Abstract
Phenobarbital and phenytoin have been the mainstay treatment modalities for neonatal seizures. Studies have revealed these agents control seizures in less than half of neonates, can cause neuronal apoptosis in vitro, and have highly variable pharmacokinetics in neonates. In contrast, there have been no reports of levetiracetam causing these neurotoxic effects. Due to its favorable side effect and pharmacokinetic profiles and positive efficacy outcomes in neonatal studies to date, there is great interest in the use of levetiracetam for neonatal seizures. This article reviews the literature regarding the safety of levetiracetam in neonates and its efficacy in neonatal seizures.
INDEX TERMS: Keppra, levetiracetam, neonates, review, seizures
BACKGROUND
Neonatal seizures occur in 1.8 per 1000 live births in the United States,1 with most seizure activity occurring in the first few days of life.2 Due to cerebral pathology, such as intraventricular hemorrhage and neurodevelopmental immaturity, premature neonates of less than 30 weeks gestation have a higher incidence of seizures than neonates older than 30 weeks.2 Neonatal seizures are rarely idiopathic.3 Hypoxic-ischemic encephalopathy (HIE) due to asphyxia4 is the most common cause of seizure activity in the neonatal population, accounting for approximately two-thirds of neonatal seizures.5 HIE seizures are generally self-limiting,1 and therefore, efficacy of agents in treatment of these seizures is questionable. Other causes include metabolic disturbances, cerebrovascular disease, infection, and congenital malformations.1,3
Synaptic and dendritic density peaks at birth and into the first month of life.6–8 Due to the immaturity of the central nervous system during this period of neurodevelopment, the cause and presentation of neonatal seizures differ from those in older children and adults. During development of the neonatal brain, excitatory (glutamate) neurotransmitters and receptors mature slightly faster than inhibitory (gamma-amino-butyric acid [GABA]) neurotransmitters and receptors.1 This imbalance, along with the increased concentration of synapses in the neonatal brain, may explain the lowered seizure threshold during the neonatal period.1 Additionally, the chloride (Cl¯) gradient in neonatal neurons is reversed compared to that in the pediatric and adult brain, with higher intracellular Cl¯ concentrations and lower extracellular Cl¯ concentrations. This reversed gradient is secondary to overexpression of the sodium-potassium-chloride Cl¯ importer (NKCC1) and underexpression of the potassium-chloride exporter (KCC2) (Figure 1). KCC2 is not fully expressed until the end of the first year of life; therefore, minimal Cl− is exported, resulting in synpatic firing.9 The combination of decreased GABA function, increased glutamate function, and reversed Cl¯ gradient potentially decreases the neonatal seizure threshold.
Figure 1.
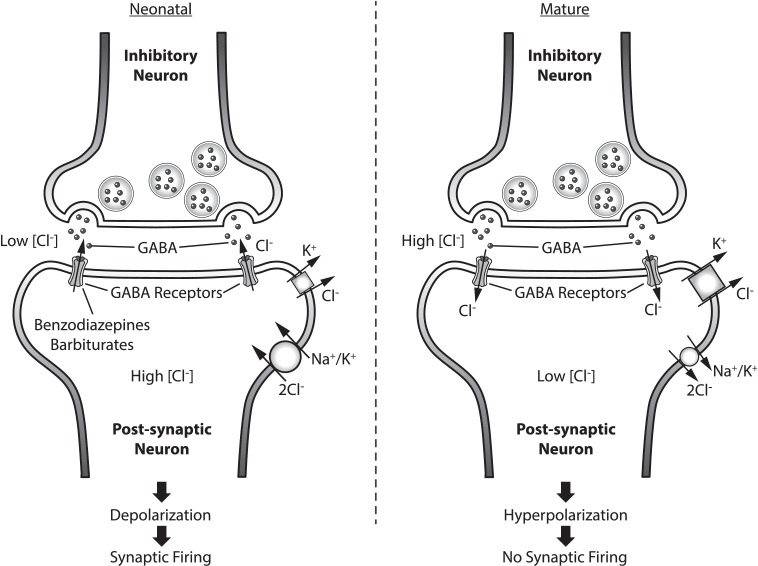
Pre and post inhibitory neuron in neonatal and mature brain. GABA neurotransmitter effect based on Cl- gradient in post-synaptic neuron. Mechanism of action of common antiepileptics depicted.  KCC2;
KCC2;  NKCC1
NKCC1
GABA, gamma-Aminobutyric acid; KCC2, Potassium-chloride transporter member 5; NKCC1, Na-K-Cl cotransporter
Although a lower seizure threshold is observed with the immature neonatal brain, the developing neurons are more resistant to the neurotoxic effects of seizures.10 In adult seizures, excessive glutamate release can lead to N-methyl-d-aspartate receptor (NMDA) activation and excessive calcium entry into cells, leading to neuronal apoptosis. In neonates, however, the increase in intracellular calcium from NMDA stimulation is much less pronounced and is thought to be a mechanism of resistance to neuronal damage due to seizures.11 Other potential mechanisms of resistance in neonates may be explained by a decrease in active synapses, decreased energy consumption, and immaturity of receptors and biochemical pathways.10
It is important to note that generalized seizures are rare in neonates due to immature myelination of the nervous system. Neonatal seizures often have subtle manifestations such as ocular changes, tongue thrusting, cycling limb movements, apnea, or blood pressure fluctuations. Clonic seizures are more common and will usually begin in one extremity then migrate to an opposite extremity.12
Due to these differences, diagnosis of neonatal seizures via clinical observation alone becomes difficult. A 2007 study reported two-thirds of clinical manifestations are unrecognized or misinterpreted as neonatal seizures by experienced neonatal staff. 13 This can lead to a misdiagnosis of seizures in this patient population. Therefore, neonates are evaluated for seizure activity through electroencephalography (EEG), video electroencephalography (vEEG), or amplitude integrated electroencephalography (aEEG). Normal EEG signals change significantly with gestational age; therefore, familiarity with age-specific norms is crucial for accurate interpretation. To diagnose an electrographic seizure in a neonate, a neuronal burst of electrical activity must be greater than or equal to 10 seconds in duration, compared to 3 seconds in older age groups.3,6,10 EEG results may demonstrate a multifocal process instead of typical coordinated seizure activity, therefore specialist interpretation is required.
