is stable and available for synthesis of the depurinating DA adducts. The adducts were produced in a much higher yield compared to those reported previously (1). The structures of N-Boc-DA-6-N3Ade and N-Boc-DA-6-N7Gua were confirmed by 1D and 2D NMR spectroscopy.
Covalent Binding of Tyrosinase-Activated Dopamine to DNA at Different pH
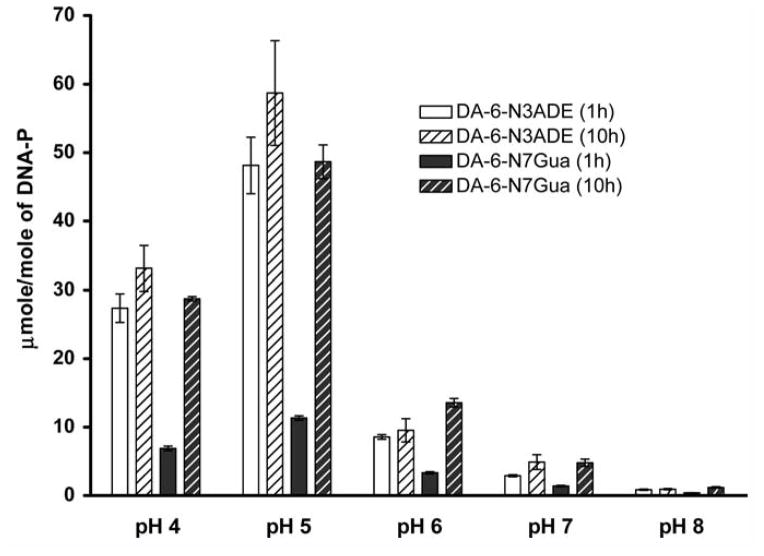
The effect of pH on the formation of depurinating adducts of DA was studied by reacting tyrosinase-activated DA with DNA for 1 or 10 H, due to the slow depurination of the N7Gua adduct (see below). The amount of depurinating adducts was measured by using ultraperformance liquid chromatography/tandem mass spectrometry (UPLC-MS/MS). The highest level of depurinating adducts (50 ± 13 for N3Ade and 49 ± 4 μmol/mol DNA-P for N7Gua) was achieved at pH 5 (Fig. 2). Interestingly, at pH 4 the level of depurinating adducts was reduced to 27 ± 4 for N3Ade and 29 ± 1 μmol/mol DNA-P for N7Gua; this probably was due to the partial solubility of the DNA in the buffer. When the pH was increased to 6, the amounts of depurinating adducts were 4–5 times lower than at pH 5, with formation of 10 ± 3 μmol/mol DNA-P for N3Ade and 14 ± 1 μmol/mol DNA-P for N7Gua adducts after 10 H. A sharp reduction was observed at pH 7 and 8, where the amounts of N3Ade adducts were 3 ± 1 and 1 ± 0.2 μmol/mol DNA-P and those of the N7Gua adduct were 5 ± 1 and 1 ± 0.2 μmol/mol DNA-P, respectively (Fig. 2). One observation is common despite differences in the reaction pH: the level of N3Ade adduct is maximized in 1 H and does not increase by 10 H, while the N7Gua adduct level is low at 1 H and increases 3- to 4-fold by 10 H.
Figure 2.
Effect of pH on the formation of depurinating adducts after reaction of tyrosinase-activated DA with DNA.
