Abstract
Objectives
To determine the safety and efficacy of 2 dose formulations of melatonin for the treatment of insomnia in patients with Alzheimer’s disease.
Design
A multicenter, randomized, placebo-controlled clinical trial of 2 dose formulations of oral melatonin coordinated by the National Institute of Aging-funded Alzheimer’s Disease Cooperative Study. Subjects with Alzheimer’s disease and nighttime sleep disturbance were randomly assigned to 1 of 3 treatment groups: placebo, 2.5-mg slow-release melatonin, or 10-mg melatonin.
Setting
Private homes and long-term care facilities.
Participants
157 individuals were recruited by 36 Alzheimer’s disease research centers. Subjects with a diagnosis of Alzheimer’s disease were eligible if they averaged less than 7 hours of sleep per night (as documented by wrist actigraphy) and had 2 or more episodes per week of nighttime awakenings reported by the caregiver.
Measurements
Nocturnal total sleep time, sleep efficiency, wake-time after sleep onset, and day-night sleep ratio during 2- to 3-week baseline and 2-month treatment periods. Sleep was defined by an automated algorithmic analysis of wrist actigraph data.
Results
No statistically significant differences in objective sleep measures were seen between baseline and treatment periods for the any of the 3 groups. Nonsignificant trends for increased nocturnal total sleep time and decreased wake after sleep onset were observed in the melatonin groups relative to placebo. Trends for a greater percentage of subjects having more than a 30-minute increase in nocturnal total sleep time in the 10-mg melatonin group and for a decline in the day-night sleep ratio in the 2.5-mg sustained-release melatonin group, compared to placebo, were also seen. On subjective measures, caregiver ratings of sleep quality showed improvement in the 2.5-mg sustained-release melatonin group relative to placebo. There were no significant differences in the number or seriousness of adverse events between the placebo and melatonin groups.
Conclusions
Based on actigraphy as an objective measure of sleep time, melatonin is not an effective soporific agent in people with Alzheimer’s disease.
INTRODUCTION
Although progressive deterioration of memory, language, and intellect are the classic hallmarks of alzheimer’s disease (ad), the degenerative process also produces neurobehavioral symptoms that can be as stressful to patients and caregivers as the dementia itself. Sleep disturbances are among the more common neurobehavioral symptoms of AD, affecting up to 45% of patients.1 Nighttime awakenings can be extremely stressful for family members and caregivers and can lead to nursing home placement.1,2 Frequently reported sleep disturbances include nighttime awakenings, early morning awakening, excessive daytime sleepiness, and on rare occasions, a diurnal reversal of the sleep-wake cycle with the main sleep period occurring in the daytime. 3–19 Memory function in people without dementia, but at high genetic risk for AD, is particularly sensitive to the adverse effects of daytime sleepiness.20 This suggests that improving nighttime sleep and daytime alertness in people with dementia can help their cognitive function. Despite the high prevalence and great impact of these symptoms, no large clinical trial of a sleep medication in AD patients has been reported in the literature.
Melatonin is in widespread use for the treatment of insomnia, and although there is scientific plausibility for its use, there are no data to support either safety or efficacy in the dementia population. The Alzheimer’s Disease Cooperative Study (ADCS) provided the administrative structure for the type of large-scale clinical trial needed to determine the safety and efficacy of the use of melatonin in patients with AD. The ADCS is funded by the National Institute on Aging, primarily to carry out clinical trials for agents in the public domain that might be useful in the treatment of patients with AD but would not be developed by industry because of a lack of patent protection. The consortium comprises the majority of the National Institute on Aging-funded AD centers in the United States, along with other centers of excellence in aging and dementia research. Since melatonin is a naturally occurring compound widely used in the AD community but is not being developed for AD because of a lack of patent protection, its testing falls within the mandate of the ADCS. The reasons to investigate the use of melatonin were especially compelling, given its potential for both soporific21–36 and chronobiologic37–44 activity. The ADCS, therefore, designed a study to determine the safety and efficacy of melatonin’s soporific effect in patients with AD and nocturnal insomnia. To our knowledge, this is the only large, multicenter, clinical trial for the treatment of sleep disorders in AD ever attempted.
METHODS
Overview
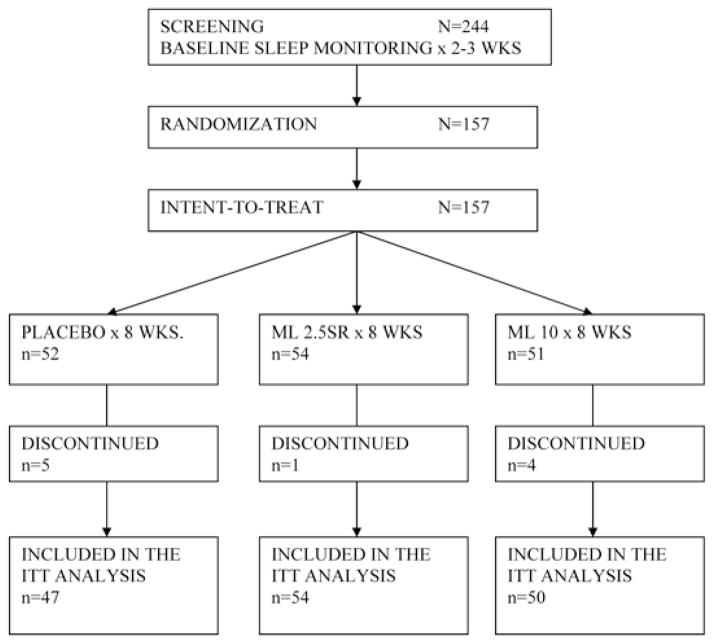
This was a multicenter, randomized, placebo-controlled study of 2 melatonin formulations (2.5 mg sustained-release (SR) and 10 mg immediate release). Subjects were randomly assigned (blocked by study site) in a double-blind fashion to 1 of 3 groups: placebo (PLA), 2.5 mg SR melatonin (ML 2.5SR), and 10 mg immediate-release melatonin (ML 10). Total nighttime sleep, defined by actigraph-determined immobility between the hours of 8:00 PM and 8:00 AM the following morning, was the primary outcome measure. The protocol schematic and subject flow are presented in Figure 1. The schedule of procedures and visits is presented in Table 1, and the primary and secondary outcome variables are described in Table 2.
Figure 1.
Design Flow Chart. ML 2.5SR refers to treatment with 2.5 mg sustained-release melatonin; ML 10, treatment with 10 mg melatonin; ITT, intent to treat.
Table 1.
Schedule of study visits.*
| Baseline | Treatment period | P/w | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Week number | −3 | −2 | −1 | 0 | 2 | 4 | 6 | 8 | 10 |
| Visit number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Review records | X | ||||||||
| Explain study | X | ||||||||
| Obtain consent | X | ||||||||
| Patient registration | X | ||||||||
| Demographics | X | ||||||||
| Electrocardiogram | X | X | |||||||
| Physical examination | X | X | |||||||
| Pill count/dispense | X | X | X | X | X | ||||
| Adverse events | X | X | X | X | X | X | |||
| Vital signs | X | X | X | X | X | X | X | ||
| Concurrent medications | X | X | X | X | X | X | X | X | X |
| Laboratory studies/melatonin | X | X | |||||||
| MMSE | X | X | |||||||
| ADAS-cog | X | ||||||||
| Hamilton Depression Rating Scale | X | X | X | ||||||
| Sleep Disorders Inventory | X | X | X | X | X | X | X | ||
| NPI | X | X | X | X | |||||
| ADCS-ADL | X | X | X | X | |||||
| Actigraph procedures | X | X | X | X | X | X | X | X | X |
| Sleep diary evaluation | X | X | X | X | X | X | X | X | |
| Blindness evaluation | X | ||||||||
There were 2 to 3 weeks of baseline actigraphy, 8 weeks of treatment, and 2 weeks of placebo washout (P/w).
MMSE refers to Mini-Mental State Examination; ADAS-cog, Alzheimer’s Disease Assessment Scale-cognitive scale; NPI, Neuropsychiatric Inventory; ADCS-ADL, Alzheimer’s Disease Cooperative Study
Table 2.
