Abstract
Background
Head and neck squamous cell carcinoma (HNSCC) is associated with tobacco use. Still, most smokers do not develop HNSCC. The mechanisms of varying susceptibility to HNSCC are poorly studied to date. Tobacco metabolite research provides insight regarding the innate metabolism and excretion of carcinogens.
Methods
Smokers with HNSCC (cases) were compared with smokers without HNSCC (controls) in a matched cohort. The tobacco metabolites studied were: 1-hydroxypyrene (1-HOP), N′-nitrosonornicotine (NNN), and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL).
Results
In 33 subjects, mean 1-HOP was 1.82 pmol/mg creatinine versus 1.08 pmol/mg creatinine (p = .004) and mean NNN was 0.10 pmol/mg creatinine versus 0.04 pmol/mg creatinine (p = .01) in cases and controls, respectively. NNAL did not differ between groups.
Conclusions
Smokers with HNSCC have elevated urinary levels of 1-HOP and total NNN compared with matched controls, suggesting an increased effective exposure to these carcinogens. Tobacco constituent metabolites may be useful in understanding tobacco-related carcinogenesis in HNSCC.
Keywords: tobacco, head and neck cancer, metabolites, biomarkers, carcinogenesis
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is one of many cancers that are strongly associated with tobacco use.1,2 The risk for HNSCC in smokers is approximately 10 times higher than that of never-smokers.3 Although tobacco cessation does reduce the risk of carcinoma, there are conflicting data as to whether a past smoker’s risk of carcinoma ever decreases to the level of a never-smoker.4 Alcohol enhances the risk of HNSCC in tobacco users but also acts as an individual risk factor.5 When considering all new presentations of HNSCC, 80% are associated with tobacco and alcohol use.5
Although smoking is an important causal factor for HNSCC, only a fraction of lifelong smokers develop HNSCC over their lifetime. This is evident given that the number of smokers in the United States is estimated at approximately 46 million, whereas there are approximately 50,000 cases of HNSCC diagnosed per year.6–8 The factors that account for the difference in susceptibility between smokers with and without HNSCC are poorly studied to date. Some smokers may be inherently more susceptible to developing carcinoma due to patterns of tobacco use, individual intrinsic metabolism of carcinogens, or altered excretion. Additionally, variability in individual immune competence can impact susceptibility to a variety of carcinomas, including those of the head and neck.9 Identifying those smokers at greatest risk for HNSCC would have great benefit through targeted smoking cessation efforts and enhanced surveillance in addition to a greater understanding of carcinogenic pathways.
One approach to better understand the extent of exposure to tobacco carcinogens is through the investigation of tobacco carcinogen metabolites. The interindividual variation in risk of smoking-related HNSCC may be determined in part by individual variability in the uptake and metabolism of tobacco carcinogens. Evaluation of tobacco-related carcinogens and their metabolites can determine exposure and extent or pattern of metabolism and perhaps ultimately characterize important differences between smokers who develop HNSCC and smokers who do not. Much of the work performed thus far on biomarkers of tobacco-related carcinogens and toxicants has addressed their role in the development of lung cancer. This work helps to inform the study in patients who develop HNSCC. There are >70 established carcinogens in cigarette smoke.10 With regard to lung carcinoma, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and polycyclic aromatic hydrocarbons (PAHs) are considered to be important causative agents. The ingestion of NNK results in metabolic conversion to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL). Administration of NNK to rodents results in the development of lung and nasal tumors. NNAL is a potent pulmonary carcinogen in rats.11 Furthermore, epidemiologic data in humans directly links NNAL to the risk of developing lung cancer.11,12 NNAL is glucuronidated in humans to produce a mixture of glucuronides, NNAL-Glucs.13 Although NNK is not detectable in human urine due to its extensive metabolism, both NNAL and NNAL-Glucs (the sum of which will be designated as total NNAL) can be quantified in human urine.14,15
Many PAHs are potent locally acting carcinogens in laboratory animals.16 The most extensively applied and reliable biomarker of PAH exposure is 1-hydroxypyrene (1-HOP). It is a urinary metabolite of pyrene, which is always present in PAH mixtures. Although 1-HOP is not tobacco specific, urinary levels are generally 2 to 3 times higher in smokers when compared with non-smokers.15,17,18 Another commonly studied member of the PAH class is benzo[a]pyrene (BaP). BaP causes skin tumors when applied to the skin of different strains of mice whereas subcutaneous injection produces malignant tumors at the injection site. Intratracheal administration alone or mixed with particulates generates benign and malignant tumors in hamsters. Administration of BaP in the diet to mice and rats of different strains led to tumor development in multiple organs including the esophagus and tongue.16 Inhalation administration of BaP to hamsters caused dose-related increases in tumors of the upper respiratory tract, including nose, larynx and trachea, and upper digestive tract including the pharynx, esophagus, and forestomach.16
