Abstract
Although ecologists believe that vertebrate herbivores must select a diet that allows them to meet their nutritional requirements, while avoiding intoxication by plant secondary metabolites, this is remarkably difficult to show. A long series of field and laboratory experiments means that we have a good understanding of the factors that affect feeding by leaf-eating marsupials. This knowledge and the natural intraspecific variation in Eucalyptus chemistry allowed us to test the hypothesis that the feeding decisions of greater gliders (Petauroides volans) depend on the concentrations of available nitrogen (incorporating total nitrogen, dry matter digestibility and tannins) and of formylated phloroglucinol compounds (FPCs), potent antifeedants unique to Eucalyptus. We offered captive greater gliders foliage from two species of Eucalyptus, E. viminalis and E. melliodora, which vary naturally in their concentrations of available nitrogen and FPCs. We then measured the amount of foliage eaten by each glider and compared this with our laboratory analyses of foliar total nitrogen, available nitrogen and FPCs for each tree offered. The concentration of FPCs was the main factor that determined how much gliders ate of E. viminalis and E. melliodora, but in gliders fed E. viminalis the concentration of available nitrogen was also a significant influence. In other words, greater gliders ate E. viminalis leaves with a particular combination of FPCs and available nitrogen that maximised the nutritional gain but minimised their ingestion of toxins. In contrast, the concentration of total nitrogen was not correlated with feeding. This study is among the first to empirically show that browsing herbivores select a diet that balances the potential gain (available nutrients) and the potential costs (plant secondary chemicals) of eating leaves. The major implication of the study is that it is essential to identify the limiting nutrients and relevant toxins in a system in order to understand feeding behaviour.
Introduction
Ecologists acknowledge that diet selection in browsing mammals involves some balance between the positive effects of nutrients and the negative effects of plant secondary metabolites (PSMs) [1,2]. It is, however, difficult to show that feeding on natural diets involves a trade-off between the acquisition of nutrients and the avoidance of toxins. This has prompted a plethora of studies over the past 30 years in which researchers attempt to relate the chemical composition of foliage to diet selection or the nutritional status of free ranging mammals e.g., [3–5], and yet, most of these studies prove inconclusive, sometimes for unknown reasons but often due to the difficulty in identifying the limiting nutrients and PSMs in the system, usually from a large number of intricately interacting possibilities. In lieu of this information, researchers often turn to the nutrients and PSMs that many regard as important. From the nutrient perspective, of most interest are often amino acids, measured as nitrogen (N) or crude protein, because there is a general consensus that they are the limiting nutrients for many herbivores [6,7]. This may be true as shown by Felton et al. [8], who found that meeting their protein requirements dictated how much spider monkeys ate. In contrast, Rothman et al. [9] found the opposite in mountain gorillas, which at times ingested more protein than they required in order to meet their energy requirements. This comparison emphasises the need to identify the limiting nutrients and the PSMs that either challenge detoxification systems or that reduce the digestibility of nutrients, rather than those believed to be important, such as total phenolics and fibre. Identifying the key factors for herbivore diet selection will prove invaluable as it will enable us to better understand their habitat choices, and, from a conservation point, conserve the correct habitats for herbivore species.
After a long series of studies, we have a good understanding of eucalypt chemistry and the factors that influence feeding on eucalypts by marsupial folivores e.g., [10–13]. Although species from the two most populous eucalypt subgenera, Symphyomyrtus and Eucalyptus (formerly Monocalyptus), commonly coexist [14] and attract different marsupial folivores [15] there are notable differences in their chemistry. Species of the subgenus Symphyomyrtus usually contain formylated phloroglucinol compounds (FPCs) [16] which do not occur in subgenus Eucalyptus. Leaves of species of the subgenus Eucalyptus typically contain about the same concentration of total N as do symphyomyrtles but only about half the concentration of available nitrogen (AvailN), due largely to the effects of tannins [17]. Tannins can reduce the digestion of nutrients in mammalian herbivores [18] largely via the formation of tannin-protein complexes, (depending on the specifics of tannin structure) and so can be a major influence on diet choice [19, 20]. If tannins make some proteins indigestible then concentrations of total nitrogen do not reflect the availability of protein to the herbivore. In contrast, available N (AvailN) measures the fraction of total N that is not bound to tannins or fibre [17,21] and thus is a more useful measure of the concentration of protein in a plant.