Description of the primary and secondary outcome variables
| NTST | Total sleep time (min) during the 12-h nocturnal epoch 8:00 PM – 08:00 AM, as calculated by the computerized algorithm |
| DTST | Total sleep time (min) during the 12-h daytime epoch 08:00 AM – 8:00 PM, as calculated by the computerized algorithm |
| DTST/NTST | Ratio of daytime to nighttime sleep |
| WASO | Wake after sleep onset or time awake (min) after sleep onset until the final awakening during the 12-h nocturnal epoch 8:00 PM – 08:00 AM |
| SE% | Percentage of time asleep during the 12-h nocturnal epoch |
| Gained > 30 min | Percentage of subjects with at least a 30-minute increase in NTST |
| MMSE39 | Mini-Mental State Examination, a 30-point measure of cognitive function (lower score indicates more severe dementia) |
| ADAS-cog40 | Alzheimer’s Disease Assessment Scale-cognitive subscale, a 70-point measure of cognitive function (higher score indicates more severe dementia) |
| ADCS-ADL Inventory41 | Rates degree of independence in 23 activities of daily living validated by the Alzheimer’s Disease Cooperative Study |
| Hamilton Depression Rating Scale42 | Rating used in this study to help rule out major depression |
| NPI43 | Neuropsychiatric Inventory rating of 3 factors (frequency, severity, and distress) for 12 different neurobehavioral symptoms in persons with dementia. The symptoms are delusions, hallucinations, agitation or aggression, depression or dysphoria, anxiety, elation or euphoria, apathy, disinhibition, irritability or lability, aberrant motor behavior, appetite and eating behaviors, and sleep. The sleep questions were deleted from this protocol in favor of an expanded format, the Sleep Disorders Inventory. |
| SDI | Sleep Disorders Inventory, a rating scale of sleep-related symptoms based on the NPI and developed specifically for this study. Consists of 3 ratings (frequency, severity, and distress) of 7 sleep variables (difficulty falling asleep, getting up during the night, nighttime wandering and pacing, awakening the caregiver, awakening during the night and thinking it is daytime, awakening too early in the morning, and sleeping excessively during the day. |
| Sleep Quality Rating | Five-point sleep-quality rating scale included in the Daily Sleep Diary that the primary caregiver completed every morning. Scores range from 1 (very poor night with no or little sleep) to 5 (outstanding night with no awakenings). |
Melatonin Formulations
The decision as to what doses of melatonin to study in this project was an important one. High doses of melatonin (50 mg) but not physiologic-range doses (0.2 mg) appeared to be effective in our pilot studies in healthy elderly subjects, in whom sleep was recorded with polysomnography.34,35 In an 11-patient placebo-controlled pilot study in AD patients, the use of 10 mg of melatonin provided an average of a 30-minute increase in actigraphically determined sleep time (unpublished pilot data), whereas a 0.5-mg dose did not affect sleep in another trial involving 7 AD subjects.36
We chose 2 pharmacologic-range doses of melatonin for this study—1 moderately high (10 mg) and the other moderately low (2.5 mg). Based on initial 1-dose pharmacokinetic studies we had conducted in 7 elderly healthy subjects and 4 elderly subjects with AD, the 10-mg preparation was shown to yield initial blood levels high enough to be sustained through the night with bedtime administration, clearing with a half-life of 40 to 60 minutes, and leaving normal low physiologic daytime levels by dawn. The 2.5-mg SR preparation was selected as the lower-dose melatonin formulation. This SR preparation produced elevated melatonin levels for several hours in young healthy subjects but with a much lower peak level than the 10-mg dose. Peak plasma levels varied from one person to another, but these preparations generally yielded peak plasma levels 10 to 100 time normal physiologic levels.45 Placebo and both melatonin preparations were supplied by Genzyme Limited (Boston, Mass) in identical capsules. Melatonin was used under a Food and Drug Administration Investigational New Drug Application number.
The time at which melatonin was given in this protocol was another important consideration. This study was a test of the soporific efficacy of melatonin in AD subjects with nighttime insomnia. We specifically wanted to minimize a potential circadian phase shift by melatonin, as this could affect sleep propensity and quality.46 A circadian phase shift would be most likely to occur if exogenous melatonin was given earlier than the subjects’ own endogenous melatonin onsets. It was not possible in this protocol to monitor circadian rhythms other than sleep. We presumed most subjects were entrained to the day-night cycle, although we did not exclude people with more daytime than nighttime sleep (who could therefore could be abnormally entrained). Although not yet studied in AD subjects, normally entrained healthy individuals have a melatonin onset that begins about 14 hours after awakening (ie “circadian time 14” or “CT14”) and about 2 hours before sleep onset.41 Using habitual sleep onset as a surrogate circadian phase marker, we assumed that our subjects’ endogenous melatonin onset would be 2 hours prior to sleep onset. The subjects and caregivers were therefore instructed to take (or give) the melatonin 1 hour prior to habitual bedtime so that the exogenous melatonin would likely be taken after the endogenous onset and, therefore, have minimal circadian phase-shifting effect but still yield high plasma levels by bedtime.
Research Subjects
Subjects with an NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke/the Alzheimer’s Disease and Related Disorders Association) diagnosis of probable AD,47 a nighttime sleep disturbance, a family caregiver or guardian able to give informed consent, and the ability of both themselves and their caregiver to comply with the protocol were eligible for the study. There is no generally accepted quantitative definition of sleep disturbance or insomnia in the AD population, although one has recently been proposed by a group of AD sleep investigators.48 For the purpose of this study, sleep disturbance was defined as averaging less than 7 hours of total time immobile (ie, 0 activity counts) between 8:00 PM and 8:00 AM during the screening period of at least 1 week plus 2 or more episodes per week of nighttime behaviors as reported by the caregiver on the Sleep Disorders Inventory (SDI), a scale developed specifically for this protocol. To create the SDI, the sleep section of the Neuropsychiatric Inventory (NPI)49 was expanded to provide a 7-item questionnaire focusing on sleep behavior disturbance. With this instrument, the caregiver was able to rate observable behaviors occurring within the previous 2 weeks. The SDI items include difficulty falling asleep, getting up during the night (other than for toileting), nighttime wandering, awakening the caregiver, awakening and thinking it is daytime, awakening too early, and excessive daytime sleeping. Caregivers rated each item for frequency (never = 0 to at least once per night = 4), severity (not present = 0 to markedly disturbing = 3), and caregiver distress (not at all distressing = 0 to extremely distressing = 5). The SDI was used in establishing eligibility as well as for monitoring symptoms throughout the study. Details of inclusion and exclusion criteria are presented in Appendix 1.
Investigators from 36 AD research centers throughout the US enrolled subjects over a 3-year period (see Appendix 2). The subjects were recruited from the investigators’ own dementia clinics, other clinics, long-term care facilities, newspaper advertisements, Alzheimer’s Association newsletters, public lectures, and calls to individual physicians.
Actigraphs
Wrist actigraphy is generally accepted as the most practical method for obtaining objective, quantitative, sleep-wake cycle data in persons with dementia.48,50–53 The actigraphs used in this study were Actiwatch AW64 series models produced by Mini-Mitter, Inc. (Sunriver, Oregon). With few exceptions, research subjects wore the same actigraph throughout the trial. In 3 cases, an actigraph had to be substituted for the original one that was misplaced by the subject or caregiver. All Mini-Mitter actigraphs are calibrated at the factory to provide standardized movement sensitivity within less than a 0.1% error, so a correction factor was not employed when a subject had worn more than 1 actigraph during the course of the study. The Actiwatch AW64 series sleep-algorithm software calculates sleep probabilities based on arm-motion data and, when the instrument is set on high-sensitivity mode, provides excellent correlation when compared to total sleep times determined by polysomnography (PSG) in healthy elderly.54 The actigraph and software also provided excellent correlation with PSG sleep (r2=0.92, P < .01) in 7 subjects with AD, although it consistently overestimated sleep relative to PSG (Figure 2 and Table 3). The high Spearman correlation between the 2 sets of observations makes us confident that actigraphs and PSG measure the same threshold phenomenon (ie, sleep or wake).
Figure 2.
Actigraph-scored and polysomnography-scored sleep in 7 subjects with Alzheimer’s disease. The Mini-Mitter Actiwatch actigraph sleep algorithm showed excellent correlation (r2 = 0.92, P <. 01) with polysomnography-scored sleep, although it consistently overestimated sleep relative to electroencephalogram-based sleep scoring. The sleep studies were carried out at the general clinical research center at Oregon Health Sciences University. PSG refers to polysomnography; ACT, actigraphy; AD, Alzheimer’s disease.
Table 3.
