Summary
Introduction
This phase II trial investigated chemoradiation followed by surgery and 2 years of adjuvant tetrathiomolybdate (TM) for resectable esophageal cancer.
Methods
Patients with resectable, locally advanced esophageal cancer received neoadjuvant cisplatin 60 mg/m2 (days 1 and 22), paclitaxel 60 mg/m2 (days 1, 8, 15, and 22), and 45 Gy hyperfractionated radiotherapy for 3 weeks followed by transhiatal esophagectomy. TM 20 mg PO QD was started 4 weeks post-op, and continued for 2 years to maintain the ceruloplasmin level between 5 and 15 mg/dl.
Results
Sixty-nine patients were enrolled (median age, 60 years). Sixty-six patients underwent surgery and 61 patients had a complete resection. Histologic complete response rate was 10 %. Twenty-one patients did not receive TM (metastases noted in the peri-operative period, prolonged post-operative recovery time, or patient refusal). Forty-eight patients started TM; 14 completed 24 months of treatment, 11 completed 10–18 months, 15 completed 2–8 months, and 8 completed ≤1 month. Twenty-seven patients had disease recurrence. With a median follow-up of 55 months, 25 patients were alive without disease, 1 was alive with disease, and 43 have died. Three-year recurrence-free survival was 44 % (95 % CI, 32–55 %) and the three-year overall survival was 45 % (95 % CI 33–56 %).
Conclusions
TM is an antiangiogenic agent that is well tolerated in the adjuvant setting. Disease-free survival and overall survival are promising when compared to historical controls treated at our institution with a similar regimen that did not include TM. However, the challenges associated with prolonged administration limit further investigation.
Keywords: Esophageal cancer, Neoadjuvant chemoradiation, Tetrathiomolybdate, Cisplatin, Paclitaxel
Introduction
Esophageal cancer is one of the leading causes of cancer-related mortality in the United States with 16,640 new cases and 14,500 deaths estimated in 2010. [1] Patients with localized disease confined to the esophagus and regional lymph nodes are most often treated with multimodality therapy. The most common approach in the United States is neoadjuvant chemoradiation [2–4].
Tetrathiomolybdate (TM) is an oral copper chelator, initially developed for the treatment of Wilson’s disease [5, 6]. After ingestion, TM is absorbed systemically and forms a tripartite complex with copper and albumin. This decreases copper bioavailability and facilitates hepatic clearance via excretion in the bile. In murine models, TM has demonstrated antiangiogenic properties and reductions in proangiogenic cytokines such as vascular endothelial growth factor (VEGF), interleukin (IL-) 6, IL-8, and basic fibroblast growth factor (bFGF), possibly due to the reduced activity of nuclear factor-κB (NF-κB) [7, 8]. A phase I study in patients with solid tumor malignancy found doses up to 120 mg/day to be very well tolerated with mild reversible neutropenia and anemia as the main dose-limiting toxicities. [9] Several phase II studies in patients with castrate resistant prostate cancer, renal cell carcinoma and colorectal cancer have confirmed safety and tolerability of TM when given as a single agent over several months [10–12].
Ceruloplasmin is a protein synthesized hepatically and functions as the main transporter of copper in the body. Animal experiments have demonstrated that serum ceruloplasmin levels are an accurate biomarker for serum copper levels. Preclinical data have also shown that TM-facilitated reduction of ceruloplasmin levels below 20 % of baseline values results in antiangiogenesis [7, 13]. Patients with malignancy typically have ceruloplasmin levels in the range of 30–65 mg/dl and, ceruloplasmin levels of 5–15 mg/dl would indicate optimal reduction in serum copper to potentially inhibit tumor neoangiogeneis [9, 14].
Despite aggressive therapy with chemoradiation and surgery for esophageal cancer, many patients will harbor residual micrometastatic disease that results in an early recurrence (most commonly in the first 1–2 years). With these considerations, we designed a phase II study of neoadjuvant cisplatin and paclitaxel plus radiation therapy prior to transhiatal esophagectomy in patients with locally advanced esophageal carcinoma, followed by 2 years of adjuvant TM. We hypothesized that after maximal cytoreduction with chemoradiation and surgical resection, TM would inhibit neoangiogenesis and thus prevent or delay progression of residual micrometastatic disease. Because the highest risk of disease recurrence is typically the first 2 years post resection, we chose to administer the TM during this period. The primary objective was to assess the 3-year recurrence-free survival and the secondary endpoints were 3-year overall survival (OS) rate and treatment toxicity.
