Abstract
The neural correlates of cognitive control for typically developing nine-year-old children were examined using dense-array ERPs and estimates of cortical activation (LORETA) during a go/no-go task with two conditions: a neutral picture condition and an affectively charged picture condition. Activation was estimated for the entire cortex after which data were exported for four regions of interest (ROIs): ventrolateral prefrontal cortex (VLPFC), dorsal anterior cingulated cortex (dACC), dorsolateral prefrontal cortex (DLPFC), and orbitofrontal/ventromedial prefrontal cortex (OFC/VMPFC). Results revealed faster reaction times, greater N2 activation, and greater prefrontal activation for the affectively charged picture condition than the neutral picture condition. The findings are discussed in reference to the impact of affective stimuli on recruitment of specific brain regions involved in cognitive control.
Keywords: cognitive control, negative emotion, children, N2, go/no-go, source analysis
1. Introduction
Our ability to monitor and control our actions adaptively from moment-to-moment depending on current goals falls under the rubric of cognitive control. The development of cognitive control, especially in the context of negative emotion, is crucial for typical socioemotional functioning. Research on the neural correlates of cognitive control has primarily been conducted on adult samples (e.g., Blasi et al. 2006; Garavan, Ross, Murphy, Roche, & Stein, 2002; Rubia, Smith, Brammer, & Taylor, 2003), and far less is known about these neural correlates in children. Furthermore, since control mechanisms may vary depending on the context of the event, we examined children’s cognitive control abilities in a relatively neutral context and an affectively charged context.
A number of cortical regions have been associated with cognitive control, including the dorsal anterior cingulate cortex (dACC; e.g., Garavan, Ross, Murphy, Roche, & Stein, 2002), the dorsolateral prefrontal cortex (DLPFC; e.g., Blasi et a., 2006), the ventrolateral prefrontal cortex (VLPFC; e.g., Aron, Robbins, & Poldrack, 2004), and the orbitofrontal/ventromedial prefrontal cortex (OFC/VMPFC; e.g., Durston, Mulder, Casey, Ziermans, & van Engeland, 2006). Furthermore, activation of the same regions associated with cognitive control, i.e., dACC, DLPFC, VLPFC, OFC/VMPFC, have also been found to vary depending on the context of the task, i.e., emotional or unemotional contexts. For example, Cremers and colleagues (2010) found that presentation of angry, sad, and fearful emotional faces, compared to neutral faces, revealed negative correlations between ACC-amygdala connectivity and neuroticism scores; Monk et al. (2003) found greater ACC, orbitofrontal cortex, and VLPFC activation to fearful faces than neutral faces during an attention task; and, Ochsner et al. (2004) found ACC, VLPFC, and DLPFC activation during emotional up-regulation and down-regulation. Thus, it may be that this prefrontal control system (ACC, VLPFC, DLPFC, OFC/VMPFC) recruited in relatively unemotional contexts is also recruited in emotional contexts.
However, few studies have investigated how cognitive control varies in the context of emotion for children. Two recent papers by Lamm et al. (2010; 2011) revealed that prefrontal cortical activation during a cognitive control task (go/no-go task) increased in the context of negative emotion, specifically when children (aged 8 to 14) were frustrated/anxious about potentially not winning a desired toy. The authors interpreted these results to reflect the need for additional neural resources to successfully control responding in the context of negative emotion. We were interested in building on these results using a substantially different go/no-go task with affectively charged pictures. Specifically, we examined how behavioral performance and prefrontal activation differed in the context of affectively charged pictures compared to relatively neutral pictures, for a sample of 9-year-old children. We measured no-go N2 activation—an ERP component associated with cognitive control (for example, Dimoska, Johnstone, Barry, & Clarke, 2003; Falkenstein, Hoormann, & Hohnsbein, 1999; Jonkman, Lansbergen, & Stauder, 2003)—using dense-array EEG and a method of estimating cortical activation (LORETA; Pascual-Marqui, Esslen, Kochi, & Lehmann, 2002). Activation was estimated for the entire cortex and subsequently activation values were exported for four ROIs: VLPFC, dACC, DLPFC, and OFC/VMPFC. Based on the Lamm et al. (2010; 2011) results, we predicted that during the affectively charged condition compared to the neutral condition children would require additional prefrontal resources to effectively control their actions.
2. Method
2.1, Participants
Seventeen typically developing nine-year old children (Mean = 9.57 years, SD= 0.28, range = 9.18–10.00 years, 7 males) were included in the current study. Participants were primarily right handed (only 1was left handed) Caucasian (48%) or African American (32%) healthy children. None of the children had any medical or psychiatric conditions. Participants were recruited through an independent mailing company that provided addresses of families with young children located in the Washington, D.C. region. Seven additional children participated but were excluded from analyses due to insufficient artifact free trials.
