Abstract
Regulatory T cells (Tregs) are known to control autoreactivity during and subsequent to the development of the peripheral immune system. Professional antigen presenting cells (APCs), dendritic cells (DCs) and monocytes, have an important role in inducing Tregs. For the first time, this study evaluated proportions and phenotypes of Tregs in canine peripheral blood depleted of professional APCs, utilizing liposomal clodronate (LC) and multicolor flow cytometry analysis.
Our results demonstrate that LC exposure promoted short term decreases followed by significant increases in the proportions or absolute numbers of CD4+CD25+FOXP3+ Tregs in dogs. In general, the LC-dependent Treg fluctuations were similar to the changes in the levels of CD14+ monocytes in Walker hounds. However, the proportions of monocytes showed more dramatic changes compared to the proportions of Tregs that were visually unchanged after LC treatment over the study period. At the same time, absolute Treg numbers showed, similarly to the levels of CD14+ monocytes, significant compensatory gains as well as the recovery during the normalization period. We confirm the previous data that CD4+ T cells with the highest CD25 expression were highly enriched for FOXP3. Furthermore, for the first time, we report that CD4+CD25lowFOXP3+ is the major regulatory T cell subset affected by LC exposure. The increases within the lowest CD25 expressers of CD4+FOXP3+ cells together with compensatory gains in the proportion of CD14+ monocytes during compensatory and normalization periods suggest the possible direct or indirect roles of monocytes in active recruitment and generation of Tregs from naïve CD4+ T cells.
Keywords: Regulatory T cells, Liposomal Clodronate, Antigen Presenting Cells, Monocytes
Introduction
Abnormalities of peripheral tolerance contribute to the pathogenesis of a number of inflammatory, autoimmune and neoplastic diseases in mammals. The discovery of a population of suppressive CD4+ T cells characterized by high constitutive expression of the IL-2Rα chain (CD25) led to remarkable progress in elucidating the phenomenon of cell-mediated suppression and its importance in self-tolerance and regulation of adaptive immune responses [1–3]. One of the key mechanisms of peripheral tolerance and a critical component of the host immune system, CD4+CD25+FOXP3+ regulatory T cells (Tregs), have been described in detail in humans and mice [4, 5]. Naturally occurring regulatory T cells (nTregs) form a distinct thymus-derived lineage in both mice [6] and humans [7], which possesses the capacity to suppress activation and function of effector T cells as well as APCs such as dendritic cells (DCs), monocytes and B cells [8, 9]. Conversely, APCs have an important role in nurturing peripheral Treg populations. It has been shown that immature DCs and alternatively activated macrophages are able to induce Tregs de novo, and that these properties are dependent on the expression of surface co-stimulatory molecules and the production of soluble factors such as IL-10 and indoleamine 2,3-dioxygenase (IDO) by the APC subpopulations [5, 10]. Similar to effector T cells, the function of nTregs has been shown to be dependent on activation via the T cell receptor complex. However, once appropriately activated, this regulatory function was found to be antigen nonspecific [2, 11]. Thus, on antigen encounter, nTregs create a tolerogenic milieu in order to down-regulate aberrant or harmful immune responses [5]. Although the majority of Tregs develop in the thymus, induction of peripheral Tregs, which is dependent on immature DCs, is thought to be a major source of adaptive regulatory T cells [10]. Utilizing transgenic and knockout mice, Anderson et al. convincingly demonstrated that Tregs could directly induce naïve CD4+ T cells to become FOXP3+ (forkhead box P3 transcription factor) induced Tregs (iTregs), and that these iTregs possess potent suppressive function both in vitro and in vivo [12]. This conversion of iTregs by nTregs is mediated by TGF-β, and empowers Tregs to maintain homeostasis, promote immune tolerance, and regulate host defense against foreign pathogens. TGF-β paralyzes cell activation and differentiation suppressing immune responses, converts naïve T cells into Tregs combating inflammation and infection, and prevents Tregs from undergoing apoptosis [13]. Multiple studies in humans and animals have shown that continuous high expression of FOXP3 is required to maintain Treg suppressive activity and divert conventional T cells into regulatory phenotypes. With limited expression of FOXP3, the recognizable immunosuppressive function can be lost [4, 14]. Several studies show that monocytes and macrophages are not limited to presenting antigens to effector T cells thus stimulating and shaping T cell-mediated immune responses: like DCs (the most potent professional APCs), they also are capable of priming naïve T cells, thus initiating adaptive immune responses [15–19]. Recently, monocytes and macrophages have been identified as important APCs directly controlling development, recruitment, and suppressive activity of Tregs in humans and mice [20–22] or differentiating into DCs that induce Tregs [23].
Although several recent studies have reported accurate phenotypic identification and functional characterization of canine Tregs, comprehensive functional information, especially on the role of professional APCs in Treg generation, has not yet been produced. While the early studies provided indirect evidence of Tregs in the dog, a number of recent studies have examined changes in the proportion of CD4+FOXP3+ T cells occurring in canine cancer, reviewed in Garden et al., 2011[24]. The proportion of CD4+FOXP3+ T cells in blood and tumor-draining lymph nodes of dogs diagnosed with a variety of neoplasms have been shown to be significantly increased compared to healthy control animals, and the number of Tregs has been shown to have a positive correlation with tumor stage and a negative correlation with the number of Th1 and cytotoxic T cells [25–27]. However, not all studies of canine tumors have yielded such a clear message [24]. Recently, several reports have provided direct evidence of the regulatory function of canine CD4+CD25highFOXP3+ T cells by inhibiting the proliferation of responder T cells in mixed leukocyte reactions or effector T cells [28, 29]. Importantly, the CD4+ T cells with the highest CD25 expression were enriched for FOXP3 [30], showing the regulatory function of highly pure CD4+CD25high T cells in classical suppression assays [11]. Current studies are focused on elucidating the mechanisms of Treg-mediated suppression and their implications in a number of canine diseases [24].
