Abstract
In this post hoc analysis of baseline responses to the CERAD Behavior Rating Scale for Dementia in a clinical trial of interventions for agitation in Alzheimer’s disease (AD), the authors investigated the distribution of, and relationships between, agitation, depression, and psychosis in 148 individuals with AD. Prevalence of depressive symptoms was highest (78.4%), followed by agitation (77.6%) and psychotic symptoms (69.3%); 51.1% of the sample had symptoms in all 3 domains. Cross-sectionally, psychotic symptoms were most closely associated with Mini-Mental State Examination (MMSE) scores, while agitation was less so. Depressive symptoms were relatively consistently prevalent across MMSE levels. After controlling for the presence of agitated symptoms, psychosis and depression were significantly associated, but neither symptoms of psychosis nor of depression were associated with agitation when depression or psychosis, respectively, was controlled for. Significant psychopathological comorbidity should be considered in the design of clinical trials targeting particular psychopathology in this disease population.
Keywords: psychopathology, Alzheimer’s, comorbidity, psychosis, agitation, depression
The behavioral symptoms associated with Alzheimer’s disease (AD) are of intense interest to researchers, clinicians, and caregivers.1 The relevance of these symptoms has increased with the growing understanding of AD, including the detection of significant psychopathology in persons with mild cognitive impairment (MCI) relative to cognitively intact elderly.2 MCI may be a prodromal stage of AD,3 and the observation of psychopathology in persons with MCI that is similar to the psychopathology of AD supports this argument.
The 3 most commonly described types of psychopathological symptoms are agitated, depressive/affective, and psychotic. These symptoms are assessed with a wide variety of instruments,4 but regardless of measurement method, recent investigations have demonstrated that such symptoms are common and may be interrelated. However, their relationships and their association with the cognitive changes of AD are not yet clearly understood. In the present report, we explored cross-sectional comorbidity of these symptom types in a cohort of AD patients participating in a clinical trial for treatment of agitation.
One factor limiting our understanding of the interrelationships between these symptom types is the inconsistency of the literature reporting comorbidity. This could be due, in part, to the heterogeneity of behavioral symptoms in persons with AD,5 although AD patients exhibiting only one symptom type are consistently reported to be relatively rare (eg, Levy et al,6 30%; Frisoni et al,7 12%; Lyketsos et al,8 18%). Between 64%9 and 97%10 of patients have been reported to have one or more behavioral symptoms at initial evaluation (see also refs 2, 6–8). Symptoms may differ in the degree of persistence over time,9,10 and some may become more prevalent as the disease worsens (eg, agitation/aggression8).
In the face of these sources of variability in the behavioral disturbances observed in persons with AD, it is not surprising that a relationship between symptoms of agitation and depression has been observed in some studies6,11 but not in others7–10,12; similarly, relationships of varying degrees between symptoms of agitation and psychosis have been reported by many groups (see refs 6, 7, 9, and 10 and to a lesser extent 12), but a lack of association has also been reported (see ref 8 and also the factor analyses in ref 11).
In summary, across large studies involving caregiver-rated behavioral symptoms in persons with AD, depressive and psychotic symptoms have been consistently reported to be independent,6–10,12 but the associations between psychosis, depression, and agitation are not clear. In an attempt to shed further light on this issue, we conducted these post hoc analyses of the baseline behavioral symptoms of a group of community-dwelling persons with AD who were recruited to a randomized, placebo-controlled study of interventions for agitation.13 The present study was undertaken to determine the distribution and prevalence rates of symptoms of psychosis and depression in a group of agitated AD patients.
By studying this “enriched” population, we hoped to establish whether depressive or psychotic symptoms are associated with agitation in AD. A secondary goal was to cross-sectionally examine the relationships of symptoms of depression, agitation, and psychosis to cognitive impairment in AD.
METHODS
The assessment instrument employed was the CERAD Behavioral Rating Scale for Dementia (BRSD14). We examined the individual BRSD item responses from the baseline visit of community-dwelling AD patients who had volunteered to participate in a clinical study. BRSD symptoms were assessed by caregivers for frequency in the month before the baseline visit; BRSD ratings were not used to determine eligibility for the study.