Mean polysomnography-scored and actigraph-scored sleep duration in 7 subjects with Alzheimer’s disease.
| Subject | No. nights | PSG, min | ACT, min | Difference, min |
|---|---|---|---|---|
| AD 1 | 3 | 376.27 | 387.00 | 10.73 |
| AD 2 | 1 | 296.50 | 296.00 | −0.50 |
| AD 3 | 3 | 429.50 | 546.33 | 116.83 |
| AD 4 | 3 | 467.17 | 518.33 | 51.17 |
| AD 5 | 3 | 228.17 | 287.00 | 58.83 |
| AD 6 | 3 | 262.13 | 353.67 | 91.53 |
| AD 7 | 2 | 281.33 | 341.00 | 59.67 |
| Total | 334.44 | 389.90 | 55.47 |
PSG refers to polysomnography; ACT, actigraphy; AD, Alzheimer’s disease
Scientific Aims
The primary goal of the study was to determine the efficacy of melatonin for improving nighttime sleep in patients with AD. The study was designed to determine if there was a change (relative to baseline) in total sleep time in any of the 3 treatment arms (PLA, ML 2.5SR, ML 10). We also sought to determine if there was a difference in time awake after sleep onset (WASO) and sleep efficiency (SE) between the 3 treatment arms.
Sleep latency (time to fall asleep) is usually a critical outcome variable in sleep research and has been shown to improve in response to melatonin, using both subjective and objective measures in previous studies of non-AD subjects.26 However, we did not think we could reliably determine sleep latency in a large multicenter trial. We knew we would be relying on caregivers to carefully record lights-out time in the sleep diary (see below) and press the internal event marker (a button on the actigraph that marks the sleep record at the time the button is pressed). In our experience, caregiver compliance with these procedures is not good enough to enable accurate determination of sleep latency in trials with dementia subjects. Fortunately, difficulty falling asleep is not usually a major problem for AD subjects, so sleep-latency data may not be as critical an outcome variable in AD sleep research as it is in other insomnia studies. For these reasons, we chose to not include sleep latency as a primary outcome measure in this study.
Caregivers were asked to complete a Daily Sleep Diary form to document bedtimes, lights-out time, time of melatonin administration, and the rare times that the actigraph was left off the subject. The sleep diary also included a 5-point rating of sleep quality (see Table 2). The Daily Sleep Diaries were reviewed by the site study coordinator at each study visit to enhance compliance.
Secondary outcomes included change in cognitive function (as measured by the Mini-Mental State Examination55 and the Alzheimer’s Disease Assessment Scale - cognitive subscale56), function in activities of daily living,57 depressive symptoms (Hamilton Depression Rating Scale),58 and neuropsychiatric symptoms (NPI and SDI). Change scores were calculated for all variables (later-earlier). To meet secondary goals, comparisons were made across groups in the ratio of actigraph-measured daytime to nighttime sleep, the proportion of subjects who gained at least 30 minutes in total nighttime sleep, and reported adverse events (AEs).
Procedures
Subjects wore the actigraphs continuously throughout the 2- to 3-week screening period and the 10-week protocol. Actigraph data for each subject were downloaded every 2 weeks during the treatment phase at each clinic or home visit. The actigraph coordinator at the Oregon Health Sciences University in Portland reviewed all data for quality control and transferred it electronically to the ADCS Data Management Core at the University of California, San Diego. The initial quality check at Oregon Health Sciences University of the 2-week data file was to confirm that wrist-activity data had been captured in that file and that actigraph procedures were correctly followed at the site from which the data had been sent. If there were any data gaps in a 24-hour segment of the 2-week data file, the entire 24-hour segment was deleted. The raw data were then transported to a master spreadsheet and sent to the ADCS. At the ADCS, a single actigraph record for the entire 8-week treatment period for each subject was created from the master data file. The data files were sorted by time-stamp and subject number and then loaded into the actigraphy-analysis program, which cut them into 24-hour (noon to noon) segments. Each day’s data were then rechecked for potential quality problems. Days with data gaps or spurious values were deleted, and the remaining segments were scored for sleep and other critical values. The products of these steps were then transferred to the master output file for later analysis.
A computerized automated scoring technique was developed at ADCS by one of the authors (AG) to translate the vast amount of wrist-movement data into sleep values. The output of the automated algorithm was identical to that of the manufacturer’s nonautomated sleep-scoring algorithm, which, as described previously, shows excellent correlation with PSG data in AD subjects, although it tends to overestimate sleep (Figure 2 and Table 3). At each of the 4 biweekly visits during the treatment phase, caregivers were queried regarding the occurrence of AEs using a standard clinical trial AE questionnaire. The determination as to whether or not a specific AE was related to study medication was made by the primary investigator at the individual site and the primary investigator of the overall study (C. Singer). A Safety Monitoring Committee reviewed safety data, such as clinical laboratory values and AEs, throughout the trial. Sealed code breakers were distributed to each site and recovered at the end of the trial. In no instance was it necessary to break the blind.
We obtained a midday plasma sample on the day after the final dose of study medication (ie, final study visit) to determine if the relatively high doses of melatonin used in this study result in high residual daytime levels after chronic administration. We drew these plasma samples on all willing subjects (n=128), including those in the placebo group, since placebo-group data could serve as control values, plus the fact that we were blinded as to who was in the placebo group. The plasma samples were sent to Oregon Health Sciences University for melatonin assay.
Statistical Methods
Based on pilot data, the study was designed to enroll 50 subjects per treatment arm (total N=150) in order to detect a 30-minute change in nocturnal total sleep time (NTST) between melatonin and placebo groups with greater than 80% power and type 1 error level of 0.05 (2-sided). A 2-sided analysis was planned in order to be conservative and so as not to assume that melatonin would be helpful rather than harmful to sleep.
In fact, more than 50 subjects were randomized to each arm. The primary analyses utilized the intent-to-treat sample (ie, based on all randomized subjects). One hundred fifty-seven subjects were randomly assigned to treatment groups, but because of technical difficulties, actigraph data from only 151 subjects were available for inclusion in the intent-to-treat analysis. No imputation scheme was used, and while 12 subjects discontinued treatment (13.1%), the data they contributed at any visit prior to discontinuation were used in the final analysis. However, because of technical difficulties, no actigraph data were available for 6 subjects, so not everyone who was randomly assigned to a treatment group could be included in intent-to-treat analysis of the primary sleep outcomes. Balance across groups and association with outcomes were assessed for each major potential covariate to determine inclusion status for the regression model. Age, duration of AD (defined by date of diagnosis recorded in the medical record or family’s best estimate), sex, dementia severity, and years of education were the variables we evaluated for balance across groups. Any covariate that was at least marginally out of balance (ie, P = .15) and had marginally significant association with outcome (ie, P = .10) would be included in the model.
Mean baseline and screening-period sleep values were included in the linear regression analyses of the treatment-period response variable. Mann-Whitney (nonparametric) t tests were used to evaluate each of the continuous outcome measures (pretreatment, treatment, and change). Chi square/Fisher exact tests were carried out for comparisons between the proportions of groups gaining at least 30 minutes in NTST. In every analysis, each of the 2 active treatment arms was compared to placebo.
The χ2/Fisher exact tests were carried out for comparisons between groups in terms of 5 AE summary variables (number of AEs per category per arm, mean number of AE reports per person, mean AE severity rating (1=mild; 2=moderate; 3=severe), mean AE seriousness rating (1=life threatening/serious; 2=not serious), and mean AE relatedness rating (1=AE definitely related to study drug; 2 =probably related to study drug; 3=possibly related to study drug; 4=remotely related to study drug; 5=not related to study drug).
Holm adjustments were planned where necessary to correct for the multiple comparisons. The significance level was set at adjusted P values of .05, but outlier analyses were carried out on outcomes where unadjusted P values were less than .25.
RESULTS
We screened 244 potential subjects and enrolled 157. Randomized assignment to the 3 treatment arms was fairly well balanced: PLA: n=52, ML 2.5SR: n=54, and ML 10: n=51. The average age of the subjects was 77.4 ± 8.9 years (Table 3). Women comprised 56.1% of the subject population, and minorities, 19.1%. Subjects had an average duration of AD at time of enrollment of 4.9 ± 3.0 years, and an average Mini-Mental State Examination score of 13.9 ± 8.8, and Alzheimer’s Disease Assessment Scale - cognitive subscale score of 38.5 ± 18.9, indicating moderate dementia, although the range of severity was quite broad.
None of the potential covariates were found to be out of balance at baseline (ie, all P >.30); therefore, no variable examined was included in the final analyses. Also, the groups did not differ on any baseline variable with the exception of NPI scores, which were highest in the ML 2.5SR group (Z = −1.989 vs placebo, unadjusted P =.012; see Table 2). The NPI scores were found not to be associated with the primary sleep variables (all unadjusted P =.15), and so were not included in any model of analysis.
Primary Outcome Measures
Table 4 provides descriptive statistics for the baseline, treatment period, and changes for the primary and secondary sleep variables. In none of the statistical analyses of the primary sleep measures (baseline vs treatment) did any P value fall below our preset level of significance. Holm-adjustments were not applied because the P values for planned analyses were all greater than .05.
Table 4.
