Abstract
After giving birth, women typically experience decreased sexual desire and increased responsiveness to infant stimuli. These postpartum changes may be viewed as a trade-off in reproductive interests, which could be due to alterations in brain activity including areas associated with reward. The goal of this study was to describe the roles of oxytocin and parity on reward area activation in response to reproductive stimuli, specifically infant and sexual images. Because they have been shown to be associated with reward, the ventral tegmental area (VTA) and nucleus accumbens (NAc) were targeted as areas of expected alterations in activity. Oxytocin was chosen as a potential mediator of reproductive trade-offs because of its relationship to both mother–infant interactions, including breastfeeding and bonding, and sexual responses. We predicted that postpartum women would show higher reward area activation to infant stimuli and nulliparous women would show higher activation to sexual stimuli and that oxytocin would increase activation to infant stimuli in nulliparous women. To test this, we measured VTA and NAc activation using fMRI in response to infant photos, sexual photos, and neutral photos in 29 postpartum and 30 nulliparous women. Participants completed the Sexual Inhibition (SIS) and Sexual Excitation (SES) Scales and the Brief Index of Sexual Function for Women (BISF-W), which includes a sexual desire dimension, and received either oxytocin or placebo nasal spray before viewing crying and smiling infant and sexual images in an fMRI scanner. For both groups of women, intranasal oxytocin administration increased VTA activation to both crying infant and sexual images but not to smiling infant images. We found that postpartum women showed lower SES, higher SIS, and lower sexual desire compared to nulliparous women. Across parity groups, SES scores were correlated with VTA activation and subjective arousal ratings to sexual images. In postpartum women, sexual desire was positively correlated with VTA activation to sexual images and with SES. Our findings show that postpartum decreases in sexual desire may in part be mediated by VTA activation, and oxytocin increased activation of the VTA but not NAc in response to sexual and infant stimuli. Oxytocin may contribute to the altered reproductive priorities in postpartum women by increasing VTA activation to salient infant stimuli.
Keywords: Postpartum, Oxytocin, Ventral tegmental area, Nucleus accumbens, Reward, Sexual and infant stimuli
Introduction
In species requiring a large amount of parental investment after birth (e.g., humans), it is assumed to be advantageous for the female to modify reproductive interests from a focus on sex to a focus on infants in order to maximize offspring care. After giving birth, postpartum women have shown increased neural responsiveness to infant stimuli, particularly to their own infant (Purhonen et al., 2001; Seifritz et al., 2003) and a decrease in sexual desire (Botros et al., 2006; De Judicibus and McCabe, 2002; Fischman et al., 1986; Glazener, 1997; Rupp et al., 2013). These changes may be viewed as a trade-off in reproductive interests. However, little is known about the mechanisms that contribute to these changes in humans. While complex neuroendocrinological factors may contribute to this shift, the effect of oxytocin on reward areas is examined here due to the hormone’s role in both parental responsiveness to infants and sexual responses (Carter et al., 2007).
Oxytocin is a key mediator of social, including parental and sexual, motivation (Gordon et al., 2011). It is known to play a role in a number of mother–infant interactions including breastfeeding (Altemus, 1995; Carter et al., 2001) and mother–infant bonding (Carter, 1998; Feldman, 2012), and may be involved in postpartum women’s increased responsiveness to infant stimuli (Carter et al., 2007; Galbally et al., 2011; Strathearn, 2011). Breastfeeding, which releases oxytocin, is associated with greater brain activation to own infant cries in postpartum women (Kim et al., 2011).
Oxytocin is also involved in the human sexual response, both centrally and peripherally (Borrow and Cameron, 2012). Plasma oxytocin levels are related to peripheral sexual arousal in women (Salonia et al., 2005) and are elevated after orgasm (Blaicher et al., 1999). Centrally, regions responsible for oxytocin production, such as the paraventricular nucleus, and regions receiving projections from those areas, including the periaqueductal gray, activate during orgasm (Borrow and Cameron, 2012).
The mesolimbic system is rich in oxytocin receptors (Loup et al., 1991). This system, including the ventral tegmental area (VTA) that sends dopaminergic projections to the nucleus accumbens (NAc), is important for reward and motivation (Carlezon and Thomas, 2009), including sexual and parental behaviors (O’Connell and Hofmann, 2011). Functional MRI studies have shown that these areas activate in response to infant (Glocker et al., 2009; Montoya et al., 2012) and sexual stimuli (Demos et al., 2012; Sabatinelli et al., 2007). Further, the mesolimbic system is thought to be critical in the evaluation of salient stimuli, leading the organism to the appropriate behavioral response (O’Connell and Hofmann, 2011), such as caregiving in the presence of an infant.
It is currently unknown whether postpartum women process rewarding reproductively relevant (e.g., infant, sexual) stimuli differently than nulliparous women. For example, infant stimuli may be more rewarding and stronger motivators for action in postpartum women than they are in nulliparous women. The reverse may be true for sexual stimuli. Brain activation may reflect this pattern which could potentially contribute to enhanced offspring care. Postpartum women have shown increased activation of reward and motivation areas during presentation of images of their own vs. other infants (Bartels and Zeki, 2004; Strathearn et al., 2008). In addition, the mesolimbic system facilitates maternal behavior in rodent models (D’Cunha et al., 2011; Numan, 2007; Olazabal and Young, 2006; Stolzenberg et al., 2010) and may play a role in maternal behaviors in humans (Rilling, 2013).
Although the NAc is known to respond to presentation of sexual images (Demos et al., 2012; Sabatinelli et al., 2007), the reactivity of reward areas to sexual stimuli in postpartum women has not previously been examined. The VTA (Frye and Rhodes, 2006; Frye et al., 2006) and NAc (Hedges et al., 2009; Pitchers et al., 2010) have also been shown to be involved in sexual behavior and reward in animal models. The mesolimbic system is involved in both sexual and parental behaviors and areas in this system activate in response to infant and sexual stimuli, which may be significant for reproductive priorities. Until this study, reward area activation in response to infant and sexual stimuli has not been compared in nulliparous and postpartum women.