Liposome encapsulated clodronate (LC) or dichloromethylene-bisphosphonate is being used in various types of research and treatments in many different fields of the scientific and medical communities [31–34]. When encapsulated in liposomes in order to promote and facilitate uptake into professional phagocytes, including both DCs and monocytes/macrophages, clodronate is metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, with the end result being the lysis of the mitochondrial membrane within the host monocytes/macrophage. This leads to the induction of apoptosis, therefore depleting the number of viable monocytes/macrophages and DCs that are available for immune responses [35, 36], which facilitates exploration of the role of APCs in various immune processes.
In this current study, we assessed the role of LC-dependent professional phagocyte depletion on the number and phenotype of CD4+CD25+FOXP3+ regulatory T cells in dogs.
Materials and methods
Animals
Four healthy adult greyhounds and four healthy Walker hounds were used for the pilot and main studies, respectively. Health was confirmed based on physical examination, complete blood count (CBC), serum biochemistry, and urinalysis. CBC and serum biochemistry were completed by the Mississippi State University College of Veterinary Medicine Diagnostic Laboratory Services (CVM-DLS). Immediately prior to the study, all dogs were also confirmed to be negative for infectious disease using a point of care test for heartworm antigen and Borrelia burgdorferi, Anaplasma phagocytophilum/Anaplasma platys and Ehrlichia canis/Ehrlichia ewingii antibodies (SNAP 4Dx Plus, IDEXX Laboratories Inc., Westbrook, ME), and by serologic (IFA) and PCR testing for Babesia canis and gibsoni. Three of the 4 greyhounds had a past history of Babesia canis positivity by either IFA or PCR testing approximately one year prior to the current study, but were confirmed to be persistently negative for Babesia over a subsequent six month period by multiple serial PCR and IFA assays performed before, during and after the current study. All animals were cared for according to guidelines approved by the Mississippi State University Institutional Animal Care and Use Committee (IACUC), and were housed in a university setting under standard conditions. The Mississippi State University animal facilities and program are accredited by the American Association for Accreditation of Laboratory Animal Care. The study was approved by the Mississippi State University IACUC in March 2012, protocol number 12-018.
Treatment
In an initial pilot study designed to obtain preliminary data, liposome encapsulated clodronate (dichloromethylenedisphosphonic acid disodium salt, SIGMA, encapsulated into liposomes at Colorado State University) was administered to the four greyhounds at a low (0.5 ml/kg) dose via slow intravenous infusion at a constant rate into an indwelling peripheral venous catheter over a 90-minute period, using an infusion pump. Two weeks later, the study was repeated at a medium LC dose (1 ml/kg over 90 minutes), and then, two months later, at a high LC dose (2 ml/kg).
In a subsequent main study, liposome encapsulated clodronate (Encapsula NanoSciences, Nashville, TN) was administered to three Walker hounds at a single medium dose (1 ml/kg) via slow intravenous infusion at a constant rate into an indwelling peripheral venous catheter over a 90-minute period, using an infusion pump.
In both studies, one Walker hound that received no treatment was used as a negative control.
Study Design
In the initial 4 dog greyhound pilot study, the dogs were administered LC as part of another unrelated project, and Tregs were measured at irregular intervals (timing as dictated by the unrelated project) in order to generate preliminary data for the subsequent main study. Briefly, Tregs were evaluated via flow cytometry in all greyhounds 3 days prior and 4 and 11 days after the low LC dose, 4, 11 and 18 days after the medium LC dose, and immediately prior to and 7 days after the high LC dose. The samples collected prior to the low and high LC doses were considered to be baseline samples, and the remaining samples were considered to be post-treatment samples.
In the subsequent 3 dog Walker hound main study, the dogs were only administered the medium dose of LC. Briefly, Tregs were evaluated via flow cytometry on multiple occasions (4 different days over a 1 week period) prior to exposure to LC in order to establish baseline values. Tregs were then re-evaluated 1, 3, 6, 8 and 10 days after administration of LC. Total blood cell counts and peripheral monocyte levels were measured in addition to Tregs.
Reagents and Antibodies
BD Pharm Lyse™ (10X) lysing solution (BD Biosciences) was used to remove red blood cells from the white blood samples collected in EDTA tubes.
Fluorescein-conjugated mouse anti-canine CD14 mAbs (LS-C43762, Lifespan Biosciences, Inc.) were used to stain monocytes. Stainings with isotype control mAbs wwere omitted due to the clear separation of the CD14+ monocyte populations. Fluorescein-conjugated rat anti-canine CD4 (LS-C127352, Lifespan Biosciences, Inc), Phycoerythrin (PE)-conjugated mouse anti-canine CD25 (P4A10) and the FOXP3 Staining Buffer Set (including Fixation/Permeabilization Diluent, Fixation/Permeabilization Concentrate, 10X Permeabilization Buffer) and allophycocyanin (APC)-conjugated rat anti-canine FOXP3 mAbs (FJK-16s) (all from eBioscience Inc.) were used to stain regulatory T cells. Appropriate Fluorescence Minus One (FMO) controls recommended for the multicolor analysis were used in all staining panels.
Cell Preparation
Monocytes
Whole blood samples collected in EDTA were incubated with FITC-conjugated anti-CD14 mAbs for 30 minutes in the dark at 4°C. To lyse and remove red blood cells, samples were incubated with BD Pharm Lyse ™ lysing buffer for 15 minutes in the dark at room temperature, gently vortexing every 5 minutes. The resulting cell populations were washed and analyzed by flow cytometry.