Subjects
This study sample has been described elsewhere.13 All participants met the National Institute of Neurologic and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria for probable or possible AD15; informed consent was obtained for all participants from them and their caregivers. The patients (N = 148) were recruited to a randomized, placebo-controlled clinical trial of pharmacologic (haloperidol, trazodone) treatment or behavior management therapy. Each patient had a history of 2 or more agitated behaviors occurring at least once per week for at least 2 weeks at a severity level that was characterized as disruptive or distressing to the caregiver.13 Their characteristics are presented in Table 1. Exclusion criteria for this study were the presence of a major psychiatric disorder in the prior 2 years, lack of reliable caregiver, and inability to withstand a 2-week washout of psychotropic medications for behavioral symptoms.
Table 1.
Demographic Variable Summaries for Alzheimer’s Disease Subjects (N = 148)
| Variable | M | SD |
|---|---|---|
| Age (years) | 74.7 | 7.1 |
| Education (years) | 12.6 | 3.5 |
| Mini-Mental State Examinationa | 13.2 | 7.5 |
| Behavior Rating Scale for Dementiab | 51.3 | 22.5 |
| Gender (% female) | 55 |
Range = 0–30 (0 = worst).
Range = 0–164 (0 = best).
Materials
The BRSD, administered as a 48-item instrument,14 was recoded according to the current 46-item algorithm,16 in which items pertaining to suicide and self-harm were eliminated due to rarity. The BRSD has established validity and reliability.11 Items are rated for frequency in the previous month by an informed caregiver. Of the 46 BRSD items, 37 are rated for their frequency in the preceding month; 8 other items elicit yes/no responses describing the symptom in the subject relative to before the onset of dementia, and 1 is nonspecific (“anything else?”). Total BRSD scores range from 0 (no symptoms in past month) to 164 (all symptoms on at least 16 days in past month). These analyses were based on the 37 frequency-rated items that characterize each symptom as occurring “not in the past month” (rated 0), “1–2 days in the past month” (rated 1), “3–8 days in the past month” (rated 2), “9–15 days in the past month” (rated 3), or “16 or more days in the past month” (rated 4).
The Mini-Mental State Examination (MMSE)17 established global cognitive function and includes questions targeting orientation, attention, memory, calculation, and linguistic capacity. Scores range from 0 (worst functioning) to 30 (normal cognitive status).
All patient-participants were administered the MMSE and BRSD at their initial visit. The MMSE scores for both groups were stratified into dementia severity levels: 21–30 (mild), 16–20, 10–15, 5–9, and 0–4 (severe).18
Statistical Methods
We assessed the presence of symptoms of depression or psychosis based on the items included on the prescribed BRSD subscales.16 Symptoms of depression included feelings of anxiety, sad appearance, feelings of hopelessness, crying, feelings of guilt, poor self-esteem, and feelings that life is not worth living (items 1, 2, 3, 4, 6, 7, and 8). Symptoms of psychosis included beliefs that the TV characters are real, a dead person is still alive, the house is not home, and auditory and visual hallucinations (items 40, 41, 42, 43, 44, and 45). If a subject endorsed at least one item in a given subscore, that psychopathological domain (eg, depressive symptoms) was considered “present.” It should be noted that 1 subject (0.67% of the sample) had “anxiety” as the only symptom of depression.
We assessed the presence of agitation by examining the endorsement of a single BRSD item: “Have there been times when [the patient] was agitated or upset?” (item 19).16 According to the BRSD manual,16 this item assesses “observable signs of … emotional distress” and is distinguished by the emotional component from another item assessing purely motoric agitation, although both may be present.16(p13) We found that 94.7% of subjects who endorsed the BRSD agitation item (ie, rated it as having occurred at least 3 days in the past month) scored in the “agitated” range (at least 15 points) on the Cohen-Mansfield Agitation Inventory (CMAI)19 at their baseline visit (see Tractenberg et al20 for derivation of the “agitated” range of CMAI scores). We also found that 83.7% of participants who scored in the agitated range of the CMAI endorsed the agitation item on the BRSD. We selected this BRSD item for our agitation indicator because it represented a measure of this domain but was the symptom that overlapped the least with the list of symptoms used as entry criteria for the study and does not appear on the CMAI. The BRSD does not have an agitation subscore, so with this item we were able to use the CMAI score to evaluate its utility and measure associations between symptoms of depression, psychosis, and agitation using a single instrument.