N′-Nitrosonornicotine (NNN) is thought to play a role in both esophageal and oral cancer.15 It can be readily quantified in human urine by assaying for free, unchanged NNN plus its metabolite NNN-N-Gluc.19 NNN and its metabolites are present in the urine of both smokers and smokeless tobacco users. The level of urinary NNN is significantly higher in smokeless-tobacco users when compared with smokers. This finding is consistent with the relatively higher levels of tobacco-specific nitrosamines in smokeless tobacco.19
This report describes a preliminary analysis of tobacco metabolites in patients with HNSCC. A cohort of smokers with HNSCC has been identified and enrolled in a control-matched study to determine the significance of urinary NNAL, NNN, and 1-HOP in this patient population.
MATERIALS AND METHODS
Study population
Approval from the University of Minnesota Institutional Review Board was obtained. Cases of smokers with a new or previous diagnosis of HNSCC presenting to the Otolaryngology Clinic at the University of Minnesota from April 2009 to June 2011 were identified for enrollment into this study. After obtaining consent, a tobacco and alcohol use questionnaire was administered. This questionnaire is comprehensive in nature and queries multiple aspects of all tobacco use (smokeless and smoked) and alcohol use including duration of use, current and previous rates of use, specific products used, attempted cessation methods, and perceived obstacles to cessation. After completion of the questionnaire, the enrollees submitted a 10-mL urine and 10-mL blood sample. The urine samples were kept in a −20°C cooler until assays were performed. The blood samples were centrifuged to allow separation of the buffy coat and then kept at −20°C for future studies.
Controls consisted of smokers enrolled in the University of Minnesota Tobacco Research Programs for smoking cessation or biomarker studies. These smokers were also given the tobacco and alcohol use questionnaire and submitted blood and urine for analysis as above. Data on control smokers are kept in databases at the Tobacco Research Programs at the University of Minnesota. The control databases contain demographics and urinary metabolite levels at the time of enrollment in smoking cessation studies. Cases and controls were matched on gender and cigarettes per day for purposes of analysis. Alcohol consumption is known to be a risk factor for HNSCC but does not directly impact the tobacco-related metabolites described in this report. It must be noted, however, that ethanol has been shown to be an effective solvent that can increase absorption of carcinogenic substances across a mucosal barrier.20 However, this effect is measureable at ethanol concentrations much higher than that seen in the most conventional alcoholic beverages (in which the ethanol concentration is generally 15% or less). It was therefore determined that alcohol intake in our study population would have little to no measureable effect on the urinary tobacco metabolites quantified here and subjects were not matched on levels of alcohol intake.
Experimental assays
Total NNAL, total NNN, and 1-HOP were determined as described previously. Briefly, the assay for total NNAL in urine was performed by a modification of a previously published method.14 The method involves solid-phase extraction of urine with Chem-Elute and Oasis MCX mixed mode cation exchange cartridges followed by quantification by gas chromatography with nitrosamine selective detection. The detection limit of NNAL was 0.04 pmol/mL. The intraday precision of the assay was 10.9% relative SD (RSD) and interday precision was 13.7% RSD.14,21 1-HOP was determined by high performance liquid chromatography with fluorescence detection, using [D9]1-HOP as internal standard. Intraday and interday precision were less than 5% RSD.22,23
For the analysis of total NNN,24–26 2-mL urine samples were mixed with [13C6]NNN internal standard and treated with 10N NaOH to convert NNN-N-Gluc to NNN.25 After the hydrolysis, the samples were adjusted to pH 6 to 8 and extracted with ethyl acetate, extracted on ChemElut cartridges, and further purified by mixed mode cation exchange and normal phase extraction, as previously described.26 The samples were analyzed by liquid chromatography-tandem mass spectrometry with selected reaction monitoring for m/z 178 → m/z 148 for NNN and m/z 184 → m/z 154 for [13C6]NNN.