Although both epidemiological and clinical methods have demonstrated that sexual desire decreases in postpartum women (Botros et al., 2006; De Judicibus and McCabe, 2002; Fischman et al., 1986; Glazener, 1997), potential changes in sexual inhibition and excitation during the postpartum period have not been explored. Sexual excitation and inhibition have been described as ‘dual control’ mechanisms, which regulate human sexual arousal and responsiveness (Bancroft and Janssen, 2000). The Sexual Inhibition and Excitation (SIS/SES) Scales (Janssen et al., 2002a,b) have been used to measure these mechanisms in several populations, but the present study is the first to use the SIS/SES in postpartum women.
The sample in the current study was used previously to examine subjective arousal and amygdala response to various stimuli types (Rupp et al., 2013; Rupp et al., 2014), based on the attempt to clarify the role of general arousal in postpartum women’s reproductive priorities. The current study explores the role of reward areas in response to infant and sexual stimuli and the effect of oxytocin on these areas. Previous findings from our lab demonstrated that nulliparous women experienced greater subjective (self-reported) general arousal to sexual images versus infant images, and postpartum women experienced greater arousal to infant images than did nulliparous women (Rupp et al., 2013). However, in the nulliparous sample, arousal to infant images was greater with oxytocin administration than without, such that their arousal ratings were the same as the postpartum sample. Postpartum compared to nulliparous women showed lower right amygdala activation to all image types (infant, sexual and neutral) regardless of oxytocin administration, suggesting that generalized decreased amygdala activation after parturition was not responsible for postpartum women’s increased subjective arousal to infant stimuli.
The present study attempted to further clarify the mechanisms involved in postpartum women’s altered arousal by examining reward areas of the brain. We compared NAc and VTA activation to infant and sexual images in postpartum and nulliparous women and investigated whether oxytocin modifies this activation. We predicted that new mothers would show increased activation compared to nulliparous women in reward areas such as the NAc and VTA to infant stimuli and nulliparous women would show higher activation to sexual stimuli than postpartum women. We also expected that oxytocin would mediate greater activation to infant images in nulliparous women as well as with their subjective arousal ratings of infant stimuli. Finally, we predicted that postpartum women would show decreased sexual excitation and increased sexual inhibition scores to account for their decrease in sexual desire.
Methods
Participants
Thirty nulliparous and 29 postpartum (16 primiparous, 13 multiparous) female participants were recruited through flyers, emails, and local organizations. We included only heterosexual women currently in relationships, aged 20–40 years, who were not depressed, using anti-depressants or other psychotropic medications, and not currently pregnant. Due to the strong magnetic fields of the MRI, women with magnetic life-support devices (e.g., pacemakers), metal prostheses or other metallic objects were prevented from participating in this study. Of the participants, 47 (25 postpartum) self-reported race/ethnicity as White, six (2 postpartum) reported Asian, three (all nulliparous) reported Black, one (postpartum) reported Hispanic/Latino, and two (one postpartum) chose ‘Other’. The mean age of nulliparous women was 23.8 years (SD = 3.74), which was significantly younger than the postpartum women who reported a mean age of 30.21 years (SD = 4.44; t57 = 6.01, p ≤ .001). Postpartum women also had higher weight (weight, lb, t53 = 2.16, p = .04; mean ± SD nulliparous = 138.21 ± 27.26 lb; mean ± SD postpartum = 153.72 ± 26.04 lb) and percent body fat (t53 = 3.59, p = .001; mean ± SD nulliparous = 25.84 ± 7.76; mean ± SD postpartum = 33.82 ± 8.89). Nulliparous and postpartum women did not differ in the number of hours of sleep they had during the previous night (t54 = 0.52, p = .61; mean ± SD nulliparous = 7.24 ± 1.46; mean ± SD postpartum = 7.45 ± 1.53). Postpartum women were included if they had given birth within the past 3–6 months and were primarily breastfeeding their infant (>75% breastfeeding vs. bottle; mean = 87.3%, SD = 17.5%). Participants were phone-screened for postpartum depression using the Edinburgh Postnatal Depression Scale (EPDS; Cox et al., 1987). Women were only eligible if they scored below 10 during the EPDS screening. This cutoff was chosen to be more inclusive of women prone to depression. All women reported that their health was ‘Good’ or ‘Excellent’ and health status did not differ between groups. Participants were assigned to the placebo or oxytocin nasal spray group in a double-blind procedure.
Procedure
All procedures of the study were approved by the university institutional review board in compliance with ethical treatment of human subject guidelines. After the initial phone screening and scheduling, the women were mailed a participant packet to complete at-home before the scheduled test session. This packet contained a consent form detailing the procedures of the study, as well as a questionnaire regarding demographics, health, menstrual cycle, motherhood, relationships with partners, and sexual function. The 45-item Sexual Inhibition/Sexual Excitation Scales (SIS/SES) Questionnaire for Women (Janssen et al., 2002a,b) was also included. The SIS includes two subscales; SIS1 measures fear of performance failure, and SIS2 measures external threats (e.g., risk of unwanted pregnancy, pain). The SES measures sexual excitation in response to situations including social interactions, visual stimuli, and sexual fantasy. Sexual behavior was assessed using The Brief Index of Sexual Function for Women (BISF-W), a 22-item questionnaire (Mazer et al., 2000). Seven dimensions were calculated from the BISF-W, which include thoughts/desires (D1), arousal (D2), frequency of sexual activity (D3), receptivity/initiation (D4), pleasure/ orgasm (D5), relationship satisfaction (D6), and problems affecting sexual function (D7). Higher scores on D1–D6 reflect higher sexual function. BISF-W scores for all dimensions are reported by Rupp et al. (2013) and only the desire dimension was used in this study.