2.2, Procedure
Upon arrival to the laboratory, the study was described and parental consent and child assent were obtained. Children were then seated in a chair 38 inches from the computer screen. Next, the electrode sensor net was applied and the go/no-go task (Zoo Game) was administered. Upon completion of the paradigm, families were paid $20.00 for their time. This study received IRB approval from the University of Maryland.
Affective and Neutral Go/No-go task: The Zoo Game (McDermott et al., in preparation)
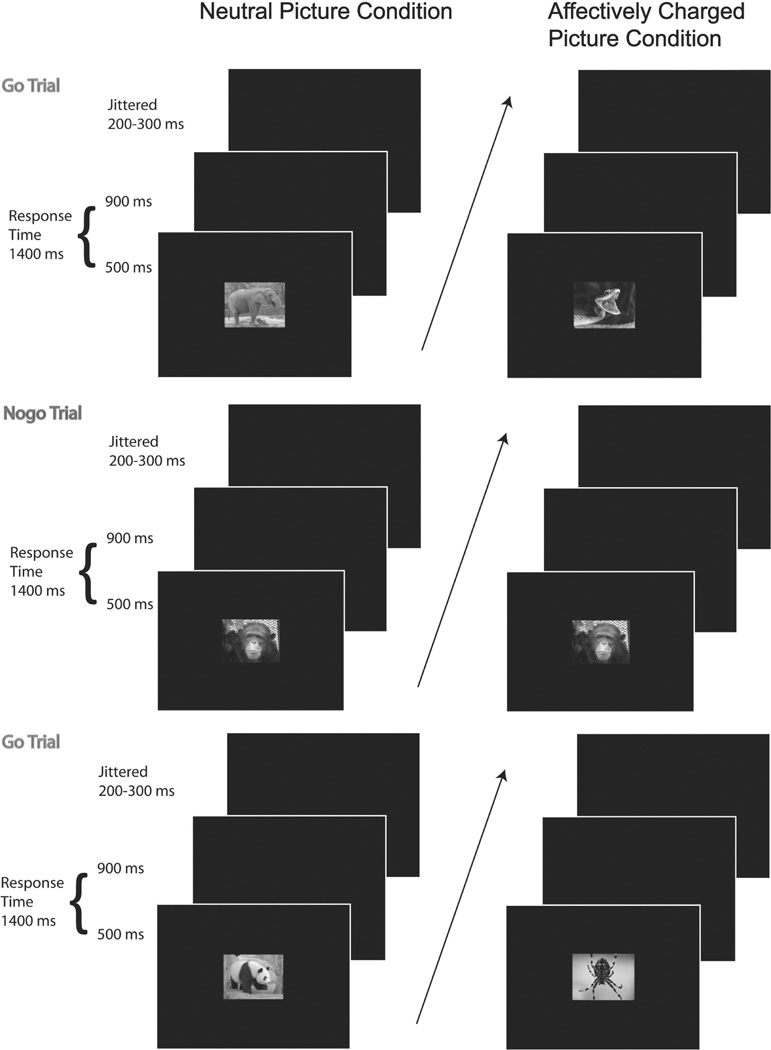
The task employed in this study was called the Zoo Game and consisted of 75% go trials and 25% no-go trials. This ratio of go to no-go trials ensures a prepotent desire to respond (i.e., requiring enhanced response control in the no-go trials). This ratio of trials occurs within two blocks, a block of non-affectively charged pictures (280 trials) and a block of affectively charged pictures (140 trials). The block presenting affectively charged pictures was limited to 140 trials to limit the duration of children’s distress. In order to limit potential affective carry-over effects the affectively charged picture block was always presented second. No-go trials, for both blocks, consisted of monkey pictures, while go trials consisted of other animal pictures. In the neutral condition, go trial stimuli consisted of non-affectively charged pictures (e.g., non-threatening looking panda bear or kangaroo). In the affectively charged condition, go trial stimuli consisted of affectively charged pictures (e.g., large dog aggressively showing teeth, large spider). Prior to completing the two blocks, children completed 12 practice trials to ensure proficiency. Children were asked to help a zoo keeper recapture escaped animals with the help of a chimpanzee referred to as the ‘monkey’. To recapture the animals, children were told to respond via button-press (as fast and accurate as possible) as soon as they saw an animal on the screen unless it was the ‘monkey’. Animal stimuli were presented on the screen for 500 ms, followed by a black screen for 900 ms or until the child responded (see Figure 1). The inter-trial interval was jittered between 200–300 ms. Images were presented on a 17-in monitor using E-prime Software (Psychology Software Tools, Inc., Pittsburgh, PA; Schneider, Eschman, & Zuccolotto, 2002).
Figure 1.