Urinary creatinine was assayed from the same urine sample to adjust for dilutional effects. Urinary creatinine was assayed by Fairview-University Medical Center Diagnostic Laboratories (Minneapolis) with a Kodak Ekta-chem 500 chemistry analyzer.
Statistical analysis
Statistical analysis was performed with the t test using Stata (College Station, TX) software with 2-sided p values < .05 considered significant. Given that this is an analysis of pilot data, we sought to strengthen the analysis and conclusions by performing control matching using a 3:1 ratio of controls to cases. This was performed in the analysis of NNAL and 1-HOP. The 3:1 matching was performed to decrease the impact of any outlier values contained in the control dataset. However, we do not currently have sufficient controls with NNN determined to allow a 3:1 match. This was due to the nature of our control database. This database represents an ongoing collection of specimens (blood, urine) from smokers recruited for smoking cessation research. Although NNAL and 1-HOP have been routinely assayed on these smokers for several years, NNN has been assayed in a consistent fashion only recently. As a result, the database contains fewer subjects with NNN values determined when compared to NNAL and 1-HOP. Therefore, the analysis of NNN was done with a 1:1 matching ratio. The SD in the control group for NNN was lower than that seen for 1-HOP and NNAL such that this did not significantly impact the power of this study. The number of subjects needed to obtain power of 80% with α = 0.5 to detect a 50% increase in creatinine-adjusted metabolite level ranged from 15 to 21 for NNN, 1-HOP, and NNAL.
RESULTS
This analysis was based on examination of the first 33 HNSCC cases enrolled in our study. The demographics and distribution of the tumor subsites in the 33 HNSCC cases is shown in Table 1. The cohort was made up of 26 men and 7 women, all of whom smoked cigarettes as their only tobacco product. The range of cigarettes per day was 4 to 60. All levels of urinary toxicant metabolites were adjusted for urinary creatinine level.
TABLE 1.
Distribution of sex and tumor subsite in study group.
| Variable | No. of patients (%) |
|---|---|
| Sex | |
| Male | 26/33 (79) |
| Female | 7/33 (21) |
| Tumor subsite | |
| Nasopharynx | 1/33 (3) |
| Oropharynx | 11/33 (33) |
| Larynx | 11/33 (33) |
| Hypopharynx | 3/33 (9) |
| Oral cavity | 7/33 (21) |
Of the 33 cases, we were able to quantify 1-HOP in 29, total NNAL in 29, and total NNN in 32. During the early stages of enrollment, some urine samples were lost or smaller than necessary such that we were not able to have all 3 metabolites assayed in some cases. For 1-HOP and total NNAL, we used a 3:1 matching ratio of controls to cases to strengthen the analysis. Given that we were able to obtain 27 matched controls for the NNN values our analysis of NNN was based on the 27 cases with available matched controls. Matching variables were gender and cigarettes per day. The metabolites studied here were not influenced by alcohol consumption.
The results of the statistical analysis are shown in Table 2. Levels of 1-HOP and NNN in cases were approximately 1.7 to 2.5 times higher than that seen in controls. In the case of 1-HOP and NNN, this difference reached statistical significance (p = .004 and p = .01, respectively). Levels of NNAL were not significantly different between case and control groups. Figures 1–3 are scatterplots of the total 1-HOP, NNN, and total NNAL values in cases and controls.
TABLE 2.
Tobacco metabolite levels in cases and controls.
| Variable | Case | Control | p value |
|---|---|---|---|
| n 1-HOP | 30 | 100 | |
| 1-HOP, pmol/mg Cr (± SE) | 1.82 (± 0.25) | 1.08 (± 0.12) | .004 |
| n NNN | 27 | 27 | |
| NNN, pmol/mg Cr (± SE) | 0.10 (± 0.02) | 0.04 (± 0.005) | .014 |
| n NNAL | 29 | 100 | |
| NNAL, pmol/mg Cr (± SE) | 1.51 (± 0.19) | 1.68 (± 0.11) | .48 |
Abbreviations: 1-HOP, 1-hydroxypyrene; NNN, nitrosonornicotine; NNAL, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol.
