Abstract
OBJECTIVES: To describe and compare off-label use and cardiovascular (CV) adverse effects of dexmedetomidine in neonates and infants in the pediatric intensive care unit (PICU).
METHODS: Patients younger than 12 months with corrected gestational ages of at least 37 weeks who were receiving continuous infusion of dexmedetomidine at a tertiary pediatric referral center between October 2007 and August 2012 were assessed retrospectively. Patients were excluded if dexmedetomidine was used for procedural sedation, postoperative CV surgery, or if postanesthesia infusion weaning orders existed at the time of PICU admission.
RESULTS: The median minimum dexmedetomidine dose was similar between infants and neonates at 0.2 mcg/kg/hr (IQR, 0.17–0.3) versus 0.29 mcg/kg/hr (IQR, 0.2–0.31), p = 0.35. The median maximum dose was higher for infants than neonates (0.6 mcg/kg/hr [IQR, 0.4–0.8] vs. 0.4 mcg/kg/hr [IQR, 0.26–0.6], p < 0.01). Additional sedative use was more common in infants than neonates (75/99 [76%] vs. 15/28 [54%], p = 0.02). At least 1 episode of hypotension was noted in 34/127 (27%) patients and was similar between groups. An episode of bradycardia was identified more frequently in infants than neonates (55/99 [56%] vs. 2/28 [7%], p < 0.01). Significant reduction in heart rate and systolic blood pressure was noted when comparing baseline vital signs to lowest heart rate and systolic blood pressure during infusion (p < 0.01).
CONCLUSIONS: Dexmedetomidine dose ranges were similar to US Food and Drug Administration–labeled dosages for intensive care unit sedation in adults. More infants than neonates experienced a bradycardia episode, but infants were also more likely to receive higher dosages of dexmedetomidine and additional sedatives.
INDEX TERMS: adverse drug event, dexmedetomidine, infant, neonate
INTRODUCTION
Dexmedetomidine (Precedex, Hospira, Lake Forest, IL) is a selective alpha2-adrenergic agonist that acts in the brainstem by inhibiting norepinephrine release.1 It is both a sedative and anesthetic with analgesic-sparing properties, and it causes minimal respiratory depression. Dexmedetomidine is approved by the US Food and Drug Administration (FDA) for use in patients 18 years of age and older for monitored anesthesia in the operating room or for up to 24 hours while intubated and undergoing mechanical ventilation in the intensive care unit (ICU).2–4 Dexmedetomidine is commonly used off-label, including use for duration greater than 24 hours and any use in pediatrics.1 Dexmedetomidine has been used in patients who are near extubation to allow for dose reduction of other sedative agents that have a tendency to cause respiratory depression.5 Dexmedetomidine has relatively few adverse events when compared to other sedative agents, with hypotension and bradycardia being most common.6 Randomized clinical trials have demonstrated safety and efficacy up to approximately 5 days in adult patients.3
While there are data to suggest efficacy in pediatric patients, some studies have shown clinically significant bradycardia and hypotension associated with the use of dexmedetomidine in this population.7,8 Neonates and infants may have a greater risk of these adverse effects than older children, especially those with underlying cardiac disease or receiving concomitant medications that may contribute to hypotension and bradycardia.5,9 In addition, dexmedetomidine is eliminated primarily through the urine after biotransformation via glucuronidation, hydroxylation (via CYP2A6), and N-methylation.2,3 Glucuronidation, which is reduced in the neonatal period and matures over the first year of life, may contribute to decreased elimination and an increase in adverse events from dexmedetomidine in this population.10 Although there are data describing the use of dexmedetomidine in infants after CV surgery or during procedural sedation, the use of dexmedetomidine in neonates and infants in the general pediatric intensive care unit (PICU) population has not been well described.11,12 This study is a retrospective, descriptive evaluation of off-label dexmedetomidine therapy, including dosage, duration, and adverse events, in neonates and infants in the PICU.