Regulatory T cells
Whole blood samples collected in EDTA were incubated with FITC-conjugated anti-CD4 and PE-conjugated anti-CD25 mAbs for 30 minutes in the dark at 4°C. To remove red blood cells, samples were incubated with BD Pharm Lyse ™ lysing buffer for 15 minutes in the dark at room temperature, gently vortexing every 5 minutes. Red blood cells were removed, and the remaining cell populations were washed and stained with anti-FOXP3 staining buffer set following the manufacturer’s instructions. Briefly, cells were incubated with fixation/permeabilization solution for 30 minutes in the dark at 4°C, washed twice with permeabilization buffer followed by incubation with APC-conjugated anti-FOXP3 mAbs for 30 minutes in the dark at 4°C. After a single wash, cells were then analyzed by flow cytometry.
Flow cytometry
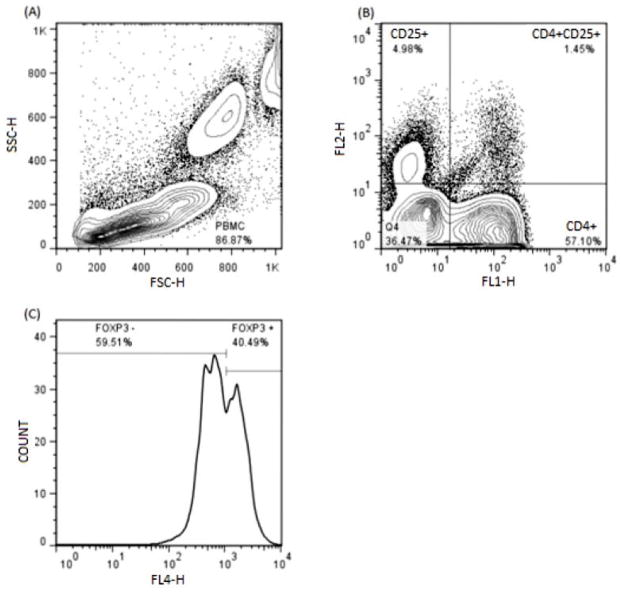
Red blood cell-depleted canine PBMCs were gated based on their relative size and granularity using forward and side scatters (FSC and SSC, respectively) with a FACSCalibur Flow Cytometer (Becton Dickinson). Immunofluorescent staining was analyzed using FlowJo 7.6.4 Software (Tree Star, Inc.). The CD14 immunofluorescent staining in canine monocytes was analyzed by using single histogram statistics (Figure 1). A three-color analysis was performed to assess the FOXP3 staining by gating on CD4+CD25+ double positive T cells, and analyzed by using single histogram statistics (Figure 2). In addition, the intensity of the CD25 fluorescence in the CD4+FOXP3+ cells was assessed by using dot plots with multiple gate statistics (Figure 3).
Figure 1.
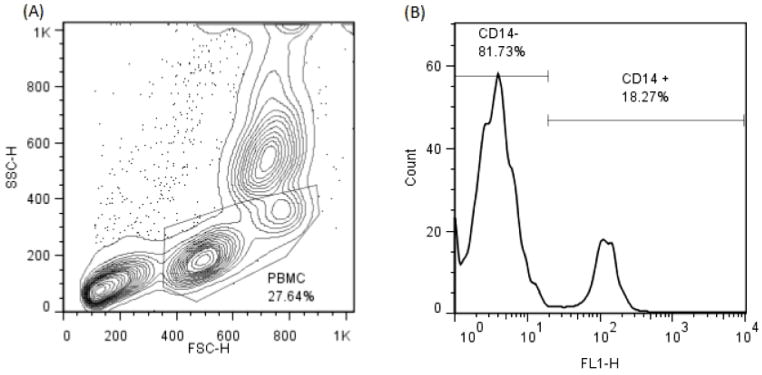
(A) – Assessment of CD14+ monocytes by flow cytometry. RBC-depleted canine cells were gated based on their relative size and granularity using forward and side scatters.
(B) – The CD14 immunofluorescent staining in canine monocytes was analyzed by using single histogram statistics.
Figure 2.
(A) – Identification of CD4+CD25+FOXP3+ regulatory T cells by three color flow cytometry approach. RBC- depleted canine cells were gated based on their relative size and granularity using forward and side scatters.
(B) – Two color analysis for the CD4+CD25+ T cells was performed by using dot plots with quadrant statistics.
(C) – The FOXP3 staining intensity was analyzed by using single histogram statistics.
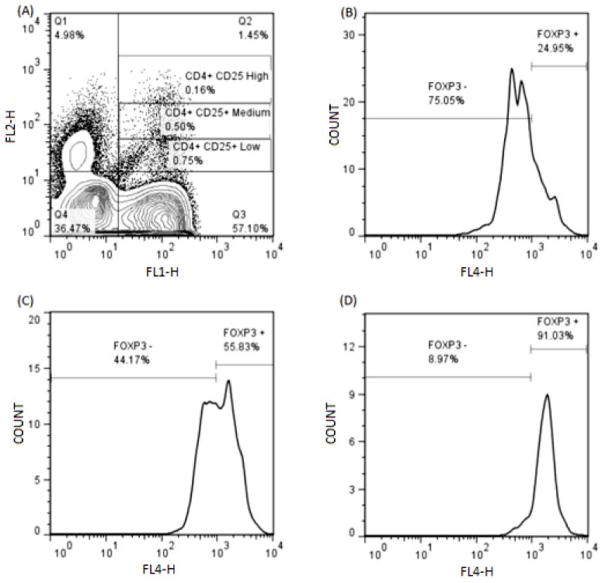
Figure 3.
(A) – FOXP3 expression in peripheral blood of CD25low, CD25medium, and CD25high CD4+ T cells. The CD4+ T cells were gated based on the brightness of CD25 staining.
(B, C, D) – FOXP3 histograms of T cells of low, medium, and high CD25 fluorescence intensity, respectively.