Patients and methods
Eligibility
Patients with histologically confirmed adenocarcinoma or squamous cell carcinoma of the esophagus or gastro-esophageal junction were entered into this study between January 2002 and January 2006. Patients had disease limited to the esophagus or regional lymph nodes that could be encompassed in tolerable radiation fields after evaluation with upper endoscopy and biopsy, barium swallow, and helical computed tomography (CT) of the chest and abdomen. Endoscopic ultrasonography was recommended but not mandatory and positron emission tomography (PET) was not routinely used at the time this trial was written. Other requirements included age 18–75, Zubrod performance status (PS) of 0–2, absolute neutrophil count (ANC) ≥1,500/mm3, platelet count ≥ 100,000/mm3, creatinine clearance ≥60 ml/min per the Cockcroft-Gault equation, [15] total bilirubin ≤1.5× the institutional upper limit of normal, and SGOT/SGPT ≤2.5× the upper limit of normal. Exclusion criteria also included prior cytotoxic chemotherapy or thoracic radiotherapy, clinically relevant hearing loss and any medical contraindication to surgery. The trial was approved by the local Institutional Review Board and written informed consent was obtained from all patients.
Treatment
Cisplatin 60 mg/m2 was given on Day 1 and 22 over 1 h and paclitaxel 60 mg/m2 was given on Days 1, 8, 15, and 22 over 1 h. Radiation therapy was delivered at a dose of 1.5 Gy twice daily on days 1–5, 8–12, and 15–19 with a minimum inter-fraction interval of 6 h, resulting in a total dose of 45 Gy. Within the three-dimensional conformal treatment planning system, a gross tumor volume (GTV) was created based on abnormalities noted in the esophagus, proximal stomach, and regional lymph nodes on pretreatment diagnostic upper endoscopy, CT scan and barium swallow. A planning target volume (PTV) was then created by expanding the GTV 5 cm superiorly and inferiorly and 1.5 cm radially. The goal of treatment planning was to encompass the PTV with the 95 % isodose surface and minimize the dose to surrounding normal structures. A four-field conformal beam arrangement was typically utilized that consisted of opposed anterior and posterior and lateral fields.
Transhiatal esophagectomy was performed on approximately Day 50. The entire thoracic esophagus was mobilized and resected from the level of the clavicles to the gastric cardia through an upper midline abdominal incision and a cervical incision. The remaining stomach was mobilized and positioned in the original esophageal bed, and alimentary continuity was re-established by means of anastomosis between the cervical esophagus and the gastric fundus above the level of the clavicles. Accessible intra-abdominal, paraesophageal, and subcarinal lymph nodes were sampled for staging purposes.
Tetrathiomolybdate (TM) was supplied by Sigma-Aldrich Co., Inc. (St. Louis, MO) in a 20 mg oral formulation. Initially, patients ingested 40 mg three times daily with meals and 60 mg before bed. Because of early gastrointestinal toxicity and data suggesting that copper depletion could be achieved at a lower dose, an amendment was instituted after 6 patients had been enrolled that reduced the initial TM dose to 20 mg once daily with a meal. Missed doses were not replaced. Treatment began 4–6 weeks after the esophagectomy and continued until disease recurrence or unacceptable toxicity for a maximum of 2 years. Patients were instructed not to ingest multivitamins or nutritional supplements which contained minerals.
A ceruloplasmin (CP) level was obtained prior to the first dose of TM, repeated once every 2 weeks for during the first 4 weeks of therapy and then every 4 weeks while the patient remained on study. A complete blood count was obtained every 2 weeks for the first 8 weeks on study and then every 4 weeks subsequently. Dose adjustments for TM were made to maintain a CP level of 5–15 mg/dl. If the CP level was not less than 15 mg/dl after the first 2 weeks of treatment, then the dose of TM was increased by 20 mg and the CP level was reassessed 2 weeks later. TM was increased by 20 mg until the target CP level was reached. Patients underwent evaluation with a physical exam, labs and a chest x-ray every 3 months after copper deficiency was achieved.
Dose adjustments for toxicity
Based upon blood counts on the day of treatment, full doses of paclitaxel and cisplatin were delivered for ANC ≥1,500/mm3 and platelets ≥100,000/mm3. Treatment drugs were decreased by 10 % for ANC of 500–999 or platelets of 50,000–99,000, and decreased by 15 % for ANC <500 or platelets <50,000. TM was held for a period of 5 days if the hematocrit dropped below 80 % of baseline, and then reintroduced at a dose decreased by 20 mg. Grade 3 or 4 toxicity attributable to TM that did not achieve a CP level of 5–15 mg/dl, resulted in removal from study.