FIGURE 1.
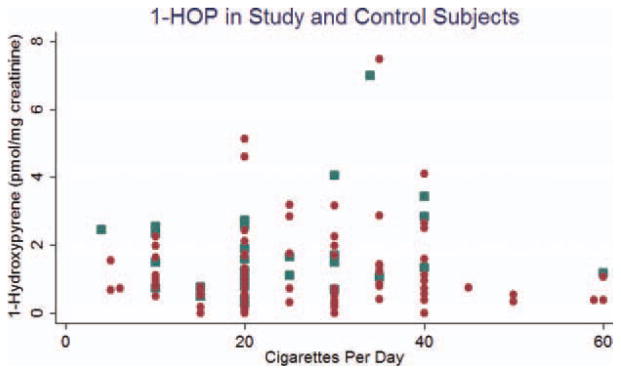
Scatterplot of 1-hydroxypyrene (1-HOP) in study (blue dot) and control (red dot) subjects. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 3.
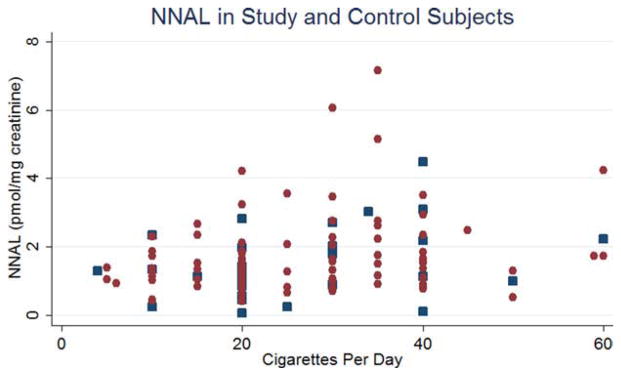
Scatterplot of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in study (blue dot) and control (red dot) subjects. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Tobacco is well known to be a source of multiple carcinogens. Chronic use of tobacco products is strongly linked to the development of multiple forms of carcinoma.2 The mucosal lining of the upper aerodigestive tract receives significant exposure to tobacco smoke as it is transmitted from the lips to the lungs. As a result, it is common to see a strong history of tobacco use in patients presenting with HNSCC. Still, a relatively small proportion of smokers go on to eventually develop head and neck cancer. One explanation for this may be variability in the uptake and processing of tobacco carcinogens and toxicants based on individual genetic differences and other factors among users.
This report represents the first investigation of tobacco-related carcinogens and their metabolites in smokers with HNSCC compared with smokers without HNSCC. The results demonstrate intriguing differences in levels of carcinogen exposure between these 2 populations despite controlling for cigarette consumption. 1-HOP is significantly elevated in the HNSCC cases compared with controls. This is notable in that a significant difference is seen in a relatively small sample size. The results indicate that PAH-related carcinogenesis may be prominent for this disease. This suggests an exciting path for further investigation as smokers with HNSCC may have an increased susceptibility to the effects of 1-HOP and other PAHs, thus resulting in their developing HNSCC while other smokers do not. The difference in susceptibility can be mediated by several mechanisms, one of which may be the previously described polymorphisms of CYP1A1, GSTM1, and GSTT1 enzymes that result in activation of PAH.27 Therefore, the data presented here point us in the direction of investigating these polymorphisms in tobacco-induced HNSCC. Given that 1-HOP is elevated in the smokers with HNSCC in this study, investigation of other PAH compounds may be warranted.
Our data demonstrating elevated NNN in those smokers with HNSCC compared with smokers without HNSCC, is interesting given that NNN has been shown to induce esophageal and nasal cavity tumors in rats.28 Like the results for 1-HOP (above), NNN is present in higher levels in smokers with HNSCC compared with smokers without HNSCC when controlling for cigarette intake. This may be due to differences at the molecular level relating to activation and/or excretion of NNN such that those smokers who are less able to detoxify this carcinogen are at increased risk for HNSCC.