Nulliparous participants were scheduled to come in for testing during the periovulatory phase of their menstrual cycle (days 8–16), to control for hormonal state and sexual desire. Participants were asked to abstain from sexual activity, alcohol, and tobacco use on the day of testing in order to remove confounding effects on the neural systems under investigation, which these behaviors are known to activate. Postpartum participants were asked to bring their infants to the test session, where childcare was provided. At the test session, postpartum women nursed their infants in a private room to enhance the comfort of mothers and infants and to control for possible changes in oxytocin that occur with breastfeeding (Altemus, 1995). Breastfeeding ended approximately 1 h and 15 min prior to fMRI scanning. All participants provided informed consent. Participants were then administered a paper version of the 10-item EPDS to confirm absence of depression (mean ± SD nulliparous = 5.42 ± 4.51; mean ± SD postpartum = 4.24 ± 2.36). Following the questionnaire, participants provided a small (20 mL) urine sample for a baseline oxytocin measurement. A second urine sample was collected following the scanning session. Urine samples were frozen at −20°C until shipment for assay by the Assay Services laboratory at the University of Wisconsin National Primate Research Center. The oxytocin assay was an enzyme linked immunosorbent assay kit used (Assay Designs. Cat no. 901-153). The antibody is specific to oxytocin and validation for human urine has been reported (Seltzer et al., 2010). Intra- and inter-coefficients of variation were determined by a human urine pool as 3.1 and 6.6, respectively. Urine oxytocin levels did not differ between parity groups (repeated-measures ANOVA, F1,55 = 1.64, p = .21; baseline mean ± SD nulliparous = 8.01 ± 14.65 pg/mL; baseline mean ± SD postpartum = 7.25 ± 3.07 pg/mL). There was an interaction of time and nasal spray (F1,55 = 10.16, p = .002) such that participants receiving oxytocin spray had higher oxytocin urine levels post-scan (mean ± SD = 56.12 ± 90.35 pg/mL) than those receiving placebo (mean ± SD = 6.89 ± 3.71 pg/mL).
Participants were then trained on the one-back matching task they would perform in the scanner. The task was chosen to ensure attention. Pictures used in the current study included sexually explicit, crying infant, smiling infant and neutral photos. Positive, negative and scrambled images were also presented but not analyzed in the current study. Neutral images were taken from the IAPS (Lang et al., 2005) and sexual and infant images were taken from publicly available websites. Sexual pictures consisted of sexual activities between heterosexual couples balanced for types of activity portrayed. The sexual photos used in this study have been used successfully elsewhere and are known to evoke sexual interest in women (Rupp and Wallen, 2007, 2009; Rupp et al., 2013).
After practicing the task, approximately 30 min prior to the first scan participants received either oxytocin or placebo nasal spray. Those in the oxytocin group received an absolute dose of 24 IU. The oxytocin nasal spray (Syntocinon®, Novartis Pharma, Switzerland) contains a synthetic nonapeptide. The placebo spray contained only the inactive carrier found in the oxytocin spray and is indistinguishable from the active spray (Meyer-Lindenber et al., 2011). The neuropeptide vasopressin, which is similar to oxytocin in structure, has been shown to enter the human central nervous system with intranasal administration (Born et al., 2002). Additionally, intranasal oxytocin administration has been shown to increase oxytocin levels in the cerebral spinal fluid of non-human primates (Chang et al., 2012), suggesting that central effects are possible.
Imaging
Imaging was performed on a Siemens Magnetom Trio 3 T whole body scanner (Siemens Healthcare, Erlangen, Germany). The one-hour MRI session includes seven whole-brain functional scans of blood oxygenation-level dependent (BOLD) imaging and one high-resolution whole-brain anatomical scan. Each of the seven functional runs was 5 min long, beginning with 12 s of rest to establish a stable baseline signal. Pictures of different categories (smiling infant, crying infant, sexual, positive, negative, neutral, and scrambled) were presented to the participants in random order (unique to each participant) using an in-house-made fMRI stimulus delivery system. Participants viewed a total of 56 images during each run, including 7 images from each of the categories above. Each stimulus was shown for 2 s with a variable inter-stimulus interval (ISI) of 2–6 s. The participants were requested to perform the one-back matching task. Participants then viewed the same stimuli as presented during the fMRI scanning on a laptop and rated them for how ‘aroused they make them feel’ (1–9). In the instructions we clarified that ‘arousal’ was not specifically sexual, but was more of an intensity measure of whatever emotion they were feeling. Data from the subjective ratings are reported in Rupp et al. (2013).
Imaging parameters and data analysis
Imaging data were processed in BrainVoyager™ QX 2.2. For each subject, the anatomical image was warped to the Talairach stereotactic space using an eight-parameter affine transformation (scaling, translation, and rotation). All functional images were motion corrected and re-aligned to a reference functional volume that was collected closest in time to the anatomical volume. Slice timing correction was then applied to all functional volumes, followed by 3-D spatial Gaussian filtering (FWHM 6 mm), and linear trend removal. After being co-registered to the corresponding anatomical volume, functional volumes were normalized to the Talairach stereotactic space based on the previous transformation of anatomical image. The functional data were re-sampled to 3 mm3 isometric resolution during normalization. Whole-brain general linear model (GLM) statistical analysis was performed on the functional data followed by ANCOVA random effect analysis. The activation map was overlaid on the average anatomical image of all the subjects.
Regions of interest (ROIs) were selected from the contrast map of negative stimuli versus fixation from GLM analysis, with the help of Talairach coordinates and neuroanatomical references. In the contrast map at p = 0.05 FDR corrected, we chose a cluster that corresponds to NAc or VTA and we selected a voxel with the strongest activation and used “Define Sphere ROI” tool to generate a ROI, which defines a spherical ROI around the voxel with 257 voxels. For NAc, we took into account the result from a recent paper (Neto et al., 2008) that the coordinate range of the right NAc is x: 3.7–15.1 mm; y: 0–11 mm; and z: −10.2–2.2 mm. The range served as the hard constraint for ROI selection for NAc. We were able to choose left NAc ROIs in the activation map while still within the anatomical constraint. We failed to do the same thing for the right NAc (due to a lack of activation there). So we decided to virtually map the left NAc to the right hemisphere. The centers of our new NAc ROIs end up with left: [−13 10 −8] mm and right: [11 10 −8] mm.