Statistical Analysis
Regulatory T cell marker-specific populations were expressed as a percentage of the total lymphocyte numbers or as absolute cell numbers. CD14+ monocyte populations were expressed as a percentage of PBMC. Then, data was subjected to a one-way analysis of variance (ANOVA) followed by Fisher’s LSD multiple comparison post hoc test and are presented as means ± SD. The level of significance for all tests of effects was set at P<0.05.
Results
Pilot Study: Liposomal clodronate exposure promotes increases in the proportions of CD4+CD25+FOXP3+ regulatory T cells in greyhounds
To develop a preliminary understanding of the effects of depletion of monocytes and other professional phagocytes on Tregs levels in dogs, we used flow cytometry to assess the proportion of peripheral blood Tregs in 4 healthy greyhounds treated with different doses of LC. The percentage of CD4+CD25+FOXP3+ Tregs increased in greyhounds treated with low, medium and high doses of LC, with an average post-treatment increase above baseline values of 30.47% (Figure 4).
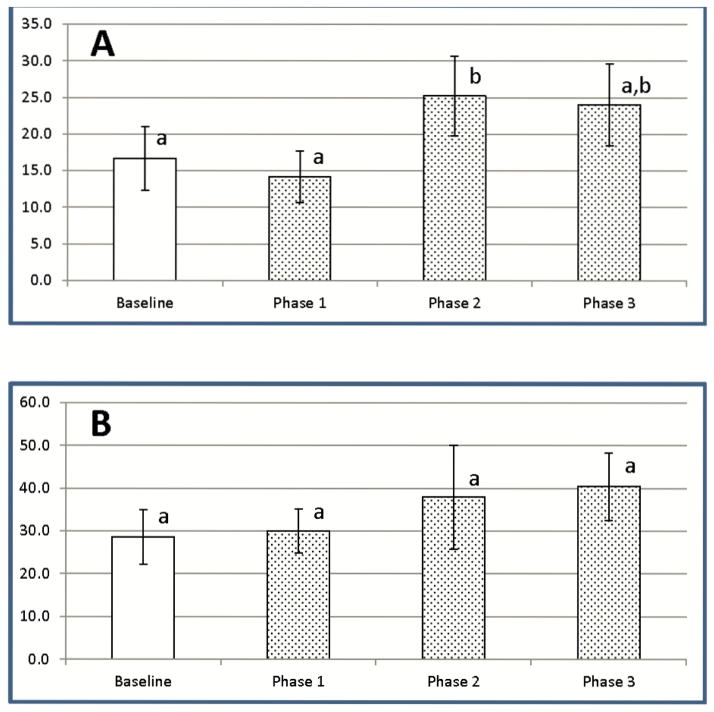
Figure 4. LC exposure promotes increases in regulatory T cell populations in dogs.
Regulatory T cells from 4 healthy greyhounds exposed to low (0.5 ml/kg), medium (1 ml/kg), and high (2 ml/kg) doses of LC in a pilot study were assessed by a three color flow cytometry approach. Data are expressed as % Tregs of total CD4+CD25+ T cells (mean ± SD), with pre-treatment results (Baseline) compared with post-treatment results at the three doses. a,b Means containing the same superscript are not significantly different from each other (P<0.05).
Minimal clinical signs or negative effects were witnessed in greyhounds at low and medium LC doses, although one dog had a mild fever of one day duration, with transient slight inappetance and mild diarrhea, after receiving the medium LC dose. At the high LC dose, the greyhounds developed more marked clinical signs, including fever of up to 2 days duration, transient vomiting and diarrhea, clear nasal discharge, and mild general malaise.
Main Study: Effects of LC treatment on peripheral blood regulatory T cells and monocytes in Walker hounds
In order to further investigate possible mechanisms of the LC-dependent increases in regulatory T cells seen in our pilot study, we assessed levels of CD4+CD25+FOXP3+ Tregs (both proportion and absolute numbers) and CD14+ monocytes in Walker hounds challenged with medium dose LC. We elected to use the medium dose in order to maximize effects on Tregs without causing side effects of the magnitude seen with higher LC doses, and we used purpose-bred Walker hounds instead of greyhounds to ensure that drug effects were not breed-specific or related to potential past exposure to infectious agents.
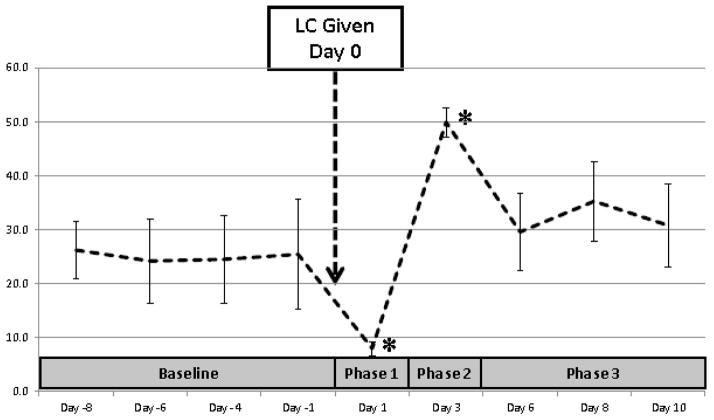
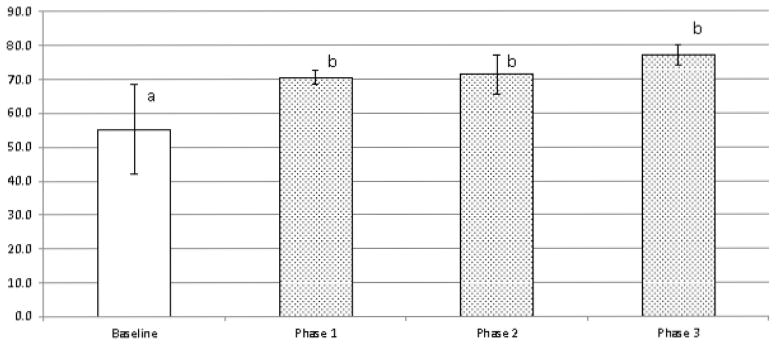
As expected, CD14+ monocyte numbers following treatment with medium doses of LC were significantly decreased in all Walker hounds within 1 day of drug exposure, and then significantly increased above baseline 3 days after treatment before then declining to numbers that were slightly above initial baseline levels by 6 days after LC challenge (Figure 5). Based on changes in CD14+ monocyte numbers in response to LC, results of associated Treg testing were divided into 4 categories: Baseline (all pre-treatment results, designated Day -8 to Day -1), Phase 1 (the first day after LC, associated with a marked decline in monocyte numbers, designated Day 1), Phase 2 (Day 3, associated with a marked increase in monocyte numbers), and Phase 3 (Days 6–10, when monocyte numbers returned to near baseline levels).