Rate of Depurination of DA-6-N7Gua From DNA
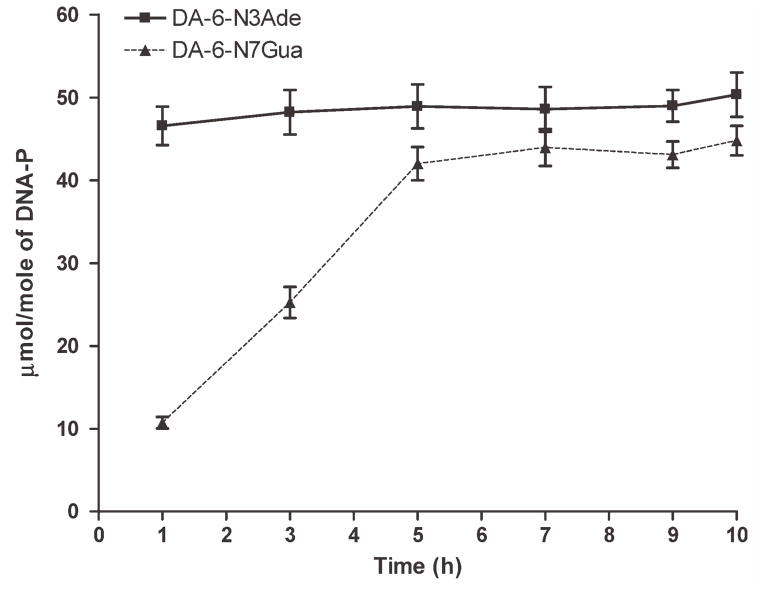
Once the conditions for the formation of depurinating DA adducts were optimized, we conducted a time-course study by reacting tyrosinase-activated DA with calf thymus DNA at pH 5. As shown in Fig. 3, the level of the N3Ade depurinating adduct (47 ± 5 μmol/mol DNA-P) reached its maximum in 1 H of incubation and after that it remained constant. On the other hand, the level of the N7Gua adduct gradually increased with time, starting with 11 ± 1 μmol/mol DNA-P at 1 H and reaching a maximum of 44 ± 4 μmol/mol DNA-P at about 5 H (Fig. 3). The half-life of DA-6-N7dG was approximately 3 H. This effect is analogous to all of our previous studies of the natural and synthetic estrogens, in which the N7Gua adduct depurinates at a slower rate than the N3Ade adduct, which depurinates instantaneously (2, 7–9).
Figure 3.
Time course of formation and depurination of the adducts DA-6-N3Ade and DA-6-N7Gua after reaction of tyrosinase-activated DA with DNA.
Preventing Effects of Antioxidants on the Formation of Depurinating Adducts of Dopamine
To prevent the formation of depurinating adducts in the reaction of tyrosinase-activated DA with DNA, we selected four natural antioxidant compounds: dihydrolipoic acid, melatonin, NAcCys, and resveratrol. To ascertain to what extent these four selected compounds can efficiently inhibit the oxidation of DA to DA-Q and/or reaction of the latter with DNA, various ratios (1:0, 1:0.1, 1:0.3, 1:1, or 1:3) of these compounds to DA were used with a constant DA concentration (87 μM).
Reduced lipoic acid (dihydrolipoic acid)
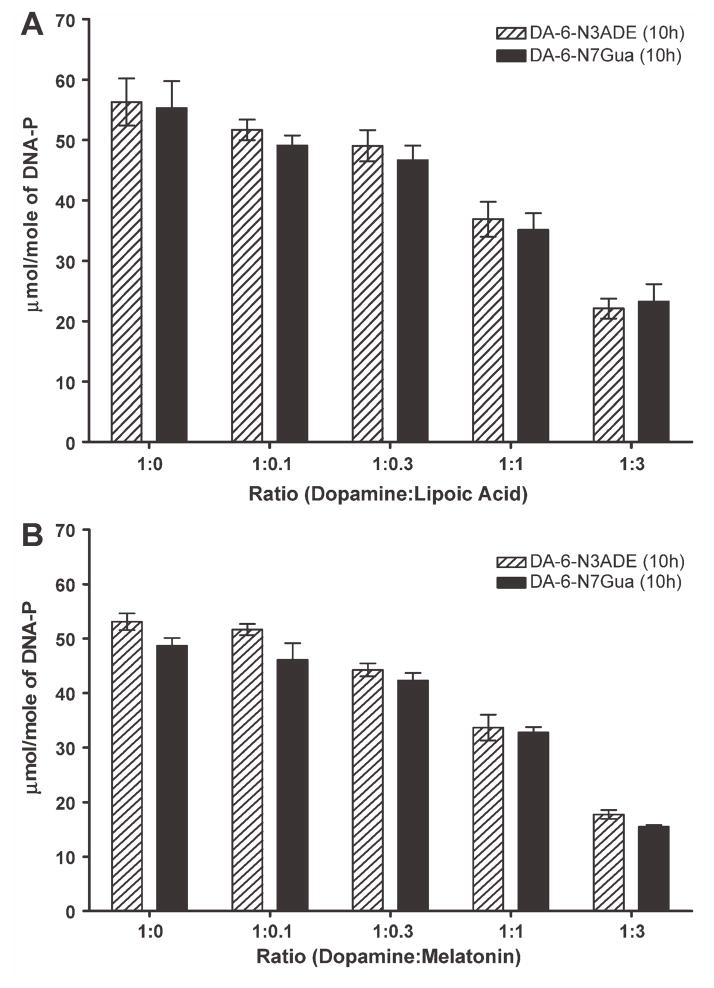
We used dihydrolipoic acid, because lipoic acid exerts its biological actions mainly when it is in the reduced form, which is obtained after ingestion of lipoic acid. Due to the presence of two sulfhydryl groups, the properties of this compound may resemble those of NAcCys for in vitro reactions. Because DA-Q is not stable due to its intramolecular cyclization, we used only tyrosinase-activated DA in this study. Dihydrolipoic acid could prevent