Structure of go/no-go task (“Zoo Game”) outlining neutral picture and affectively charged picture conditions.
2.3, EEG data collection and analysis
EEG was recorded using a 64-channel Geodesic Sensor Net and sampled at 250 Hz, using EGI software (Net Station; Electrical Geodesic, Inc., Eugene, OR [data were also processed using Net Station]). Once the impedance values for all EEG channels were reduced to below 50 kΩ, data acquisition was started. During recording, all channels were referenced to Cz and after acquisition, data were re-referenced using an average reference.
Data were filtered using a FIR bandpass filter with a lowpass frequency of 50 Hz and a highpass frequency of .3 Hz. To best approximate the data, eye blink artifact thresholds were set to 140 µV. Furthermore, signal activation change exceeding 120 µV across the entire trial were marked as bad and removed after visual inspection.
Scalp analysis
Waveforms for correct go and no-go trails were segmented into epochs from 200 ms before to 600 ms after stimulus onset and baseline corrected for the 200 ms preceding stimulus onset. Mediofrontal N2 activation was maximal between 250 and 390 ms; thus, peak activation was exported for this time. To eliminate trials characterized by attentional lapses or chronic non-responding, no-go trials that did not have a correct go trial preceding and following them were removed from analyses. Due to this strict criterion the mean number of trials comprising correct no-go ERPs was 25.06 (ranging from 10–56; mean neutral = 34.18, mean affectively charged = 15.94). Mean number of trials comprising go ERPs was 134.03 (ranging from 56–251; mean neutral = 182.71, mean affectively charged = 85.35). Because the number of trials comprising an ERP can effect ERP activation, trial count was entered as a covariate to all ERP analyses (scalp and source). Furthermore, since a prior t-tests revealed no gender differences for scalp or source space analyses, Gender was not added as a covariate.
Source-space analysis
A distributed inverse model that incorporates the change in activation from one electrode to another (in this case 65 electrodes) was used to calculate the source-space activation. This type of algorithm estimates activation voxel-by-voxel and sample-by-sample and does not require any dipoles to be “fit”, thereby limiting the influence of user bias. The specific algorithm used in the current study was LORETA (Low Resolution Brain Electromagnetic Tomography) which applies a constraint to the minimum-norm solution in order to minimizes the discrepancy between values of adjacent voxels (to achieve the most realistic model) within the GeoSource interface (Electrical Geodesic, Inc., Eugene, OR; for a review of these constraints and other minimum norm solutions, see Michel, Murray, Lantz, Gonzalez, Spinelli, & Grave de Peralta, 2004). A regularization constant (indicating how much noise is modeled) of 10−4 was applied This amount of regularization revealed current flow patterns that matched (via visual inspection) the grand-averaged scalp topography (collapsing across conditions to prevent biasing solutions) better than other levels.
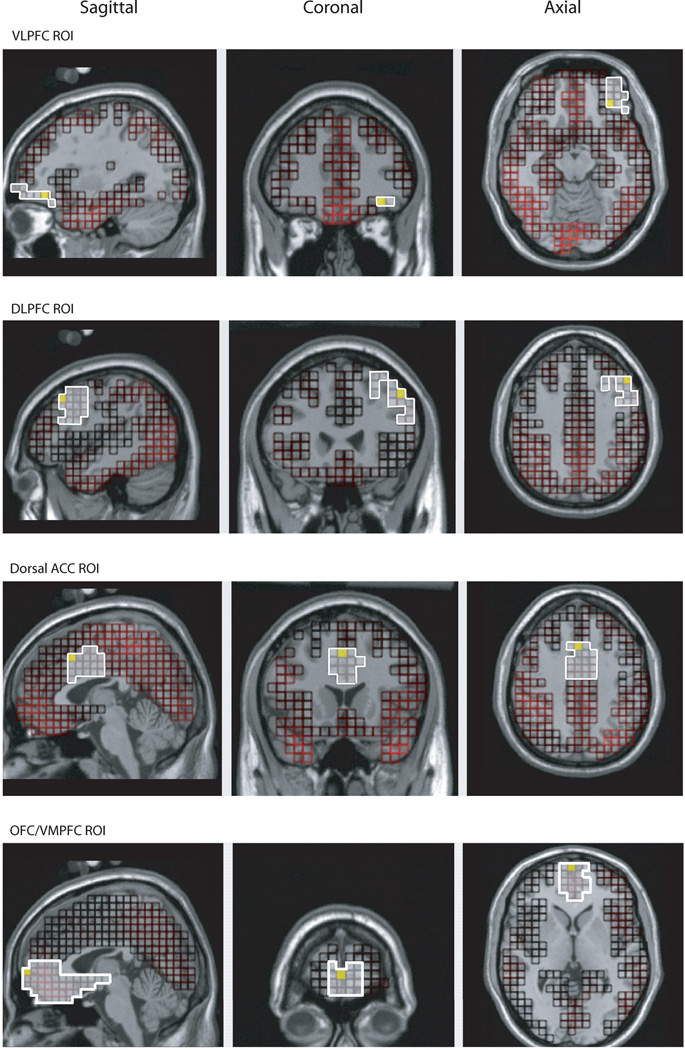
After the data were modeled (LORETA) for the entire cortex (2447 voxels), morphology-based regions of interest (ROIs) were generated using the Montreal Neurological Institute (MNI) average adult MRI (pediatric head models no available yet). We were interested in four ROIs: the VLPFC ROI (comprised of 44 voxels; lateral part of BA 11 and 47), the dACC ROI (comprised of 50 voxels; dorsal part of BA 24 and 32), the DLPFC ROI (comprised of 126 voxels; BA 9 and dorsal part of BA 46), and the OFC/VMPFC ROI (comprised of 147 voxels; ventromedial parts of BAs 11, 10, 14, and 13; see Figure 2). Source waveform amplitudes (nA) for all voxels within an ROI were extracted for 200 ms before stimulus onset to 600 ms after stimulus onset and baseline corrected using the 200 ms before stimulus onset. To ensure that each participant’s maximal activation was analyzed, we chose the voxel and moment in time (within the 140 ms during which the scalp N2 was maximal) that showed the most activation for each ROI.