Primary sleep outcomes measured via actigraph*
| Variable | PLACEBO | ML 2.5SR | ML 10 | OVERALL |
|---|---|---|---|---|
| NTST PRE, min | 344.7 ± 86.7 | 359.8±85.7 | 346.0 ± 76.8 | 350.5 ± 83.0 |
| NTST TX, min | 349.4 ± 88.4 | 375.7 ± 76.2 | 357.8 ± 70.6 | 361.4 ± 79.0 |
| Δ in NTST, min | 3.1 ± 38.6 | 15.9 ± 53.9 | 12.6 ± 44.2 | 10.7 ± 46.3 |
| Gained ≥30 min | 20.4% | 24.0% | 36.7% | 27.0% |
| DTST PRE, min | 128.2 ± 71.6 | 161.8 ± 108.2 | 162.6 ± 100.9 | 151.2 ± 96.1 |
| DTST TX, min | 142.1 ± 79.8 | 152.7 ± 91.8 | 167.0 ± 102.0 | 153.9 ± 91.6 |
| Δ in DTST, min | 9.5 ± 55.1 | −9.0 ± 52.7 | 4.8 ± 50.6 | 1.4 ± 53.1 |
| SE PRE, % | 0.70 ± 0.1 | 0.69 ± 0.11 | 0.68 ± 0.10 | 0.69 ± 0.11 |
| SE TX, % | 0.69 ± 0.1 | 0.69 ± 0.11 | 0.68 ± 0.09 | 0.69 ± 0.10 |
| Δ in SE, % | −0.01 ± 0.05 | 0.01 ± 0.06 | 0.01 ± 0.07 | 0.003 ± 0.06 |
| WASO PRE, min | 149.2 ± 53.3 | 167.2 ± 64.0 | 170.3 ± 59.0 | 162.4 ± 59.4 |
| WASO TX, min | 155.9 ± 45.0 | 163.0 ± 61.2 | 166.7 ± 48.0 | 161.9 ± 52.0 |
| Δ in WASO, min | 5.8 ± 34.7 | −4.1 ± 40.0 | −3.8 ± 38.1 | −0.82 ± 37.8 |
| DTST/NTST PRE | 0.80 ± 2.2 | 0.66 ± 0.89 | 0.55 ± 0.41 | 0.67 ± 1.4 |
| DTST/NTST TX | 0.75 ± 1.3 | 0.50 ± 0.39 | 0.58 ± 40 | 0.61 ± 0.83 |
| Δ in DTST/NTST | −0.05 ± 1.3 | −0.16 ± 0.78 | 0.03 ± 0.30 | −0.07 ± 0.88 |
Data are expressed as mean ± SD unless otherwise noted. Averages are overall daily or nightly observations.
ML 2.5SR refers to treatment with 2.5 mg sustained-release melatonin; ML 10, treatment with 10 mg melatonin; PRE, Pretreatment or baseline; TX, Treatment; D, change in values TX-PRE; NTST, nocturnal total sleep time; DTST, daytime total sleep time; SE, sleep efficiency (ie, TST/time in bed x 100); WASO: time awake after sleep onset until the final awakening
While no primary outcome measure reached significance for any tested hypothesis, NTST increased by 16 ± 54 minutes in the ML 2.5SR group and 13 ± 44 minutes in the ML 10 group compared to 3 ± 39 minutes for placebo, indicating a weak trend for more nighttime sleep in the melatonin groups. There was also a strong trend for the proportion of the group gaining at least 30 minutes of NTST to be greatest in the ML 10 group: 37% versus 20% for PLA (unadjusted P =.07) and 24% for ML 2.5SR. The analysis comparing the ratio of daytime to nighttime sleep in the placebo arm showed no difference versus either ML 2.5SR (unadjusted P =.20 when analyzed without the “free-running” subject, see next paragraph) or ML 10 (unadjusted P =.12).
A subject in the ML 2.5SR arm was subsequently discovered to have what appeared to be a free-running circadian sleep-wake cycle with a period of about 24.5 hours. At the start of baseline monitoring, this subject’s primary sleep period was during the daytime, with about a 30-minute phase delay in sleep onset per day throughout the baseline-monitoring period. By the third night of study medication (study day #17), his primary sleep period had shifted to a more normal phase relationship to nighttime and remained so entrained through the remaining study period, including the washout phase. Melatonin was administered in this subject, as in the others, 1 hour before the time his family said was his habitual bedtime. We did not discover until the actigraph data were analyzed at the end of the study that his primary sleep-period times were drifting later each night. This subject’s actigraph data suggested much improved sleep during the period of melatonin administration and washout (when he appeared to have a normally entrained sleep-wake cycle), with NTST increasing from a mean of 5.4 hours at baseline to 6.6 hours during treatment, and sleep efficiency increasing from a mean of 47.2% at baseline to 78.2% during treatment.59 This unusual subject will be discussed in further detail in a subsequent report. Because the nature of both the sleep disturbance and response to melatonin of this subject were unique relative to others in this large cohort of AD subjects, when the primary outcome measures of the ML 2.5SR group were compared to placebo and the P values were less than .25, the analyses were rerun without this subject’s atypical response. Excluding this individual resulted in virtual elimination of any effect of treatment in the ML 2.5SR group relative to PLA.
Secondary Measures
Table 5 presents the demographic data and baseline and change scores for the secondary measures. Change scores in Mini-Mental State Examination, Alzheimer’s Disease Assessment Scale - cognitive sub-scale, activities of daily living, Hamilton Depression Rating Scale, NPI, and SDI were calculated for all variables (later-earlier). Because of the high baseline NPI scores in the ML 2.5SR group, a significant difference in change in NPI scores between baseline and end of treatment was seen between ML 2.5SR and PLA (P = .05). No other differences were found, and the change in NPI in the ML 2.5SR group was not associated with change in sleep variables (all unadjusted P = .15).
Table 5.
Demographic and descriptive background variables and baseline, and 8-week change scores by arm for the secondary outcome measures.
| Variable | PLACEBO | ML 2.5SR | ML 10.0 | OVERALL |
|---|---|---|---|---|
| Age, y | 77.0 ± 8.5 | 78.4 ± 8.2 | 76.5 ± 10.1 | 77.4 ± 8.9 |
| Duration AD | 5.1 ± 2.9 | 4.6 ± 2.6 | 5.1 ± 3.4 | 4.9 ± 3.0 |
| Education, y | 12.5 ± 3.7 | 13.1 ± 4.1 | 12.4 ± 3.3 | 12.7 ± 3.7 |
| MMSE at baseline | 14.2 ± 9.0 | 12.8 ± 9.0 | 14.6 ± 8.5 | 13.9 ± 8.8 |
| MMSE change | 0.34 ± 2.7 | 0.33 ± 2.8 | −0.20 ± 3.4 | 0.16 ± 3.0 |
| ADAS | 38.0 ± 18.3 | 38.9 ± 19.1 | 38.8 ± 19.6 | 38.5 ± 18.9 |
| ADAS change | 1.4 ± 4.9 | 0.25 ± 5.4 | 0.97 ± 5.5 | 0.87 ± 5.3 |
| ADL (23 item) | 36.5 ± 23.5 | 30.5 ± 23.7 | 35.6 ± 23.2 | 34.1 ± 23.5 |
| ADL change* | −0.98 ± 4.7 | −0.65 ± 6.0 | −0.49 ± 6.7 | −0.71 ± 5.8 |
| Hamilton | 7.2 ± 3.4 | 7.8 ± 3.5 | 7.8 ± 3.7 | 7.6 ± 3.5 |
| Hamilton change* | −1.7 ± 4.5 | −1.2 ± 4.6 | −1.9 ± 3.9 | −1.6 ± 4.4 |
| NPI | 18.5 ± 17.1 | 24.9 ± 20.0† | 15.5 ± 15.8 | 19.7 ± 18.1 |
| NPI change* | −0.17 ± 14.1 | −6.4 ± 15.1‡§ | 0.46 ± 11.9 | −2.1 ± 14.0 |
| SDI (product of averages) | 3.3 ± 1.8 | 3.7 ± 2.2 | 3.8 ± 2.4 | 3.6 ± 2.2 |
| SDI change* | −1.6 ± 1.4 | −1.9 ± 2.6 | −2.3 ± 2.3 | −1.9 ± 2.2 |
| Sleep Quality Rating | 3.0 ± 0.7 | 3.0 ± 0.7 | 3.2 ± 0.7 | 3.1 ± 0.7 |
| Sleep Quality Rating Change | 0.3 ± 0.6 | 0.41 ± 0.7§ | 0.2 ± 0.4 | 0.30 ± 0.6 |
Negative score = improvement.
Mann-Whitney test: unadjusted P = .012 for ML 2.5SR mg vs ML 10 mg.