the reaction of DA-Q with DNA by reacting with the quinone. The ability of dihydrolipoic acid to inhibit formation of depurinating DA adducts was nominal at a 1:0.1 ratio, where it showed 8% inhibition for N3Ade and 11% for N7Gua (Fig. 4A), while this inhibitory effect increased with a higher ratio of lipoic acid. At a 1:1 ratio, the inhibition was 34% for N3Ade and 36% for N7Gua, and it reached its maximum at a 1:3 ratio with 61% inhibition for N3Ade and 58% for N7Gua (Fig. 4A).
Figure 4.
Effect of (A) lipoic acid or (B) melatonin on the formation of depurinating adducts in the reaction of tyrosinase-activated DA with DNA.
Melatonin
The main antioxidant property of melatonin occurs via reduction of semiquinones (10). We observed its antioxidant effect at all ratios when tyrosinase-activated DA was incubated with DNA: approximately 4% inhibition at 1:0.1, 15% inhibition at 1:0.3, 34% inhibition at 1:1, and 67% inhibition at 1:3 (Fig. 4B).
N-Acetylcysteine
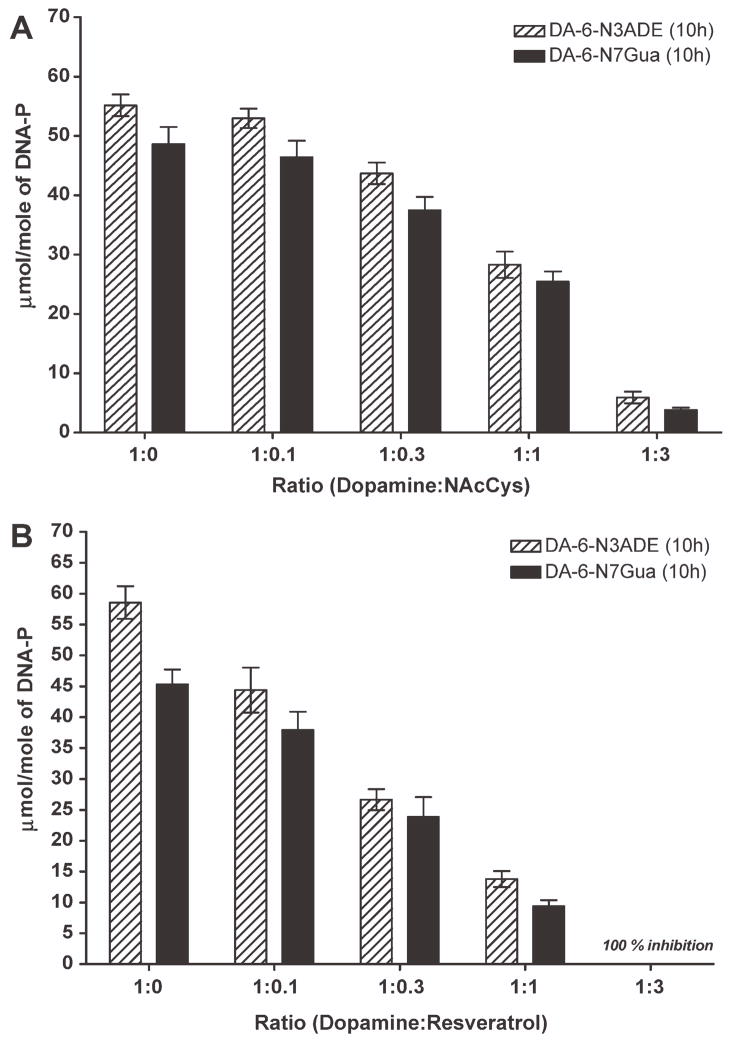
This compound has chemical properties similar to those of lipoic acid, and its predicted inhibitory effects occur in the reduction of DA semiquinone to DA and, second, the reaction of the antioxidant with DA-Q. At lower ratios, such as 1:0.1 or 1:0.3, NAcCys showed inhibitory effects on the formation of depurinating DA adducts of about 4.5% and 22%, respectively (Fig. 5A). When the ratio of NAcCys increased with respect to DA, the inhibitory effect was much larger: at a 1:1 ratio the inhibitory effect was 49% and at a ratio of 1:3 the inhibition of depurinating DA adducts was 89–92% (Fig. 5A).
Figure 5.
Effect of (A) NAcCys or (B) resveratrol on the formation of depurinating adducts in the reaction of tyrosinase-activated DA with DNA.
Resveratrol
Like melatonin, resveratrol does not react with the ortho-quinone of DA to form a conjugate, but because of the high energy of resonance in its oxidized form it reduces the intermediate of DA semiquinone to its catechol very effectively (11, 12). When tyrosinase-activated DA was reacted with calf-thymus DNA in the presence of different ratios of resveratrol, it showed a