Figure 2.
Morphology-based regions of interest (ROIs) generated using the Montreal Neurological Institute (MNI) average adult MRI.
3. Results
3.1.Picture salience
To verify that children perceived the affectively charged pictures as more frightening than the neutral pictures, we administered a survey asking “how scary is this animal” to a different sample (n=9) of typically developing 9-year-old children. Results revealed that indeed children perceived the affectively charged pictures to be scarier than the neutral pictures, t(8) = 9.59, p < .001.
3.2, Behavioral data analyses
Separate repeated-measures ANOVAs were conducted to examine condition differences in go reaction times (RT) and performance accuracy (see Table 1). To examine accuracy rate, we collapsed over trial type (go, no-go); since perseverative responding leads to high accuracy on go trials and low accuracy on no-go trials, whereas chronic non-responding leads to high accuracy on no-go trials and low accuracy on go trials, and thus the combination provides a more comprehensive picture of task performance. To examine possible effects of gender, t-tests were conducted on both performance accuracy and RT. There were gender differences in performance accuracy (tneutral(15) = −2.49, p=.03; temotional (15) = −3.66, p=.002), where females had significantly higher accuracy rates than males. As such, Gender was entered as a covariate in the performance accuracy analysis. Males and females did not differ on reaction times and thus Gender was not entered as a covariate in the RT analysis.
Table 1.
Means and standard deviations for reaction times and performance accuracy by condition.
| Neutral Picture Condition | Affectively Charged Picture Condition | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Reaction Time | ||||
| Go |
399.28 ms | 67.95 ms | 383.64 ms | 70.33 ms |
| Performance Accuracy | ||||
| Go | 98.83 % | 01.16 % | 98.82 % | 01.95 % |
| No-go | 55.48 % | 17.34 % | 52.27 % | 14.45 % |
| Combined Go and No-go | 77.15 % | 08.88 % | 75.55 % | 07.51 % |
Analyses examining condition differences revealed faster RTs for the affectively charged picture condition than the neutral condition, F(1,16)=8.76, p=.009, but no condition differences in performance accuracy.
Since the affectively charged picture condition was always presented after the neutral condition, an additional analysis was conducted to determine if condition differences in RTs (i.e., faster RTs for the affectively charged picture condition) were due to practice effects. Given that the neutral condition (280 trials) consisted of twice as many trials as the affectively charged picture condition (140 trials), trials comprising the neutral condition were subdivided into first half and second half, and a t-test was conducted comparing the RTs for these two sub-blocks. Results revealed no significant difference between the two sub-blocks of the neutral condition, t(16) = −.36, p=.72 (neutral sub-block one mean: 336.76, neutral sub-block two mean: 340.43 [i.e., no practice effect over 280 trials], affectively charged picture block mean: 328.61), suggesting that our RT condition effect (i.e., the affectively charged picture condition revealing faster RTs than the neutral condition) is probably not due to a practice effect.