Logistic regression was used to evaluate the likelihood that subjects exhibiting symptoms in one domain would also exhibit symptoms in another. We controlled for 3 covariates: age, MMSE, and gender, as well as the presence of symptoms in the third behavioral domain in each regression analysis.
RESULTS
We found that 69.3% of this group of AD patients had one or more symptoms of psychosis, 78.4% had one or more symptoms of depression, and 77.6% had agitation as assessed by the BRSD. (The BRSD was not used as an intake instrument for this study, so we would not anticipate that 100% of the subjects would appear agitated by BRSD-based criteria.) Only 18.0% of subjects had symptoms in only 1 domain; 27.3% had symptoms in 2 domains, and 51.1% had all 3 symptom types, supporting the decision to control for the presence of symptoms in the third domain when assessing the association between symptoms in the other two.
The results of the regression analyses are presented in Table 2. After controlling for the presence of agitation and the other covariates, we found a significant association between symptoms of psychosis and depression (χ2 = 10.0, P < .01). Individuals with psychotic symptoms were nearly 5 times more likely than individuals without psychosis to have symptoms of depression (odds ratio [OR] = 4.9; 95% confidence interval [CI] = 1.8–13.1). After controlling for the presence of depressive symptoms and the other covariates, the likelihood of psychotic symptoms was higher, but not significantly so, in persons with agitation than in those without (χ2 = 2.78, P = .1; OR = 2.2; 95% CI = 0.87–5.4). After controlling for the presence of psychosis and the other covariates, no association between symptoms of depression and agitation was found (χ2 = 0.46, P = .50; OR = 1.4, 95% CI = 0.53–3.7).
Table 2.
Odds of Symptoms in One Domain Given Symptoms in a Second After Controlling for Age, Gender, Mini-Mental State Examination Score, and Presence of Symptoms in a Third Domain
| Controlling for Covariates Plus: | To Examine Relationship Between: | And Found:
|
With Significance:
|
||
|---|---|---|---|---|---|
| 95% Odds Ratio | Confidence Interval | χ2 | P Value | ||
| Agitation | Depressive and psychotic symptoms | 4.9 | 1.8–13.1 | 10.0 | P<.01 |
| Depressive symptoms | Agitation and psychotic symptoms | 2.2 | 0.87–5.4 | 2.74 | P=.10 |
| Psychotic symptoms | Agitation and depressive symptoms | 1.4 | 0.53–3.7 | 0.46 | P=.50 |
We also found that when symptoms of agitation and depression and the other covariates were controlled for, higher MMSE score (less dementia) conferred a significantly but only marginally lower risk of psychotic symptoms (χ2 = 27.6, P < .001; OR = 0.90; 95% CI = 0.85–0.96). After controlling for the other symptoms and covariates, associations between the MMSE and agitation or depressive symptoms were not significant.
We next examined each of the 8 possible combinations of symptoms. Table 3 indicates the relative prevalences of each symptom type and their combinations. Symptoms of psychosis alone were rare (2.9%), while similar proportions of patients exhibited only agitation (7.9%) or only depressive symptoms (7.2%). The most common combination (51.1%) was the presence of symptoms in all 3 domains.
Table 3.