Mann-Whitney test: unadjusted P = .047 for ML 2.5SR vs placebo.
Unadjusted P = .023 for ML 2.5SR vs ML 10.
Adjusted P = .03 for ML 2.5SR without data from subject with free-running circadian rhythm vs placebo.
ML 2.5SR refers to treatment with 2.5 mg sustained-release melatonin; ML 10, treatment with 10 mg melatonin; AD, Alzheimer’s disease; MMSE, Mini-Mental State Examination; ADAS, Alzheimer’s Disease Assessment Scale; NPI, Neuropsychiatric Inventory; SDI, Sleep Disorders Inventory.
Dementia caregivers are highly stressed and, with the exception of the sleep-quality rating, the compliance with Daily Sleep Diaries was poor. Quality of sleep ratings from the Daily Sleep Diaries showed that the average gain in sleep quality was significantly greater in the ML 2.5SR group than in PLA (Z = −2.44, adjusted P = .03) but not for ML 10 (unadjusted P = .36). The mean gain in sleep quality was a change from “difficult night” to “fair night” for ML 2.5SR, while placebo ratings did not change from “fair night,” although the average rating increased slightly. The average gain in sleep quality for ML 10 was less than that for PLA. There were no significant differences in SDI scores, and, on average, improvement was observed in each group: ML 10 had the greatest improvement, followed by ML 2.5SR and PLA.
Adverse Events
There were no differences in the mean number, severity, seriousness, or relatedness ratings of spontaneously reported AEs across the 3 groups (ie, the proportions of each treatment group reporting any AE were equal). Table 6 presents summaries of the AEs for this study. The unadjusted P value (P = .04) for comparisons of average seriousness of reported AEs suggested that AEs in the placebo group were more serious than those in the ML 10 group. We also found that, while the proportion of the active treatment groups reporting at least 1 AE did not differ significantly, more AEs were reported in the ML 2.5SR group than in the ML 10 group (unadjusted P =.04).
Table 6.
Descriptives: Adverse Events
| Variable | PLACEBO | ML 2.5SR | ML 10 | OVERALL |
|---|---|---|---|---|
| Reporting at least 1 AE, % | 69.2 | 79.6 | 74.0 | 74.4 |
| Mean # AE reports x person* | 2.4 ± 2.7 | 3.4 ± 3.4 | 2.0 ± 1.9 | 2.6 ± 2.8 |
| Mean AE Severity†‡ | 1.4 ± 0.4 | 1.5 ± 0.6 | 1.5 ± 0.5 | 1.5 ± 0.5 |
| Mean AE Serious§¦ | 2.0 ± 0.0 | 1.9 ± 0.3 | 1.9 ± 0.2 | 2.0 ± 0.21 |
| Mean AE Relatedness¶ | 4.6 ± 0.6 | 4.5 ± 0.7 | 4.6 ± 0.6 | 4.6 ± 0.61 |
ML 2.5SR refers to treatment with 2.5 mg sustained-release melatonin; ML 10, treatment with 10 mg melatonin; AE, adverse events
2.5 vs ML 10: Z = −2.01, P = .04;
Severity: 1 = mild; 3 = severe.
PLA vs 2.5, Z = −1.87, P = 0.06;
Serious: 1= serious; 2 = not serious.
PLA vs ML 10: Z= −2.10, P = .04 (unadjusted P values)
Relatedness: 1 = definitely related; 5 = not related.
Most frequently reported AE’s (>5% of total AEs for group):
Placebo: Abnormal behavior, ache/pain, falls, fatigue, gastrointestinal distress, infection, respiratory/pulmonary symptom, skin/subcutaneous tissue, urinary symptoms.
ML 2.5SR: Abnormal behavior, ache/pain, falls, gastrointestinal distress, infection, respiratory/pulmonary symptom, skin/subcutaneous tissue, urinary symptoms.
ML 10: Abnormal behavior, ache/pain, falls, gastrointestinal distress, respiratory/pulmonary symptom, skin/subcutaneous tissue, urinary symptoms.
Residual Daytime Plasma Melatonin Levels
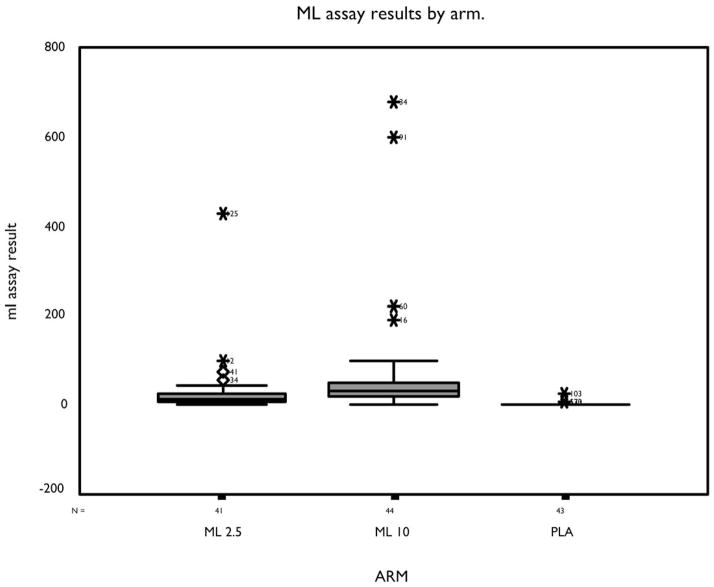
The melatonin groups had elevated daytime plasma melatonin levels relative to the PLA group, P < .05 by Holm-adjusted Mann-Whitney t test (Figure 3). Residual daytime melatonin levels in the ML 10 group (mean ± SD) were highest (67.7 ± 281 ng/dL), followed by ML 2.5SR (28.7 ± 67 ng/dL), and PLA (2.7 ± 4 ng/dL). Two subjects in the ML 10 group had midday melatonin levels greater than 600 ng/dL, and 1 subject in the ML 2.5SR group had a level greater than 400 ng/dL. All subjects in the placebo group had melatonin levels that were normal midday values (<10 ng/dL).
Figure 3.
Results of blood assays for melatonin on first day of washout, by treatment arm. Subject ID number is shown by the outlier data points. ML refers to melatonin; ML 2.5SR, treatment with 2.5 mg sustained-release melatonin; ML 10, treatment with 10 mg melatonin; PLA, placebo.
DISCUSSION
We have reported the results of the first multicenter clinical trial of a therapeutic agent for sleep disturbance in AD. No significant treatment effects were seen in the actigraph data, although 2 positive trends were detected. There were trends for increased NTST in the melatonin groups relative to the PLA group and in the percentage of subjects in the ML 10 group versus those in the PLA group to experience at least a 30-minute increase in NTST (see Table 4). There was also a very weak trend for decreased day-night sleep ratio (ML 2.5SR vs PLA).
Baseline NTST was 351 ± 83 minutes (5.8 ± 1.4 hours) and daytime total sleep time was 151 ± 96 minutes (2.5 ± 1.6 hours). That is, average total sleep time in a 24-hour cycle at baseline was more than 8 hours, with almost one-third occurring during daytime hours. This is evidence that the primary sleep disturbance for many of these patients was in the diurnal distribution rather than decreased amount of sleep. However, given the tendency for actigraphs to overestimate sleep by 27%, it is possible that many of the subjects were sleep deprived even with compensatory daytime sleep.
Although more AEs were reported in the ML 2.5SR group than in the ML 10 group (Table 5), there was no difference between groups in the likelihood of AEs being related to study medication. No AEs were definitely related to study medication. In general, melatonin appears to be as well tolerated as placebo.
The subject who had what appeared to be a free-running sleep-wake cycle during the baseline monitoring period had a large gain in NTST and sleep efficiency with ML 2.5SR.46 We believe it was reasonable to exclude this subject’s data from the final analysis because his sleep disturbance and melatonin response were atypical for this group of subjects. His response to melatonin most likely depended on a chronobiologic mechanism that may not be relevant to AD patients without free-running sleep rhythms. That is, it is likely that melatonin improved sleep quality in this subject by anchoring his primary sleep period at the appropriate time, thereby synchronizing sleep-wake and day-night cycles.
Night-to-night variability in all sleep measures, especially wake after sleep onset, was very large in our study population. The mean change in sleep measures observed in the PLA group were small, but the range of these changes was large. This variability was also seen in the melatonin groups. We had hoped melatonin would reduce night-to-night variability, but this effect was not seen in our analyses. Investigators need to anticipate this high level of variability in their power analyses for future studies involving sleep in AD subjects.