3.3, ERP N2 data analysis
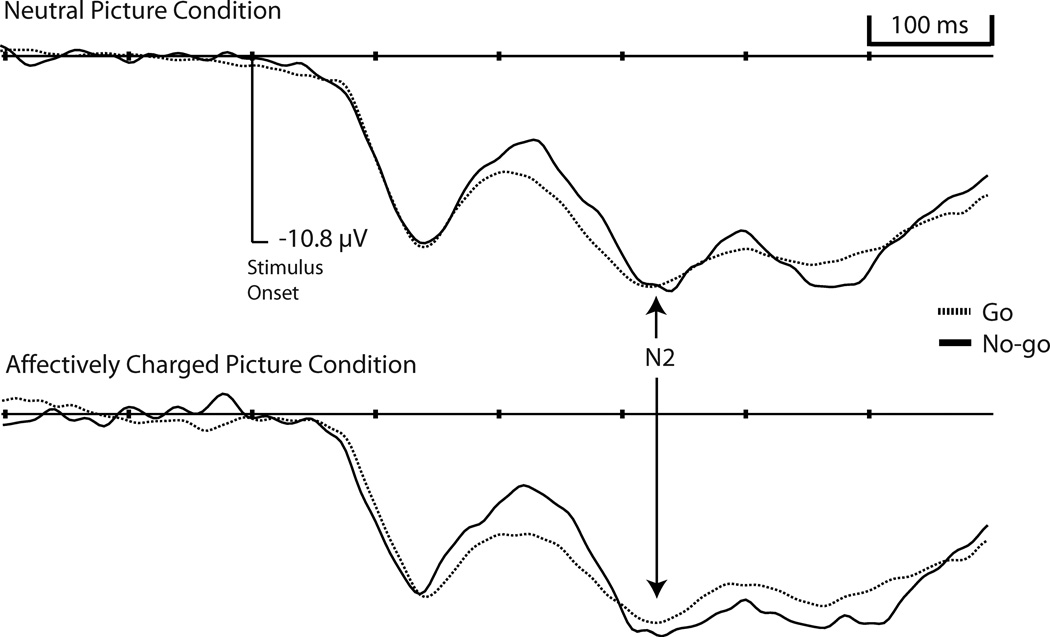
Visualization of the correct go and no-go stimulus-locked waveforms revealed clear N1, P2, and N2 components for all mediofrontal electrodes, however, component activation overlapped between P2 and N2 activation preventing the P2 from revealing positive activation values (see Figure 3).
Figure 3.
Grand averaged event-related potentials, at electrode Fz (µV; positive activation up), by Condition and Trial Type.
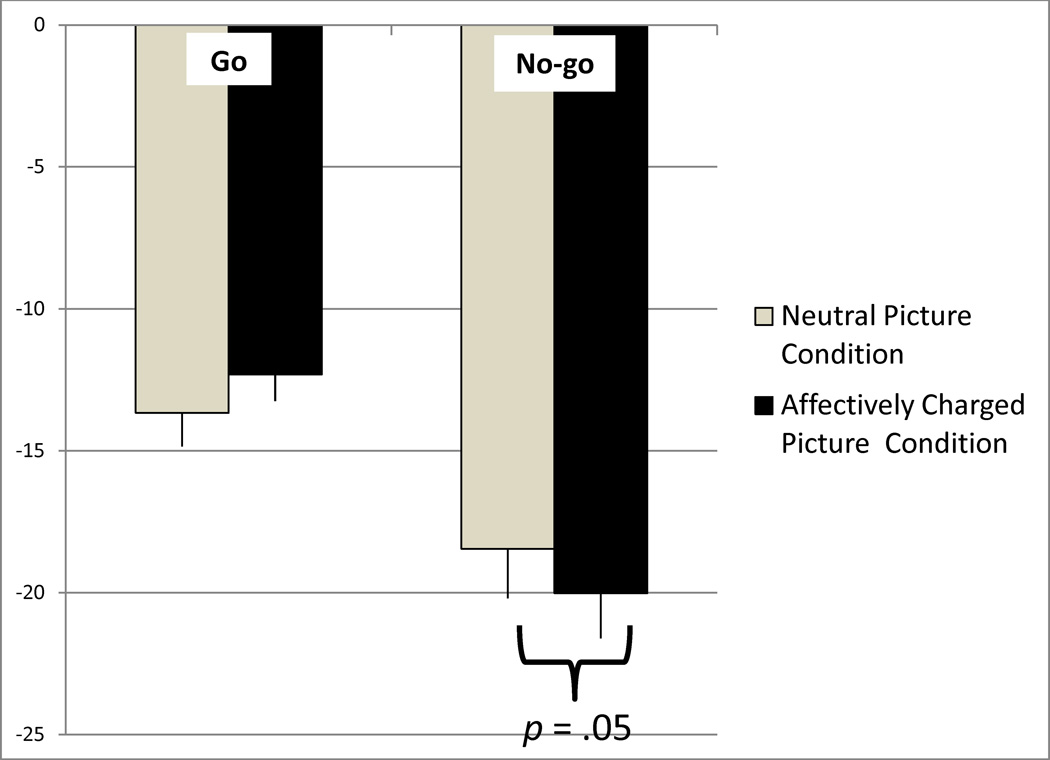
Scalp N2 activation was exported for 7 mediofrontal electrodes: three canonical midline electrodes (Fz, FCz, and Cz) as well as 4 flanking electrodes (two on each side; EGI electrode numbers: 6, 4, vref, 9, 7, 3, 54). Because of individual differences in peak N2 activation across electrodes, each participant’s minimum (most negative) activation was analyzed. A priori t-tests revealed no gender differences for either the scalp or source space activation so Gender was not entered as a covariate. Analyses of N2 activation revealed no significant Condition effects. Because visualization of the scalp stimulus-locked waveform showed P2 and N2 components that were not morphologically independent (both had negative amplitudes), P2 activation was subtracted from N2 activation (N2-P2) for all subsequent scalp activation analyses (see Table 2). A 2 (Condition: neutral pictures, affectively charged pictures)×2 (Trial Type: go, no-go) repeated-measures ANOVA was conducted revealing a main effect of Trial Type, F(1,16) = 26.53, p < .001 (no-go more negative activation than go), and a Condition-by-Trial Type interaction, F(1,16) = 6.58, p = .02 (see Figure 4). Contrasts revealed more negative N2 activation for the affectively charged picture condition than the neutral condition but only for the no-go trials (p = .05).