Figure 5.
Effects of LC treatment on CD14+ monocyte levels in the peripheral blood of Walker hounds. Monocytes from 3 healthy dogs exposed to a medium dose of LC (1 ml/kg) were assessed by one color flow cytometry analysis. Data are expressed as % CD14+ monocytes of total PBMC (mean ± SD) both before (Day -8 to -1) and after (Day 1 to Day 10) administration of LC on Day 0. Based on changes in CD14+ monocyte numbers in response to LC, results of associated Treg testing were divided into 4 categories: Baseline (all pre-treatment results), Phase 1 (Day 1, associated with a marked decline in monocyte numbers), Phase 2 (Day 3, associated with a marked increase in monocyte numbers), and Phase 3 (Days 6–10, when monocyte numbers returned to near baseline levels). * indicates group differences from baseline (P<0.05).
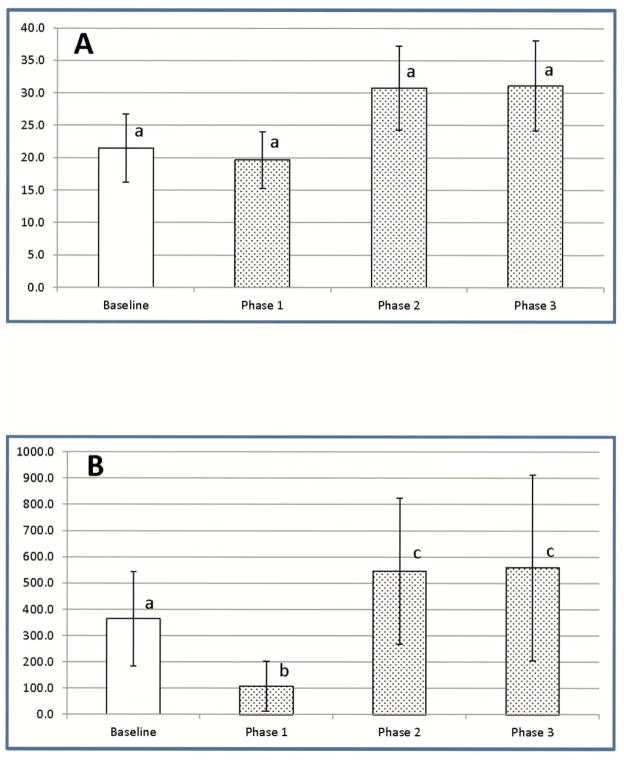
After administration of medium doses of LC, the percentage of CD4+CD25+FOXP3+ Tregs as a proportion of all CD4+CD25+ cells dropped slightly below baseline values on the first day after LC exposure, before rising above baseline values for the remainder of the study (Figure 6A). Changes in Treg percentages did not, however, achieve statistical significance. However, when total blood cell counts were used to calculate total numbers of CD4+CD25+FOXP3+ Tregs after medium dose LC, total Treg numbers dropped markedly below baseline values on Day 1 (Phase 1), before rising to levels significantly above baseline levels for the remainder of the study (Phases 2 and 3) (Figure 6B).
Figure 6.
Effects of LC treatment on levels of CD4+CD25+FOXP3+ regulatory T cells in the peripheral blood of Walker hounds. Regulatory T cells from 3 healthy dogs exposed to a medium dose of LC (1 ml/kg) were assessed by three color flow cytometry analysis at Baseline (all pre-treatment results), Phase 1 (Day 1 post-treatment), Phase 2 (Day 3), and Phase 3 (Days 6–10). (A) – % Tregs of total CD4+CD25+ T cells (B) – Absolute numbers of Tregs. Data are expressed as mean ± SD. a,b,c Means containing the same superscript are not significantly different from each other (P<0.05).
Main Study: CD4+CD25lowFOXP3+ are the major regulatory T cell subset affected by LC exposure
Previously reported data in humans and dogs has demonstrated that, although the population of CD4+CD25+ T cells contains Tregs, other cells such as recently activated pathogenic T cells may also fall in this phenotypic subset [37, 38]. Amongst CD4+CD25+ T cells, the cells that stain brightest for CD25 have been shown to be the most highly enriched FOXP3+ Tregs [37, 38]. To identify the population of Tregs selectively targeted by exposure to LC, we applied multiple gate statistics for the assessment of CD25 fluorescence intensity in CD4+CD25+FOXP3+ T cells (Figure 7). There was an increase in the percentage of CD4+CD25+FOXP3+ T cells expressing low, medium and high levels of CD25 3 days after treatment, compared to baseline levels, and this effect persisted for the duration of the study, although the increase was statistically significant only in CD4+CD25lowFOXP3+ Tregs on during Day 3 (Phase 2).
Figure 7.