There is the possibility for electroclinical dissociation, in which electrical seizure activity on EEG does not correlate with the clinical seizure activity, and in many cases, patients may not express visible signs of seizures.1 This dissociation typically occurs in neonates with severe brain dysfunction, neonates receiving paralytic medications, or neonates with controlled clinical seizures on antiepileptic drugs (AEDs).14 Mizrahi and Kellaway14 investigated the correlation of clinical seizure activity with simultaneous electrical seizure activity. Their findings revealed seizures from diffuse processes such as HIE may not have an EEG correlate.14 These findings are important because many AED efficacy studies use EEGs to define seizure cessation and treatment.
Seizure diagnosis and treatment are essential, especially in refractory seizures or HIE, as patients with continued seizures have a poor prognosis1 and significant sequelae such as mental retardation and motor deficits.3 These sequelae may still occur despite the innate resistance to neurotoxic damage in the neonatal brain as described above. A prospective study by Ronen et al15 evaluated 82 neonates with the diagnosis of neonatal seizures over a 5-year period with follow-up at 10 years of age. Causes of seizures included encephalopathy, infection, and congenital malformation. Phenobarbital was used as the first line agent in 79 patients, 23 of whom required additional treatment with phenytoin for refractory seizures. Thirty-six of the 61 surviving infants had neurological disability; 27% had epilepsy, 25% had cerebral palsy, 20% had mental retardation, and 27% had learning disorders. Furthermore, 18 of the 23 patients who required additional treatment with phenytoin were found to have deficits greater than learning disabilities at follow-up.15 In the subgroup analysis, 42% of the preterm neonates died versus 16% of the term neonates (median age of death was 13 months), and 46% of preterm neonates had impairments versus 39% of their term counterparts. Results showed that preterm infants had worse outcomes than their term counterparts (p = 0.003). The authors stated that the differences in pathologic effects on neurodevelopmental outcome may be due to metabolic stress and injury caused by not only the seizure but also the presence of hypoxia.15
TRADITIONAL ANTICONVULSANT THERAPY
Traditionally, the preferred agent for treatment of neonatal seizures has been phenobarbital, followed by phenytoin or fosphenytoin, and then benzodiazepines. The evidence for treatment with these agents was extrapolated from data in adults and children. However, it is evident these therapies alone are not adequate to control neonatal seizures. Painter et al16 reported phenobarbital and phenytoin relieved seizures in only 43% and 45% of neonates, respectively, when used as the primary agent and up to 62% of the time in combined therapy. The authors found that the severity and progression of the seizures (increasing or decreasing severity) were better predictors of successful treatment rather than the AED used.16
As stated above, phenobarbital is the preferred first-line agent in most neonatal seizures, compared to pediatrics and adults, where phenytoin use is more common. This preference is related to greater historical experience with phenobarbital,17 and the difficulties with phenytoin dosing and monitoring in the neonatal population. Challenges with phenytoin dosing in this population include reduced protein binding compared to that in adults (60%–90% compared to >90% bound to albumin, respectively18); competitive binding with bilirubin, endogenous corticosteroids, and free fatty acids (resulting in increased free-drug concentration or increased free bilirubin and possible kernicterus), lower serum albumin concentrations compared to that in adults (possible increase in free-drug concentrations), and varying adipose tissue (altering distribution and clearance).18–20 Additionally, due to incomplete maturation of the CYP2C9 enzyme and saturable metabolism, phenytoin half-life is prolonged from 8 hours in patients older than 2 weeks to 20 hours in term infants and 75 hours in preterm neonates.17,21
Although dosage regimens for phenobarbital and benzodiazepines are less complex than those for phenytoin in the neonatal population, they may be less effective due to the receptor and ion gradient variations in the neonate described previously (Figures 1 and 2). A decreased response with benzodiazepines and phenobarbital may be expected as the inhibitory GABA receptors targeted are underexpressed in the neonatal brain. Immature GABA receptors overexpress the α4 subunit compared to the α1, which has been shown to decrease responsiveness to benzodiazepine therapy. Consideration has to be given to the reversed Cl¯ gradient. Activation of the GABA receptor in a mature brain allows for the opening of a Cl¯-selective pore, the influx of Cl¯ along its gradient, and the hyperpolarization of the cell. However, in the immature neonatal brain, GABA activation by an agonist leads to an efflux of Cl¯ due to the high intracellular concentrations, which may cause depolarization of the membrane resulting in neuronal firing.6 These variations may be reason for the limited efficacy of phenobarbital and benzodiazepines observed in neonatal studies.
Figure 2.
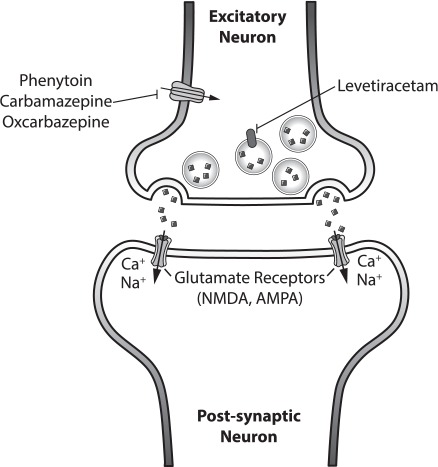
Pre and post excitatory neuron with neurotransmitter, glutamate. Mechanism of action of common antiepileptics depicted.
 Glutamate;
Glutamate;  SV2a Receptor;
SV2a Receptor;  Na+ Ion Gated Channel;
Na+ Ion Gated Channel;  Glutamate Receptors (NMDA, AMPA)
Glutamate Receptors (NMDA, AMPA)
AMPA, a-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; NMDA, N-methyl-D-aspartate receptor; SV2A, Synaptic vesicle glycoprotein 2A
There is concern regarding the potential adverse effects traditional AEDs may have on neurodevelopment. Medications such as NMDA receptor antagonists (i.e., ketamine), GABA agonists (i.e., lorazepam or phenobarbital) and sodium channel blockers (i.e., phenytoin or carbamazepine) have been observed to potentiate neurodegeneration in the developing brain in animal models.22 Neuronal death by apoptosis is one possible mechanism explaining cognitive impairment and reduced brain mass in animals treated with these agents. Bittigau et al23 studied the effects of multiple AEDs in animal models at relevant human doses. Study results revealed that phenobarbital caused neuronal apoptosis in the brains of rats at therapeutic serum concentrations of 25 to 35 mcg/mL, which is within the usual therapeutic window of 15 to 40 mcg/mL used in clinical practice. Phenytoin triggered apoptotic neurodegeneration starting at a dose of 20 mg/kg or a plasma concentration of 10 to 15 mcg/mL; however, its toxicity was found to be dose dependent, unlike phenobarbital and diazepam.23 Unlike other AEDs, levetiracetam (LEV) has shown improved neurodevelopment outcomes and lack of neurodegenerative effects in early animal studies, making LEV an attractive treatment option in neonatal seizures.