Apart from the substantial differences between the eucalypt subgenera, there is also large intraspecific variation in eucalypts in both the concentration of AvailN (but not total N) [22] and in FPCs [23] providing scope for studying trade-offs between nutritive factors and plant toxins known to be important. For example, studies of captive koalas (Phascolarctos cinereus), common ringtail possums (Pseudocheirus peregrinus) and common brushtail possums (Trichosurus vulpecula) indicate that FPCs are potent feeding deterrents [11,13,24,25]. Likewise, Moore and Foley [26] showed that wild koalas favour trees with lower concentrations of FPCs. In a different approach, DeGabriel et al. [27] studied a wild population of brushtail possums eating eucalypts of the subgenus Symphyomyrtus that surprisingly did not produce FPCs. They showed that females inhabiting home ranges with higher concentrations of AvailN (but not total N) had higher reproductive success and faster growing offspring than did females inhabiting poorer home ranges. In another field experiment, Youngentob et al. [28] found that greater gliders preferred monocalypts producing leaves with higher concentrations of digestible dry matter, total N and AvailN but preferred symphyomyrtles with lower concentrations of the FPC, sideroxylonal.
These data allowed us to test the hypothesis that food consumption depends on the balance between the potential gain (AvailN and digestible dry matter) and the potential costs (FPCs and tannins) associated with eating leaves. We chose the greater glider for this study because it feeds almost exclusively on young leaves of eucalypts with the preferred species often the dominant tall eucalypts in the habitat. Usually the diet consists of 3–6 different species of Eucalyptus over a year [29–32] but in some locations (e.g. SE NSW), a single species such as E. viminalis can comprise a large part of the diet year-round. Thus in captivity we provided 4–6 species for general maintenance of the species but focussed our studies on the single species. E. viminalis and E. melliodora because we have a good understanding of chemical variation of both species and in the case of E. melliodora we aimed to make comparisons with other folivores [25]. Another advantage of E. melliodora is that individuals vary widely in their production of sideroxylonals which allowed us to offer foliage with a wide range of FPC:AvailN concentrations.
Methods
Capture and housing
Four female and four male greater gliders (Petauroides volans) (mean body mass 1096 g; sd 195 g) were captured in Bungongo State Forest, Tumut, NSW (35° 02' S 148° 28' E) in March 2009—the non-breeding season. We used a spotlight to locate animals on exposed branches and a 0.222 calibre rifle to break the branch on which the animal perched. We captured the animal as it glided to the ground, placed it in an open weave sack and transported it to the animal house facilities at the Australian National University, Canberra, Australia. This research was approved by the Animal Experimentation Ethics Committee of the Australian National University (Permit number F.BTZ.33.08) and conforms to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. This research was conducted with the permission of State Forests New South Wales (Permit XX43632) and NSW National Parks and Wildlife Service (Permit S12812).
The animals were held in individual metabolism cages (60 x 80 x 60 cm) as described by Foley and Hume [33]. The cages were in a room maintained at 20 ± 3°C under a 12h:12h light:dark cycle with a gradual change in intensity over 30 min to simulate dawn and dusk. Each cage contained a T-shaped timber perch, a water source and a wooden nest box. Stems (ca 40 cm and 350 g) of Eucalyptus foliage were placed in a tube of water outside the cage with the foliage protruding inside. Animals were weighed at the beginning and end of each experimental period.
Diet and feeding
When first caught and when not being used in experiments, gliders were maintained on a mixed diet of Eucalyptus radiata and E. dives (sub-genus Eucalyptus), and E. bridgesiana, E. melliodora, E. viminalis, and E. grandis (sub-genus Symphyomyrtus) offered ad libitum. We collected branches of experimental foliage from individually identified E. melliodora (N = 16) and E. viminalis (N = 15) trees, whose chemistry we knew, at sites within 50 km of Canberra and stored them for up to 10 d by placing them in large polythene bags with the ends of branches in tubs of water in a cool-room (4° C).
The daily routine remained the same in all feeding experiments. We prepared bunches of foliage at 14:00 h and left them to equilibrate in water before re-weighing and placing them in cages at 16:00 h. Buds or fruits were removed to ensure measurements of intake were for foliage only. Changes in the dry matter content of the foliage were assessed with control bunches of foliage kept in the same room as the animals. Uneaten foliage was removed from the cages and weighed at 09:30 h, while spilled foliage was collected and dried to constant mass at 40° C for 48 h. This information enabled us to determine how much the gliders ate, expressed as dry matter intake (DMI).
Design of feeding experiments
We measured feeding by greater gliders in response to FPCs and AvailN using E. viminalis and E. melliodora. We selected trees based on their concentration of FPCs and AvailN to give a wide range of FPC:AvailN values. E. viminalis contains several FPCs, including sideroxylonals and various macrocarpals [23]. In contrast, sideroxylonals are the only FPCs produced by E. melliodora so this species is ideal for testing the hypothesis proposed by Youngentob et al. [28] that sideroxylonals rather than other FPCs determine the trees that greater gliders visit.
We chose to use single-choice protocols to increase our statistical power to detect the role of FPCs and AvailN in feeding choices. We used cyclical changeover designs that varied depending on the number of animals available but in all cases the animals were offered the leaves of a treatment tree for one night and thus could choose only how much they ate as opposed to selecting individual trees or species. In the first study, with E. viminalis, we fed 15 trees to seven or eight animals with four or five measures of food intake per tree. In the experiment with E. melliodora, we fed the leaves of 16 trees to eight gliders giving four measures of food intake for each tree. Each eucalypt species was offered to the gliders over the course of two experimental periods in which experimental nights alternated with rest nights and a rest period of one week separated the experimental blocks. On these rest nights and during the rest periods, we offered the gliders a mixture of foliage from several species described previously under “Diet and feeding”.