Assessment of response and toxicity
Patients were considered evaluable for toxicity assessment if treatment with neoadjuvant chemoradiation was started. Patients underwent a CT scan of the chest and abdomen approximately 1 week prior to the planned esophagectomy to evaluate for progressive or metastatic disease that would preclude surgery. Cisplatin, paclitaxel, radiation therapy or TM were discontinued if a patient developed progressive disease or life-threatening/irreversible toxicity that was not manageable with symptomatic care or dose reduction and/or delay. All toxicity was graded according to the NCI Common Toxicity Criteria version 2.0 (http://ctep.cancer.gov/reporting/ctc.html).
Statistical analysis
To be evaluable for efficacy, the patient had to receive at least one dose of chemotherapy and one dose of radiation. Accrual continued until 69 evaluable subjects were enrolled, which provided 80 % power to detect a significant difference between the hypothesized 3-year recurrence-free survival probability for TM of 40 % versus our historical 3-year recurrence-free survival probability of 26 % in this population.
Recurrence-free survival was defined as the time from the initiation of neoadjuvant chemoradiation to the documentation of disease progression or death due to any cause. Of the seven patients who were never disease-free, they were considered as having recurrence and their time-to-recurrence is zero. Overall survival was defined as the time from the initiation of neoadjuvant chemoradiation to death from any cause. Kaplan-Meier estimates were used to explore the distributions for overall survival time and the disease free survival duration. [16] The SAS System (Cary, NC) was used for all analyses.
Results
69 patients were enrolled onto the study between January 2002 and January 2006 (Table 1). Data were collected until May 2010. The median age at study entry was 60 years (range, 42–74), and 90 % were males. All patients enrolled had a Zubrod PS of 0 or 1. Eighty percent of tumors were adenocarcinoma, 20 % were squamous cell carcinoma and all primary tumors were localized to the middle to lower third of the esophagus. Fifty seven patients underwent a preoperative endoscopic ultrasound with stage IIA, IIB, III disease identified in 12 %, 12 %, 76 % of these patients, respectively.
Table 1.
Patient characteristics
| Characteristic | No. | Percentage |
|---|---|---|
| Sex | ||
| Male | 62 | 90 |
| Female | 7 | 10 |
| Age, years | ||
| Median | 60 | |
| Range | 42–74 | |
| Performance Status | ||
| 0 | 67 | 97 |
| 1 | 2 | 3 |
| Histology | ||
| Adenocarcinoma | 56 | 81 |
| Barrett’s Changes | 25 | 44 |
| Squamous Cell Carcinoma | 13 | 19 |
| Stage by EUS | ||
| IIA (T2/T3, N0, M0) | 7 | 10 |
| IIB (T1/T2, N1, M0) | 7 | 10 |
| III (T3, N1, M0) | 43 | 63 |
| No EUS performed | 12 | 17 |
Neoadjuvant chemoradiation
Treatment was well tolerated: 53 patients received 100 % of the planned cisplatin dose, and 16 received 50 % of the dose. In addition, 30, 32 and 7 patients received 100 %, 75 % and 50 % of the planned paclitaxel dose, respectively. All patients completed the planned radiation dose of 45 Gy. The main toxicities that required dose modifications were grade 3/4 leucopenia in 15 patients (22 %) and grade 3/4 vomiting in 4 patients (6 %) (Table 2). Seventeen patients (25 %) required tube feedings during or after neoadjuvant therapy based on the inability to maintain adequate hydration and/or nutrition. Three patients demonstrated progressive disease on the restaging CT scan after chemoradiation and were removed from study.
Table 2.
Worst toxicity from neoadjuvant chemoradiation experienced per patient (n=69) grade
| Toxicity | 1–2 | 3 | 4 |
|---|---|---|---|
| Anemia | 31 | 0 | 0 |
| Leucopenia | 39 | 15 | 0 |
| Neutropenia | 5 | 14 | 0 |
| Thrombocytopenia | 12 | 0 | 0 |
| Nausea | 28 | 1 | 1 |
| Vomiting | 18 | 3 | 1 |
| Diarrhea | 7 | 0 | 0 |
| Constipation | 18 | 1 | 0 |
Surgery results
Sixty-six patients underwent surgery. Four patients had metastatic disease or gross residual disease at the time of resection and 1 patient had a positive surgical margin on pathologic review. Therefore, 61 patients ultimately underwent a complete resection. Seven patients (10 %) demonstrated a complete histologic response to the neoadjuvant therapy and 5 patients (8 %) subsequently developed an anastomotic leak. There were no deaths in the 30-day postoperative period.