Mean daytime melatonin levels at the study’s end were higher in the ML groups than in the PLA group (Figure 3). Melatonin is a nocturnal hormone, and, normally, melatonin levels are nearly undetectable during the day.60 The high melatonin doses used in this trial were selected because lower doses were ineffective in our pilot studies, whereas higher doses looked promising for enhancing sleep maintenance. At the time we designed this trial, lower doses had never been shown to improve sleep maintenance in any subjects, although a recent report of a small sample of subjects suggests that a “physiologic-range” dose of 0.3 mg can improve sleep efficiency in older patients with insomnia.61
With nightly administration for 8 weeks, pharmacologic-range melatonin produced elevated daytime levels at the last study visit in several of our subjects, especially at the 10-mg dose (Figure 3). High residual melatonin levels could have a daytime soporific effect that adversely impacts nighttime sleep. Apart from this potential daytime soporific effect, there may also be a counter-therapeutic chronobiologic effect from these high daytime plasma levels, since the circadian pacemaker is sensitive to the phase-shifting effects of melatonin during the day as well as at night.38 In 1 study, high doses of melatonin (20 mg) given to a free-running blind person failed to entrain the subject, whereas a low dose (0.5 mg) succeeded, presumably because of high residual daytime melatonin levels interacting with the phase-advanced portion of the melatonin phase-response curve normally not exposed to endogenous melatonin.62 A recently published, trial of high-dose, slow-release melatonin (6 mg, described as low dose by the investigators) in 25 subjects with mixed dementia diagnoses was also negative. Although they did not report daytime melatonin levels, it is conceivable that high daytime residual levels of melatonin also contributed to the negative findings of the well-designed, high-dose trial of Serfaty et al.63
We do not know whether elevated daytime melatonin levels affected the outcome of this trial. The subject in the ML 10 group with the highest residual daytime level (680 ng/dL at 12:00) gained an average of 49 minutes of daytime total sleep time, suggesting a counter-therapeutic effect in this individual. A subject in the ML 2.5SR group had a plasma melatonin level of 427 ng/dL at 3:00 PM on the last day of the study. This subject had nearly 15 minutes less nighttime sleep and 18 minutes more daytime sleep on average during the melatonin treatment period relative to baseline. In fact, 2 of the 3 subjects in the ML arms with the highest residual afternoon melatonin levels had significant deterioration in nighttime sleep and day-night sleep ratio. Elevated daytime melatonin levels, through interaction with the phase-advance portion of the melatonin phase-response curve, could have induced a phase advance in circadian rhythms that resulted in more daytime sleep and less evening sleep. This may have contributed to the mixed results we observed in the whole study sample. However, total sleep time during the day did not increase more in the melatonin groups as a whole than in the PLA group (Table 4), nor did subjects receiving melatonin report more daytime fatigue or other AEs than the other groups (Table 6).
We chose a 12-hour nighttime epoch to define the nocturnal period for automated scoring. We did not want to impose sleep times on the subjects, and we therefore had to use a large nocturnal window to encompass the subjects’ variable nocturnal sleep periods. However, by arbitrarily defining nighttime sleep and daytime sleep periods, we may have misrepresented the sleep of subjects who either went to bed before 8:00 PM or who sleep past 8:00 AM for their primary nocturnal sleep period. Any potential improvement in NTST as a result of study medication coming before 8:00 PM or after 8:00 AM would be interpreted as a negative outcome in this analysis. A review of the sleep-diary data indicated that the 12-hour nocturnal window did capture all of the subjects’ primary sleep periods.
A second potential problem with this method of analysis is that many subjects may actually have had improved sleep during 1 segment of the 12-hour nocturnal epoch, say between midnight and 4:00 AM, that would also go undetected by our method of analysis because of more wakefulness during another segment of the nocturnal epoch, say between 4:00 AM and 8:00 AM. This is an important issue because our analysis may have missed a change in sleep that would be much appreciated by families and caregivers, such as improved middle-of-the night sleep. We intend to address this question in future analyses.
Although this study was the first multicenter trial of melatonin in this population, and was large by sleep-research standards, it was small in size compared to most multicenter trials of psychotropic medications. For example, trials of antipsychotic medications for psychosis or agitation in AD often require several hundred subjects to show efficacy because of very modest treatment-effect size,64 yet these medications are in widespread clinical use in AD patients and are generally considered to be efficacious. Subjective measures did show change in this trial, and in the case of caregiver sleep-diary assessments of sleep, there was a significant improvement in the ML 2.5SR group relative to the PLA group and trends for the ML groups relative to the PLA group to show improvement on the SDI. Nevertheless, without significant change in objective sleep measures, there is little doubt that this is a negative trial. We powered this study to detect a 30-minute increase in NTST (á =0.8), which is not only the treatment effect seen in our pilot study, but, arguably, is a minimum effect size for clinical significance. We observed roughly half this increase in NTST for 2 two active-treatment arms (16 ± 54 minutes in the ML 2.5SR group and 13 ± 44 minutes in the ML 10 group) versus essentially no change in the PLA group (3 ± 39 minutes). Although our methodology had certain limitations, our failure to detect a 30-minute increase in NTST in the ML groups suggests that melatonin does not have a clinically significant treatment effect on objective measures of sleep maintenance, sleep duration, or day-night sleep ratio in most patients with AD.
We believe that these data can be helpful in advising our patients who ask us about melatonin. It is reasonable to conclude that melatonin is very well tolerated, even at a high dose. It is also reasonable to expect some people to experience improved sleep from doses in the 2.5-mg to 10-mg range, although clinical response is not predictable or robust. Finally, AD patients with circadian rhythm sleep disturbances, such as 1 of our subjects, may have more positive responses. Nevertheless, the essentially negative results of this trial leave us without any proven therapy for most AD patients with insomnia, and clinicians are left to make intuitive choices of sleep therapies until more conclusive data are available.
Acknowledgments
We wish to thank all the subjects, their families, and caregivers for their willingness to participate and contribute so much to this project. We also thank the study monitors, staff at the ADCS, and the many study coordinators and investigators at centers around the country who recruited and tended to the many details of this complex protocol. Eric Colling, BSN, did an outstanding job of providing oversight of actigraph data quality and serving as actigraphy consultant to the sites. The assistance of Dr. Sonia Ancoli-Israel and Dr. Alfred Lewy, who provided very helpful comments on the manuscript, is deeply appreciated. Finally, both the ADCS Minority Recruitment Committee, headed by Dr. Mary Sano, and the ADCS Safety Data Monitoring Board, headed by Dr. Karl Kieburtz, made substantial contributions to this research effort. This work was supported by Grant AG 10483 from the NIA.