Diet analysis
Two leaf samples representing the foliage eaten by the gliders were taken from each control bunch and placed in paper bags. We placed one sample, weighing approximately 10 ± 1 g wet matter, in a 40° C oven for 48 h to calculate DM while the other sample, destined for analysis, was stored at -20° C before being freeze-dried and ground to pass a 1 mm screen in a Tecator cyclone mill.
Formylated phloroglucinol compounds
We extracted and quantified sideroxylonals (the only FPCs in E. melliodora) following the method of Wallis and Foley [34], which uses sonication followed by isocratic HPLC separation (93% acetonitrile, 7% water, both with 0.1% trifluoroacetic acid) lasting 12 min. We used the same method to extract FPCs from E. viminalis but because this species contains several FPCs, we used an 80 min HPLC procedure to separate and analyse the FPC compounds Extracts were eluted under gradient conditions with 0.1% TFA in acetonitrile (A) and 0.1% TFA in water (B) as follows: 60% A/40% B for 5 min, linear gradient to 90% A/10% B at 60 min, hold for 10 min and return to starting conditions by 80 min. We used a Waters Alliance Model HPLC fitted with photo diode array detector for all separations. The analytical column was a Wakosil 250 × 4 mm GL 3C18RS (SGE) held at 37° C and the flow rate was 0.75 ml min-1. We measured the peak response at 275 nm.
Available nitrogen
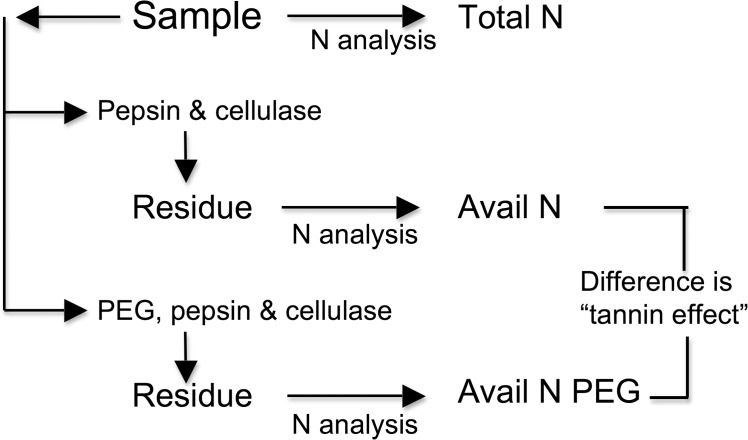
We determined in vitro AvailN of all leaf samples using the method of DeGabriel et al. [22] as modified by Wallis et al. [17] (Fig 1). Briefly, we incubated 800 ± 10 mg samples in ANKOM F57 filter bags (Macedon, U.S.A.) in a series of buffers and enzymes, at 35° C with constant stirring. At the start, we incubated two bags per sample in 0.05M Tris-Base buffer (for AvailN) and another two bags in buffer containing 33.3 g.L-1 of polyethylene glycol (PEG) 4000 (for AvailNPEG). PEG can disrupt tannin-protein complexes and thus its inclusion in the in vitro procedure allows an estimate of the extent to which tannins are limiting in vitro dry matter and N digestibility. After 24 h we washed the samples before drying them to constant mass and reweighing them. We then digested the samples in pepsin (2 g.L-1 1:10 000 pepsin in 0.1N HCl) for 24 h, followed by cellulase (6.25 g.L-1 cellulase in acetate buffer, pH 4.8) for 48 h. Finally, after thorough washing, they were dried to a constant mass at 50° C, weighed and the contents were analysed for N. All N analyses were done on a Leco Truspec Carbon/Nitrogen Determinator (Leco, St. Joseph, U.S.A.) using 200 ± 10 mg of leaf sample and 100 ± 10 mg of sample from the in vitro digestion bags. From this, we calculated in vitro dry matter digestibility and N digestibility (%) as the proportional losses of dry matter and N from the original sample respectively, while AvailN (% DM) is the product of the concentration of total N and in vitro N digestibility. The difference in available N between samples incubated with (AvailNPEG) and without PEG (AvailN) is estimated as the effect of tannins [17,21] (Fig 1).
Fig 1. Schematic diagram showing the relationship between the Total N, Available N and Available N PEG as measured in vitro.
The difference between the concentration of Available N and Available N PEG, is an estimate of the effect of tannins complexing with proteins in the sample.