Adjuvant tetrathiomolybdate
Forty-eight (70 %) of the initial 69 patients began adjuvant TM. Twenty-one patients (30 %) did not receive any TM for the following reasons: 7 patients demonstrated metastatic disease after chemoradiation or at the time of surgery; 1 had an extensively positive surgical margin; 1 developed metastatic disease in the post-operative period; 6 had a prolonged post-operative recovery period, and 6 refused therapy. Six patients were treated with the initially planned dose of 40 mg TID and 60 QHS of TM. However, grade 2 diarrhea was reported in 3 patients during the first month of TM therapy, and this unanticipated level of toxicity precipitated an amendment for dose de-escalation to 20 mg daily for the rest of the patients enrolled.
Fourteen of the 48 patients who began TM (29 %) completed the planned 24 months of adjuvant therapy; 11 patients (23 %) completed 10–18 months; 15 patients (31 %) completed 2–8 months and 8 patients (17 %) completed 1 month of therapy. Thirty-eight of the patients had at least 4 Cp levels assessed. Of these, 25, 15, and 10 patients had at least 60 %, 70 % and 80 % of the Cp levels in the therapeutic range of 5–15 mg/dl, respectively.
At the lower dose, the most common grade 3 toxicity included nausea (6 %) and dizziness (6 %) with no grade 4 toxicity identified (Table 3). Disease recurrence was the main reason for discontinuation of TM (n=13) (Table 4). Other reasons for treatment cessation included: nausea (n=5), dizziness (n=3), patient request (n=4), diarrhea (n=2), prolonged grade 3 neutropenia (n=1), non-compliance (n=1), inadequate CP levels (n=1), and lethargy (n=1).
Table 3.
Worst toxicity from tetrathiomolybdate experienced per patient (n=48) grade
| Toxicity | 1 | 2 | 3 |
|---|---|---|---|
| Anemia | 7 | 5 | 0 |
| Neutropenia | 3 | 1 | 1 |
| Nausea/Vomiting | 1 | 6 | 3 |
| Neuropathy/parasthesia | 1 | 1 | 0 |
| Diarrhea | 3 | 3 | 3 |
| Dizziness | 1 | 0 | 3 |
| Neuropathy | 1 | 1 | 0 |
| Fatigue/Decline in PS | 2 | 0 | 0 |
Table 4.
Reasons for discontinuing TM (n=48)
| Completed Treatment Plan | 14 |
| Disease Recurrence | 13 |
| Toxicity | 12 |
| Requested discontinuation | 4 |
| Noncompliance | 1 |
| Inadequate CP level | 1 |
| Other | 3 |
Time to recurrence and survival
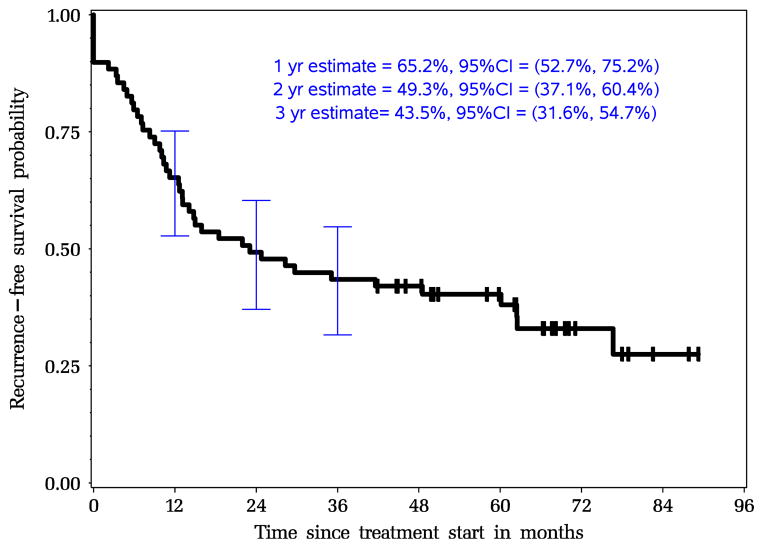
All 69 patients were included in the survival analysis and the median follow-up time was 55 months. Twenty-six patients (38 %) were still alive, 25 patients were disease free and 1 was alive with recurrent disease. Forty-three patients (62 %) died of disease or other causes and no treatment-related deaths were identified. The 1-, 2- and 3-year recurrence-free survival probabilities were 65 % (95 % CI, 53–75 %), 49 % (95 % CI, 37–60 %), and 44 % (95 % CI, 32–55 %), respectively. After surgical resection, recurrent disease was identified in 27 patients; 22 patients demonstrated distant metastases, 4 patients developed a local recurrence and 1 one patient had both.