Given the anatomic proximity and histological similarity of the esophagus to the upper aerodigestive tract, this finding would seem to be intuitive as it is reasonable to expect a similar mechanism of carcinogenesis in tumors of the esophagus and upper aerodigestive tract. Animal studies suggest a prominent role for NNN in the development of a variety of cancers.29 NNN given in drinking water or by subcutaneous injection induces predominantly nasal tumors in rats.11 The tumors induced include squamous cell carcinoma, rhabdomyosarcoma, olfactory neuroblastoma, as well as papillomas. Both the esophageal and nasal mucosa can metabolically activate NNN, and DNA adducts are detected in both tissues. Those rats receiving NNN in drinking water only are more likely to develop esophageal tumors than those receiving subcutaneous injection. This finding is hypothesized to be related to direct contact of NNN with the esophageal mucosa in those rats given the compound in drinking water. In smokers, urinary total NNN was found to be strongly associated with the risk of developing esophageal cancer in a prospective study based on a cohort of 18,244 Chinese men in Shanghai, China.30 In that study, urinary total NNN was measured in samples collected before diagnosis in 77 patients with esophageal cancer and 223 individually matched controls, all current smokers. The levels of total NNN were significantly higher in cases as compared to controls. Odds ratios (95% confidence intervals) of esophageal cancer for the second and third tertiles of total NNN were 3.99 (1.25–12.7) and 17.0 (3.99–72.8), respectively, compared to the first tertile after adjustment for urinary total NNAL, total cotinine, and smoking intensity and duration (Ptrend < 0.001). These findings, along with data from previous experimental studies, strongly suggest a significant and unique role of NNN in esophageal carcinogenesis in humans. This is consistent with the data presented here in that total NNN is elevated in smokers with HNSCC.
In this study, total NNAL did not differ between cases and controls. This suggests a less significant role for NNK (precursor of NNAL) in the carcinogenic pathway of HNSCC. On the other hand, total NNAL is associated with the risk of developing lung cancer. A recent study by Church et al31 prospectively examined serum levels of total NNAL in smokers with and without lung cancer. Before adjustment for confounders, the variables of age, duration of smoking, and serum total NNAL were statistically significantly associated with the risk of lung cancer. After logistic regression analysis, only age and total NNAL level remained statistically significant. A second recent study examined prediagnostic levels of tobacco metabolites and the risk of lung cancer in 2 prospective cohorts of cigarette smokers.12 Patients with total urinary NNAL in the second and third tertiles had an increased risk of developing lung cancer (RR, 1.43 and 2.11, respectively). When considering levels of cotinine in combination with total NNAL, an interesting result was observed. Those subjects who were in the highest tertile of total NNAL and total cotinine had an 8.5-fold risk of developing lung cancer compared with smokers with a comparable smoking history but total cotinine and NNAL levels in the lowest tertile. Similar more extensive studies need to be conducted among individuals with HNSCC.
We are encouraged by the potential implications of the data presented here as it supports the identification of the tobacco-related carcinogens that are most important in the development of HNSCC. This information will be useful in targeting the metabolism of those specific carcinogens that are most responsible for HNSCC. In this way, the genetic polymorphisms that account for varying metabolism, and therefore varying degrees of risk for tobacco-induced HNSCC, may be identified. Ultimately, this information has the potential to identify those who are at highest risk for developing tobacco-induced HNSCC. It is our hope that, with further study of the metabolites investigated in this report, as well as additional metabolites and markers, we can eventually describe tobacco constituent metabolite profiles that indicate “standard increasd risk” or “highest risk” for developing HNSCC. Given that the assays performed in this study are relatively inexpensive, it is conceivable that they may eventually be used in screening for those smokers who are most likely to develop HNSCC. Last, our data suggest an important difference between the carcinogenic pathways of HNSCC and lung carcinoma in that NNAL levels have been shown to indicate risk for lung carcinoma but were not elevated in our patients with HNSCC.