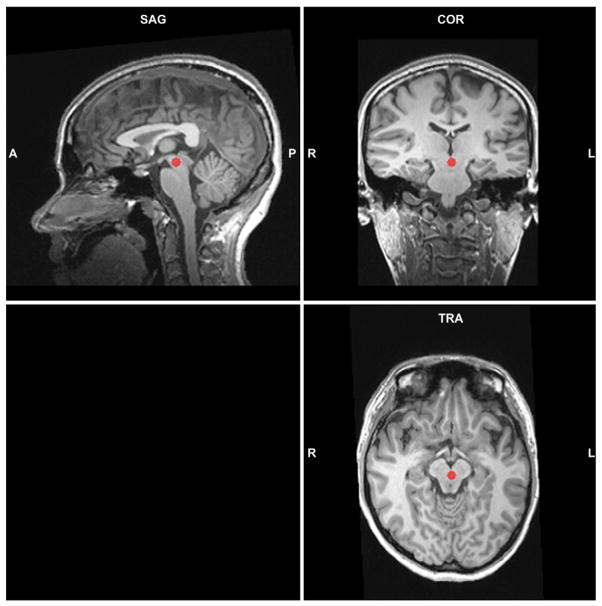
For VTA, the anatomical reference was from the paper of Murty et al. (2014). The average coordinates for the center of mass in MNI space for VTA were y = −15.9 mm and z = −13.9 mm, which were equivalent to Talairach coordinates of y = −16.1 mm and z = −11.0 mm. We chose the new ROI for VTA in the activation map but adjusted the center to [0 −20 −8] so that it was closest to the average coordinates of VTA of anatomical reference (Fig. 1). BOLD signal changes were extracted from group ROIs using the ANCOVA table tool in BrainVoyager’s volume of interest module.
Fig. 1.
Sagittal (SAG), coronal (COR), and horizontal (TRA) slices on the anatomical image of a subject showing functionally-defined VTA ROI determined from main effect of negative > fixation, collapsed across parity cohorts and spray condition. The Talairach coordinates are (0 −20 −8) with 257 voxels in total.
Multiple ANOVAs were performed with OT group (OT vs. placebo) and parity cohort as the between subjects factors. All statistical analyses were performed using SPSS 22 (IBM SPSS, Inc., IL). For our analyses, p-values less than 0.05 are considered significant. Effect size estimates are reported as eta-squared (η2) for ANOVAs and Cohen’s d for pairwise comparisons.
Results
ROIs
Imaging data were analyzed using activation change from baseline (fixation point). Imaging data from four postpartum participants were excluded due to motion artifacts and incomplete data. Excluded postpartum participants (N = 4) did not differ from included postpartum participants (N = 25) in age, weight, percent body fat, percent breast vs. bottle feeding, or hours of sleep (ps > .15).
VTA
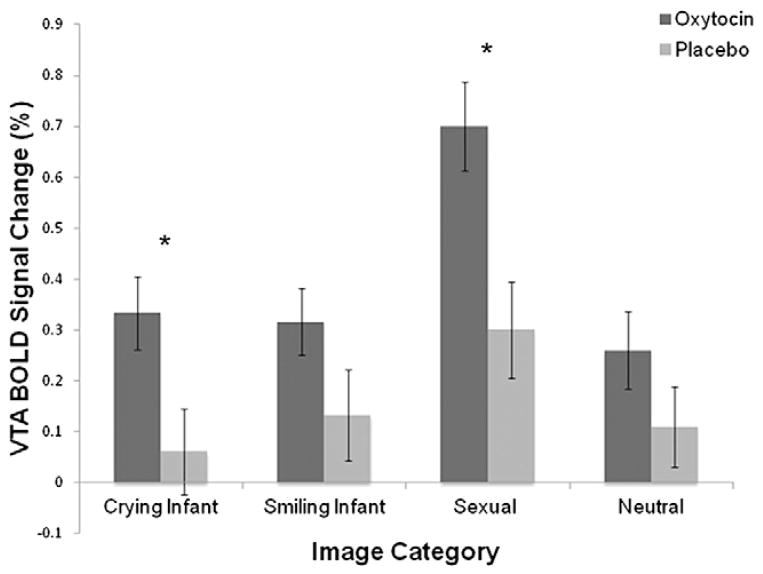
In the OT administration comparison, there was a main effect for crying infant (F1,51 = 5.45, p = .02, η2 = .10) and sexual images (F1,51 = 9.17, p = .004, η2 = .15; Fig. 2), such that women receiving OT nasal spray had higher VTA activation in response to both types of images. There was no main effect for the nasal spray group for neutral (F1,51 = 1.56, p = .22, η2 = .03) or smiling infant images (F1,51 = 2.56, p = .12, η2 = .05). There was no main effect of parity for neutral (F1,51 = 3.87, p = .06, η2 = .07), crying infant (F1,51 = 0.18, p = .67, η2 = .003), smiling infant (F1,51 = 2.05, p = .16, η2 = .04), or sexual (F1,51 = 0.013, p = .91, η2 = .0002) images. No interaction effects were observed for any image category (ps > .51).
Fig. 2.
VTA BOLD signal change as a function of image category and nasal spray group. There were significant main effects of nasal spray for crying infant and sexual images such that women receiving OT versus placebo showed higher VTA activation to both image types. Error bars indicate ±SE.
NAc
Contrary to expectations, there were no significant differences in the spray group or parity for any image category for the right NAc (all ps > .18) or the left NAc (ps > .17; Table 1).