APPENDIX 1. Inclusion and exclusion criteria for the melatonin trial
A. Inclusion Criteria
Diagnosis of probable Alzheimer’s disease by National Institute of Neurological and Communicative Disorders and Stroke/the Alzheimer’s Disease and Related Disorders Association criteria53
Mini-Mental State Examination score of 0 to 26
Hachinski Ischemia Scale score less than 5
Two-week history of 2 or more sleep disorder behaviors, occurring at least once weekly, as reported by the caregiver on the Sleep Disorder Inventory
Computed tomography or magnetic resonance imaging since the onset of memory problems showing no more than 1 lacunar infarct in a nonstrategic area and no clinical events suggestive of stroke or other intracranial disease since the computed tomography or magnetic resonance imaging
Physically acceptable for the study as confirmed by medical history and examination, clinical laboratory results, and electrocardiogram
Actigraph evidence of a mean time immobile of less than 7 hours per night based on at least 5 nights of complete actigraph data collected over a single week
Stable home situation with no planned move during the 13-week investigation period
Residing with a responsible spouse, family member, or professional caregiver who is present during the night and would agree to assume the role of the principal caregiver for the 13-week protocol, including arranging transportation for the patient to and from the investigator’s clinic, answering questions regarding the patient’s condition, and assuming responsibility for medication and actigraph procedures
Ability to ingest oral medication and participate in all scheduled evaluations
Six grades of education or a work history sufficient to exclude mental retardation
Fifty-five years of age or older
Hamilton Depression Rating Scale score of 15 or less
Stable medications for nonexcluded concurrent medical conditions for 4 weeks prior to the screening visit
B. Exclusion Criteria
Acute sleep disturbance, developing within 2 weeks of screening
Sleep disturbance associated with an acute illness or delirium
Clinically significant movement disorder, such as akinesia, that would affect actigraphic differentiation of sleep and wakefulness
Not having a mobile upper extremity to which to attach an actigraph
Severe agitation
Pain syndrome affecting sleep
Unstable medical condition
Use of an investigational or unapproved medications within 4 weeks of the screening visit
Discontinuation of psychotropic or sleep medications within 2 weeks of the screening visit
Patient unwilling to maintain caffeine abstinence after 2:00 PM for the duration of the protocol
Patient unwilling to comply with the maximum limit of 2 alcoholic drinks per day, and only 1 alcoholic drink after 6:00 PM for the duration of the protocol
Use of melatonin within 2 weeks of the screening visit
Clinically significant abnormal laboratory findings that have not been approved by the Project Director
Residing in a facility without a consistent caregiver present during the night who could function as the primary informant
Caregiver deemed too unreliable to supervise the wearing of the actigraph, to administer melatonin at the proper time, to maintain the sleep diary, or to bring the patient to the scheduled visits
Autoimmune disease such as rheumatoid arthritis or polymyalgia rheumatica that could be contraindications to melatonin administration
APPENDIX 2. Participating sites and investigators
| Site Name Protocol | Principal Investigator | No. Screened/Enrolled |
|---|---|---|
| Oregon Health Sciences University | Jeffrey Kaye, MD | 8/6 |
| University of Southern California | Lon Schneider, MD | 7/4 |
| University of California, San Diego | Michael Grundman, MD, MPH | 6/6 |
| University of Michigan | Norman Foster, MD | 4/4 |
| Mayo Clinic, Rochester | Bradley Boeve, MD | 3/2 |
| Baylor College of Medicine | Rachelle Smith-Doody, MD, PhD | 15/8 |
| Columbia University | Mary Sano, PhD | 6/4 |
| Washington University | John Morris, MD | 8/6 |
| University of Minnesota | David Knopman, MD | 4/2 |
| Wien Center, University Miami | Ranjan Duara, MD | 9/6 |
| University Hospitals of Cleveland | Alan Lerner, MD | 7/6 |
| Suncoast, University of South Florida | Eric Pfeiffer, MD | 8/5 |
| New York University | Emile Franssen, MD | 2/1 |
| University of Pennsylvania | Christopher Clark, MD | 3/3 |
| University of Pittsburgh | Daniel Kaufer, MD | 5/3 |
| University of Rochester Medical Center | Anton Porsteinsson, MD | 14/9 |
| University of California, Irvine | Carl Cotman, PhD | 6/4 |
| University of Texas, Southwestern | Myron Weiner, MD | 6/5 |
| Emory University | Allan Levey, MD, PhD | 8/4 |
| Kansas University | Charles DeCarli, MD | 3/2 |
| Vanderbilt University | Richard Margolin, MD | 3/3 |
| University of California, Los Angeles | Jeffrey Cummings, MD | 11/7 |
| Augusta VA Medical Center | Edward Zamrini, MD | 2/2 |
| Brown University | Brian Ott, MD | 5/5 |
| Yale University | Christopher van Dyck, MD | 9/5 |
| University of California, Davis | William Jagust, MD | 2/1 |
| Arizona Health Sciences Center | Geoffrey Ahern, MD, PhD | 6/4 |
| Fletcher Allen Health Care, University of Vermont | Paul Newhouse, MD | 2/1 |
| Southwestern Vermont Medical Center | Paul Solomon, PhD | 2/2 |
| University of Nevada, Las Vegas | Charles Bernick, MD | 4/3 |
| Medical University of South Carolina | Jacobo Mintzer, MD | 13/9 |
| ClinSearch, Incorporated, New Jersey | Mark Roffman, PhD | 9/7 |
| Alzheimer’s Research Corporation, New Jersey | Joel Ross, MD | 10/5 |
| Memorial VA Hospital, Boston University | Ladislav Volicer, MD, PhD | 3/3 |
| Memory Disorders Institute (Medwise), New Jersey | Joshua Shua-Haim, MD | 19/9 |
| University of Virginia | Robert Brashear, MD | 3/1 |
Footnotes
Disclosure Statement
No significant financial interest/other relationship to disclose.
References
- 1.Swearer J, Drachman D, O’Donnell B, Mitchell A. Troublesome and disruptive behaviors in dementia. J Am Geriatr Soc. 1988;36:784–90. doi: 10.1111/j.1532-5415.1988.tb04260.x. [DOI] [PubMed] [Google Scholar]
- 2.Pollack CP, Perlick D. Sleep problems and institutionalization of the elderly. J Geriatri Psychiatry Neurol. 1991;4:204–10. doi: 10.1177/089198879100400405. [DOI] [PubMed] [Google Scholar]
- 3.Prinz PN, Vitaliano PP, Vitiello MV, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging. 1982;3:299–309. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 4.Vitiello MV, Prinz PN. Alzheimer’s disease: sleep and sleep/wake patterns. Clin Geriatr Med. 1989;5:289–99. [PubMed] [Google Scholar]
- 5.Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol: Med Sci. 1990;45:M131–8. doi: 10.1093/geronj/45.4.m131. [DOI] [PubMed] [Google Scholar]
- 6.Vitiello MV, Poceta JS, Prinz PN. Sleep in Alzheimer’s disease and other dementing disorders. Can J Psychol. 1991;45:221–39. doi: 10.1037/h0084283. [DOI] [PubMed] [Google Scholar]
- 7.Aaron-Peretz J, Masiah A, Pillar T, Epstein R, Tzischinsky O, Lavie P. Sleep-wake cycle in multi-infarct dementia and dementia of the Alzheimer’s type. Neurology. 1991;41(10):1616–9. doi: 10.1212/wnl.41.10.1616. [DOI] [PubMed] [Google Scholar]
- 8.Vitiello MV, Bliwise DL, Prinz PN. Sleep in Alzheimer’s disease and the sundowning syndrome. Neurology. 1992;42(Suppl 6):83–94. [PubMed] [Google Scholar]
- 9.Bliwise D. Sleep in normal aging and dementia. Sleep. 1993;16(1):40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Klauber MR, Gillin JC, Campbell SS, Hofstetter CR. Sleep in non-institutionalized Alzheimer’s disease patients. Aging Clin Exp Res. 1994;6:451–8. doi: 10.1007/BF03324277. [DOI] [PubMed] [Google Scholar]
- 11.Bliwise DL, Hughes M, McMahon PM, Kutner N. Observed sleep/wakefulness and severity of dementia in an Alzheimer’s disease special care unit. J Gerontol: Biol Med Sci. 1995;50(6):M303–6. doi: 10.1093/gerona/50a.6.m303. [DOI] [PubMed] [Google Scholar]
- 12.Moe KE, Vitiello MV, Larsen LH, Prinz PN. Sleep/wake patterns in Alzheimer’s disease: Relationships with cognition and function. J Sleep Res. 1995;4:15–20. doi: 10.1111/j.1365-2869.1995.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Klauber MR, Jones DW, et al. Variations in circadian rhythms of activity, sleep and light exposure related to dementia in nursing-home patients. Sleep. 1997;20(1):24–7. [PubMed] [Google Scholar]
- 14.Pollak CP, Stokes PE. Circadian rest-activity rhythms in demented and nondemented older community residents and their caregivers. J Am Geriatr Soc. 1997;45(4):446–52. doi: 10.1111/j.1532-5415.1997.tb05169.x. [DOI] [PubMed] [Google Scholar]
- 15.Bliwise DL. Sleep and circadian rhythm disorders in aging and dementia. In: Turek FW, Zee PC, editors. Regulation of Sleep and Circadian Rhythms. New York: Marcel Decker Inc; 1999. pp. 487–525. [Google Scholar]