Proportion of Alzheimer’s Disease Cohort Exhibiting Single or Combinations of Psychopathological Symptoms (n = 139)
| Domain | Percentage of Group |
|---|---|
| No symptoms | 3.6 |
| Psychosis only | 2.9 |
| Depression only | 7.2 |
| Agitation only | 7.9 |
| Depression and agitation | 11.5 |
| Depression and psychosis | 8.6 |
| Psychosis and agitation | 7.2 |
| Depression and psychosis and agitation | 51.1 |
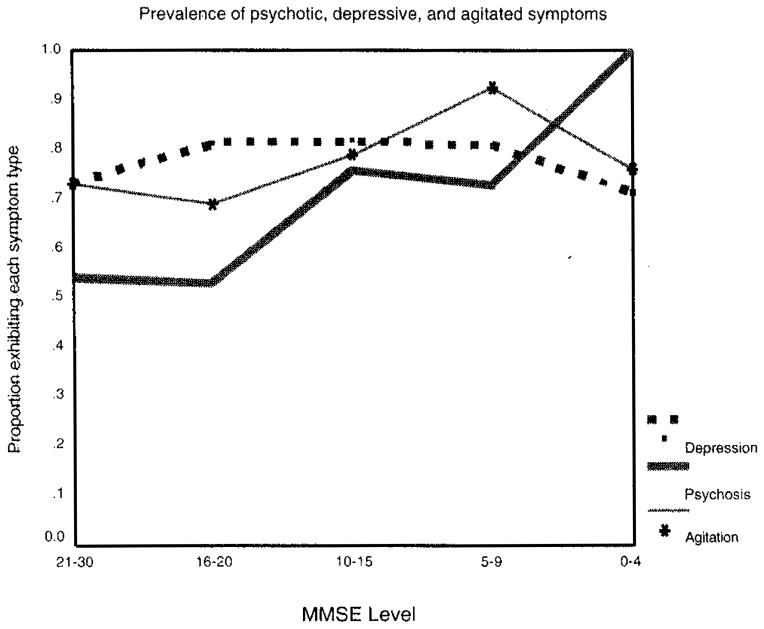
Table 4 presents each domain by MMSE level. The prevalence of psychotic symptoms increases fairly monotonically as MMSE decreases, as can be seen in Figure 1.
Table 4.
Prevalence of Symptoms of Depression, Psychosis, and Agitation by Mini-Mental State Examination (MMSE) Level
| Domain | Overall
|
MMSE Score
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21–30
|
16–20
|
10–15
|
5–9
|
0–4
|
||||||||
| % | n | % | n | % | n | % | n | % | n | % | n | |
| Psychosis | 66.7 | 97 | 53.8 | 14 | 53.1 | 17 | 75.8 | 25 | 70.4 | 19 | 100 | 21 |
| Depression | 78.4 | 116 | 74.1 | 20 | 81.8 | 27 | 82.9 | 29 | 75.9 | 22 | 72.7 | 16 |
| Agitation | 77.6 | 114 | 70.4 | 19 | 69.7 | 23 | 80.0 | 28 | 89.3 | 25 | 77.3 | 17 |
Figure 1.
Cross-sectional prevalence of symptoms by Mini-Mental State Examination level.
Figure 1 shows that the proportion of the group endorsing symptoms of agitation and depression are fairly stable across MMSE levels.
In Table 5, the basis of the association between the presence of psychotic symptoms and MMSE can be seen. Greater proportions of the cohort with lower MMSE-based severity levels exhibited less comorbidity, and only individuals with the lowest MMSE scores had symptoms of psychosis without comorbid symptomatology.
Table 5.
Cognitive Level (Mini-Mental State Examination [MMSE]) and Symptom Distribution in an Alzheimer’s Disease Cohort
| Domain | MMSE Score
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21–30
|
16–20
|
10–15
|
5–9
|
0–4
|
||||||
| % | n | % | n | % | n | % | n | % | n | |
| No symptoms | 7.7 | 2 | 6.3 | 2 | 3.0 | 1 | 0 | 0 | ||
| Psychosis only (PS) | 0 | 0 | 0 | 3.8 | 1 | 14.3 | 3 | |||
| Depression only (DE) | 7.7 | 2 | 18.8 | 6 | 6.1 | 2 | 0 | 0 | ||
| Agitation only (AG) | 19.2 | 5 | 9.4 | 3 | 3.0 | 1 | 7.7 | 2 | 0 | |
| DE + AG | 11.5 | 3 | 12.5 | 4 | 12.1 | 4 | 19.2 | 5 | 0 | |
| DE + PS | 11.5 | 3 | 6.3 | 2 | 12.1 | 4 | 3.8 | 1 | 9.5 | 2 |
| PS + AG | 0 | 3.1 | 1 | 12.1 | 4 | 7.7 | 2 | 14.3 | 3 | |
| DE + PS + AG | 42.3 | 11 | 43.8 | 14 | 51.5 | 14 | 57.7 | 15 | 61.9 | 13 |
Mini-Mental State Exam at baseline.