Statistical Analysis
We analysed the data in GenStat Release 10.2, Lawes Agricultural Trust (Rothamsted Experimental Station). First we examined relationships between the chemical measures of foliage using simple linear regression. We then used the restricted maximum-likelihood algorithm (REML) to analyse the factors that influenced feeding by gliders. For gliders fed both E. viminalis and E. melliodora, we tested the following model:
Response Dry matter intake
Fixed model: Constant + Period + AvailN + FPC + AvailN*FPC + Body Mass
Random model: Glider + Period + Glider*Period
“Period” refers to the two experimental periods that were part of each feeding study (see experimental design), while AvailN and FPC refers to the concentration of these entities in the leaves we fed. We progressively dropped non-significant terms (P>0.1) from the model leaving a reduced model on which we based the results.
We tested the normality of the residual plots after developing each model and the second order Akaike information coefficient (AICc) to guide selection of the models to ensure, that dropping a term resulted in a better model. The body mass of the gliders did not affect how much they ate so we present results of DMI as g per animal per day.
Results
Variation in the concentrations of AvailN and FPCs in leaves from experimental trees
Leaves from individual E. melliodora and E. viminalis varied widely in their concentrations of FPCs (coefficient of variation, CV = 39% and 36%, respectively) and AvailN (CV = 23% and 26%) but there was less variation in the concentration of total N (CV = 13% and 10%). There was even less variation in in vitro dry matter digestibility (CV = 6% and 6%) (Table 1) with a seven-fold range in the ratio of the concentrations of FPCs to AvailN in E. viminalis and an eleven-fold range in E. melliodora (Table 1).
Table 1. Concentrations of nitrogen and formylated phloroglucinol compounds in the leaves of Eucalyptus melliodora (N = 16) and Eucalyptus viminalis (N = 15) fed to greater gliders.
| Species | Statistics | Sideroxylonal (mg.g-1 DM) | Other FPCs (mg.g-1 DM) | Total N (% DM) | AvailN 1 (% DM) | AvailNPEG 2 (% DM) | DMD 3 (%) | FPC: AvailN 4 |
|---|---|---|---|---|---|---|---|---|
| E. melliodora | ||||||||
| Mean | 29.6 | 0 | 1.53 | 0.84 | 1.02 | 64.4 | 38.0 | |
| SD | 11.4 | 0 | 0.20 | 0.20 | 0.21 | 4.1 | 22.4 | |
| CV % 5 | 39 | 0 | 13 | 23 | 21 | 6 | 59 | |
| Minimum | 6.2 | 0 | 1.23 | 0.44 | 0.71 | 58.3 | 7.5 | |
| Maximum | 55.7 | 0 | 1.92 | 1.24 | 1.53 | 70.1 | 84.6 | |
| E. viminalis | ||||||||
| Mean | 3.7 | 15.6 | 1.50 | 0.85 | 1.13 | 54.2 | 25.3 | |
| SD | 5.5 | 6.0 | 0.16 | 0.22 | 0.18 | 3.0 | 13.1 | |
| CV % | 151 | 36 | 10 | 26 | 16 | 6 | 51 | |
| Minimum | 0.6 | 7.0 | 1.24 | 0.60 | 0.83 | 48.8 | 6.9 | |
| Maximum | 22.8 | 23.7 | 1.76 | 1.32 | 1.45 | 59.1 | 48.6 |
1, 2 Available N and available N in the presence of PEG measured in an in vitro assay
3 Dry matter digestibility measured in vitro assay
4 The ratio of the concentrations of FPC and available N shown to indicate the range of values
5 Coefficient of variation
The concentration of AvailN was related to the concentration of total N in both E. melliodora (AvailN = -0.27 + 0.73 x Total N; r2 = 0.56; F 1,14 = 18.0; P < 0.001) and in E. viminalis (AvailN = -0.63 +0.99 x Total N; r2 = 0.50; F1,13 = 13.2; P = 0.003). The concentration of AvailNPEG was also related to that of total N in both species. The relationship, however, compared to that for AvailN was weaker in E. melliodora (AvailNPEG = -0.07 + 0.71 x Total N; r2 = 0.44; F 1,14 = 11.4; P = 0.004) but stronger in E. viminalis (AvailNPEG = -0.38 + 1.00 x Total N; r2 = 0.72; F 1,14 = 34.4; P < 0.001).
We found no relationship between the concentrations of FPCs and N or AvailN in either E. melliodora (P = 0.99, P = 0.29) or in E. viminalis (P = 0.92, P = 0.46) and between FPCs and AvailNPEG in E. viminalis (P = 0.38). There was, however, a relationship between FPCs and AvailNPEG in E. melliodora (FPC = 61.3–30.9 x AvailNPEG; r2 = 0.34; F 1,14 = 7.2; P = 0.017).
Feeding by greater gliders in response to plant chemistry
Greater gliders willingly ate leaves from both species of eucalypt and gained a little weight (mean = 0.79%; se = 1.42) during the E. viminalis experiments but lost weight (mean = -5.16%; se = 0.91) while eating E. melliodora. The body mass of the gliders had no effect on how much they ate (E. melliodora P = 0.49; E. viminalis P = 0.90; REML).