MATERIALS AND METHODS
The study was performed as a retrospective chart review at a tertiary pediatric referral center with 36 PICU beds. The study was reviewed by the university's institutional review board and granted approval under exempt status. A report of all patients younger than 12 months, admitted to the PICU with an order for continuous infusion dexmedetomidine between October 2007 and August 2012, was generated by using the pharmacy order entry system.
Patients were included in this study if they had a corrected gestational age (CGA) of at least 37 weeks and were younger than 12 months at the time dexmedetomidine was ordered and administered. Patients were excluded from the study if they received dexmedetomidine for procedural sedation, immediately after CV surgery, or if postanesthesia weaning orders existed at admission to the PICU. Included subjects were reviewed in reverse chronological order. Neonates included term newborns who initiated dexmedetomidine on days 0 to 28 of life and pre-term newborns who initiated dexmedetomidine within 28 days of reaching a CGA of 37 weeks. All other children younger than 12 months were classified as infants.
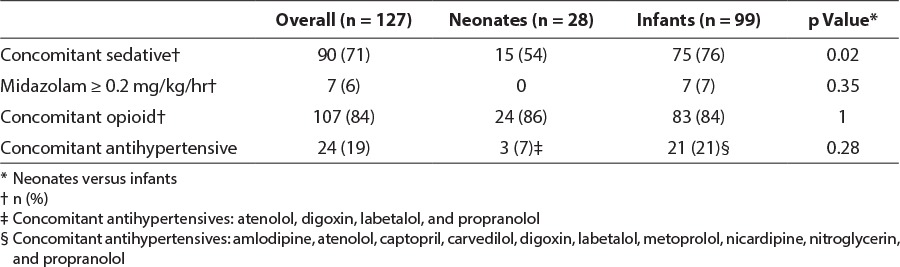
The primary objective of this study was to describe the off-label use of dexmedetomidine in infants and neonates in the PICU. Secondary objectives included describing the occurrence of at least 1 episode of bradycardia or hypotension and comparing dosing and adverse effects between infants and neonates. Each patient's chart was manually reviewed to extract and record demographic data, reason for admission, vital signs and concomitant receipt of antihypertensives, intravenous opioids, fluid boluses, vasoactive medications, atropine, or other sedatives (i.e., lorazepam and midazolam continuous infusions or as-needed boluses). Specific dosage information was collected, including dosage range, use of a loading dose, duration of use, and indication for dexmedetomidine use.
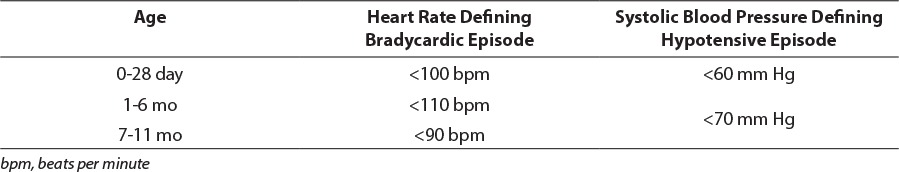
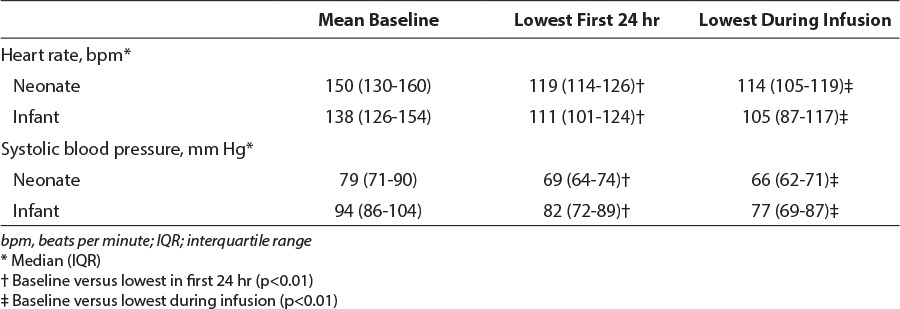
Patients were classified as having an episode of bradycardia or hypotension if their lowest heart rate or blood pressure during infusion was below the normal range based on their CGA according to the US National Institutes of Health (Table 1).14 Additionally, the change in heart rate and systolic blood pressure was assessed by comparing each patient's baseline vital signs (average of documented heart rate or systolic blood pressure in the 12 hours immediately before initiation of dexmedetomidine) to their lowest heart rate or systolic blood pressure in the first 24 hours and during the entire duration of infusion of dexmedetomidine.