Table 1.
Mean (SD) activation of VTA and left and right NAc in response to crying infant, smiling infant, sexual and neutral photos by parity and nasal spray group. Participants receiving OT nasal spray showed higher VTA activation for crying infant and sexual images. No significant differences were found for NAc.
| Nulliparous
|
Postpartum
|
|||
|---|---|---|---|---|
| OT (n = 14) | Placebo (n = 16) | OT (n = 13) | Placebo (n = 12) | |
| VTA | ||||
| Crying infant* | .35 (.39) | .009 (.44) | .32 (.37) | .13 (.45) |
| Smiling infant | .23 (.36) | .07 (.38) | .41 (.30) | .21 (.58) |
| Sexual** | .72 (.40) | .30 (.51) | .68 (.51) | .31 (.50) |
| Neutral | .15 (.42) | .03 (.37) | .38 (.34) | .23 (.47) |
| Left NAc | ||||
| Crying infant | −.05 (.46) | −.12 (.35) | −.14 (.39) | −.07 (.38) |
| Smiling infant | −.23 (.54) | −.13 (.30) | −.09 (.56) | −.19 (.35) |
| Sexual | −.09 (.50) | −.29 (.36) | −.34 (.54) | −.23 (.54) |
| Neutral | .0008 (.47) | −.23 (.44) | −.15 (.45) | −.26 (.42) |
| Right NAc | ||||
| Crying infant | .02 (.55) | −.08 (.39) | −.17 (.42) | −.16 (.53) |
| Smiling infant | −.37 (.55) | −.12 (.45) | −.27 (.58) | −.15 (.54) |
| Sexual | −.16 (.58) | −.19 (.35) | −.43 (.46) | −.20 (.59) |
| Neutral | .03 (.44) | −.10 (.40) | −.25 (.38) | −.12 (.42) |
Significant differences across nasal spray groups: *p ≤ .05; **p ≤ .01.
Questionnaires
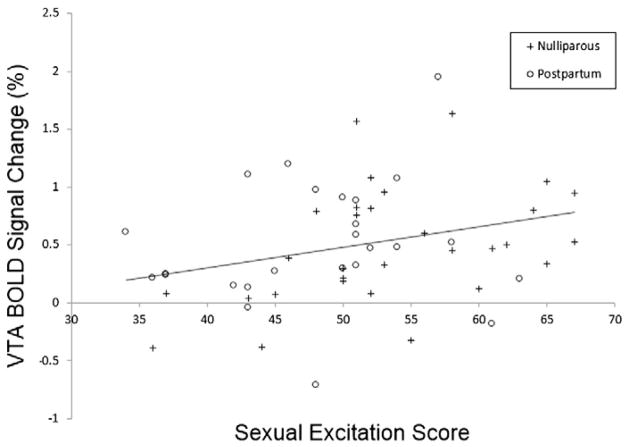
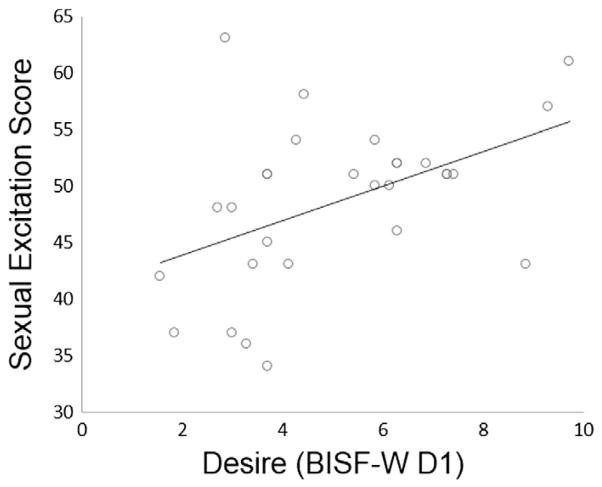
We also expected that postpartum women’s lower sexual desire could be related to changes in sexual inhibition and excitation. Postpartum women showed lower sexual desire than nulliparous women as indicated by the BISF-W D1 (Desire Scale; t57 = 4.77, p < .001, d = 1.24; mean ± SD nulliparous = 7.83 ± 2.2; mean ± SD postpartum = 5.11 ± 2.2). Postpartum women also scored lower than nulliparous women on the SES (t57 = 2.36, p = .02, d = .61; mean ± SD nulliparous = 53.4 ± 8.2; mean ± SD postpartum = 48.7 ± 7.2) and higher on the SIS1 (t57 = 2.41, p = .02, d = .63; mean ± SD nulliparous = 30.3 ± 3.9; mean ± SD postpartum = 33.1 ± 5.1) but not on the SIS2 (t57 = 1.27, p = .21, d = .33; mean ± SD nulliparous = 31.6 ± 3.8; mean ± SD postpartum = 32.8 ± 3.8). Thus, postpartum women indicated lower sexual excitation and greater sexual inhibition due to threat of performance failure. When both groups of women were combined, SES scores were significantly correlated with VTA activation to sexual images (N = 55, r = .29, p = .03; Fig. 3), subjective arousal ratings for sexual images (N = 56, r = .36, p = .006), and the Desire Scale (N = 59, r = .63, p < .001). Desire Scale scores were also positively correlated with VTA activation to sexual images (N = 55, r = .37, p = .006). SIS1 scores were negatively correlated with the Desire Scale (N = 59, r = −.26, p = .043). In postpartum women, sexual desire scores were correlated with VTA activation to sexual images (N = 25, r = .48, p = .01) and SES (N = 29, r = .47, p = .01; Fig. 4).
Fig. 3.
VTA BOLD signal change in response to sexual images in relation to sexual excitation score (SES). There was a significant positive relationship between VTA response and sexual excitation scores (N = 55, r = .29, p = .03).
Fig. 4.
Sexual desire correlated with SES. In postpartum women, sexual desire scores were positively correlated with SES (n = 29, r = .47, p = .01).