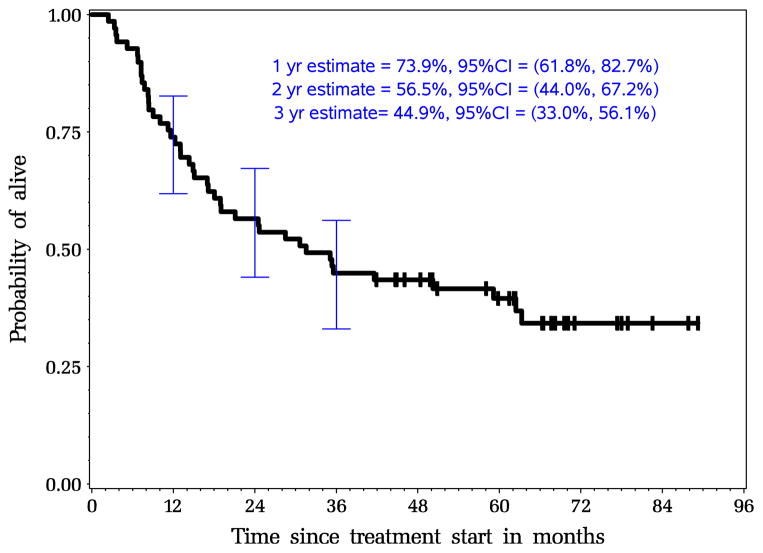
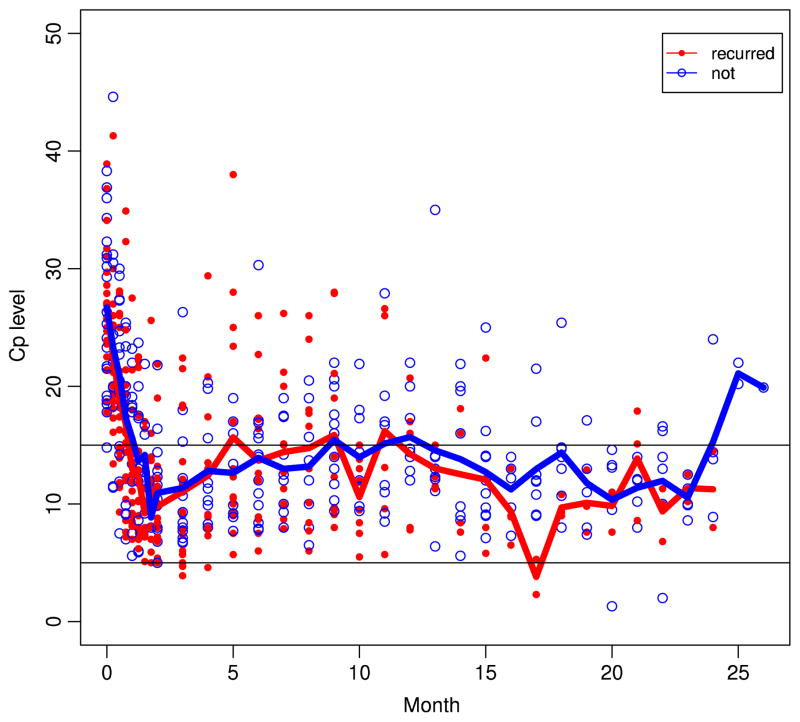
The 1-, 2- and 3-year overall survival probabilities were 74 % (95 % CI, 62–83 %), 57 % (95 % CI, 44–67 %), and 45 % (95 % CI, 33–56 %), respectively. The median recurrence-free survival and overall survival from the start of neoadjuvant therapy were 23.1 months (95 % CI, 12.7–60.1) and 31.5 months (95 % CI, 17.1–62.4 months), respectively. Based on histology, median overall survival was 29 months for the patients with adenocarcinoma and 43 months for the 13 patients with squamous cell carcinoma. The Kaplan-Meier curves for recurrence-free survival and overall survival are shown in Figs. 1 and 2. There was no association between decreased level of ceruloplasmin with recurrence free survival or overall survival (Fig. 3). The recurrence free survival did not appear to improve with the duration of therapeutic Cp levels either.