Table 1.
Adverse Event Definitions14
There was a single data extractor for the study who used a standardized data collection form and organized data in Microsoft Excel (Microsoft Corporation, Redmond, WA). Descriptive statistics were performed by using Microsoft Excel; data are reported using the appropriate measure of central tendency based on distribution. Statistical analysis of the data was performed with MiniTab (MiniTab Statistical Software: 16.2.1 [2010], MiniTab, Inc, State College, PA). Categorical data were analyzed by using χ2 or Fisher exact test, and Mann-Whitney U test was used for non-parametric continuous data.
RESULTS
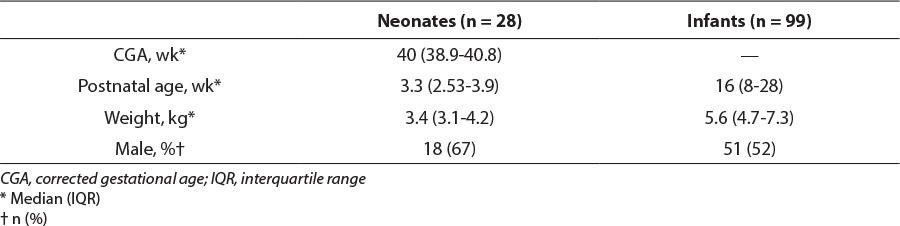
One hundred and twenty-seven patients were included in this study. Data were collected for the first 28 neonates and 99 infants in reverse chronological order who met inclusion and exclusion criteria (Figure). Patients were stratified by CGA, and baseline characteristics are shown in Table 2. Thirteen of the 28 neonates were born before 37 weeks' gestation. On the basis of admission notes, 42/99 (42%) infants were admitted due to respiratory distress, which was most commonly due to viral infection including rhinovirus and respiratory syncytial virus. Sepsis or septic shock led to admission of 18/99 (18%) infants, 15/99 (15%) were admitted post operatively, and the remainder of admissions were related to trauma, failure to thrive, intracranial hemorrhage, or seizures. Similarly, the most common reason for admission among neonates was respiratory distress (16/28 [57%]), followed by sepsis or septic shock (7/28 [25%]). The remainder of neonatal admissions related to failure to thrive, intracranial hemorrhage, or seizures. Age and weight recorded were from the time of initiation of dexmedetomidine.
Figure.
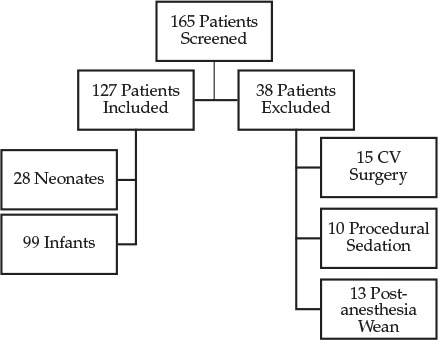
Patient selection: inclusion and exclusion criteria.
Table 2.