LEVETIRACETAM
Labeled Indications
LEV was approved by the US Food and Drug Administration (FDA) in November 1999 for use in adult patients with myoclonic seizures, juvenile myoclonic epilepsy, or primary generalized tonic-clonic seizures.24 It was not until 2012 that the FDA approved LEV for use as adjunctive therapy for partial onset seizures in infants and children 1 month of age and older. Approval was based on a multicenter, double-blind, placebo-controlled study in 116 randomized pediatric patients (8 of these patients were less than 6 months of age) with partial onset seizures, demonstrating tolerability and seizure reduction of 43.6% with LEV versus 7.1% with placebo (p < 0.001).25 In 2013, LEV gained monotherapy indications with new level I, II, and III evidence for use in adult partial onset seizures, adult tonic-clonic seizures, and children with benign childhood epilepsy with centrotemporal spikes.26 Despite lack of studies supporting its use at that time, a 2007 survey demonstrated 47% of pediatric neurologists recommended LEV off-label for the treatment of neonatal seizures.27
Mechanism of Action
The mechanism of action of LEV continues to be evaluated and has not been fully elucidated. LEV is a pyrrolidine derivative antiepileptic that binds to the synaptic vesicle protein SV2a, which is expressed throughout the brain. LEV binding to SV2a impedes neurotransmitter release and vesicle transport within the neuron.28,29 SV2a receptor appears to be important in both partial and generalized seizure disorders.30 Targeting the SV2a protein is unique to LEV and, therefore, provides a novel mechanism of action for neonatal patients for whom primary and secondary treatments have already failed. Because the SV2a is found in all areas of the brain, it can treat partial seizures that arise in various regions of the brain, as seen in neonatal seizures. In addition, LEV may inhibit synaptic high-voltage-operated calcium channels31 and potassium-gated channels32; however, most of the current research focuses on its effect on the SV2a receptor. By targeting the SV2a receptor, LEV circumvents the problems other AEDs face with the overexpression of glutamate receptors, under development of the GABA receptors, and inversion of the Cl− gradient seen in the neonatal brain (Figure 2).
Pharmacokinetic Data
In adults, LEV exhibits high bioavailability (>95%), quickly reaches peak and steady state concentrations in 1.3 hours, and displays linear time-dependent kinetics.33 Because LEV is metabolized by type-B esterases in whole blood to inactive metabolites, it undergoes minimal hepatic metabolism, resulting in fewer drug-drug interactions.33,34 LEV has lower protein binding (~10%) than medications such as phenytoin (~90%), resulting in less serum drug variability in neonates.33,34 Sixty-six percent of the drug is eliminated in the urine, and clearance is dependent on renal function.33–35 Table 1 outlines the neonatal pharmacokinetic studies of LEV published in the current literature.
Table 1.
Pharmacokinetics of Levetiracetam in Neonates
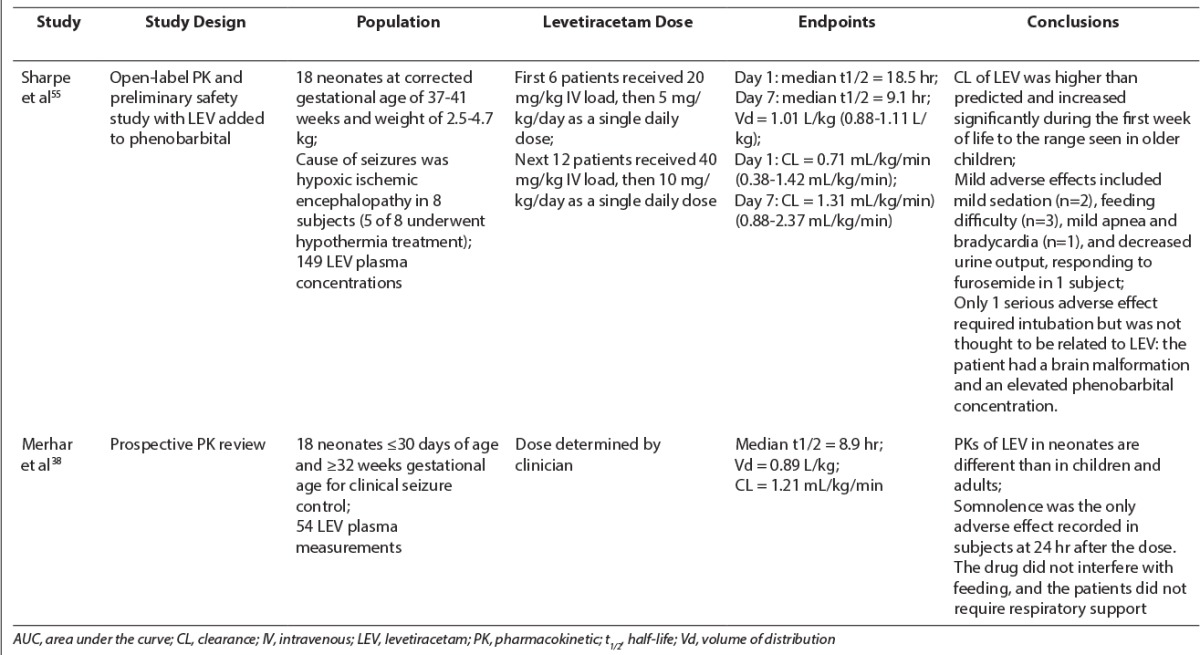