Table 2.
Means and standard deviations for P2 and N2 (go and no-go) activation by condition.
| Neutral Pictures | Affectively Charged Pictures | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| P2 Activation (µV) | ||||
| Go | −1.82 | 3.70 | −2.15 | 4.30 |
| No-go |
1.10 | 4.25 | 2.97 | 4.27 |
| N2 Activation (µV) | ||||
| Go | −15.49 | 5.34 | −14.46 | 4.38 |
| No-go | −17.36 | 6.73 | −16.04 | 6.82 |
More positive activation is greater for the P2. More negative activation is greater for the N2.
Figure 4.
Condition (neutral picture, affectively charged picture) and Trial Type (go, no-go) differences in N2 activation (N2-P2 activation µV). Positive activation is up (error bars = standard error).
3.4, Source-space data marked by the N2
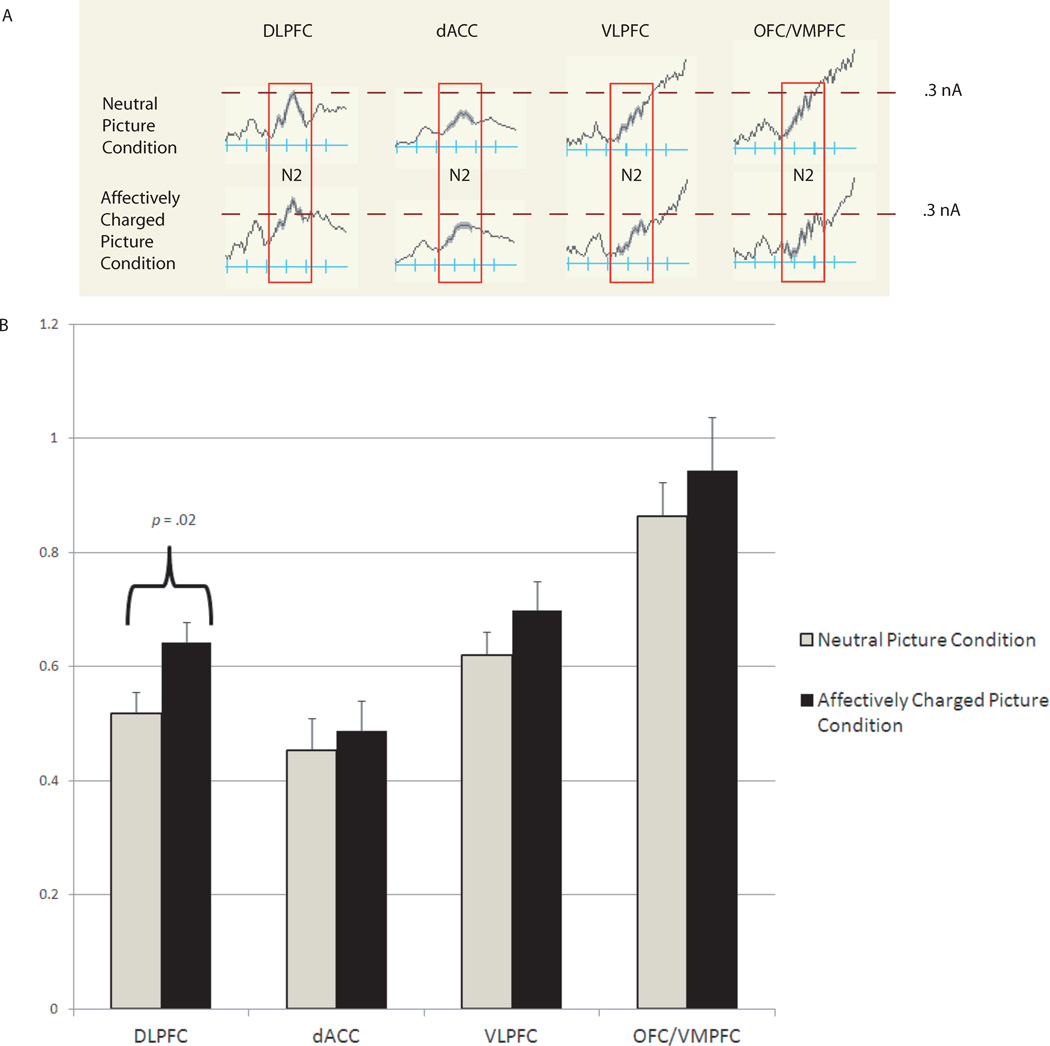
A 2 (Condition: neutral pictures and affectively charged pictures)×4 (Region: dACC, VLPFC, DLPFC, VMPFC) repeated-measures ANOVA was conducted for go and no-go trials separately. Results for the go trials revealed no significant effects. Results for the no-go trials revealed a Region-by-Condition interaction, F(3,42) = 7.25, p = .003 (see Figure 5). Contrasts revealed greater activation for the affectively charged picture condition than the neutral condition for DLPFC (p = .02).
Figure 5.
Condition (neutral picture, affectively charged picture) differences in source-space activation marked by the N2 (nA; greater magnitude of activation up). Part A shows grand-averaged source space waveforms. Part B shows bar graph of mean differences (error bars = standard error).
An additional 2 (Condition: neutral pictures and affectively charged pictures)×4 (Region: dACC, VLPFC, DLPFC, VMPFC) repeated-measures ANOVA was conducted on mean activation during the 200 ms prior to no-go stimulus onset to test for condition differences in arousal. Results revealed no Condition-by-Region interaction suggesting that the pattern of results for the source space activation underlying the no-go N2 cannot be simply due to condition differences in arousal.
Lastly, to determine that condition differences were specific to regions related to cognitive control and not all brain regions, an additional repeated-measures ANOVA was conducted on a medioparietal ROI. Results revealed no condition differences, F(1,14) = .62, p = .44.
4. Discussion
4.1, General Discussion