very promising inhibitory effect on the formation of depurinating DA adducts at all ratios. At a 1:0.1 ratio, the inhibition of adducts was 24%, which increased to 54% when the ratio was 1:0.3. This inhibitory effect was much better at a 1:1 ratio, where inhibition of N3Ade was 76% and N7Gua was 79%, respectively. When resveratrol was present at a ratio three times that of DA, the inhibition was 100% for both adducts (Fig. 5B).
DISCUSSION
One of the important functions of the neurotransmitter DA is the synthesis of neuromelanin. This occurs by oxidation of DA to its ortho-quinone, followed at neutral pH by intramolecular cyclization of the nucleophilic amino group via 1,4-Michael addition (Fig. 1). The product obtained, leukochrome, is further oxidized to aminochrome, which polymerizes to neuromelanin. When the pH of the reaction decreases to 6 or below, a competitive intermolecular 1,4-Michael addition between DA-Q and DNA can occur, with formation of the depurinating adducts, DA-6-N3Ade and DA-6-N7Gua (Figs. 1 and 2). The apurinic sites formed in the DNA can generate mutations that, we hypothesize, could lead, via several steps, to neurodegeneration (Fig. 1).
Formation of DA-DNA adducts was greatest at pH 5 (Fig. 2). A recent report showed that an excessive amount of glutamate, as it occurs in excitotoxicity, can give rise to a stable pH of 5.5 during glutamate and DA corelease from synaptic vesicles (3). Mitochondria are dense in presynaptic regions and mitochondrial DNA (mtDNA) encodes 7 of 49 proteins in Complex I of the electron transport chain. Complex I is disrupted in Parkinson’s disease (13, 14), and inhibitors of Complex I (MPTP, trichlorethylene, paraquat, rotenone) result in many symptoms of Parkinson’s disease in animal and human studies (15–18). We hypothesize that DA-Q-generated mutations in mtDNA could, potentially, disrupt Complex I. Therefore, our next experimental steps should assess the concentrations of DA-Q formed intracellularly in the presence of compounds that cause excitotoxicity and determine whether DA-Q crosses the mitochondrial membranes to react with mtDNA as in Fig. 1.