Fig. 1.
Intent-to-treat Kaplan-Meier estimates for recurrence-free survival for all patients N=69 (median RFS, 49.1 months)
Fig. 2.
Intent-to-treat Kaplan-Meier estimates for overall survival for all patients N=69 (median OS, 31.7 months)
Fig. 3.
Ceruloplasmin levels for patients alive compared to patients who died from recurrent disease. Blue line indicates the average Cp levels for patients who were alive. Red line indicates the average Cp levels for patients who died of recurrent disease. Blue circles indicate individual patient Cp levels at the given time point who were alive. Red dots indicate Cp levels for those who died of recurrent disease
Discussion
Neoangiogenesis is a vital process in malignancy and inhibitors of pro-angiogenic cytokines such as VEGF have demonstrated a clinical benefit in a wide range of solid tumors [17–19]. Micrometastatic disease is often present after surgical resection of locally advanced esophageal cancer as evidenced by recurrence rates of approximately 70 % even after aggressive tri-modality therapy. Progression to clinically apparent metastatic disease requires angiogenesis and the inhibition of this process is an attractive target in the adjuvant setting. Preclinical studies suggest that TM lowers pro-angiogenic cytokines VEGF, IL-6, IL-8, and bFGF in vitro and in vivo [7, 8]. A murine xenograft model of squamous cell carcinoma of the head and neck demonstrated a reduction in the number of distant metastasis in the TM group compared to controls that supports the hypothesis that this agent may demonstrate optimal clinical benefit in patients with sub-clinical disease. [20] In our study, serum markers associated with tumor angiogenesis (bFGF, VEGF, V-CAM1, IL-6, and IL-8) were drawn pre-operatively, postoperatively, at the onset of copper deficiency, 12 and 24 weeks after copper deficiency, and upon relapse and the results will be reported in a separate manuscript.
Clinical trials that evaluated TM as a single agent in patients with grossly metastatic solid tumors have been disappointing. Redman et al. investigated the use of single agent TM in patients with advanced kidney cancer. [11] No responses were identified in 15 treated patients; however, disease stability was noted in 5 patients. Henry et al. evaluated single agent TM in patients with hormone-refractory prostate cancer and no delay in disease progression was identified [12].
TM may require a prolonged lead-in phase to attain the desired antiangiogenic effect and the evaluation of TM in patients with minimal tumor burden may be preferable. Adjuvant treatment with TM in patients with early-stage mesothelioma supports this hypothesis. [21] Thirty patients with resected stage I–III mesothelioma were subsequently treated with TM for a median of 15 months. When compared with historic controls, no improvement in time to progression was identified in patients with resected stage III disease; however, the time to progression in resected stage I and II patients was intriguingly superior (20 months versus 10 months; p=0.046) which again suggests TM may be more effective in patients with a low tumor burden.
In our study, neoadjuvant treatment with cisplatin and paclitaxel plus hyperfractionated radiation was well tolerated without any substantial delay in surgical resection. This supports the tolerability of platinum-taxane combinations with radiation therapy prior to esophagectomy, and the 3-year recurrence-free survival of 47 % is encouraging. Many trials have demonstrated that a histologic complete response is associated with better long-term survival compared to patients with residual disease. The histologic complete response rate in our study was disappointing (10 %) even compared to our historical controls (22 %). The reason for this is unclear, but it should be noted that although the majority had residual disease at the time of resection, the 3-year recurrence-free survival of the whole group was still very favorable compared to previous studies.
We compared our current results utilizing adjuvant TM to a group of 69 historical controls treated at our institution in a very similar preoperative chemoradiation protocol, but without TM. Those patients were treated with cisplatin 75 mg/m2 on day 1 plus paclitaxel 60 mg/m2 on days 1, 8, 15, and 22, with an identical hyperfractionated radiation schedule of 45 Gy2. Long-term results from our previous trial indicate the 1-, 2-, and 3-year disease-free survival probabilities were 55 %, 39 %, and 32 %, respectively. The 1-, 2- and 3-year overall survival probabilities were 75 % (95 % CI, 65–86 %), 50 % (95 % CI, 39–63 %), and 34 % (95 % CI, 23–46 %), respectively. Compared to the current trial where the 1-, 2-, and 3-year overall survival probabilities were 74 % (95 % CI, 62–83 %), 57 % (95 % CI, 44–67 %), and 45 % (95 % CI, 44–56 %), respectively, although not statistically significant, the addition of TM offers a slight survival advantage as seen by the 3-year overall survival probability. Unfortunately, the current trial is underpowered which contributed to wide confidence intervals in survival estimates. While these results are not randomized and we cannot make an absolute comparison, the higher survival percentages in our current trial suggests that adjuvant TM may offer clinical benefit. Furthermore, the median overall survival time for current trial is 31.5 months (95 % CI, 17.1–62.3 months) compared to 24 months (95 % CI, 16–33 months) with the historical controls. This provides further evidence that adjuvant TM is potentially beneficial.