Even though they ate roughly similar amounts of the two species, the gliders responded differently to the plant chemistry, with the concentration of sideroxylonals being the only significant (P = 0.007) attribute of plant chemistry that influenced feeding in gliders offered E. melliodora. Total N (P = 0.25), AvailN (P = 0.60), AvailNPEG (0.76) and dry matter digestibility (P = 0.50) all had no bearing on how much the animals ate (see to Eq 1).
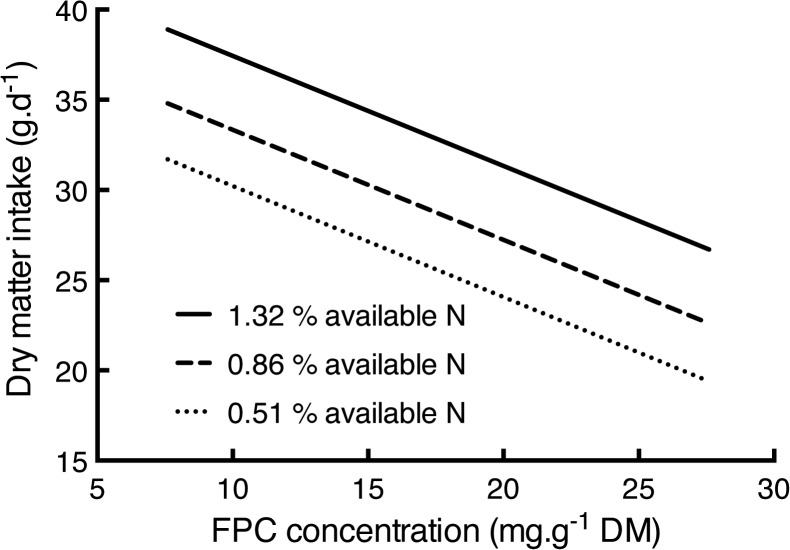
In contrast, the amount of E. viminalis that gliders ate depended on the concentrations of AvailN (P = 0.018) and of FPCs (P < 0.001) (Fig 2). The model from REML indicated positive effects on food intake for AvailN countered by the negative response to total FPCs (see Eq 2 for the model with the covariates centred (mean AvailN = 0.859; mean FPC = 17.55). Furthermore, separating the FPCs into sideroxylonals and non-sideroxylonal FPCs did not improve the model indicating that greater gliders respond similarly to all FPCs (ΔAICc = 0.85). Also, in vitro dry matter digestibility, which ranged from 49 to 59% did not influence food intake (P = 0.45).
Fig 2. The predicted amounts of E. viminalis eaten by greater gliders in relation to observed concentrations of formylated phloroglucinol compounds.
The lines represent predicted dry matter intakes at three concentrations of AvailN. Minimum observed (small dashed line; 0.51% DM), mean (larger dashed line; 0.86% DM) and maximum (solid line; 1.32% DM). Confidence intervals were omitted to simplify the graph but errors around the measures of the constant, AvailN and FPCs appear in Eq 2.
| Eq 1 |
| Eq 2 |
Discussion
In this study, we confirmed our first hypothesis that the amount of E. viminalis foliage that greater gliders ate depended on the balance between the potential gain of nutrients (AvailN) and the potential costs (FPCs) associated with eating leaves. This seems intuitive but demonstrating the point required a long and arduous series of experiments on arboreal folivores with two key breakthroughs that identified explanatory traits. The first of these was the discovery of the primary feeding deterrent, the FPCs, in Eucalyptus ovata [12] and subsequent survey that showed they occur in species of the subgenus Symphyomyrtus but not in the subgenus Eucalyptus [16]. A series of feeding experiments with captive animals demonstrated the potency of these compounds [25], while a field survey indicated their importance in tree-selection by several species [13,26]. The second breakthrough was tackling the role of tannins from a functional angle, which allowed the protein-binding capacity of tannins to be incorporated into the measure of AvailN [17,21].
Expressed differently, a key finding of this study was that total N had no bearing on food intake because the total N in the leaves we fed the gliders did not reflect its availability largely due to the influence of tannins. The ubiquity of tannins means that the findings extend much further than marsupials eating eucalypt leaves; they are relevant to any vertebrate herbivore eating tannin-rich material. Thus, in these cases, we see no value in using total N or broad measures of tannins, such as total phenolics, as explanatory variables and instead urge researchers to measure what is important to the animal.