The antioxidants reduced lipoic acid and melatonin demonstrated weak activity in blocking formation of the DA-DNA adducts (Figs. 4A and 4B), although lipoic acid has been found to reduce neurodegenerative progression in Parkinson’s disease (19). NAcCys and resveratrol were previously found to block formation of the depurinating estrogen-DNA adducts (12, 20–22) and transformation of human and mouse breast epithelial cells (12, 23), suggesting that these two antioxidants could be used as cancer-preventing agents. Similarly, NAcCys and resveratrol efficiently block formation of depurinating DA adducts formed by the reaction of tyrosinase-activated DA with DNA (Figs. 5A and 5B). NAcCys was already shown to block DA-induced apoptosis in N27 rat dopaminergic cells (24) and increase Complex I activity in aged mice (25). Resveratrol has been suggested as a therapeutic agent for Parkinson’s and other neurodegenerative diseases (26).
CONCLUSIONS
Slightly acidic conditions have been found to have the ability to render the conversion of DA to neuromelanin less efficient and the reaction of DA-Q with DNA to form depurinating DA-DNA adducts competitive (Fig. 1). Formation of adducts could lead to mutations in critical genes to initiate the series of events leading to neurodegenerative diseases such as Parkinson’s disease. If this hypothesized mechanism is correct, use of specific antioxidants, such as NAcCys and resveratrol, as dietary supplements may be a strategy to prevent initiation and development of neurodegenerative diseases.
Acknowledgments
This research was supported by Prevention LLC. Core support at the Eppley Institute was provided by grant CA P30 36727 from the National Cancer Institute.
Abbreviations
- Boc
di-tert-butyl-dicarbonate
- DA
dopamine
- DA-Q
dopamine quinone
- dG
2′-deoxyguanosine
- DNA-P
DNA-phosphate
- ESI
electrospray ionization
- NacCys
N-acetylcysteine
- PI
positive ion
- TFA
trifluoroacetic acid
- UPLC-MS/MS
ultraperformance liquid chromatography/tandem mass spectrometry
References
- 1.Cavalieri EL, Li KM, Balu N, Saeed M, Devanesan P, et al. Catechol ortho-quinones: the electrophilic compounds that form depurinating DNA adducts and could initiate cancer and other diseases. Carcinogenesis. 2002;23:1071–1077. doi: 10.1093/carcin/23.6.1071. [DOI] [PubMed] [Google Scholar]
- 2.Zahid M, Saeed M, Rogan EG, Cavalieri EL. Benzene and dopamine catechol quinones could initiate cancer or neurogenic disease. Free Radic Biol Med. 2010;48:318–324. doi: 10.1016/j.freeradbiomed.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hnasko TS, Chuhma N, Zhang H, Goh GY, Sulzer D, et al. Vesicular glutamate transport promotes dopamine storage and glutamate corelease in vivo. Neuron. 2010;65:643–656. doi: 10.1016/j.neuron.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalieri EL, Rogan EG. Depurinating estrogen-DNA adducts in the etiology and prevention of breast and other human cancers. Future Oncol. 2010;6:75–91. doi: 10.2217/fon.09.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakravarti D, Mailander PC, Li KM, Higginbotham S, Zhang HL, et al. Evidence that a burst of DNA depurination in SEN-CAR mouse skin induces error-prone repair and forms mutations in the H-ras gene. Oncogene. 2001;20:7945–7953. doi: 10.1038/sj.onc.1204969. [DOI] [PubMed] [Google Scholar]
- 6.Mailander PC, Meza JL, Higginbotham S, Chakravarti D. Induction of A.T to G.C mutations by erroneous repair of depurinated DNA following estrogen treatment of the mammary gland of ACI rats. J Steroid Biochem Mol Biol. 2006;101:204–215. doi: 10.1016/j.jsbmb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Saeed M, Zahid M, Gunselman SJ, Rogan E, Cavalieri E. Slow loss of deoxyribose from the N7deoxyguanosine adducts of estradiol-3,4-quinone and hexestrol-3′,4′-quinone. Implications for mutagenic activity. Steroids. 2005;70:29–35. doi: 10.1016/j.steroids.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Zahid M, Kohli E, Saeed M, Rogan E, Cavalieri E. The greater reactivity of estradiol-3,4-quinone versus estradiol-2,3-quinone with DNA in the formation of depurinating adducts. Implications for tumor-initiating activity. Chem Res Toxicol. 2006;19:164–172. doi: 10.1021/tx050229y. [DOI] [PubMed] [Google Scholar]