Discussion
Despite significant behavioral implications for postpartum women, reward area activation to infant and sexual stimuli has not been researched in this population. The goals of this study were to describe the roles of oxytocin and parity on reward area activation in response to reproductive stimuli, specifically infant and sexual images, and to compare SIS, SES and sexual desire in postpartum and nulliparous women. We predicted that postpartum women would show greater activation of the VTA and NAc in response to infant images and nulliparous women should show greater activation of these areas to sexual images. We also expected oxytocin to increase nulliparous women’s reward system activation to infant stimuli, due to the hormone’s role in mother–infant relationships and the increase in subjective arousal seen in nulliparous women receiving oxytocin (Rupp et al., 2013).
Previous work demonstrated that the postpartum women involved in this study showed lower amygdala activation to all stimuli types (Rupp et al., 2013). The authors suggested that an overall decrease in amygdala activation may buffer postpartum women from the stresses of new motherhood. Surprisingly, in the present study there were no effects of parity on reward area activation. Although reward areas in the maternal brain have been shown to activate in response to infant stimuli, especially their own infant (Swain, 2011), few studies have compared mothers and non-mothers. To our knowledge, our study is the first to describe VTA and NAc activation in postpartum and nulliparous women. The infants in the images shown in this study were unknown to the participants, which likely lowered the salience for the postpartum women and may have contributed to the lack of significant differences between the examined cohorts. Other studies have demonstrated stronger activation in regions related to emotion in mothers viewing photos of their own vs. other infants (Leibenluft et al., 2004) which was additionally associated with positive mood (Barrett et al., 2012; Nitschke et al., 2004). On the other hand, women of this age may be primed to respond similarly to general infant stimuli, since it fits with the reproductive life history phase they occupy. Future postpartum studies may benefit from using photos of the participants’ own infants, which for the nulliparous women would still be general infant stimuli, and examining the relationship between positive emotion, reward, and associated brain areas, including the orbitofrontal cortex, amygdala, and insula.
In this study, oxytocin administration resulted in greater VTA activation in both postpartum and nulliparous women to crying infant and sexual images. This is consistent with previous data showing that oxytocin mediated VTA activation to socially rewarding cues (Groppe et al., 2013). Higher VTA activation to crying, but not smiling, infant images may also reflect a greater motivation to approach and care for the infant, as oxytocin in the VTA has been shown to activate maternal caretaking behaviors in postpartum rats (Pedersen et al., 1994). In other areas such as the amygdala, oxytocin has been found to reduce activation to auditory infant stimuli (Riem et al., 2011, 2012), although oxytocin did not reduce amygdala activation to visual stimuli in our sample (Rupp, et al., 2013). Mode of presentation (audio versus visual) is likely to influence the effect of oxytocin on brain activation, in part because of the amount and quality of information communicated to the adult.
It was unexpected that oxytocin would increase VTA activation to sexual images as it did not increase subjective arousal to sexual images. However, intranasal oxytocin has been shown to improve sexual functions such as libido, erection and orgasm in males (MacDonald and Feifel, 2012) and is released during rewarding sexual activity (paced mating) in female rats (Nyuyki et al., 2011) and orgasm in humans (Blaicher et al., 1999). Oxytocin may play a role in the rewarding aspects of sex or at least in the processing of sexually explicit material. One study found that males receiving oxytocin experienced heightened arousal while viewing an erotic film and masturbating (Burri et al., 2008). It is possible that oxytocin does not cause the same increase in subjective arousal in females or its effects may only be perceived during more intense sexual stimulation (films) than was used in our study (slides).
It was expected that postpartum women would have higher SIS, lower SES and lower desire scores than nulliparous women. This hypothesis was supported for desire, SES and SIS1, but not SIS2. Low correlations have been found between age and SES and SIS1 (Janssen et al., 2002a), and age was not correlated with these measures in our sample. Therefore the differences seen in postpartum women in SES and SIS1 are not likely due to their greater age.
Postpartum women had higher SIS1 scores, measuring sexual inhibition based on threats related to sexual performance and functioning (Janssen et al., 2002a). Postpartum women may be particularly concerned about pleasing their partner after going through the physical changes that accompany parturition and the typical exclusion of sexual interactions in late pregnancy. Postpartum women did not have higher inhibition scores due to external threats such as risk of being caught, unwanted pregnancy, or pain, which suggests that these factors play similar roles in this sample of postpartum and nulliparous women’s sexual arousal. Sexual excitation scores were also lower in postpartum women, supporting past data and the finding that postpartum women were less likely to become aroused in the presence of sexual stimuli and reported lower arousal ratings for sexual images.
SES and desire scores were modestly positively correlated with VTA activation, so lower sexual desire in postpartum women may in part be mediated by VTA activation in response to sexual stimuli. SES scores were also correlated with subjective arousal ratings of sexual images. This fits with our expectations, as the SES includes items about arousal in response to visual sexual stimuli. Future longitudinal research examining SIS/SES and sexual desire in women before and after pregnancy would improve our understanding of the changes that take place after parturition, and within-subjects designs would be useful in documenting actual multilevel complex changes in the neuroendocrinological profiles of participants.
One limitation of the current study is that women were asked about their arousal level and not about how rewarding or pleasurable they found each image. Information on subjective reward and whether the women felt motivation to care for the presented infants would be helpful in determining the relationship between these factors and oxytocin administration and mesolimbic system activity. It is not known to what extent these women perceived the images they were viewing as positive. While a sexual or infant image may be specifically or generally arousing, it is not necessarily rewarding. An additional limitation is that the women viewed several types of stimuli, only some of which were analyzed here. The complexity of the experimental design may have impacted the specific stimuli affects being measured. Finally, the study was limited by the lack of existing data on oxytocin levels after breastfeeding. The rate at which oxytocin returns to baseline levels is unclear, as results vary based on the method of assessment (blood vs. saliva vs. urine; Stuebe et al., 2013; White-Traut et al., 2009) and measurement has not extended past 30 min post-breastfeeding.