Baseline Characteristics
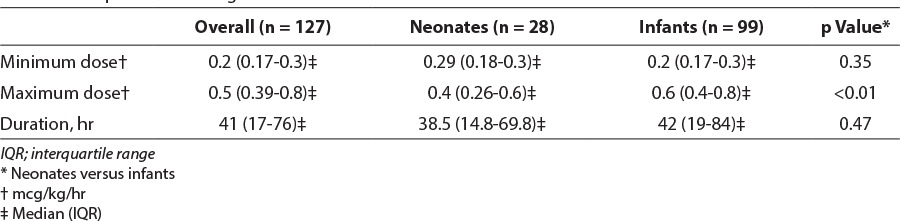
Dexmedetomidine was used for sedation during invasive ventilation in 92/99 (93%) infants and 26/28 (93%) neonates in this study. The remainder of patients received dexmedetomidine during noninvasive ventilation. Several patients were transitioned to dexmedetomidine from other sedatives for a short period before extubation. The median minimum infusion rate for the entire population was 0.2 mcg/kg/hr (interquartile range [IQR], 0.17–0.3) and median maximum rate was 0.5 mcg/kg/hr (IQR, 0.39–0.8). During the study, minimum dosages were similar between infants and neonates; however, the median maximum dosage was higher in infants than neonates at 0.6 mcg/kg/hr (IQR, 0.4–0.8) versus 0.4 mcg/kg/hr (IQR, 0.26–0.6; p < 0.01). Maximum infusion rate was 1.5 mcg/kg/hr in infants and 1 mcg/kg/hr in neonates; only 1 infant and neonate received these dosages, respectively. Median duration of use for all patients was 41 hours (IQR, 17–76) and was similar between groups. Maximum durations of use in infants and neonates were 338 hours and 121 hours, respectively. Four infants received loading doses; 2 received loading doses of 0.5 mcg/kg and 2 received 1 mcg/kg. Three of the infants (75%) did not have documented bradycardia or hypotension after bolus administration. The infant received a 1 mcg/kg bolus infused over 10 minutes, had a heart rate of 103 beats per minute (bpm) before the bolus, which decreased 19% to 83 bpm 30 minutes later. The next documented heart rate 2 hours later was increased to 95 bpm (7% from baseline). Detailed dosage data can be found in Table 3.
Table 3.
Comparison of Dosage and Duration of Dexmedetomidine Use Between Neonates and Infants
During the study 57/127 (45%) patients experienced at least 1 episode of bradycardia; this occurred more frequently in infants than neonates (55/99 [56%] vs. 2/28 [7%], p < 0.01]. Hypotension was noted in 34/127 (27%) of the study population. There was no difference between infants and neonates in patients experiencing hypotension (30/99 [30%] vs. 4/28 [14%], p = 0.15). Significant reduction in heart rate and systolic blood pressure occurred among both neonates and infants, based on comparison of mean baseline vital signs to the lowest heart rate and systolic blood pressure during the first 24 hours or entire infusion (p < 0.01). Cardiovascular adverse events are described in Tables 4 and 5.
Table 4.
Cardiovascular Adverse Events in Neonates Versus Infants

Table 5.
Cardiovascular Adverse Events
Data regarding several surrogate markers of adverse events were collected. Among infants, 12/99 (12%) received at least 1 fluid bolus during dexmedetomidine infusion, which may represent the occurrence of an adverse event. Discontinuation due to bradycardia or hypotension was described in the charts of 3/99 (3%) infants. No vasopressors were used in infants. Among neonates, 3/28 (11%) received a fluid bolus, and 4/28 (14%) had CV adverse events documented as the reason for dexmedetomidine discontinuation in their charts. Dopamine was used in 3/28 (11%) neonates during dexmedetomidine infusion; however, dopamine use preceded dexmedetomidine in 2 of the 3 patients. Atropine was not administered to any patients during the study period.