Most individuals with MMSE scores ≤ 10 (52.5%) had all 3 symptom types, as did nearly 43% of the subjects with MMSE scores between 16 and 30.
DISCUSSION
In this sample of AD patients with significant levels of agitation at baseline, we found that symptoms of depression and psychosis were significantly associated but depressive symptoms and agitation were not. We also found that agitation and symptoms of psychosis were not significantly associated (see Table 2). The relationships of agitation and depressive or psychotic symptoms have been inconsistently characterized across the literature describing persons with mild to moderate AD. However, symptoms of psychosis and depression have consistently been reported to be analytically independent6–10,12; in this sample, we found considerable comorbidity as well as significant association between these 2 types of symptoms.
Among the large studies involving symptoms in these 3 domains, prevalence of at least 1 symptom ranged from 64.3%9 to 97%10 (see also Lyketsos et al2,8; Levy et al6; Frisoni et al7). Only 3.6% of the cohort described here had no symptom in any of the BRSD-based symptom domains, placing these subjects among the most behaviorally disturbed in the literature to date. Some degree of comorbidity of these symptoms in persons with AD has been reported by other groups6–9; Kunik et al21 reported comorbidity in persons with dementia (not AD specifically). While we expected agitation to be prevalent in the cohort (given the study into which they were recruited), we did not anticipate nearly 70% comorbidity with symptoms of depression, psychosis, or both (Table 3).
We found evidence of higher prevalence of psychopathology (psychosis and agitation) at more severe dementia (Tables 4 and 5). Conversely, depressive symptoms were found not to be associated with MMSE level, which is consistent with the findings of Devanand et al9(p261) and, to some extent, Fitz and Teri.22(p191) Our findings also tend to support the conclusion of Marin et al10 that the tendency toward depressive symptomatology in persons with AD may not be associated with disease progression. However, no conclusions as to the association between behavioral disturbance and the progression of AD can be drawn because this study was cross-sectional. These cross-sectional trends must be confirmed with longitudinal evaluations.
There is some evidence in the literature that at least one aspect of agitated behaviors, physical aggression, is related to depression. In their population-based study, Lyketsos and colleagues23 found that elderly persons with dementia residing in the community who had more depressive symptoms were also significantly more likely to exhibit symptoms of physical aggression. Furthermore, after adjusting for the presence of mild to severe depression (using an instrument for assessing depression in persons with dementia), they found that symptoms of psychosis and physical aggression were not significantly associated.
Conversely, Kunik et al21 reported that baseline (intake) levels of aggressive behaviors were associated with symptoms of psychosis and not depression, while nonaggressive symptoms of agitation were associated with depression in geropsychiatric inpatients with diagnoses of dementia (not AD per se). In the present study, reports of aggressive behavior were extremely rare (ie, mean ratings near “absent” in the month prior to the baseline interview) and so were not specifically examined. It remains to be determined whether the physical aggression signals a distinct aspect of agitation; both were found to be significantly associated with more severe dementia in a recent population-based study2, however, agitation and aggression were treated as one symptom class in that study.
Our results suggest that agitation tends to occur with symptoms in other psychopathological domains, but the association between agitation and other symptoms is accounted for by the presence of depressive or psychotic symptoms and other covariates. Agitation may be the most easily observable type of behavioral or psychologic symptom in persons with AD,5 but our observation of agitation occurring alone (7.9%) in this agitated sample is very close to the population-based estimate of 9% reported by Lyketsos et al.2 This similarity in prevalence was observed even though persons with 1 symptom from a list of 7 were counted as “agitated” by Lyketsos et al (according to administration rules for the instrument they used) and persons with only 1 symptom were counted as “agitated” in this study. Thus, agitated behaviors in AD patients should be assessed and treated in the context of their other psychopathologic symptoms.