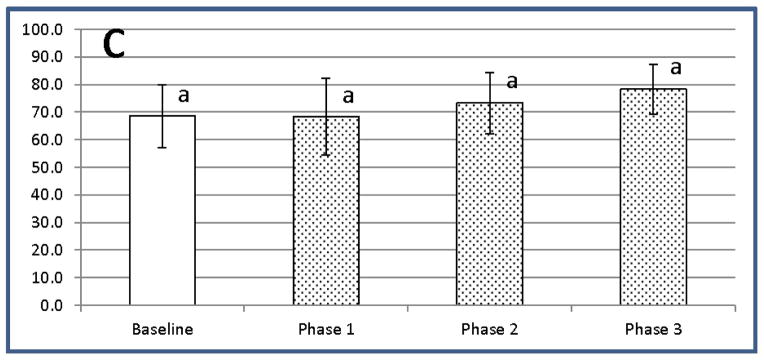
LC exposure promotes significant increases in the most recently activated CD4+CD25lowFOXP3+ Tregs. CD4+FOXP3+ T cells from the dogs exposed to LC were gated based on brightness of CD25 staining resulting in separation into low, medium, and high expressers of CD25. (A) – Low CD25 expressors (B) – Medium CD25 expressors (C) – High CD25 expressors. Data are presented as % FOXP3+Tregs of CD4+T cells with low, medium and high CD25+ expression (mean ± SD). a,b Means containing the same superscript are not significantly different from each other (P<0.05).
Discussion
Regulatory T cells (CD4+CD25+FOXP3+ T cells) have been well described in both humans and mice, and have been shown to be essential for healthy function of the mammalian immune system. Regulatory T cells are critical for regulation of tolerance, and they have an important role in suppressing pathological immune responses in autoimmune disease, transplantation, and graft-versus-host disease. Monocytes and macrophages have an important relationship with T cell function: in addition to presenting antigens to effector T cells and stimulating T cell mediated immune responses, monocytes and macrophages have also been shown to play an essential role in the production of regulatory T cells. Recent studies have demonstrated that monocytes and macrophages are responsible for initiating adaptive immunity by the priming of naïve T cells, triggering the generation of new Tregs, a critical subset of T cells [20–22]. Furthermore, specific Tregs acquire suppressive activity through activation by DCs and their progenitor monocytes and expression specific antigens by these cells [39].
Compared to humans and mice, less is known about the functional purpose and clinical relevance of Tregs in dogs. Recent studies have provided initial phenotypic and functional characterization of Tregs within the canine system [26, 29, 30]. Furthermore, data from recent work indicate that either increased or decreased numbers of Tregs are associated with various disease conditions or vaccination [26, 29, 40–44]. Our current study expands on previous research by assessing the possible role of professional APCs on the proportions and phenotypes of regulatory T cells in healthy dogs treated with liposome encapsulated clodronate.
Liposome encapsulated clodronate has many uses, both in research and for the treatment of autoimmune diseases, based on the drug’s ability to cause transient marked depletion of macrophages [32–34, 45–47]. In dogs, LC has been used to treat immune-mediated hemolytic anemia and malignant histiocytosis [34, 48]. The goal in utilizing LC in our study was to transiently deplete monocyte, macrophages and immature DCs in dogs, thus allowing for evaluation of the role of these APCs on levels and phenotypes of regulatory T cells.
Contrary to the data in humans and mice, the role of APCs in the generation of Tregs within canine system is still unknown. For the first time, this study evaluated proportions and phenotypes of canine regulatory T cells in canine peripheral blood depleted of professional cell APCs, utilizing LC. Our results demonstrate that treatment with liposome encapsulated clodronate causes a transient marked decline in numbers of circulating CD14+ monocytes in experimental dogs, a finding that reflects the well described mechanism of action of LC. A comparable transient decline was also observed in absolute Treg numbers as well as in total PBMC numbers following LC exposure. The effects of LC on non-phagocytic white blood cells are not known, and our data do not exclude possible contact-dependent pro-apoptotic mechanisms triggered by LC in non-phagocytic PBMCs, in particular T cells. However, the transient marked decline in circulating monocytes was not associated with a concurrent significant decline in the proportion of circulating Tregs. Persistence of a stable proportion of CD4+CD25+FOXP3+ peripheral blood cells in LC-treated dogs during the initial phase of the treatment timeline is in agreement with previously reported evidence that, on antigen encounter or during any changes in homeostasis, Tregs are present in sufficient numbers to create and maintain a tolerogenic milieu [5]. Our data suggest that LC exposure created an aberrant and potentially harmful environment due to the observed short term 20–30% decrease in viable white blood cell numbers as observed on CBC. Our results also demonstrate that, after an initial decline associated with LC treatment, circulating CD14+ monocyte numbers then recover and, in fact, transiently increase to above baseline levels. In parallel with the increase in monocyte numbers, absolute Treg numbers also increase above baseline levels. This rise in Treg numbers may be a compensatory increase that reflects initiation of adaptive immune responses by production of new Tregs via activation from naïve T cells in the periphery.
We report that CD4+CD25lowFOXP3+ Tregs are the major regulatory T cell subset affected by LC exposure, and that proportions of these particular Tregs significantly increase by 3 days after treatment with LC. Previous studies in dogs [30] and humans [37, 38, 49, 50] have demonstrated that CD4+ T cells with the highest CD25 expression are the most enriched for FOXP3. Pinheiro et al. showed that isolated CD4+CD25highFOXP3+ Tregs, which are frequently considered to be the canonical nTregs, were alone able to effectively suppress the proliferation of responder CD4+ T cells in vitro [30]. However, although the top CD25bright gate most reliably identifies a highly enriched FOXP3+ population of Tregs, focusing on these cells alone significantly underestimates the frequency and functional importance of FOXP3+ cells in most individuals [38]. Chen et al. reported that human peripheral blood FOXP3+ cells are present in CD25high, CD25low and even CD25− subsets of CD4+ cells, and that co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ Tregs than does focusing on the top CD25bright T cells [49]. A complete understanding of the functional properties of Tregs with low and intermediate expression of CD25 is still lacking, and the utilization of the degree of CD25 expression alone to define functional Tregs is not sufficient, as this IL-2R α-chain does not discriminate regulatory from activated effector or memory T cells. Therefore, more reliable surface markers selectively expressed on functional Tregs are required [49].