The present study examined behavioral measures of performance as well as N2 activation and source-space (LORETA) activation marked by the N2 to investigate the mechanisms underlying effective cognitive control in a sample of 9-year-old children. Furthermore, this study explored how cognitive control mechanisms differed in an affectively challenging context compared to a relatively neutral context. As predicted, results revealed faster reaction times and greater N2 activation in the affectively charged picture condition than the neutral condition. More specifically, results revealed greater DLPFC activation—a cortical region associated with cognitive control (e.g., Blasi et al., 2006)—in the affectively charged picture condition compared to the neutral condition.
These results are in line with previous studies examining N2 activation evoked during an emotionally challenging go/no-go task with children and adolescents (e.g., Lewis, Lamm, Segalowitz, Stieben, & Zelazo, 2006). In these studies, emotion was induced by removing valued points. Lewis et al. (2006) showed greater N2 activation for the emotionally challenging condition than the neutral condition but only for adolescents, not children. Lamm and Lewis (2010), on the other hand, revealed greater activation for the emotional condition, compared to the neutral condition, for both children and adolescents but only for source space data (underlying the N2) and not scalp N2 data. In the current study of a sample of 9 year old children, we found greater activation for the affectively charged picture condition than the neutral condition. This pattern of results supports the Lamm and Lewis (2010) findings and suggests that children may require additional prefrontal resource to effectively control their actions in the context of emotion.
The current study found greater DLPFC activation (during the time period marked by the N2) in the affectively charged picture condition than the neutral condition. Elevated DLPFC activation has been linked with controlling emotional memories (Anderson et al., 2004) and controlling thoughts during emotional reappraisal (Ochsner et al., 2004). Thus, DLPFC activation may be part of a cognitive control network, which is recruited more or less depending on the emotional climate.
Furthermore, results also revealed faster reaction times for the affectively charged picture condition than the neutral condition. However, the cause for this altered behavioral performance is not clear, i.e., were reaction times faster due to less vigilant and more impulsive responding or as result of purposeful removal of the affectively charged pictures. Given that we did not find any condition effects in performance accuracy, it may be that children responded faster during the affectively charged picture condition compared to the neutral condition to eliminate/remove the emotional perturbation (i.e., remove the affectively charged picture from the computer screen).