- 16.Zee PC, Grujic ZM. Neurological disorders associated with disturbed sleep and circadian rhythms. In: Turek FW, Zee PC, editors. Regulation of Sleep and Circadian Rhythms. New York: Marcel Decker Inc; 1999. pp. 557–96. [Google Scholar]
- 17.McCurry SM, Logsdon RG, Teri L, et al. Characteristics of sleep disturbance in community-dwelling Alzheimer’s disease patients. J Geriatr Psychiatry Neurol. 1999;12(2):53–9. doi: 10.1177/089198879901200203. [DOI] [PubMed] [Google Scholar]
- 18.Werth E, Savaskan E, Knoblauch V, et al. Decline in long-term circadian rest-activity cycle organization in a patient with dementia. J Geriatr Psychiatry Neurol. 2002;15:55–9. doi: 10.1177/089198870201500111. [DOI] [PubMed] [Google Scholar]
- 19.Yesavage JA, Taylor JL, Kraemer H, et al. Sleep/wake cycle disturbance in Alzheimer’s disease: how much is due to an inherent trait? Int Psychogeriatr. 2002;14:73–81. doi: 10.1017/s1041610202008293. [DOI] [PubMed] [Google Scholar]
- 20.Caselli JR, Reiman EM, Hentz JG, Osborne D, Alexander GE, Boeve BF. A distinctive interaction between memory and chronic daytime somnolence in asymptomatic APOE e4 homozygotes. Sleep. 2002;25(4):447–53. [PubMed] [Google Scholar]
- 21.Brzezinski A. Melatonin in humans. N Engl J Med. 1997;336:186–95. doi: 10.1056/NEJM199701163360306. [DOI] [PubMed] [Google Scholar]
- 22.Sack RL. Melatonin. Sci Med. 1998;5(5):8–17. [Google Scholar]
- 23.Sack RL, Hughes RJ, Edgar DM, Lewy AJ. Sleep-promoting effects of melatonin: At what dose, in whom, under what conditions, and by what mechanisms? Sleep. 1997;20:908–15. doi: 10.1093/sleep/20.10.908. [DOI] [PubMed] [Google Scholar]
- 24.Singer C, Wild, Sack R, Lewy A. High dose melatonin is well tolerated by the elderly. Sleep Res. 1994c;23:86. [Google Scholar]
- 25.Singer C, MacArthur A, Hughes R, Sack R, Lewy A. High dose melatonn and sleep in the elderly. Sleep Res. 1995;24A:151. [Google Scholar]
- 26.Zhdnova IV, Wurtman RJ. Efficacy of melatonin as a sleep-promoting agent. J Biol Rhythms. 1997;12(6):644–50. doi: 10.1177/074873049701200620. [DOI] [PubMed] [Google Scholar]
- 27.Mendelson WB. Efficacy of melatonin as a hypnotic agent. J Biol Rhythms. 1997;12(6):651–6. doi: 10.1177/074873049701200621. [DOI] [PubMed] [Google Scholar]
- 28.Hughs RJ, Sack RL, Lewy AJ. The role of melatonin and circadian phase in age-related sleep maintenance insomnia: assessment in a clinical trial of melatonin replacement. Sleep. 1998;21:52–68. [PubMed] [Google Scholar]
- 29.Dawson D, Rogers NL, van den Heuvel CJ, Kennaway DJ, Lushington K. Effect of sustained nocturnal transbuccal melatonin administration on sleep and temperature in elderly insomniacs. J Biol Rhythms. 1998;13(6):532–8. doi: 10.1177/074873098129000354. [DOI] [PubMed] [Google Scholar]
- 30.Zisapel N. The use of melatonin for the treatment of insomnia. Biol Signals Recept. 1999;8(1–2):84–9. doi: 10.1159/000014574. [DOI] [PubMed] [Google Scholar]
- 31.Stone BM, Turner C, Mills SL, Nicholson AN. Hypnotic activity of melatonin. Sleep. 2000;23(5):663–9. [PubMed] [Google Scholar]
- 32.Monti JM, Cardinali DP. A critical assessment of the melatonin effect on sleep in humans. Biol Signals Recept. 2000;9(6):328–39. doi: 10.1159/000014656. [DOI] [PubMed] [Google Scholar]
- 33.Andrade C, Srihari BS, Reddy KP, Chandramma L. Melatonin in medically ill patients with insomnia: a double blind, placebo-controlled study. J Clin Psychiatry. 2001;62(1):41–5. doi: 10.4088/jcp.v62n0109. [DOI] [PubMed] [Google Scholar]
- 34.Singer C, McArthur A, Hughes R, Sack R, Lewy A. Physiologic melatonin administration and sleep in the elderly. Sleep Res. 1995;24A:152. [Google Scholar]
- 35.Singer C, MacArthur A, Hughes R, Sack R, Lewy A. High dose melatonin and sleep in the elderly. Sleep Res. 1995;24A:151. [Google Scholar]
- 36.Singer C, Moffit M, Colling E, et al. Low dose melatonin administration and nocturnal activity levels in patients with Alzheimer’s disease. Sleep Res. 1997;26:752. [Google Scholar]
- 37.Sack RL, Stevenson J, Lewy AJ. Entrainment of a previously free-running blind human with melatonin. Sleep Res. 1990;19:404. [Google Scholar]
- 38.Lewy AJ, Ahmed S, Jackson JML, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–92. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 39.Zaidan R, Geoffriau M, Brun J, et al. Melatonin is able to influence its secretion in humans: description of a phase-response curve. Neuroendocrinology. 1994;60:105–12. doi: 10.1159/000126726. [DOI] [PubMed] [Google Scholar]
- 40.Lapierre O, Dumont M. Melatonin treatment of a non-24-hour sleep-wake cycle in a blind retarded child. Biol Psychiatry. 1995;38:119–22. doi: 10.1016/0006-3223(95)00072-O. [DOI] [PubMed] [Google Scholar]
- 41.Lewy AJ, Sack RL. Melatonin as a chronobiotic: treatment of circadian desychrony in night workers and the blind. J Biol Rhythms. 1997;12(6):595–603. doi: 10.1177/074873049701200615. [DOI] [PubMed] [Google Scholar]
- 42.Czeisler CA. Evidence for melatonin as a circadian phase-shifting agent. J Biol Rhythms. 1997;12:618–23. doi: 10.1177/074873049701200617. [DOI] [PubMed] [Google Scholar]
- 43.Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med. 2000;343:1070–7. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 44.Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system in blind subjects. J Endocrinol. 2000;164:R1–6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- 45.Proprietary data on file.
- 46.Lockley SW, Skene DJ, Arendt J. Changes in sleep in relation to circadian phase in the blind. In: Touitou Y, editor. Biological Clocks: Mechanisms and Applications. Amsterdam: Elsevier; 1998. pp. 247–52. [Google Scholar]
- 47.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 48.Yesavage JA, Friedman L, Ancoli-Israel S, et al. Development of diagnostic criteria for defining sleep disturbance in Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2003 doi: 10.1177/0891988703255684. in press. [DOI] [PubMed] [Google Scholar]
- 49.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 50.Ancoli-Israel S, Clopton P, Klauber MR, et al. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep. 1997;20(1):24–7. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sadeh A, Hauri PJ, Kripke DF, Lavie P. The role of actigraphy in the evaluation of sleep disorders. Sleep. 1995;18:288–302. doi: 10.1093/sleep/18.4.288. [DOI] [PubMed] [Google Scholar]
- 52.Sadeh A, Acebo C. The role of actigraphy in sleep medicine. Sleep Med Rev. 2002;6:113–24. doi: 10.1053/smrv.2001.0182. [DOI] [PubMed] [Google Scholar]
- 53.Yesavage JA, Friedman L, Kraemer HC, et al. A follow-up study of actigraphic measures in home-residing Alzheimer disease patients. J Geriatr Psychiatry Neurol. 1998;11:7–10. doi: 10.1177/089198879801100103. [DOI] [PubMed] [Google Scholar]
- 54.Colling E, Wright M, Lahr S, Schmedlen L, DeJongh L, Singer C. A comparison of wrist actigraphy with polysomnography as an instrument of sleep detection in elderly persons. Sleep. 2000;23:A378. [Google Scholar]
- 55.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1974;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 56.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 57.Galasko D, Bennet D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer’s disease. Alzheimer’s Disease and Associated Disorders. 1997;11:S33–9. [PubMed] [Google Scholar]
- 58.Hamilton M. A rating scale for depression. J Neurol, Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singer C, Tractenberg R, Kaye J, et al. ADCS clinical trial of melatonin for the sleep disturbance of Alzheimer’s disease: case report of an unusual sleep/wake cycle and response to melatonin. Am J Geriatr Psychiatry. 2002;2(Suppl 1):92. [Google Scholar]
- 60.Lewy AJ, Cutker NL, Sack RL. The endogenous melatonin profile as a marker for circadian phase position. J Biol Rhythms. 1999;14(3):227–36. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- 61.Zhdanova IV, Wurtman RJ, Regan MM, Taylor JA, Ping Shi J, Leclair OU. Melatonin for treatment of age-related insomnia. J Clin Endocrinol Metab. 2001;86(10):4727–30. doi: 10.1210/jcem.86.10.7901. [DOI] [PubMed] [Google Scholar]
- 62.Lewy AJ, Emens JS, Sack RL, Hasler BP, Bernert RA. Low, but not high doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol Int. 2002;19(3):649–58. doi: 10.1081/cbi-120004546. [DOI] [PubMed] [Google Scholar]
- 63.Serfaty M, Kennell-Webb S, Warner J, Blizard, Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int J Geriatr Psychiatry. 2002;17:1120–7. doi: 10.1002/gps.760. [DOI] [PubMed] [Google Scholar]
- 64.Tariot PN, Ryan M, Porteinsson AP, Loy R, Schneider LS. Pharmocologic therapy for behavioral symptoms of Alzheimer’s disease. Clin Geriatr Med. 2001;17:359–76. doi: 10.1016/s0749-0690(05)70073-2. [DOI] [PubMed] [Google Scholar]