To date, no strong evidence supports the use of adjuvant therapy after neoadjuvant chemoradiation followed by esophagectomy regardless of the post-operative pathologic stage. Many patients are unable to tolerate further therapy given the toxicity of the prior treatment and this is apparent even when peri-operative chemotherapy alone is used. [22] Given that the majority of patients with resected disease will relapse during the first 2 years after surgery, we postulated that adjuvant TM during this critical time period would be beneficial. Unfortunately, the addition of adjuvant TM for 2 years was challenging. Although TM is an oral agent, patients required close monitoring which included a clinical evaluation every 3 months plus monthly blood draws to assess ceruloplasmin levels. Several patients withdrew from treatment simply based on travel-related issues. Unexpected toxicity in 3 of the first 6 patients resulted in a marked dose reduction from 180 mg to 20 mg daily. We postulated that because this group of patients had an esophagectomy and therefore decreased acid secretion in the gastrointestinal tract, the TM was absorbed much more rapidly and completely than anticipated. Thus, a much lower dose of TM was effective for achieving the targeted dose of ceruloplasmin, with less toxicity. Because most of the side effects were relatively mild, patients might have been willing to tolerate this degree of toxicity for a short period of time. However, because the duration of therapy was 2 years, some patients elected to stop the drug rather than endure even a mild side effect for a prolonged period of time.
In conclusion, the combination of cisplatin and paclitaxel with hyperfractionated radiation was well tolerated. Adjuvant tetrathiomolybdate appeared to improve the recurrence-free probability compared to historical controls although no firm conclusions can be made. Although TM was generally well tolerated, the mild but persistent toxicity and frequent monitoring of ceruloplasmin levels made the full 2 year treatment course difficult to complete. Because of the challenges associated with prolonged administration of this therapy, further investigation of TM would be difficult in this patient population.
Acknowledgments
Supported in part by a grant NIH CA-67112 and by the Breast cancer Research Foundation
Footnotes
Presented in part at the Forty-Fifth Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, June 2009.
Ethical standards The trial was approved by the local Institutional Review Board and written informed consent was obtained from all patients.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Bryan J. Schneider, Email: bjs2004@med.cornell.edu, Division of Hematology/Oncology, Department of Internal Medicine, Weill Cornell Medical College, 1305 York Avenue, 7th floor, New York, NY 10021, USA
Julia Shin-Jung Lee, Email: julialee@umich.edu, Biostatistics Core Facility, University of Michigan Cancer Center, Ann Arbor, MI, USA. Institute for Social Research Program in Survey Methodology, University of Michigan, Room 4050, 426 Thompson Street, Ann Arbor, MI 48104-2321, USA.
James A. Hayman, Email: hayman@umich.edu, Department of Radiation Oncology, University of Michigan, 1500 E Medical Ctr Dr, SPC 5010, UH B2C490, Ann Arbor, MI 48109-5010, USA
Andrew C. Chang, Email: andrwchg@umich.edu, Department of Thoracic Surgery, University of Michigan, A. Alfred Taubman Health Care Center, 1500 East Medical Center Drive, Room 2120, Ann Arbor, MI 48109-5344, USA
Mark B. Orringer, Email: morrin@umich.edu, Department of Thoracic Surgery, University of Michigan, A. Alfred Taubman Health Care Center, 1500 East Medical Center Drive, Room 2120, Ann Arbor, MI 48109-5344, USA
Allan Pickens, Email: apicke3@emory.edu, Department of Thoracic Surgery, Emory University Hospital,, Midtown 550 Peachtree Street, NE Atlanta, GA 30308, USA.