Other medications used concomitantly may have contributed to CV adverse events and affected outcomes in this study. Concomitant intravenous opioids were used in 107/127 (84%) patients. These included fentanyl, methadone, morphine, and hydromorphone. Most patients received concomitant opioids. Among patients documented as receiving opioids, 56/107 (52%) received a continuous infusion with or without additional opioid as needed. The remainder received opioids only on an as-needed basis. Antihypertensive agents were used in 24/127 (19%) patients, with similar use between the 2 groups of patients. Use of concomitant medications is presented in Table 6.
Table 6.
Use of Opioids and Antihypertensives in Patients Experiencing Bradycardia or Hypotension
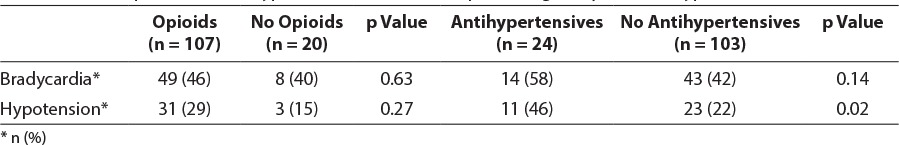
Cardiovascular adverse events were further assessed to determine if opioid or antihypertensive use was associated with hypotension or bradycardia. During the study, 31/107 (29%) patients who received opioids experienced hypotension, and only 3/20 (15%) patients who did not receive opioids experienced hypotension; however, this difference did not reach statistical significance (p = 0.27). Use of opioids was not associated with bradycardia (Table 6). Antihypertensives were associated with an increase in hypotension; hypotension occurred in 11/24 (46%) patients who received antihypertensives versus 23/103 (22%) patients who did not receive antihypertensives (p = 0.02). Use of concomitant sedatives was not associated with CV adverse events.
Additional sedative agents may have confounded dosage requirements and were used in 90/127 (71%) patients. Use was more common in infants than in neonates (75/99 [76%] vs. 15/28 [54%], p = 0.02). Concomitant sedatives used in the study population included midazolam and lorazepam, which were used as continuous infusions and/or bolus doses as needed. Seven infants received greater than 0.2 mg/kg/hr of midazolam. Use of phenobarbital as an adjunct sedative is rare in this institution, and thus was not considered in this evaluation. Use of concomitant medications is presented in Table 7.
Table 7.
Use of Concomitant Medications
DISCUSSION
Dexmedetomidine is a unique sedative that offers many benefits over other currently available agents. Few studies have assessed safety and efficacy in the general PICU population; available studies describe dexmedetomidine use after CV surgery and for procedural sedation.2,3 Hypotension and bradycardia, two of the most clinically significant adverse effects associated with dexmedetomidine, may limit its use in hemodynamically unstable adult patients in the ICU.1,2 These adverse effects are not as well described in pediatric patients. Because CV surgery patients are at risk for tachyarrhythmias, and use for procedural sedation is generally of short duration, rates of hypotension and bradycardia seen in these patient populations may underestimate adverse event rates in a broad infant and neonatal PICU population.7
Practitioners at this institution dosed dexmedetomidine in infants and neonates in the PICU similarly to FDA-labeled mcg/kg dosages for adults. Many patients received dexmedetomidine concomitantly with other sedatives. Rates of hypotension and bradycardia at these doses in adults have been reported to be up to 50% and 14%, respectively.2,7 Our study of infants younger than 1 year identified higher rates of bradycardia and lower rates of hypotension, when classified as any heart rate or blood pressure outside of the published normal ranges, than reported previously in adult patients. According to this conservative definition, 45% of patients experienced bradycardia and 27% experienced hypotension. However, there is notable variation among studies in their definition of these adverse events. Additionally, owing to the retrospective nature of this study and the many clinical situations that could result in vital sign changes, it is important to recognize the inability to establish causation between dexmedetomidine administration and these changes.