A second advantage of the AvailN assay is that it accounts for the N-binding effects of tannins and fibre and so precludes the need to concoct ratios, such as total N:fibre or total N:total phenolics to weight the good and the bad attributes of a food. Such ratios, often of traits that researchers think are important, are commonplace in ecology. Frequently this approach is inconclusive e.g., [35], while in other cases it seems to work. For instance, Cork and Catling [36] predicted the abundance of arboreal folivores in eucalypt forests from the concentrations of N and total phenolics. These relationships, however, are simply correlative and without further experimentation to identify explanatory traits may merely indicate some unmeasured factor. The nitrogen-to-fibre ratio proposed by Milton [37] to explain leaf selection by primates and since used to predict the biomass of folivorous monkeys at local [38,39] and regional [40–43] scales is another example. Wallis et al. [21] suggested that this ratio might give the “right answer for the wrong reason” because the concentration of total N in plants often does not reflect its availability to animals. This critique garnered support from Gogarten et al. [44] who did not find higher densities of red colobus monkeys in regenerating forests where leaves had more favourable ratios of protein to fibre. Another risk that ratios pose is that they make something appear significant when in fact it is not. In the current study, the ratio of FPCs to total N had a significant influence on the quantity of E. viminalis leaves that greater gliders ate (F1,62 = 25.6; P < 0.001), but total N had no effect when included in a model with FPCs (F1,56 = 0.05; P = 0.82)
In contrast, AvailN influenced the amount gliders ate and while the effect may appear subtle, relative to that of the FPCs, DeGabriel and colleagues [27] showed that common brushtail possums (Trichosurus vulpecula) inhabiting home ranges where the trees produced leaves with lower mean concentrations of AvailN (0.30% DM) had lower reproductive success and slower growing offspring than did possums occupying better home ranges (AvailN = 0.40% DM). Similarly, McArt et al. [45] reported lower fecundity and twinning rates in moose where the AvailN concentration in the summer food was lower. In both of these studies, tannins were mainly responsible for limiting the availability of N and our data supports those findings. Furthermore, Felton et al. [8] showed that spider monkeys tightly regulate their intake of AvailN but not of total N.
The apparently subtle effect of AvailN is the likely explanation for the different responses of gliders to E. melliodora and E. viminalis. Both eucalypt species have similar mean values of AvailN but the higher concentrations of AvailNPEG (E. viminalis = 1.13% DM; E. melliodora = 1.02% DM) indicate that tannins are more active in binding protein in E. viminalis. This variation in E. viminalis provides the scope for gliders eating the species to select their diet using both FPCs and AvailN.
Our study identifies the tenuous balance between nutrients and plant secondary compounds in one system—the greater glider consuming eucalypt foliage. Nevertheless our findings have much wider implications. Ecologists wanting to explain the uneven distribution of animals across landscapes often seek nutritional explanations. Identifying nutritional factors that predict abundance, however, has proven remarkably difficult. While most researchers agree that the nutritional value of a landscape depends on both positive (concentrations of available nutrients) and negative (concentration of plant defences) traits, identifying these traits and understanding their interactions is much harder. This study confirms this thesis, shows the importance of identifying the crucial factors, in this case AvailN and FPCs and confirms what we might expect and what others report—that, due to secondary compounds, there may be a very fine balance between nutrient gain and nutrient loss.
Acknowledgments
We thank Jeff Whiting, Suat Hui Yeoh and Kara Youngentob for help with catching the gliders. Hannah Windley and Tyrie Starrs assisted with leaf collections and Karen Marsh and Amanda Padovan who provided many useful comments on the manuscript. The authors declare no conflict of interest.
Data Availability
All relevant data are within the paper.
Funding Statement
The work was supported by an Australian Research Council Discovery Grant (DP0986142) to IRW and WJF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Iason G, Villalba J (2006) Behavioral strategies of mammal herbivores against plant secondary metabolites: the avoidance tolerance continuum. Journal of Chemical Ecology 32: 1115–1132. [DOI] [PubMed] [Google Scholar]