4.2, Limitations
There are limitations to the current study. First, the use of source-space analyses allowed us to ask region specific questions which scalp ERPs do not. However, activation patterns are estimated effects and therefore should be interpreted with caution. Furthermore, since activation is estimated, measuring activation differences for small ROIs or regions close together is difficult. Thus, rostral/subgenual ACC activation was not measured. Second, the current study had a relatively small sample size. Future work should expand on both the number and age range of children.
4.3, Conclusions
In sum, the present study revealed faster reaction times and greater prefrontal activation in an affectively charged condition compared to a relatively neutral condition, during a cognitive control task. In the future, contextually varying (i.e., more or less negative emotion) measures of regulatory neural activation should be tracked in conjunction with varying cognitive demands to determine if 1) variation in emotional salience differentially impacts neural cognitive demands and 2) emotion impacts neural resources differentially based on varying cognitive demands.
Highlights of study.
-
-
Typically-developing 9 year old children played a go/no-go task
-
-
The N2—an ERP that measures cognitive control—was measured
-
-
Cortical activation underlying the N2 was estimated using the LORETA method
-
-
Results showed greater activity and faster response times in the context of emotion
Acknowledgements
This research was supported by grants from the National Institutes of Health (HD17899) to Nathan A. Fox and (P50 MH078105-01A2) to Megan R. Gunnar.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron A, Robbins T, Poldrack R. Inhibition and the right inferior frontal cortex. TRENDS in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Anderson M, Ochsner K, Kuhl B, Cooper J, Robertson E, Gabrieli S, Glover G, Gabrieli J. Neural Systems Underlying the Suppression of Unwanted Memories. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- Blasi G, Goldberg T, Weinberger D, Mattay V. Brain regions underlying response inhibition and interference monitoring and suppression. European Journal of Neuroscience. 2006;23:1658–1664. doi: 10.1111/j.1460-9568.2006.04680.x. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. TRENDS in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cremers H, et al. Neuroticism modulates amygdala—prefrontal connectivity in response to negative emotional facial expressions. NeuroImage. 2010;49:963–970. doi: 10.1016/j.neuroimage.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Dimoska A, Johnstone S, Barry R, Clarke Adam. Deficit/hyperactivity disorder: event-related potentials in the stop-signal paradigm. Society of Biological Psychiatry. 2003;53:1345–1354. doi: 10.1016/s0006-3223(03)00703-0. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain Systems Mediating Cognitive Interference by Emotional Distraction. The Journal of Neuroscience. 2006;26(7):2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. Erp components in go/nogo tasks and their relation to inhibition. Acta Psychologica. 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche R, Stein E. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Jonkman L, Lansbergen M, Stauder J. Developmental differences in behavioral and eventrelated brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40:752–761. doi: 10.1111/1469-8986.00075. [DOI] [PubMed] [Google Scholar]
- Lamm C, Granic I, Zelazo PD, Lewis MD. Magnitude and chronometry of neural mechanisms of emotion regulation in subtypes of aggressive children. Brain and Cognition. doi: 10.1016/j.bandc.2011.06.008. (in press). [DOI] [PubMed] [Google Scholar]
- Lamm C, Lewis M. Developmental Change in the Neurophysiological Correlates of Self-Regulation in High- and Low-Emotion Conditions. Developmental Psychology. 2010;35(2):156–176. doi: 10.1080/87565640903526512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Lamm C, Segalowitz S, Stieben J, Zelazo P. Neurophysiological Correlates of Emotion Regulation in Children and Adolescents. Journal of Cognitive Neuroscience. 2006;18(3):430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Henderson HA, Degnan KA, Fox NA. Tempermental behavioral inhibition and inhibitory control predict children’s social competence. (in preparation). [Google Scholar]
- Michel C, Murray M, Lantz G, Gonzalez S, Spinelli L, Grave de Peralta R. Eeg source imaging. Clinical Neurophysiology. 2004;115:2195–2222. doi: 10.1016/j.clinph.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Monk C, McClure E, Nelson E, Zarahn E, Bilder R, Leibenluft E, Charney D, Ernst M, Pine D. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Knierim K, Ludlow D, Hanelin J, Ramachandran T, Glover G, Mackey S. Reflecting upon feelings: an fmri study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD, Esslen M, Kochi K, Lehmann D. Functional imaging with low resolution brain electromagnetic tomography (LORETA): review, new comparisons, and new validation. Japanese Journal of Clinical Neurophysiology. 2002;30:81–94. [PubMed] [Google Scholar]
- Rubia K, Smith A, Brammer M, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage. 2003;20:351–358. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime getting started guide. Psychology Software Tools, Inc., University of Pittsburgh; 2002. [Google Scholar]
- Shaw P, Kabani N, Wise S. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience. 2008;28(14):3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]