- 9.Saeed M, Rogan E, Cavalieri E. Mechanism of metabolic activation and DNA adduct formation by the human carcinogen diethylstilbestrol: the defining link to natural estrogens. Int J Cancer. 2009;124:1276–1284. doi: 10.1002/ijc.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ianas O, Olivescu R, Badescu I. Melatonin involvement in oxidative processes. Rom J Endocrinol. 1991;29:117–123. [PubMed] [Google Scholar]
- 11.Stivala LA, Savio M, Carafoli F, Perucca P, Bianchi L, et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J Biol Chem. 2001;276:22586–22594. doi: 10.1074/jbc.M101846200. [DOI] [PubMed] [Google Scholar]
- 12.Lu F, Zahid M, Wang C, Saeed M, Cavalieri EL, et al. Resveratrol prevents estrogen-DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev Res. 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim Biophys Acta. 2009;1792:651–653. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, et al. Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun. 1989;163:1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- 16.Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, et al. Mitochrondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- 17.Gash DM, Rutland K, Hudson NL, Sullivan PG, Bing G, et al. Trichloroethylene: Parkinsonism and complex I mitochondrial neurotoxicity. Ann Neurol. 2008;63:184–192. doi: 10.1002/ana.21288. [DOI] [PubMed] [Google Scholar]
- 18.Peng J, Peng L, Stevenson FF, Doctrow SR, Andersen JK. Iron and paraquat as synergistic environmental risk factors in sporadic Parkinson’s disease accelerate age-related neurodegeneration. J Neurosci. 2007;27:6914–6922. doi: 10.1523/JNEUROSCI.1569-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Araujo DP, de Lobato RF, Cavalcanti JR, Sampaio LR, Araujo PV, et al. The contributions of antioxidant activity of lipoic acid in reducing neurogenerative progression of Parkinson’s disease: a review. Int J Neurosci. 2011;121:51–57. doi: 10.3109/00207454.2010.535934. [DOI] [PubMed] [Google Scholar]
- 20.Zahid M, Gaikwad N, Ali MF, Lu F, Saeed M, et al. Prevention of estrogen-DNA adducts in MCF-10F cells by resveratrol. Free Radic Biol Med. 2008;45:136–145. doi: 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zahid M, Saeed M, Ali MF, Rogan EG, Cavalieri EL. N-acetylcysteine blocks formation of cancer-initiating estrogen-DNA adducts in cells. Free Radic Biol Med. 2010;49:392–400. doi: 10.1016/j.freeradbiomed.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zahid M, Saeed M, Beseler C, Rogan EG, Cavalieri EL. Resveratrol and N-acetylcysteine block the cancer-initiating step in MCF-10F cells. Free Radic Biol Med. 2011;50:78–85. doi: 10.1016/j.freeradbiomed.2010.10.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venugopal D, Zahid M, Mailander PC, Meza JL, Rogan EG, et al. Reduction of estrogen-induced transformation of mouse mammary epithelial cells by N-acetylcysteine. J Steroid Biochem Mol Biol. 2008;109:22–30. doi: 10.1016/j.jsbmb.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zafar KS, Inayat-Hussain SH, Ross D. A comparative study of proteasomal inhibition and apoptosis induced in N27 mesencephalic cells by dopamine and MG132. J Neurochem. 2007;102:913–921. doi: 10.1111/j.1471-4159.2007.04637.x. [DOI] [PubMed] [Google Scholar]
- 25.Banaclocha MM. N-Acetylcysteine elicited increase in complex I activity in synaptic mitochondria from aged mice: implications for treatment of Parkinson’s disease. Brain Res. 2000;859:173–175. doi: 10.1016/s0006-8993(00)02005-9. [DOI] [PubMed] [Google Scholar]
- 26.Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–383. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]