A meta-analysis of 24 adult studies showed that dexmedetomidine did not increase the risk of bradycardia requiring intervention when used at FDA-labeled doses of 0.2–0.7 mcg/kg/hr, although the use of loading doses and infusion rates above 0.7 mcg/kg/hr increased the risk of bradycardia.1 In addition, dexmedetomidine did not increase the risk of hypotension requiring intervention. A recent study by Chrysostomou and colleagues13 evaluated the use of dexmedetomidine among 42 preterm and term neonates. Neonates received dexmedetomidine at dosages of 0.05, 0.1, and 0.2 mcg/kg/hr for a maximum of 24 hours. Although the primary outcome was efficacy, baseline hemodynamic values were compared with the lowest values during the 24-hour infusion. A 12% ± 9% reduction in heart rate after 7.7 ± 7.3 hours of dexmedetomidine infusion was identified. Systolic blood pressure was reduced 14% ± 12% at 6.5 ± 7 hours. Although the present study identified a greater reduction in heart rate in the first 24 hours of infusion (Table 5), the median dexmedetomidine dosage was also higher, and infants were more likely to receive concomitant medications that affected the cardiovascular system. Although bolus administration of dexmedetomidine has been associated with hypertension, owing to initial peripheral vasoconstriction, or tachycardia, 1 infant in this study experienced bradycardia with rapid bolus administration.2,5 Reduced heart rate in the 1 infant may have been related to the rapid rate of bolus administration. Systolic blood pressure reduction was similar. Chrysostomou and colleagues13 identified a prolonged half-life and increased unbound concentration of dexmedetomidine in neonates, suggesting that lower doses may be required to achieve the same level of sedation and minimize adverse events.
Owing to the retrospective design of this study, it was difficult to assess the need for intervention due to severity of hypotension or bradycardia. Administration of atropine, vasopressors, and fluid boluses were used as surrogate markers of adverse events, but it is important to note that circumstances other than dexmedetomidine use may have lead to these interventions, especially in light of sepsis and septic shock accounting for a portion of the reason for admission in this patient population. If these interventions are used to determine clinically significant events, 16/127 (12.6%) patients required a fluid bolus or initiation of vasopressor after dexmedetomidine initiation. Although assessing clinically significant cardiovascular adverse events in this neonatal and infant population was difficult owing to the retrospective design, a notable proportion of patients were found to have had dexmedetomidine discontinued owing to cardiovascular adverse events. As this documentation was incidental in progress notes, and not required, it likely underestimates the number of patients who experience clinically significant cardiovascular adverse events but does indicate that they are likely common in this population.
There are limitations to this study. First, the nature of a retrospective study limits the control of confounding factors. This makes it difficult to interpret the use of concomitant sedatives as well as the possible contribution of concomitant medications to adverse effects. Other agents that are commonly administered to patients in the ICU, such as intravenous opioids, may increase the risk of CV adverse events and may have confounded the assessment of adverse events related to dexmedetomidine in this study. Individual clinicians dictated use and dosage of dexmedetomidine, and there were no standard clinical criteria for dexmedetomidine use. It is possible that only those patients considered at low risk for CV adverse events were selected for dexmedetomidine therapy. Several outcomes assessed in this study found no difference between neonates and infants; however, these assessments are at risk of type 2 error given the size of this study. It is possible, that a larger patient population may expose greater differences between groups. Lastly, this study was not able to assess long-term outcomes, such as the impact of dexmedetomidine and other sedatives on long-term developmental outcomes.
In conclusion, dexmedetomidine dosages used in infants and neonates in the PICU were similar to FDA-labeled dosage for ICU sedation in adults. More infants experienced a bradycardic episode than neonates, but they were also more likely to receive higher dosages of dexmedetomidine and additional sedatives. With careful patient selection and a conservative approach to dosing, dexmedetomidine may be safe for use in the infant and neonatal PICU population, but all aspects of a patient's medication regimen and clinical picture must be thoroughly evaluated when determining if the benefits of dexmedetomidine therapy outweigh the risks.
ACKNOWLEDGMENTS
This work was completed while the authors were afiliated with Riley Hospital for Children at Indiana University Health, Indianapolis, Indiana.