One reason for a lack of convergence in findings may be the variety of instruments used and of patients from whom responses were collected across these studies. In addition, different definitions of “presence” of symptoms and even different definitions of symptomatology in the 3 domains studied here could lead to, or account for, different results in terms of the interrelationships between them. Our analyses focused on the presence of symptoms in these 3 domains and not on their severity. This is a similar approach to that used in other studies2,7,9,10 and so is not likely the reason for divergent results. We also used the same threshold (at least 1 symptom) for detecting symptoms of psychosis or depression in our study as others have.2,6–10
The fact that our sample was recruited to a study for the treatment of agitation in AD may limit the generalizability of our findings or be one factor in the differences between our findings and those of others. It is possible that our analyses revealed the associations between symptom types only in AD patients for whom treatment for agitation is likely to be sought; this may suggest that the greatest degree of agitation is observed in AD patients who have psychotic and/or depressive symptoms. We have reported elsewhere24 that 2 similar groups of patients with AD (1 “nondisturbed” and 1 “behaviorally disturbed”) exhibited essentially the same agitated behaviors and that significant differences in their levels of agitation arose from the frequency of this “core” group of agitated behaviors. This suggests that the relationships we described here are not unique to the patients we analyzed in the present report.
Our findings, together with a growing body of literature, suggest that the possible contribution of mood disturbance and psychosis should be considered in the treatment for AD patients with behavioral disturbance. Recent efforts at deriving classifications for various neuropsychological symptoms in persons with AD7,8 have pointed out that it is inappropriate to lump these together; it may similarly not serve to create syndromes or subgroups that may lead future study designers to overlook the cooccurrence of important symptom types. Furthermore, it is possible that interventions along multiple symptom dimensions may be more efficacious than treatment of one type. For example, Kunik et al21 reported improvement in behavioral disturbance associated with improvement in depressive and psychotic symptoms. Comorbid psychopathology can represent significant confounding in clinical trials or studies of single behavioral domains, but it can easily be assessed with instruments such as the BRSD and other behavioral measures (reviewed inWeiner25) and thereby controlled in recruitment, accounted for in analysis, or exploited in the treatment of this dimension of dementing illness.
Acknowledgments
This work was supported by grant AG 10483 from the National Institute on Aging. We would like to acknowledge the contributions of Lon Schneider, MD, on early drafts of this article.
Contributor Information
Dr. Rochelle E. Tractenberg, Center for Population and Health, Georgetown University, Washington, DC.
Dr. Myron F. Weiner, Departments of Psychiatry and Neurology, University of Texas Southwestern Medical Center, Dallas, Texas.
Dr. Marian B. Patterson, Department of Psychiatry, University Hospitals of Cleveland, Cleveland, Ohio.
Dr. Linda Teri, Departments of Psychosocial and Community Health, Psychiatry and Behavioral Sciences, and Psychology, University of Washington, Seattle, Washington.
Dr. Leon J. Thal, Department of Neurosciences, University of California, San Diego, La Jolla, California.
References
- 1.Finkel SI. Behavioral and psychological symptoms of dementia: a current focus for clinicians, researchers and caregivers. J Clin Psychiatry. 2001;62(suppl 21):3–6. [PubMed] [Google Scholar]
- 2.Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 3.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 4.Weiner MF, Koss E, Wild KV, et al. Measures of psychiatric symptoms in Alzheimer patients: a review. Alzheimer’s Disease and Associated Disorders. 1996;10(1):20–30. [PubMed] [Google Scholar]
- 5.Devanand DP. The interrelations between psychosis, behavioral disturbance, and depression in Alzheimer’s disease. Alzheimer’s Disease and Associated Disorders. 1999;13(suppl 2):S3–S8. [PubMed] [Google Scholar]