Conclusion
In sum, our findings contribute new data regarding the role of oxytocin on reward area activation to sexual and infant stimuli in postpartum and nulliparous women. We demonstrated that exogenous oxytocin appears to influence a reward area, specifically VTA, activation in response to reproductive stimuli. Oxytocin may influence maternal behavior by increasing VTA activation to salient infant stimuli, potentially leading to greater motivation to approach and care for infants. However, oxytocin also increased VTA activation in response to sexual stimuli. This research is the first to show that changes in sexual excitation and inhibition may contribute to the decreased sexual desire often experienced by postpartum women, and that these changes may in part be mediated by VTA activation in response to sexual stimuli. These results add to our understanding of the mechanisms that underlie the altered reproductive priorities in postpartum women, and encourage further hypothesis-driven research. Such work may uncover factors that influence optimal infant care, as well as informing cases, such as postpartum depression, in which care may be compromised.
Acknowledgments
This study was supported by grant #NIMHR21MH082925 to J. Heiman and H. Rupp. The authors would like to thank Diane Ebling at the Indiana University Health Center for safety monitoring of the nasal spray. We are grateful to Bloomington Area Birth Services for the help with recruitment. Colleague Tom James was valuable in developing fMRI methods and technology. We thank the following for assistance with data collection: L. Reckley, B. Keller, C. Anderson, C. White, and Q. Class. We thank Toni Ziegler and the Assay Services laboratory at the University of Wisconsin National Primate Research Center for completing our hormone analysis. Finally we want to thank the MR operators B. Ward and T. Atwood.
References
- Altemus M. Neuropeptides in anxiety disorders — effects of lactation. Ann N Y Acad Sci. 1995;771:697–707. doi: 10.1111/j.1749-6632.1995.tb44721.x. [DOI] [PubMed] [Google Scholar]
- Bancroft J, Janssen E. The dual control model of male sexual response: a theoretical approach to centrally mediated erectile dysfunction. Neurosci Biobehav Rev. 2000;24:571–579. doi: 10.1016/s0149-7634(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Barrett J, Wonch KE, Gonzales A, Ali N, Steiner M, Hall GB, Fleming AS. Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Soc Neurosci. 2012;7:252–268. doi: 10.1080/17470919.2011.609907. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Blaicher W, Gruber D, Bieglmayer C, Blaicher AM, Knogler W, Huber JC. The role of oxytocin in relation to female sexual arousal. Gynecol Obstet Investig. 1999;47:125–126. doi: 10.1159/000010075. [DOI] [PubMed] [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Borrow AP, Cameron NM. The role of oxytocin in mating and pregnancy. Horm Behav. 2012;61:266–276. doi: 10.1016/j.yhbeh.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Botros SM, Abramov Y, Miller JJR, Sand PK, Gandhi S, Nickolov A, Goldberg RP. Effect of parity on sexual function: an identical twin study. Obstet Gynecol. 2006;107:765–770. doi: 10.1097/01.AOG.0000207677.03235.76. [DOI] [PubMed] [Google Scholar]
- Burri A, Heinrichs M, Schedlowski M, Kruger THC. The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology. 2008;33:591–600. doi: 10.1016/j.psyneuen.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56:122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the postpartum period. Prog Brain Res. 2001;133:241–249. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Oxytocin: behavioral associations and potential as a salivary bio-marker. Ann N Y Acad Sci. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Chang SWC, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- D’Cunha TM, King SJ, Fleming AS, Lévy F. Oxytocin receptors in the nucleus accumbens shell are involved in the consolidation of maternal memory in postpartum rats. Horm Behav. 2011;59:14–21. doi: 10.1016/j.yhbeh.2010.09.007. [DOI] [PubMed] [Google Scholar]
- De Judicibus MA, McCabe MP. Psychological factors and the sexuality of pregnant and postpartum women. J Sex Res. 2002;39:94–103. doi: 10.1080/00224490209552128. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32:5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Horm Behav. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Fischman SH, Rankin EA, Soeken KL, Lenz ER. Changes in sexual relationships in postpartum couples. J Obstet Gynecol Neonatal Nurs. 1986;15:58–63. doi: 10.1111/j.1552-6909.1986.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME. Infusions of 5a-Pregnan-3a-Ol-20-One (3a,5a-THP) to the ventral tegmental area, but not the substantia nigra, enhance exploratory, anti-anxiety, social and sexual behaviours and concomitantly increase 3a,5a-THP concentrations in the hippocampus, diencephalon and cortex of ovariectomised oestrogen-primed rats. J Neuroendocrinol. 2006;18:960–975. doi: 10.1111/j.1365-2826.2006.01494.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Rhodes ME, Petralia SM, Walf AA, Sumida K, Edinger KL. 3a-Hy-droxy-5a-pregnan-20-one in the midbrain ventral tegmental area mediates social, sexual, and affective behaviors. Neuroscience. 2006;138:1007–1014. doi: 10.1016/j.neuroscience.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbally M, Lewis AJ, van IJzendoorn M, Permezel M. The role of oxytocin in mother–infant relations: a systematic review of human studies. Harv Rev Psychiatry. 2011;19:1–14. doi: 10.3109/10673229.2011.549771. [DOI] [PubMed] [Google Scholar]
- Glazener CMA. Sexual function after childbirth: women’s experiences, persistent morbidity and lack of professional recognition. Br J Obstet Gynaecol. 1997;104:330–335. doi: 10.1111/j.1471-0528.1997.tb11463.x. [DOI] [PubMed] [Google Scholar]