- 2. McLean S, Duncan AJ (2006) Pharmacological perspectives on the detoxification of plant secondary metabolites: implications for ingestive behavior of herbivores. Journal of Chemical Ecology 32: 1213–1228. [DOI] [PubMed] [Google Scholar]
- 3. Barthelmess EL (2001) The effects of tannin and protein on food preference in eastern grey squirrels. Ethology 13: 115–132. [Google Scholar]
- 4. Degabriele R (1981) A relative shortage of nitrogenous food in the ecology of the koala (Phascolarctos cinereus). Australian Journal of Ecology 6: 139–141. [Google Scholar]
- 5. Kool KM (1992) Food selection by the silver leaf monkey, Trachypithecus auratus sondaicus, in relation to plant chemistry. Oecologia 90: 527–533. [DOI] [PubMed] [Google Scholar]
- 6. White TCR (1993) The inadequate environment: Nitrogen and the abundance of animals Berlin: Springer-Verlag. [Google Scholar]
- 7. Mattson WJ (1980) Herbivory in relation to plant nitrogen content. Annual Review of Ecology and Systematics 11: 119–161. [Google Scholar]
- 8. Felton AM, Felton A, Raubenheimer D, Simpson SJ, Foley WJ, Wood JT, et al. (2009) Protein content of diets dictates the daily energy intake of a free-ranging primate. Behavioral Ecology 20: 685–690. [Google Scholar]
- 9. Rothman JM, Raubenheimer D, Chapman CA (2011) Nutritional geometry: gorillas prioritize non-protein energy while consuming surplus protein. Biology Letters 7: 847–849. 10.1098/rsbl.2011.0321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawler IR, Foley WJ, Eschler BM, Pass DM, Handasyde K (1998) Intraspecific variation in Eucalyptus secondary metabolites determines food intake by folivorous marsupials. Oecologia 116: 160–169. [DOI] [PubMed] [Google Scholar]
- 11. Moore BD, Foley WJ, Wallis IR, Cowling A, Handasyde KA (2005) Eucalyptus foliar chemistry explains selective feeding by koalas. Biology Letters 1: 64–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pass DM, Foley WJ, Bowden B (1998) Vertebrate herbivory on Eucalyptus—Identification of specific feeding deterrents for common ringtail possums (Pseudocheirus peregrinus) by bioassay-guided fractionation of Eucalyptus ovata foliage. Journal of Chemical Ecology 24: 1513–1527. [Google Scholar]
- 13. Wallis IR, Watson ML, Foley WJ (2002) Secondary metabolites in Eucalyptus melliodora: field distribution and laboratory feeding choices by a generalist herbivore, the common brushtail possum. Australian Journal of Zoology 50: 507–519. [Google Scholar]
- 14. Pryor LD (1959) Species distribution and association in Eucalyptus In: Keast A, Crocker RL, Christian CS, editors. Biogeography and Ecology in Australia. The Hague: W. Junk; pp. 461–471. [Google Scholar]
- 15. Moore BD, Wallis IR, Marsh KJ, Foley WJ (2004a) The role of nutrition in the conservation of the marsupial folivores of eucalypt forests In: Lunney D, editor. Conservation of Australia’s Forest Fauna. 2nd ed. Mosman, NSW, Australia: Royal Zoological Society of New South Wales. [Google Scholar]
- 16. Eschler BM, Pass DM, Willis R, Foley WJ (2000) Distribution of foliar formylated phloroglucinol derivatives amongst Eucalyptus species. Biochemical Systematics and Ecology 28: 813–824. [DOI] [PubMed] [Google Scholar]
- 17. Wallis IR, Nicolle D, Foley WJ (2010) Available and not total nitrogen in leaves explains key chemical differences between the eucalypt subgenera. Forest Ecology and Management 260: 814–821. [Google Scholar]
- 18. Robbins CT, Hanley TA, Hagerman AE, Hjeljord O, Baker DL, Schwartz CC, et al. (1987) Role of tannins in defending plants against ruminants: reduction in protein availability. Ecology 68: 98–107. [DOI] [PubMed] [Google Scholar]
- 19. Barbehenn RV, Constabel CP (2011) Tannins in plant–herbivore interactions. Phytochemistry 72: 1551–1565. 10.1016/j.phytochem.2011.01.040 [DOI] [PubMed] [Google Scholar]
- 20.Foley WJ, Iason G, McArthur C (1999) Role of plant secondary metabolites in the nutritional ecology of mammalian herbivores—how far have we come in 25 years? In: Jung H-JG, Fahey GCJ, editors. Vth International Symposium on the Nutrition of Herbivores. Savoy IL: American Society of Animal Science. pp. 203–274
- 21. Wallis IR, Edwards MJ, Windley H, Krockenberger AK, Felton A, Quenzer M, et al. (2012) Food for folivores: nutritional explanations linking diets to population density. Oecologia 169: 281–291. 10.1007/s00442-011-2212-9 [DOI] [PubMed] [Google Scholar]
- 22. DeGabriel JL, Wallis IR, Moore BD, Foley WJ (2008) A simple, integrative assay to quantify nutritional quality of browses for herbivores. Oecologia 156: 107–116. 10.1007/s00442-008-0960-y [DOI] [PubMed] [Google Scholar]
- 23. Moore BD, Wallis IR, Pala-Paul J, Brophy JJ, Willis RH, Foley WJ (2004b) Antiherbivore chemistry of Eucalyptus—cues and deterrents for marsupial folivores. Journal of Chemical Ecology 30: 1743–1769. [DOI] [PubMed] [Google Scholar]