The main limitation of our study was that it was preliminary in nature and thus contained a small cohort. However, we feel that the potential implications of the data are nonetheless significant, as discussed above. An additional limitation was that due to the small sample size we were unable to perform an analysis based on tumor subsite within the upper aerodigestive tract. We anticipate performing this analysis as our cohort increases in size.
In summary, we have presented the first report of urinary tobacco-related carcinogen metabolites in patients with head and neck cancer. This preliminary analysis has identified statistically significant differences between smokers with HNSCC and smokers without HNSCC. We now plan to study these metabolites in a larger group of subjects. We anticipate that further study along this line will improve our understanding of tobacco-associated carcinogenesis in head and neck cancer and provide a potential opportunity for prediagnosis risk assessment and prevention.
FIGURE 2.
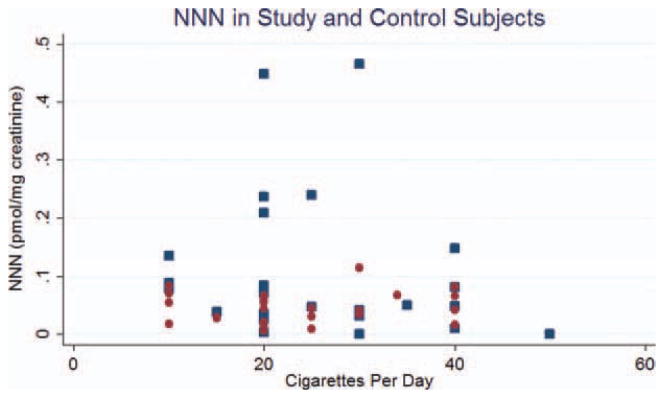
Scatterplot of nitrosonornicotine (NNN) in study (blue dot) and control (red dot) subjects. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Acknowledgments
Contract grant sponsor: The Minnesota Medical Foundation.
This publication was supported by Grant Number 1UL1RR033183 from the National Center for Research Resources (NCRR) of the National Institutes of Health (NIH) to the University of Minnesota Clinical and Translational Science Institute (CTSI). Its contents are soley the responsibility of the authors and do not necessarily represent the official views of the CTSI or the NIH. The University of Minnesota CTSI is part of a national Clinical and Translational Science Award (CTSA) consortium created to accelerate laboratory discoveries into treatments for patients.
References
- 1.Peto R, Lopez AD, Boreham J, Thun M, Heath C, Jr, Doll R. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Tobacco or health: a global status report. Geneva: World Health Organization; 1997. [Google Scholar]
- 3.Schlecht NF, Franco EL, Pintos J, Kowalski LP. Effect of smoking cessation and tobacco type on the risk of cancers in the upper aerodigestive tract in Brazil. Epidemiology. 1999;10:412–418. doi: 10.1097/00001648-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Day GL, Blot WJ, Shore RE, et al. Second cancers following oral and pharyngeal cancers: role of tobacco and alcohol. J Natl Cancer Inst. 1994;86:131–137. doi: 10.1093/jnci/86.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Sturgis EM, Wei Q, Spitz MR. Descriptive epidemiology and risk factors for head and neck cancer. Semin Oncol. 2004;31:726–733. doi: 10.1053/j.seminoncol.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). . Tobacco use among adults—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1145–1148. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). . Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1227–1232. [PubMed] [Google Scholar]
- 9.Duray A, Demoulin S, Hubert P, Delvenne P, Saussez S. Immune suppression in head and neck cancers: a review. Clin Dev Immunol. 2010;2010:701657. doi: 10.1155/2010/701657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 11.Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- 12.Yuan JM, Koh WP, Murphy SE, et al. Urinary levels of tobacco-specific nitrosamine metabolites in relation to lung cancer development in two prospective cohorts of cigarette smokers. Cancer Res. 2009;69:2990–2995. doi: 10.1158/0008-5472.CAN-08-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmella SG, Le Ka KA, Upadhyaya P, Hecht SS. Analysis of N- and O-glucuronides of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Chem Res Toxicol. 2002;15:545–550. doi: 10.1021/tx015584c. [DOI] [PubMed] [Google Scholar]
- 14.Carmella SG, Han S, Fristad A, Yang Y, Hecht SS. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003;12(11 Pt 1):1257–1261. [PubMed] [Google Scholar]