LEV maximum concentration (Cmax) and area under the curve (AUC) in children differ from those in adults. A study by Pellock et al35 in pediatric patients 6 to 12 years of age receiving doses of 20 mg/kg LEV showed a 30% to 40% reduction in Cmax and AUC and a 60% increase in clearance of LEV compared to those in adults.36 Similar results were found in patients 1 month to less than 4 years of age.37 However, Merhar et al38 observed that the clearance of LEV in premature infants receiving 14.4 to 39.9 mg/kg of LEV was reduced compared to that in children and adults (half-life of approximately 9 hours compared to 5–7 hours and 7–8 hours, respectively). This decreased clearance may be secondary to a decreased glomerular filtration rate or decreased esterase activity. The authors recommended extending the dose interval to twice daily in premature neonates compared to 3 times daily in older children. The volume of distribution (VD) in neonates in this study was 0.89 L/kg compared to 0.6–0.7 L/kg in children and 0.5–0.7 L/kg in adults.38 Because neonates have a higher total body water content than children and adults, a larger VD is expected because LEV is water soluble and therefore has a distribution that reflects total body water.38
Evidence of Levetiracetam Use
Decreased efficacy and adverse neurodevelopmental outcomes of traditional therapies have generated an interest in the use of LEV for the treatment of neonatal seizures. Manthley et al39 demonstrated that LEV lacked neurotoxic effects at all studied doses (5, 10, 25, 50, and 100 mg/kg per dose, similar to doses in humans) in 7-day-old rats, making LEV an attractive treatment option. LEV given prophylactically to HIE-induced neonatal rats was found to significantly reduce hypoxic seizure activity as well as duration of ictal EEG activity in a dose-related manner.28 LEV appeared to exert a disease-modifying effect on hypoxic-ischemic seizures that may potentially attenuate seizures later in life. Kilicdag et al40 evaluated the effects of LEV on neuronal apoptosis in rat pups with induced hypoxic ischemic brain injury. LEV was administered intraperitoneally at a loading dose of 80 mg/kg and a maintenance dose of 40 mg/kg/day after 7 days of hypoxia. Investigators demonstrated administration of LEV after hypoxia significantly reduced the number of apoptotic cells in the hippocampus and cerebral cortex compared to placebo (p < 0.006).40 Possessing both disease-modifying effects and lack of neuronal apoptosis during hypoxic ischemic brain injury, LEV may be an attractive antiepileptic agent for the treatment of seizures in HIE.
Despite published data in children, there are few studies evaluating the safety and efficacy of LEV in neonatal seizures (Tables 2 and 3). The first reports of LEV used for neonatal seizures were published as several case reports.41,42 Hmaimess et al41 demonstrated the efficacy of LEV in a neonate with malignant migrating partial seizures refractory to phenytoin, clonazepam, phenobarbital, and lamotrigine. The patient received an initial dose of LEV, 10 mg/kg/day, which was increased to 30 mg/kg/day without adverse effects. Within 8 days, LEV therapy resulted in improvement in clinical status and decreased seizure activity confirmed via EEG recordings.41 Shoemaker et al42 discussed the use of LEV in 3 infants (2 days to 3 months of age) for whom conventional AED therapy had failed. Patients were treated with LEV dosages ranging from 30 to 60 mg/kg/day divided into 2 to 3 doses daily. Despite the fact that all 3 patients' seizures had different causes (infarction, hydrocephalus, and meningitis), each neonate was safely and effectively treated with LEV as adjunct therapy without adverse effects.
Table 2.
Levetiracetam Case Reports in Neonates
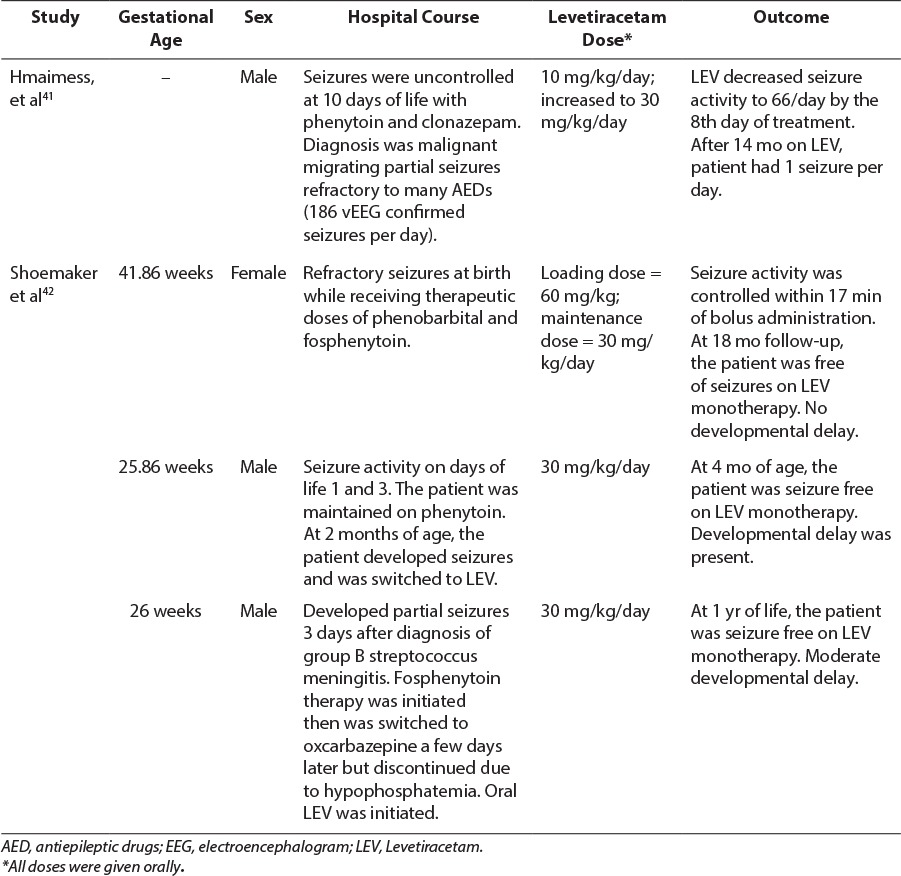
Table 3.