- 24. Lawler IR, Foley WJ, Eschler BM (2000) Foliar concentration of a single toxin creates habitat patchiness for a marsupial folivore. Ecology 81: 1327–1338. [Google Scholar]
- 25. Jensen LM, Wallis IR, Marsh KJ, Moore BD, Wiggins NL, Foley WJ (2014) Four species of arboreal folivore show differential tolerance to a secondary metabolite. Oecologia 176: 251–258. 10.1007/s00442-014-2997-4 [DOI] [PubMed] [Google Scholar]
- 26. Moore BD, Foley WJ (2005) Tree use by koalas in a chemically complex landscape. Nature 435: 488–490. [DOI] [PubMed] [Google Scholar]
- 27. DeGabriel JL, Moore BD, Foley WJ, Johnson CN (2009) The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal. Ecology 90: 711–719. [DOI] [PubMed] [Google Scholar]
- 28. Youngentob KN, Wallis IR, Lindenmayer DB, Wood JT, Pope ML, Foley WJ (2011) Foliage chemistry influences tree choice and landscape use of a gliding marsupial folivore. Journal of Chemical Ecology 37: 71–84. 10.1007/s10886-010-9889-9 [DOI] [PubMed] [Google Scholar]
- 29. Foley WJ, Kehl JC, Nagy KA, Kaplan IR, Borsboom AC (1990) Energy and water metabolism in free-living greater gliders, Petauroides volans . Australian Journal of Zoology 38: 1–9. [Google Scholar]
- 30. Kavanagh RP, Lambert MJ (1990) Food selection by the greater glider, Petauroides volans: is foliar nitrogen a determinant of habitat quality? Australian Wildlife Research 17: 285–299. [Google Scholar]
- 31. Comport SS, Ward SJ, Foley WJ (1996) Home ranges, time budgets and food-tree use in a high-density tropical population of greater gliders, Petauroides volans minor (Pseudocheiridae: Marsupialia). Wildlife Research 23: 401–419. [Google Scholar]
- 32. Harris JM, Maloney SK (2010) Petauroides volans (Diprotodontia: Pseudocheiridae). Mammalian Species 42: 207–219. [Google Scholar]
- 33. Foley WJ, Hume ID (1987) Passage of digesta markers in two species of arboreal folivorous marsupials—the greater glider (Petauroides volans) and the brushtail possum (Trichosurus vulpecula). Physiological Zoology 60: 103–113. [Google Scholar]
- 34. Wallis IR, Foley WJ (2005) The rapid determination of sideroxylonals in Eucalyptus foliage by extraction with sonication followed by HPLC. Phytochemical Analysis 16: 49–54. [DOI] [PubMed] [Google Scholar]
- 35. Cork SJ, Pahl L (1984) The possible influence of nutritional factors on diet and habitat selection by the ringtail possum (Pseudocheirus peregrinus) In: Smith A, Hume ID, editors. Possums and Gliders. Sydney: Surrey Beatty & Sons and Australian Mammal Society; pp. 269–276. [Google Scholar]
- 36. Cork SJ, Catling PC (1996) Modelling distributions of arboreal and ground-dwelling mammals in relation to climate, nutrients, plant chemical defences and vegetation structure in the eucalypt forests of southeastern Australia. Forest Ecology and Management 85: 163–175. [Google Scholar]
- 37. Milton K (1979) Factors influencing leaf choice by howler monkeys: A test of some hypotheses of food selection by generalist herbivores. American Naturalist 114: 362–378. [Google Scholar]
- 38. Chapman CA, Chapman LJ, Bjorndal KA, Onderdonk DA (2002) Application of protein-to-fiber ratios to predict colobine abundance on different spatial scales. International Journal of Primatology 23: 283–310. [Google Scholar]
- 39. Ganzhorn JU (2002) Distribution of a folivorous lemur in relation to seasonally varying food resources: integrating quantitative and qualitative aspects of food characteristics. Oecologia 131: 427–435. [DOI] [PubMed] [Google Scholar]
- 40. Chapman CA, Chapman LJ, Naughton-Treves L, Lawes MJ, McDowell LR (2004) Predicting folivorous primate abundance: Validation of a nutritional model. American Journal of Primatology 62: 55–69. [DOI] [PubMed] [Google Scholar]
- 41. Ganzhorn JU (1992) Leaf chemistry and the biomass of folivorous primates in tropical forests—test of a hypothesis. Oecologia 91: 540–547. [DOI] [PubMed] [Google Scholar]
- 42. Oates JF, Whitesides GH, Davies AG, Waterman PG, Green SM, Dasilva GL, et al. (1990) Determinants of variation in tropical forest primate biomass—new evidence from West-Africa. Ecology 71: 328–343. [Google Scholar]
- 43. Waterman PG, Ross JAM, Bennett EL, Davies AG (1988) A comparison of the floristics and leaf chemistry of the tree flora in two Malaysian rain forests and the influence of leaf chemistry on populations of colobine monkeys in the old world. Biological Journal of the Linnean Society 34: 1–32. [Google Scholar]
- 44. Gogarten JF, Guzman M, Chapman CA, Jacob AL, Omeja PA, Rothman JM (2012) What is the predictive power of the colobine protein-to-fiber model and its conservation value? Tropical Conservation Science 5: 381–393. [Google Scholar]
- 45. McArt SH, Spalinger DE, Collins WB, Schoen ER, Stevenson T, Bucho M (2009) Summer dietary nitrogen availability as a potential bottom-up constraint on moose in southcentral Alaska. Ecology 90: 1400–1411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.