The results of this study have been presented at the following professional meetings: 1) Great Lakes Pharmacy Resident Conference, West Lafayette, Indiana, April 26, 2013; 2) 22nd Annual PPAG Meeting and 2013 Pediatric Pharmacy Conference, Indianapolis, Indiana, Pediatric Pharmacy Advocacy Group, May 5, 2013.
ABBREVIATIONS
- bpm
beats per minute
- CGA
corrected gestational age
- CV
cardiovascular
- FDA
US Food and Drug Administration
- ICU
intensive care unit
- IQR
inter-quartile range
- PICU
pediatric intensive care unit
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36(6):926–939. doi: 10.1007/s00134-010-1877-6. [DOI] [PubMed] [Google Scholar]
- 2.Greenwood Village, CO: Thomson Micromedex; Dexmedetomidine. DrugPoint Summary. http://thomsonhc.com. Accessed October 12, 2014. [Google Scholar]
- 3.Hudson, OH: Lexi-Comp, Inc; Dexmedetomidine. Lexi-Drugs Online. Lexi-Comp Online. http://online.lexi.com/crlonline. Accessed October 12, 2014. [Google Scholar]
- 4.Phan H, Nahata MC. Clinical uses of dexmedetomidine in pediatric patients. Pediatr Drugs. 2008;10(1):49–69. doi: 10.2165/00148581-200810010-00006. [DOI] [PubMed] [Google Scholar]
- 5.Su F, Hammer G. Dexmedetomidine: pediatric pharmacology, clinical uses and safety. Expert Opin Drug Saf. 2011;10(1):55–66. doi: 10.1517/14740338.2010.512609. [DOI] [PubMed] [Google Scholar]
- 6.Riker RR, Shehabi Y, Bokesch PM et al. Dexmedetomidine vs. midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 7.Tobias JD, Gupta P, Naguib A, Yates AR. Dexmedetomidine: applications for the pediatric patient with congenital heart disease. Pediatr Cardiol. 2011;32(8):1075–1087. doi: 10.1007/s00246-011-0092-8. [DOI] [PubMed] [Google Scholar]
- 8.Reiter PD, Pietras M, Dobyns E. Prolonged dexmedetomidine infusions in critically ill infants and children. Indian Pediatr. 2009;46(9):767–773. [PubMed] [Google Scholar]
- 9.Jones GM, Murphy CV, Gerlach AT et al. High-dose dexmedetomidine for sedation in the intensive care unit: an evaluation of clinical efficacy and safety. Ann Pharmacother. 2011;45(6):740–747. doi: 10.1345/aph.1P726. [DOI] [PubMed] [Google Scholar]
- 10.Allegaert K, Vanhaesebrouck S, Verbesselt R, van der Anker JN. In vivo glucuronidation activity of drugs in neonates: extensive interindividual variability despite their young age. Ther Drug Monit. 2009;31(4):411–415. doi: 10.1097/FTD.0b013e3181a8cc0a. [DOI] [PubMed] [Google Scholar]
- 11.Buck ML, Willson DF. Use of dexmedetomidine in the pediatric intensive care unit. Pharmacotherapy. 2008;28(1):51–57. doi: 10.1592/phco.28.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Lin H, Faraklas I, Sampson C et al. Use of dexmedetomidine for sedation in critically ill mechanically ventilated pediatric burn patients. J Burn Care Res. 2011;32(1):98–103. doi: 10.1097/BCR.0b013e318203332d. [DOI] [PubMed] [Google Scholar]
- 13.Chrysostomou C, Schulman SR, Castellanos MH et al. A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J Pediatr. 2014;164(2):276–282. doi: 10.1016/j.jpeds.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. Pediatric basic and advanced life support. 2010. AHA. http://chemm.nlm.nih.gov/pals.htm. Accessed December 28, 2014.