Levetiracetam Studies in Neonates
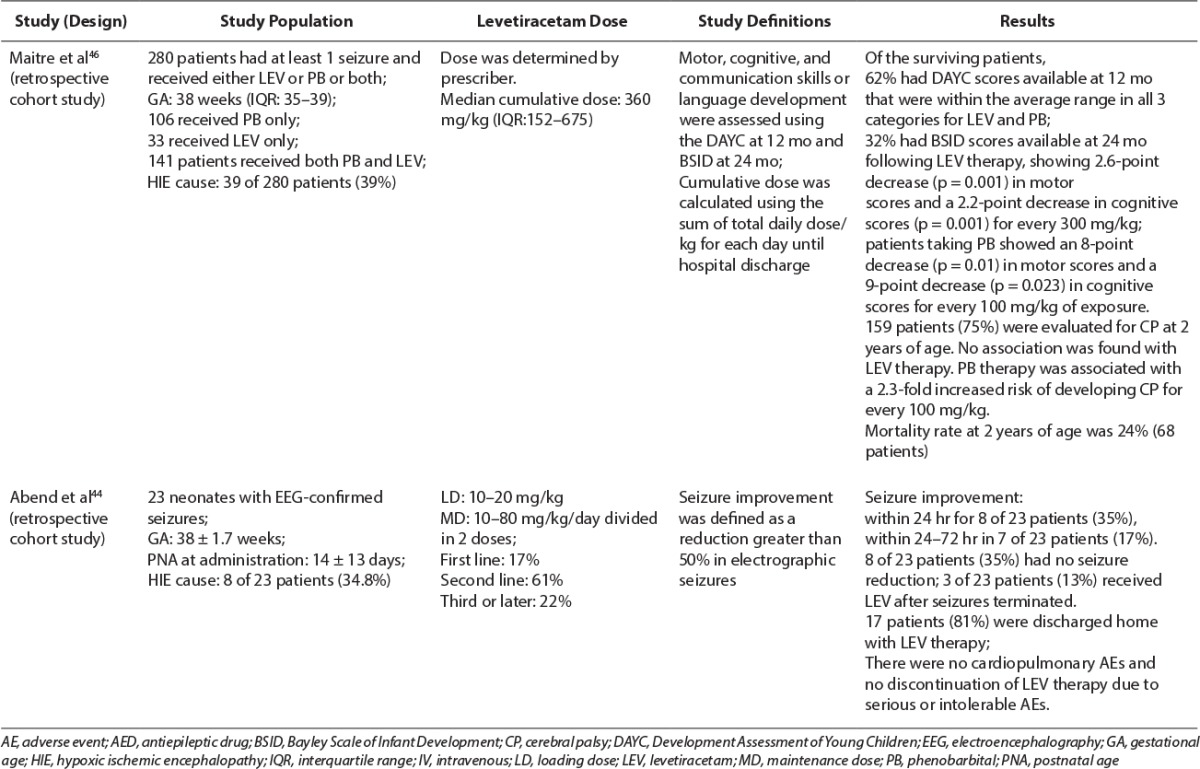
Table 3.
Levetiracetam Studies in Neonates (cont.)
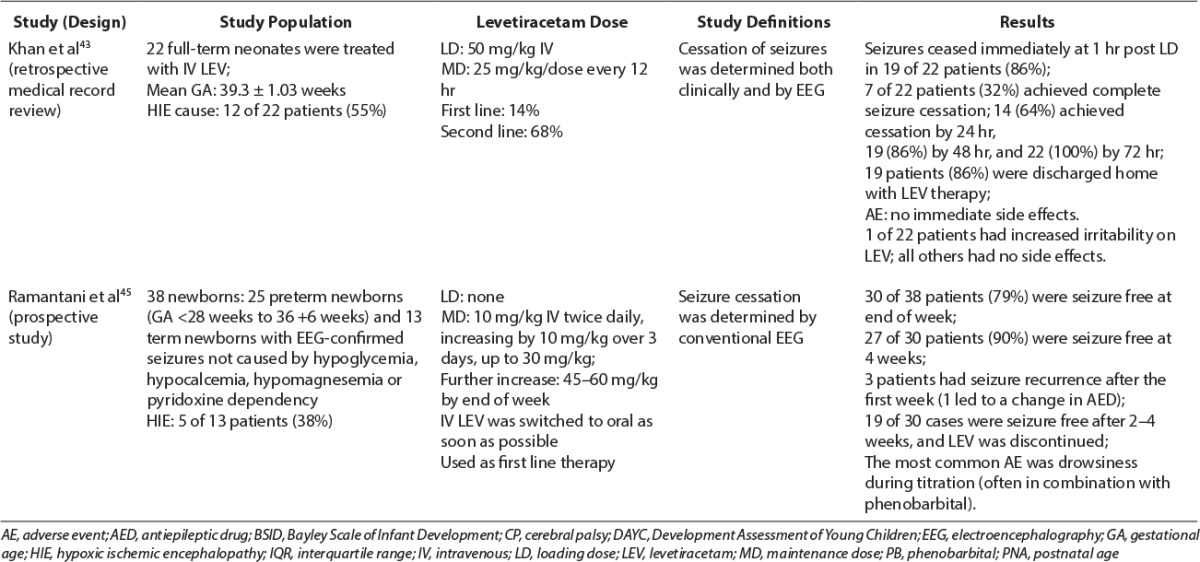
A retrospective study by Khan et al43 evaluated the use of intravenous LEV for acute neonatal seizures as a second-line agent after phenobarbital therapy failure. All patients were term infants (gestational age ≥37 weeks) with 12 of 22 (55%) having HIE as the cause of the seizure. Seventy-two percent of patients received 1 AED, 9% received 2 AEDs, and 5% received 3 AEDs before initiation of LEV therapy. The majority of patients (68%) received LEV due to continued seizures while receiving phenobarbital therapy. Patients received an intravenous loading dose of 50 mg/kg LEV and then maintenance therapy of 25 mg/kg/dose every 12 hours. Seven of the 22 neonates (32%) had complete seizure cessation by EEG at 1 hour after the loading dose, and 100% had complete cessation at 72 hours. Minimal side effects were reported in the study. One patient developed increased irritability but improved with pyridoxine, 50 mg daily. Eighty-six percent of patients were discharged home with an oral LEV regimen, and 9% were discharged with an additional oral AED. This trial demonstrated LEV was safe and effective in the management of acute seizures in term neonates.