- 6.Levy ML, Cummings JL, Fairbanks LA, et al. Longitudinal assessment of symptoms of depression, agitation, and psychosis in 181 patients with Alzheimer’s disease. Am J Psychiatry. 1996;153:1438–1443. doi: 10.1176/ajp.153.11.1438. [DOI] [PubMed] [Google Scholar]
- 7.Frisoni GB, Rozzini L, Gozzetti A, et al. Behavioral syndromes in Alzheimer’s disease: description and correlates. Dement Geriatr Cogn Disord. 1999;10:130–138. doi: 10.1159/000017113. [DOI] [PubMed] [Google Scholar]
- 8.Lyketsos CG, Sheppard J-ME, Steinberg M, et al. Neuropsychiatric disturbance in Alzheimer’s disease clusters into three groups: the Cache County study. Int J Geriatr Psychiatry. 2001;16:1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 9.Devanand DP, Jacobs DM, Tan MX, et al. The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry. 1997;54:257–263. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- 10.Marin DB, Green CR, Schmeidler J, et al. Noncognitive disturbances in Alzheimer’s disease: frequency, longitudinal course, and relationship to cognitive symptoms. J Am Geriatr Soc. 1997;45:1331–1338. doi: 10.1111/j.1532-5415.1997.tb02932.x. [DOI] [PubMed] [Google Scholar]
- 11.Mack JL, Patterson MB, Tariot PN. The Behavior Rating Scale for Dementia (BRSD): development of test scales and presentation of data for 555 individuals with Alzheimer’s disease. J Geriatr Psychiatry Neurol. 1999;12:211–223. doi: 10.1177/089198879901200408. [DOI] [PubMed] [Google Scholar]
- 12.Weiner MF, Koss E, Patterson M, et al. A comparison of the Cohen-Mansfield Agitation Inventory with CERAD behavioral rating scale for dementia in community-dwelling persons with Alzheimer’s disease. J Psych Res. 1998;32:347–351. doi: 10.1016/s0022-3956(98)00027-2. [DOI] [PubMed] [Google Scholar]
- 13.Teri L, Logsdon RG, Peskind E, et al. Treatment of agitation in Alzheimer’s disease patients: as randomized placebo controlled clinical trial. Neurology. 2000;55:1271–1278. doi: 10.1212/wnl.55.9.1271. [DOI] [PubMed] [Google Scholar]
- 14.Tariot PN, Mack JL, Patterson MB, et al. The CERAD Behavioral Rating Scale for Dementia. Am J Psychiatry. 1995;152:1349–1357. doi: 10.1176/ajp.152.9.1349. [DOI] [PubMed] [Google Scholar]
- 15.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 16.Mack JL, Patterson MB. Manual: CERAD behavior rating scale for dementia. 2. Durham, NC: Consortium to Establish a Registry for Alzheimer’s Disease; 1996. [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Ferris SH, Mackell JA, Mohs R, et al. A multicenter evaluation of new treatment efficacy instruments for Alzheimer’s disease clinical trials: overview and general results. Alzheimer’s Disease and Associated Disorders. 1997;11(suppl 2):S1–S12. [PubMed] [Google Scholar]
- 19.Cohen-Mansfield J. Instruction manual for the Cohen-Mansfield Agitation Inventory (CMAI) Rockville, MD: Research Institute of the Hebrew Home of Greater Washington; 1991. [Google Scholar]
- 20.Tractenberg RE, Weiner MF, Thal LJ. Estimating the prevalence of agitation and behavioral disturbance in community-dwelling persons with Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2002;14:11–18. doi: 10.1176/jnp.14.1.11. [DOI] [PubMed] [Google Scholar]
- 21.Kunik ME, Snow-Turek AL, Iqbal N, et al. Contribution of psychosis and depression to behavioral disturbances in geropsychiatric inpatients with dementia. J Gerontology Medical Sciences. 1999;54A:M157–M161. doi: 10.1093/gerona/54.3.m157. [DOI] [PubMed] [Google Scholar]
- 22.Fitz AG, Teri L. Depression, cognition, and functional ability in patients with Alzheimer’s disease. J Am Geriatr Soc. 1994;42(2):186–191. doi: 10.1111/j.1532-5415.1994.tb04950.x. [DOI] [PubMed] [Google Scholar]
- 23.Lyketsos CG, Steele C, Galik E, et al. Physical aggression in dementia patients and its relationship to depression. Am J Psychiatry. 1999;156:66–71. doi: 10.1176/ajp.156.1.66. [DOI] [PubMed] [Google Scholar]
- 24.Tractenberg RE, Gamst A, Weiner MF, et al. Frequency of behavioral symptoms characterizes agitation in Alzheimer’s disease. Int J Geriatr Psychiatry. 2001;16:886–891. doi: 10.1002/gps.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiner MF. Clinical diagnosis of cognitive function and dementing illness. In: Weiner MF, Lipton AM, editors. The dementias: diagnosis, treatment and research. 3. Washington, DC: American Psychiatric Publishers; 2003. pp. 49–76. [Google Scholar]