- Glocker ML, Langleben DD, Ruparel K, Loughead JW, Valdez JN, Griffin MD, Sachser N, Gur RC. Baby schema modulates the brain reward system in nulliparous women. Proc Natl Acad Sci. 2009;106:9114–9119. doi: 10.1073/pnas.0811620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Martin C, Feldman R, Leckman JF. Oxytocin and social motivation. Dev Cogn Neurosci. 2011;1:471–493. doi: 10.1016/j.dcn.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppe SE, Gossen A, Rademacher L, Hahn A, Westphal L, nder GG, Spreckelmeyer KN. Oxytocin influences processing of socially relevant cues in the ventral tegmental area of the human brain. Biol Psychiatry. 2013;74:172–179. doi: 10.1016/j.biopsych.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Hedges VL, Chakravarty S, Nestler EJ, Meisel RL. FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes Brain Behav. 2009;8:442–449. doi: 10.1111/j.1601-183X.2009.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen E, Vorst H, Finn P, Bancroft J. The sexual inhibition (SIS) and sexual excitation (SES) scales: I. Measuring sexual inhibition and excitation proneness in men. J Sex Res. 2002a;39:114–126. doi: 10.1080/00224490209552130. [DOI] [PubMed] [Google Scholar]
- Janssen E, Vorst H, Finn P, Bancroft J. The sexual inhibition (SIS) and sexual excitation (SES) scales: II. Predicting psychophysiological response patterns. J Sex Res. 2002b;39:127–132. doi: 10.1080/00224490209552131. [DOI] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J Child Psychol Psychiatry. 2011;52:907–915. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. University of Florida; Gainesville: 2005. International Affective Picture System (IAPS): affective ratings of pictures and instruction manual. [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mothers’ neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 1991;555:220–232. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- MacDonald K, Feifel D. Dramatic improvements in sexual function induced by intranasal oxytocin. J Sex Med. 2012;9:1407–1410. doi: 10.1111/j.1743-6109.2012.02703.x. [DOI] [PubMed] [Google Scholar]
- Mazer NA, Leiblum SR, Rosen RC. The Brief Index of Sexual Functioning for Women (BISF-W): a new scoring algorithm and comparison of normative and surgically menopausal populations. Menopause. 2000;7:350–363. doi: 10.1097/00042192-200007050-00009. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsh P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature. 2011;12:524–535. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Montoya JL, Landi N, Kober H, Worhunsky PD, Rutherford HJV, Mencl E, Mayes LC, Potenza MN. Regional brain responses in nulliparous women to emotional infant stimuli. PLoS One. 2012;7:1–11. doi: 10.1371/journal.pone.0036270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA. Resting state networks distinguish human ventral tegmental area from substantia nigra. NeuroImage. 2014;100:580–589. doi: 10.1016/j.neuroimage.2014.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto LL, Oliveira E, Correia F, Ferreira AG. The human nucleus accumbens: where is it? A stereotactic, anatomical and magnetic resonance imaging study. Neuromodulation. 2008;11:13–22. doi: 10.1111/j.1525-1403.2007.00138.x. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. NeuroImage. 2004;21:583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Nyuyki KD, Waldherr M, Baeuml S, Neumann ID. Yes, I am ready now: differential effects of paced versus unpaced mating on anxiety and central oxytocin release in female rats. PLoS One. 2011;6:e23599. doi: 10.1371/journal.pone.0023599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J Comp Neurol. 2011;519:3599–3639. doi: 10.1002/cne.22735. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. FosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav. 2010;9:831–840. doi: 10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purhonen M, Kilpelainen-Lees R, Paakkonen A, Ypparila H, Lehtonen J, Karhu J. Effects of maternity on auditory event-related potentials to human sound. Neuroreport. 2001;12:2975–2979. doi: 10.1097/00001756-200109170-00044. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, van Ijzendoorn MH, Rombouts SA. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol Psychiatry. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Riem MM, van IMH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ. No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology. 2012;37:1257–1266. doi: 10.1038/npp.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK. The neural and hormonal bases of human parental care. Neuropsychologia. 2013;51:731–747. doi: 10.1016/j.neuropsychologia.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Rupp HA, Wallen K. Sex differences in viewing sexual stimuli: an eye tracking study in men and women. Horm Behav. 2007;51:524–533. doi: 10.1016/j.yhbeh.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Rupp HA, Wallen K. Sex-specific content preferences for visual sexual stimuli. Arch Sex Behav. 2009;38:417–426. doi: 10.1007/s10508-008-9402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Ditzen B, Heiman JR. Lower sexual interest in postpartum women: Relationship to amygdala activation and intranasal oxytocin. Horm Behav. 2013;63:114–121. doi: 10.1016/j.yhbeh.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp HA, James TW, Ketterson ED, Sengelaub DR, Ditzen B, Heiman JR. Amygdala response to negative images in postpartum vs nulliparous women and intranasal oxytocin. Soc Cogn Affect Neurosci. 2014;9:48–54. doi: 10.1093/scan/nss100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ, Costa VD, Versace F. Pleasure rather than salience activates human nucleus accumbens and medial prefrontal cortex. J Neurophysiol. 2007;98:1374–1379. doi: 10.1152/jn.00230.2007. [DOI] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rigatti P, Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, Bardeleben Uv, Radue EW, Cirillo S, Tedeschi G, Salle FD. Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. Proc R Soc B. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Zhang KY, Luskin K, Ranker L, Bress J, Numan M. Dopamine D1 receptor activation of adenylyl cyclase, not phospholipase C, in the nucleus accumbens promotes maternal behavior onset in rats. Horm Behav. 2010;57:96–104. doi: 10.1016/j.yhbeh.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Strathearn L. Maternal neglect: oxytocin, dopamine and the neurobiology of attachment. J Neuroendocrinol. 2011;23:1054–1065. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. J Womens Health. 2013;22:352–361. doi: 10.1089/jwh.2012.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE. The human parental brain: in vivo neuroimaging. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1242–1254. doi: 10.1016/j.pnpbp.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A, Carter CS. Detection of salivary oxytocin levels in lactating women. Dev Psychobiol. 2009;51:367–373. doi: 10.1002/dev.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]