Abend et al44 reported results in seizure control in a retrospective cohort study that included 23 neonates with EEG-confirmed seizures with a mean gestational age of 38.7 ± 1.7 weeks. The most common cause of neonatal seizures was HIE (34.8%). Patients received an initial LEV bolus dose of 10 to 20 mg/kg intravenously over 15 minutes. No information was provided regarding the titration of maintenance dose, but the mean maximum daily dosage was 45 ± 19 mg/kg/day (range, 10–80 mg/kg/day) in 2 divided doses. Seizure improvement (defined as seizure termination or reduction of >50% in seizures) was seen in less than 24 hours after LEV initiation in 8 of 23 neonates (35%) and between 24 to 72 hours in an additional 4 patients (17%). The impact of seizure cessation could not be evaluated in 3 of the 23 subjects (13%) because LEV was started after seizures had already terminated. The remaining 8 of 23 infants (35%) had no seizure reduction with the use of LEV.44 This could reflect the lower loading dose used in this study compared to that in the previous study. Of the 23 subjects in this trial, LEV was used as first-line therapy in 4 patients, second-line after phenobarbital in 14 patients, and third-line or later in the remaining 5 patients.44 This study demonstrated the added benefit of LEV in ameliorating seizure treatment as a second-line agent without increased risk of major adverse events. Because few patients received LEV as a first-line agent, it is difficult to determine its benefit as a monotherapy or a first-line agent based on these data.
Ramantani et al45 performed a prospective evaluation of the use of LEV as first-line therapy in 38 preterm and term neonates. HIE was diagnosed in 1 (5%) of 19 extremely premature infants (<28 weeks gestation), 3 (50%) of 6 premature infants (28–36 weeks gestation), and 5 (38%) of 13 term newborns (≥37 weeks gestation). LEV was administered as the first-line agent within 8 hours of seizure manifestation at an initial dose of 10 mg/kg/dose, intravenously, twice daily with a dosage increase of 10 mg/kg/day over 3 days to a dosage of 30 mg/kg/day. Dosages were further increased up to 45 to 60 mg/kg/day at the end of the first week if needed for persistent seizures. LEV trough sera concentrations ranged from 12.5 to 55 mcg/mL. Patients were allowed up to 2 doses of phenobarbital during LEV dose titration for prolonged or repetitive breakthrough seizures. In addition, all patients received pyridoxine, 100 mg intravenously (up to a cumulative dose of 300 mg), to mitigate pyridoxine deficiency as a cause of the seizure disorder. Thirty of the neonates treated with LEV were seizure-free according to clinical presentation and EEG interpretation by the end of the first week, and 27 remained seizure-free at 1 month.45 After the first week, 3 infants presented with seizure recurrence, and 1 extremely premature infant required an AED change to phenobarbital. At 6 months follow-up, patients who remained seizure free at 1 month, 27% of extremely premature, 33% of premature infants, and 17% term newborns, developed post-neonatal epilepsy; 55% of extremely premature, 33% premature, and 42% of term newborns presented with developmental delay; and 45% of extremely premature infants, none of the premature infants, and 8% of term newborns had comorbidities. Drowsiness was the only adverse effect observed during the titration period, often in the neonates who received phenobarbital doses for breakthrough seizures. Limitations of this study were the administration of the long-acting antiepileptic phenobarbital during LEV dose titration, and no simultaneous vEEG monitoring was performed. Authors concluded that LEV was safe in neonates, including premature neonates; however, LEV monotherapy may not achieve seizure control because adjunctive phenobarbital therapy was used in more than 50% of the study population.45 Giving a loading dose of LEV as described in other studies may have mitigated the use of phenobarbital for breakthrough seizures and achieved seizure cessation earlier.
Maitre et al46 conducted a retrospective study evaluating neurodevelopmental outcomes (death, cerebral palsy [CP] at 2 years of age, Developmental Assessment of Young Children [DAYC] score at 12 months, and Bayley Scales of Infant Development [BSID] score at 24 months) after 280 infants (median gestational age of 38 weeks) were exposed to both phenobarbital and LEV intravenously or orally for neonatal seizures. A total of 106 neonates received only phenobarbital, 33 neonates received LEV alone, and 141 neonates received both phenobarbital and LEV. The most common cause of seizures was hypoxia or ischemia (39%). Doses were calculated as the cumulative AED dose in mg/kg from the time of transport and admission to the neonatal intensive care unit through hospital discharge. Median doses were 60 mg/kg for phenobarbital and 360 mg/kg for LEV. Seizure severity was determined by the number of EEG-documented seizures instead of the seizure type. Seizure severity in those who received phenobarbital was similar to those who received LEV. Twenty-four percent of patients died by 2 years of age, but this outcome was not associated with either AED exposure. DAYC scores for motor, cognitive, and communication status were available for 62% of surviving patients, all of which were within the average range at 12 months. However, correction for gestational age and seizure severity did suggest that both phenobarbital and LEV were associated with decreased motor scores. BSID scores were reported for 32% of the surviving patients at 24 months. Phenobarbital demonstrated an 8-point cognitive score decrease and 9-point motor score decrease for every 100 mg/kg, whereas LEV demonstrated 2.2- and 2.6-point decreases, respectively, for every 300 mg/kg.46 BSID communication scores were also decreased with phenobarbital and LEV use; however, those scores had less clinical significance. Of the surviving 159 patients, there was no association found between CP and LEV exposure. However, authors found that for every 100 mg/kg increase of phenobarbital, patients had a 2.3-fold increase in the probability of developing CP by 2 years of age. This is the first and largest pediatric study to date evaluating neurodevelopmental outcomes from AED exposure during the neonatal period. Results of this study reflect previous findings documented in animal models, that is, neuro-toxicity and poor neurodevelopmental outcomes with phenobarbital and reduction in neuronal apoptosis and improved outcomes with LEV. Limitations of this study include the use of LEV as a second-line agent in most of the cohort and a few patients receiving only 1 AED, making it difficult to draw individual conclusions about the association of phenobarbital and LEV.
Larger prospective studies in this patient population are still needed to investigate LEV as a safe and effective treatment option for neonatal seizures. There are currently ongoing studies in the neonatal population that will hopefully help elucidate the appropriate place in therapy of LEV for the treatment of neonatal seizures.