Charlie C. Pan, Email: panch@bellin.org, Bellin Health Care System, 1580 Commanche Avenue, Green Bay, WI 54313, USA
Sofia D. Merajver, Email: smerajve@umich.edu, Division of Hematology/Oncology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA. Department of Internal Medicine, University of Michigan Comp Cancer Ctr, 7217 CCGC, 1500 E Medcl Ctr Dr, Ann Arbor, MI 48109-0948, USA
Susan G. Urba, Email: surba@umich.edu, Division of Hematology/Oncology, Department of Internal Medicine, University of Michigan, Ann Arbor, MI, USA. Department of Internal Medicine, University of Michigan Comp Cancer Ctr, C347 MIB, SPC 5848, 1500 E Medcl Ctr Dr, Ann Arbor, MI 48109-5848, USA
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Urba SG, Orringer MB, Ianettonni M, et al. Concurrent cisplatin, paclitaxel and radiotherapy as preoperative treatment for patients with locoregional esophageal carcinoma. Cancer. 2003;98:2177–2183. doi: 10.1002/cncr.11759. [DOI] [PubMed] [Google Scholar]
- 3.Gaast AV, van Hagen P, Hulshof M, et al. Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable esophageal or esophagogastric junction cancer: Results from a multicenter randomized phase III study. J Clin Oncol. 2010;28(Suppl):15s. abstr 4004. [Google Scholar]
- 4.Sharma R, Yang GY, Nava HR, et al. A single institution experience with neoadjuvant chemoradiation (CRT) with irinotecan (I) and cisplatin (C) in locally advanced esophageal carcinoma (LAEC) J Clin Oncol. 2009;27(Suppl):15s. abstr 15619. [Google Scholar]
- 5.Brewer GJ, Hedera P, Kluin KJ, et al. Treatment of Wilson’s disease with tetrathiomolybdate III. Initial therapy in a total of 55 neurology affected patients and follow-up with zinc therapy. Arch Neurol. 2003;60:378–385. doi: 10.1001/archneur.60.3.379. [DOI] [PubMed] [Google Scholar]
- 6.Brewer GJ, Askari F, Lorincz MT, et al. Treatment of Wilson’s disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double blind study of treatment of neurologic presentation of Wilson’s disease. Arch Neurol. 2006;63:521–527. doi: 10.1001/archneur.63.4.521. [DOI] [PubMed] [Google Scholar]
- 7.Pan Q, Kleer CG, van Golen KL, et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 8.Pan Q, Bao LW, Merajver SD. Tetrathiomolybdate inhibits angiogenesis and metastasis through suppression of the NFκB signaling cascade. Mol Canc Res. 2003;1:701–706. [PubMed] [Google Scholar]
- 9.Brewer GJ, Dick RD, Grover DK, et al. Treatment of metastatic cancer with tetrathiomolybdate, and anticopper, antiangiogenic agent: Phase I study. Clin Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 10.Henry NL, Dunn R, Merajver S, et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology. 2006;71:168–175. doi: 10.1159/000106066. [DOI] [PubMed] [Google Scholar]
- 11.Redman BG, Esper P, Pan Q, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res. 2003;9:1666–1672. [PubMed] [Google Scholar]
- 12.Gartner EM, Griffith KA, Pan Q, et al. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-fluorouracil and leucovorin for metastatic colon cancer. Investig New Drugs. 2009;27:159–165. doi: 10.1007/s10637-008-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewer GJ. Tetrathiomolybdate anticopper therapy for Wilson’s disease inhibits angiogenesis, fibrosis and inflammation. J Cell Mol Med. 2003;7:11–20. doi: 10.1111/j.1582-4934.2003.tb00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brewer GJ, Merajver S. Treatment of metastatic cancer with the anticopper, antiangiogenic, tetrathiomolybdate. J Investig Med. 1999;47:223A. [PubMed] [Google Scholar]
- 15.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephro. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 18.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2007;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 19.Miller K, Wang M, Gralow J, et al. Paclitaxel Plus Bevacizumab Versus Paclitaxel Alone for Metastatic Breast Cancer. N Engl J Med. 2007;35:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 20.Hassouneh B, Islam M, Nagel T, et al. Tetrathiomolybdate promotes tumor necrosis and prevents distant metastases by suppressing angiogenesis in head and neck cancer. Mol Cancer Ther. 2007;6:1039–1045. doi: 10.1158/1535-7163.MCT-06-0524. [DOI] [PubMed] [Google Scholar]
- 21.Pass HI, Brewer GJ, Dick R, et al. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann Thorac Surg. 2008;86:383–390. doi: 10.1016/j.athoracsur.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastro-esophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]