- 15.Hecht SS. Human urinary carcinogen metabolites: biomarkers for investigating tobacco and cancer. Carcinogenesis. 2002;23:907–922. doi: 10.1093/carcin/23.6.907. [DOI] [PubMed] [Google Scholar]
- 16.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum. 2010;92:1–853. [PMC free article] [PubMed] [Google Scholar]
- 17.Carmella SG, Chen M, Han S, et al. Effects of smoking cessation on eight urinary tobacco carcinogen and toxicant biomarkers. Chem Res Toxicol. 2009;22:734–741. doi: 10.1021/tx800479s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hecht SS, Yuan JM, Hatsukami D. Applying tobacco carcinogen and toxicant biomarkers in product regulation and cancer prevention. Chem Res Toxicol. 2010;23:1001–1008. doi: 10.1021/tx100056m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stepanov I, Hecht SS. Tobacco-specific nitrosamines and their pyridine-N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol Biomarkers Prev. 2005;14:885–891. doi: 10.1158/1055-9965.EPI-04-0753. [DOI] [PubMed] [Google Scholar]
- 20.Du X, Squier CA, Kremer MJ, Wertz PW. Penetration of N-nitrosonornicotine (NNN) across oral mucosa in the presence of ethanol and nicotine. J Oral Pathol Med. 2000;29:80–85. doi: 10.1034/j.1600-0714.2000.290205.x. [DOI] [PubMed] [Google Scholar]
- 21.Carmella SG, Akerkar SA, Richie JP, Jr, Hecht SS. Intraindividual and interindividual differences in metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers’ urine. Cancer Epidemiol Biomarkers Prev. 1995;4:635–642. [PubMed] [Google Scholar]
- 22.Carmella SG, Le KA, Hecht SS. Improved method for determination of 1-hydroxypyrene in human urine. Cancer Epidemiol Biomarkers Prev. 2004;13:1261–1264. [PubMed] [Google Scholar]
- 23.Hochalter JB, Zhong Y, Han S, Carmella SG, Hecht SS. Quantitation of a minor enantiomer of phenanthrene tetraol in human urine: correlations with levels of overall phenanthrene tetraol, benzo[a]pyrene tetraol, and 1-hydroxypyrene. Chem Res Toxicol. 2011;24:262–268. doi: 10.1021/tx100391z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stepanov I, Carmella SG, Briggs A, et al. Presence of the carcinogen N′-nitrosonornicotine in the urine of some users of oral nicotine replacement therapy products. Cancer Res. 2009;69:8236–8240. doi: 10.1158/0008-5472.CAN-09-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porubin D, Hecht SS, Li ZZ, Gonta M, Stepanov I. Endogenous formation of N′-nitrosonornicotine in F344 rats in the presence of some antioxidants and grape seed extract. J Agric Food Chem. 2007;55:7199–7204. doi: 10.1021/jf0712191. [DOI] [PubMed] [Google Scholar]
- 26.Stepanov I, Hecht SS. Detection and quantitation of N′-nitrosonornicotine in human toenails by liquid chromatography-electrospray ionization-tandem mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2008;17:945–948. doi: 10.1158/1055-9965.EPI-07-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexandrie AK, Warholm M, Carstensen U, et al. CYP1A1 and GSTM1 polymorphisms affect urinary 1-hydroxypyrene levels after PAH exposure. Carcinogenesis. 2000;21:669–676. doi: 10.1093/carcin/21.4.669. [DOI] [PubMed] [Google Scholar]
- 28.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–744. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann D, Hecht SS. In: Handbook of Experimental Pharmacology. Cooper CS, Grover PL, editors. Heidelberg: Springer–Verlag; 1990. pp. 63–102. [Google Scholar]
- 30.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary levels of the tobacco-specific carcinogen N′-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis. 2011;32:1366–1371. doi: 10.1093/carcin/bgr125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Church TR, Anderson KE, Caporaso NE, et al. A prospectively measured serum biomarker for a tobacco-specific carcinogen and lung cancer in smokers. Cancer Epidemiol Biomarkers Prev. 2009;18:260–266. doi: 10.1158/1055-9965.EPI-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]