Tolerability and Monitoring
LEV is generally well tolerated by patients. An open label study demonstrated long term tolerance with minimal behavioral and cognitive effects in children 4 to 16 years of age when LEV was used as adjunctive therapy for partial onset seizures.47 The adverse effects most commonly reported in this group of patients were headache (24%), pyrexia (22%), and upper respiratory tract infection (21%). Similar levels of tolerability were reported in a subgroup analysis of children 1 month to less than 4 years of age.25 Clinically, it remains difficult to assess these side effects in the neonatal population.
Due to the limited side effect profile and drug interactions of LEV, routine monitoring is not necessary. However, monitoring may be considered in patients presenting with seizures resistant to high doses of LEV or exhibiting adverse reactions. The sera reference range for LEV has not been well established and may vary from 5 to 65 mcg/mL in pediatrics and adults.38,45,48,49 Some studies have failed to establish a correlation between therapeutic efficacy of LEV and its serum concentrations.50 Monitoring should be discussed on a case-by-case basis and decided based on provider judgment. If monitoring is used, it may be possible to use saliva samples instead of sera, as saliva and sera concentrations of LEV have been shown to be highly correlated.51 The required saliva sample is 0.25 mL, which the authors recommend to be collected by plastic pipette in infants. Contamination of saliva samples must be considered when patients are receiving liquid oral doses. Finally, although saliva monitoring presents multiple benefits to serum monitoring, particularly in premature infants, this test may not be available at all care centers.
Place in Therapy
In currently published guidelines,52,53 LEV is not listed as a therapeutic option for the treatment of neonatal seizures. The 2011 World Health Organization (WHO) guidelines for neonatal seizures and the 2010 Queensland Maternity and Neonatal Clinical Guidelines for neonatal seizures recommend phenobarbital as the first-line agent for treatment of neonatal seizures, followed by phenytoin, and then benzodiazepines.52,53 As discussed earlier, there are known difficulties with establishing dosages of phenytoin due to variable pharmacokinetics, questionable neurodevelopmental outcomes with treatment of phenobarbital and benzodiazepines, and ineffectiveness with other AEDs due to underdeveloped biochemical pathways. LEV has recently been recommended by pediatric neurologists for neonatal seizure control due to its favorable pharmacokinetic profile extrapolated from older children and small neonatal pharmacokinetic studies.4,25,27,38,41,43–45,47,54–56 Based on a review of publications, LEV appears to be safe and effective in treating several types of neonatal seizures, but data are lacking for its use as a first-line agent or as monotherapy. LEV could be used as a second-line agent to phenobarbital as LEV's overall pharmacokinetic and adverse event profiles appear to be more beneficial than those of phenobarbital or phenytoin in neonates.
CONCLUSIONS
While LEV is not currently listed as a therapeutic option in the treatment of neonatal seizures, many studies report its use in treatment of neonatal seizures. LEV is safe and effective in pediatric patients and adults, and the current research suggests its additional safety and efficacy in neonates as second-line therapy after phenobarbital. Dosage information provided from the studies suggest that loading doses of 10 to 20 mg/kg are appropriate and effective in neonates, with a maintenance dose range of 10 to 80 mg/kg/day divided twice daily. The decision to use LEV should be directed by seizure presentation and EEG findings, which in some cases may warrant higher loading doses of LEV for resistant seizure types. Monitoring, although available for resistant cases, is not warranted in most cases as LEV has a large therapeutic window and minimal side effect profile. Continued studies investigating LEV as monotherapy in neonatal seizures and its use in patients specifically presenting with seizures secondary to HIE are warranted.
ACKNOWLEDGMENT
We acknowledge Brian Gardner, PharmD, for review of the article.
ABBREVIATIONS
- AED
antiepileptic drugs
- aEEG
amplitude integrated electroencephalography
- AUC
area under the curve
- BSID
Bayley Scales of Infant Development
- Cl−
chloride
- Cmax
maximum concentration
- CP
cerebral palsy
- DAYC
Developmental Assessment of Young Children
- EEG
electroencephalography
- FDA
US Food and Drug Administration
- GABA
gamma-amino-butyric acid
- HIE
hypoxic-ischemic encephalopathy
- LEV
levetiracetam
- NMDA
N-methyl-d-aspartate
- VD
volume of distribution
- vEEG
video electroencephalography
- WHO
World Health Organization
Footnotes
Disclosures The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
REFERENCES
- 1.Jensen FE. Neonatal seizure: an update on mechanisms and management. Clin Perinatol. 2009;36(4):881–900. doi: 10.1016/j.clp.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scher MS, Aso K, Beggarly ME et al. Electrographic seizures in preterm and full-term neonates: clinical correlates, associated brain lesions, and risk for neurologic sequelae. Pediatrics. 1993;91(1):128–134. [PubMed] [Google Scholar]
- 3.Hill A. Neonatal seizures. Pediatr Rev. 2000;21(4):117–121. doi: 10.1542/pir.21-4-117. [DOI] [PubMed] [Google Scholar]
- 4.van Rooij LG, van den Broek MP, Rademaker CM et al. Clinical management of seizures in newborns: diagnosis and treatment. Paediatr Drugs. 2013;15(1):9–18. doi: 10.1007/s40272-012-0005-1. [DOI] [PubMed] [Google Scholar]