Our study demonstrated that the significant increase in the proportion of the lowest CD25 expressers of amongst the population of CD4+CD25+FOXP3+ cells seen during recovery from LC exposure occurs concurrently with an increase in CD14+ monocyte numbers, and suggests a possible direct or indirect role for monocytes in the active recruitment of peripheral blood memory Tregs and/or generation of Tregs from naïve CD4+ peripheral blood T cells. Our data are in agreement with multiple studies that identified monocytes as important professional APCs capable of priming naive CD4+ T cells into regulatory T cell subsets, either directly or indirectly, by differentiating into DCs [20–23].
Conclusion
The increases within the lowest CD25 expressers of CD4+FOXP3+ cells together with significant compensatory gains in the proportion of CD14+ monocytes during compensatory and normalization periods suggest the possible direct or indirect roles of monocytes in active recruitment and generation of Tregs from naïve CD4+ peripheral blood T cells. Further research is essential in creating a working understanding of the mechanisms of canine CD4+CD25+FOXP3+ Treg induction, and the role of professional APCs monocytes and DCs in their generation.
In conclusion, APCs such as monocytes, macrophages and DCs play a major role in shaping protective adaptive immune responses in dogs, as has been demonstrated in humans and mice, and a drug-induced decrease and subsequent increase in APC numbers appears to lead to an associated conversion of naïve T cells into suppressive FOXP3-expressing Tregs. Further research is essential to create a working understanding of canine CD4+CD25+FOXP3+ Tregs and the role of professional APCs in their generation.
Acknowledgments
The authors would like to thank Dr. Steven Dow and Miles McKenna of Colorado State University for generously preparing and providing the liposome encapsulated clodronate used in the initial phase of our study.
Abbreviations
- APCs
Antigen presenting cells
- Tregs
regulatory T cells
- nTregs
naturally occurring regulatory T cells
- iTregs
induced regulatory T cells
- FOXP3
forkhead box P3
- DCs
dendritic cells
- LC
liposomal clodronate
- IL-R
interleukin receptor
- CBC
complete cell count
- FITC
fluorescein
- PE
Phycoerythrin
- APC
allophycocyanin
- FSC
forward scatter
- SSC
side scatter
- PBMC
peripheral mononuclear cells
- ANOVA
analysis of variance
Footnotes
Conflict of Interest
No financial and competing interests are declared
References
- 1.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 3.Sakaguchi S, Toda M, Asano M, Itoh M, Morse SS, Sakaguchi N. T cell-mediated maintenance of natural self-tolerance: its breakdown as a possible cause of various autoimmune diseases. J Autoimmun. 1996;9:211–220. doi: 10.1006/jaut.1996.0026. [DOI] [PubMed] [Google Scholar]
- 4.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmetterer KG, Neunkirchner A, Pickl WF. Naturally occurring regulatory T cells: markers, mechanisms, and manipulation. FASEB J. 2012;26:2253–2276. doi: 10.1096/fj.11-193672. [DOI] [PubMed] [Google Scholar]
- 6.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 7.Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, et al. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–2744. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 8.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 9.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahnke K, Bedke T, Enk AH. Regulatory conversation between antigen presenting cells and regulatory T cells enhance immune suppression. Cell Immunol. 2007;250:1–13. doi: 10.1016/j.cellimm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 12.Anderson AC, Chandwaskar R, Lee DH, Kuchroo VK. Cutting edge: the Idd3 genetic interval determines regulatory T cell function through CD11b+CD11c- APC. J Immunol. 2008;181:7449–7452. doi: 10.4049/jimmunol.181.11.7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran DQ. TGF-beta: the sword, the wand, and the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol. 2012;4:29–37. doi: 10.1093/jmcb/mjr033. [DOI] [PubMed] [Google Scholar]
- 14.Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R, et al. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153–166. doi: 10.1016/j.cell.2012.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajtay Z, Csomor E, Sandor N, Erdei A. Expression and role of Fc- and complement-receptors on human dendritic cells. Immunol Lett. 2006;104:46–52. doi: 10.1016/j.imlet.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Helmy KY, Katschke KJ, Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 18.Olazabal IM, Martin-Cofreces NB, Mittelbrunn M, Martinez del Hoyo G, Alarcon B, Sanchez-Madrid F. Activation outcomes induced in naive CD8 T-cells by macrophages primed via “phagocytic” and nonphagocytic pathways. Mol Biol Cell. 2008;19:701–710. doi: 10.1091/mbc.E07-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr Opin Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong H, Yazdanbakhsh K. Differential control of Helios(+/−) Treg development by monocyte subsets through disparate inflammatory cytokines. Blood. 2013;121:2494–2502. doi: 10.1182/blood-2012-11-469122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter GJ, Evans HG, Menon B, Gullick NJ, Kirkham BW, Cope AP, et al. Interaction with activated monocytes enhances cytokine expression and suppressive activity of human CD4+CD45ro+CD25+CD127(low) regulatory T cells. Arthritis Rheum. 2013;65:627–638. doi: 10.1002/art.37832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189:5602–5611. doi: 10.4049/jimmunol.1201018. [DOI] [PubMed] [Google Scholar]
- 23.Faivre V, Lukaszewicz AC, Alves A, Charron D, Payen D, Haziot A. Human monocytes differentiate into dendritic cells subsets that induce anergic and regulatory T cells in sepsis. PLoS One. 2012;7:e47209. doi: 10.1371/journal.pone.0047209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garden OA, Pinheiro D, Cunningham F. All creatures great and small: regulatory T cells in mice, humans, dogs and other domestic animal species. Int Immunopharmacol. 2011;11:576–588. doi: 10.1016/j.intimp.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Biller BJ, Elmslie RE, Burnett RC, Avery AC, Dow SW. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol. 2007;116:69–78. doi: 10.1016/j.vetimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Tominaga M, Horiuchi Y, Ichikawa M, Yamashita M, Okano K, Jikumaru Y, et al. Flow cytometric analysis of peripheral blood and tumor-infiltrating regulatory T cells in dogs with oral malignant melanoma. J Vet Diagn Invest. 2010;22(3):438–441. doi: 10.1177/104063871002200317. [DOI] [PubMed] [Google Scholar]
- 27.O’Neill K, Guth A, Biller B, Elmslie R, Dow S. Changes in regulatory T cells in dogs with cancer and associations with tumor type. J Vet Intern Med. 2009;23:875–881. doi: 10.1111/j.1939-1676.2009.0333.x. [DOI] [PubMed] [Google Scholar]
- 28.Abrams VK, Hwang B, Lesnikova M, Gass MJ, Wayner E, Castilla-Llorente C, et al. A novel monoclonal antibody specific for canine CD25 (P4A10): selection and evaluation of canine Tregs. Vet Immunol Immunopathol. 2010;135:257–265. doi: 10.1016/j.vetimm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knueppel A, Lange S, Sekora A, Altmann S, Freund M, Junghanss C. Phenotypic and functional characterization of freshly isolated and expanded canine regulatory T cells. Exp Anim. 2011;60:471–479. doi: 10.1538/expanim.60.471. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro D, Singh Y, Grant CR, Appleton RC, Sacchini F, Walker KR, et al. Phenotypic and functional characterization of a CD4(+) CD25(high) FOXP3(high) regulatory T-cell population in the dog. Immunology. 2011;132:111–122. doi: 10.1111/j.1365-2567.2010.03346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt-Weber CB, Rittig M, Buchner E, Hauser I, Schmidt I, Palombo-Kinne E, et al. Apoptotic cell death in activated monocytes following incorporation of clodronate-liposomes. J Leukoc Biol. 1996;60:230–244. doi: 10.1002/jlb.60.2.230. [DOI] [PubMed] [Google Scholar]
- 32.Everhart MB, Han W, Parman KS, Polosukhin VV, Zeng H, Sadikot RT, et al. Intratracheal administration of liposomal clodronate accelerates alveolar macrophage reconstitution following fetal liver transplantation. J Leukoc Biol. 2005;77:173–180. doi: 10.1189/jlb.1203647. [DOI] [PubMed] [Google Scholar]
- 33.Summan M, Warren GL, Mercer RR, Chapman R, Hulderman T, Van Rooijen N, et al. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1488–1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 34.Hafeman S, London C, Elmslie R, Dow S. Evaluation of liposomal clodronate for treatment of malignant histiocytosis in dogs. Cancer Immunol Immunother. 2010;59:441–452. doi: 10.1007/s00262-009-0763-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brack A, Labuz D, Schiltz A, Rittner HL, Machelska H, Schafer M, et al. Tissue monocytes/macrophages in inflammation: hyperalgesia versus opioid-mediated peripheral antinociception. Anesthesiology. 2004;101:204–211. doi: 10.1097/00000542-200407000-00031. [DOI] [PubMed] [Google Scholar]
- 36.Weisser SB, van Rooijen N, Sly LM. Depletion and reconstitution of macrophages in mice. J Vis Exp. 2010;66:4105. doi: 10.3791/4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Q, Krummel MF. Imaging the function of regulatory T cells in vivo. Curr Opin Immunol. 2006;18:496–502. doi: 10.1016/j.coi.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 38.Tang Q, Bluestone JA. Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev. 2006;212:217–237. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 39.Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells--ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18:103–110. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim JH, Chon SK, Im KS, Kim NH, Cho KW, Sur JH. Infiltrating Foxp3+ regulatory T cells and histopathological features in canine classical and spermatocytic seminomas. Reprod Domest Anim. 2013;48:218–222. doi: 10.1111/j.1439-0531.2012.02135.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Hur JH, Lee SM, Im KS, Kim NH, Sur JH. Correlation of Foxp3 positive regulatory T cells with prognostic factors in canine mammary carcinomas. Vet J. 2012;193:222–227. doi: 10.1016/j.tvjl.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell L, Thamm DH, Biller BJ. Clinical and immunomodulatory effects of toceranib combined with low-dose cyclophosphamide in dogs with cancer. J Vet Intern Med. 2012;26:355–362. doi: 10.1111/j.1939-1676.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 44.Junginger J, Schwittlick U, Lemensieck F, Nolte I, Hewicker-Trautwein M. Immunohistochemical investigation of Foxp3 expression in the intestine in healthy and diseased dogs. Vet Res. 2012;43:23. doi: 10.1186/1297-9716-43-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fink K, Ng C, Nkenfou C, Vasudevan SG, van Rooijen N, Schul W. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur J Immunol. 2009;39:2809–2821. doi: 10.1002/eji.200939389. [DOI] [PubMed] [Google Scholar]
- 46.Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin WJ, Walton M, Harper J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum. 2009;60:281–289. doi: 10.1002/art.24185. [DOI] [PubMed] [Google Scholar]
- 48.Mathes M, Jordan M, Dow S. Evaluation of liposomal clodronate in experimental spontaneous autoimmune hemolytic anemia in dogs. Exp Hematol. 2006;34:1393–1402. doi: 10.1016/j.exphem.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Chen W, Konkel JE. TGF-beta and ‘adaptive’ Foxp3(+) regulatory T cells. J Mol Cell Biol. 2010;2:30–36. doi: 10.1093/jmcb/mjp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]