- 5.Tekgul H, Gauvreau K, Soul J et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117(4):1270–1280. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- 6.Takashima S, Chan F, Becker LE, Armstrong DL. Morphology of the developing visual cortex of the human infant: a quantitative and qualitative Golgi study. J Neuropathol Exp Neurol. 1980;39(4):487–501. doi: 10.1097/00005072-198007000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Rakic P, Bourgeois JP, Eckenhoff MF et al. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232(4747):232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 8.Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human cortex—evidence of synapse elimination during normal development. Neurosci Lett. 1982;33(3):247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- 9.Silverstein FS, Jensen FE. Neonatal seizures. Ann Neurol. 2007;62(2):112–120. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- 10.Holmes GL, Ben-Ari Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr Res. 2001;49(3):320–325. doi: 10.1203/00006450-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Marks JD, Friedman JE, Haddad GG. Vulnerability of CA1 neurons to glutamate is developmentally regulated. Brain Res Dev Brain Res. 1996;97(2):194–206. doi: 10.1016/s0165-3806(96)00149-6. [DOI] [PubMed] [Google Scholar]
- 12.Stafstrom CE. Neonatal seizures. Pediatr Rev. 1995;16(7):248–255. doi: 10.1542/pir.16-7-248. [DOI] [PubMed] [Google Scholar]
- 13.Murray DM, Boylan GB, Ali I et al. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93(3):F187–191. doi: 10.1136/adc.2005.086314. [DOI] [PubMed] [Google Scholar]
- 14.Mizrahi EM, Kellaway P. Characterization and classification of neonatal seizures. Neurology. 1987;37(12):1837–1844. doi: 10.1212/wnl.37.12.1837. [DOI] [PubMed] [Google Scholar]
- 15.Ronen GM, Buckley D, Penney S et al. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology. 2007;69(19):1816–1822. doi: 10.1212/01.wnl.0000279335.85797.2c. [DOI] [PubMed] [Google Scholar]
- 16.Painter MJ, Scher MS, Stein AD et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341(7):485–489. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 17.Loughnan PM, Greenwald A, Purton WW et al. Pharmacokinetic observations of phenytoin disposition in the newborn and young infant. Arch Dis Child. 1977;52(4):302–309. doi: 10.1136/adc.52.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Painter MJ, Minnigh B, Gaus L et al. Neonatal phenobarbital and phenytoin binding profiles. J Clin Pharmacol. 1994;34(4):312–317. doi: 10.1002/j.1552-4604.1994.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 19.Mirkin BL. Perinatal pharmacology: placental transfer, fetal localization, and neonatal disposition of drugs. Anesthesiology. 1975;43(2):156–170. doi: 10.1097/00000542-197508000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Assael BM. Pharmacokinetics and drug distribution during postnatal development. Phamacol Ther. 1982;18(2):159–197. doi: 10.1016/0163-7258(82)90066-3. [DOI] [PubMed] [Google Scholar]
- 21.Skinner AV. Neonatal pharmacology. Anaesthesia and Intensive Care Medicine. 2011;12(3):79–84. [Google Scholar]
- 22.Ikonomidou C. Triggers of apoptosis in the immature brain. Brain Dev. 2009;31(7):488–492. doi: 10.1016/j.braindev.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Bittigau P, Sifringer M, Ikonomidou C. Antiepileptic drugs and apoptosis in the developing brain. Ann N Y Acad Sci. 2003;993(23):103–114. doi: 10.1111/j.1749-6632.2003.tb07517.x. [DOI] [PubMed] [Google Scholar]
- 24.Smyrna, GA, IN: UCB, Inc.; 2011. Levetiracetam [package insert] [Google Scholar]
- 25.Pina-Garza JE, Nordli DR, Jr, Rating D et al. Adjunctive levetiracetam in infants and young children with refractory partial-onset seizures. Epilepsia. 2009;50(5):1141–1149. doi: 10.1111/j.1528-1167.2008.01981.x. [DOI] [PubMed] [Google Scholar]
- 26.Glauser T, Ben-Menachem E, Bourgeois B et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54(3):551–563. doi: 10.1111/epi.12074. [DOI] [PubMed] [Google Scholar]
- 27.Silverstein FS, Ferriero DM. Off-label use of antiepileptic drugs for the treatment of neonatal seizures. Pediatr Neurol. 2008;39(2):77–79. doi: 10.1016/j.pediatrneurol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Talos DM, Chang M, Kosaras B et al. Antiepileptic effects of levetiracetam in a rodent neonatal seizure model. Pediatr Res. 2013;73(1):24–30. doi: 10.1038/pr.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang XF, Rothman SM. Levetiracetam has a time- and stimulation-dependent effect on synaptic transmission. Seizure. 2009;18(9):615–619. doi: 10.1016/j.seizure.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Kaminski RM, Matagne A, Leclercq K et al. SV2A protein is a broad-spectrum anticonvulsant target: functional correlation between protein binding and seizure protection in models of both partial and generalized epilepsy. Neuropharmacology. 2008;54(4):715–720. doi: 10.1016/j.neuropharm.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 31.Vogl C, Mochida S, Wolff C et al. The synaptic vesicle glycoprotein 2A ligand levetiracetam inhibits presynaptic Ca2+ channels through an intracellular pathway. Mol Pharmacol. 2012;82(2):199–208. doi: 10.1124/mol.111.076687. [DOI] [PubMed] [Google Scholar]
- 32.Madeja M, Margineanu DG, Gorji A et al. Reduction of voltage-operated potassium currents by levetiracetam: a novel antiepileptic mechanism of action? Neuropharmacology. 2003;45(5):661–671. doi: 10.1016/s0028-3908(03)00248-x. [DOI] [PubMed] [Google Scholar]
- 33.Patsalos PN. Clinical pharmacokinetics of levetiracetam. Clin Pharmacokinet. 2004;43(11):707–724. doi: 10.2165/00003088-200443110-00002. [DOI] [PubMed] [Google Scholar]
- 34.Patsalos PN. Levetiracetam: pharmacology and therapeutics in the treatment of epilepsy and other neurological conditions. Rev Contemp Pharmacother. 2004;13(1):1–168. [Google Scholar]
- 35.Pellock JM, Glauser TA, Bebin EM et al. Pharmacokinetic study of levetiracetam in children. Epilepsia. 2001;42(12):1574–1579. doi: 10.1046/j.1528-1157.2001.41300.x. [DOI] [PubMed] [Google Scholar]
- 36.Johannessen Landmark C, Baftiu A, Tysse I et al. Pharmacokinetic variability of four newer antiepileptic drugs, lamotrigine, levetiracetam, oxcarbazepine, and topiramate: a comparison of the impact of age and comedication. Ther Drug Monit. 2012;34(4):440–445. doi: 10.1097/FTD.0b013e31825ee389. [DOI] [PubMed] [Google Scholar]
- 37.Glauser TA, Mitchell WG, Weinstock A et al. Pharmacokinetics of levetiracetam in infants and young children with epilepsy. Epilepsia. 2007;48(6):1117–1122. doi: 10.1111/j.1528-1167.2007.01090.x. [DOI] [PubMed] [Google Scholar]
- 38.Merhar SL, Schibler KR, Sherwin CM et al. Pharmacokinetics of levetiracetam in neonates with seizures. J Pediatr. 2011;159(1):152–154. doi: 10.1016/j.jpeds.2011.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manthey D., Asimiadou S, Stefovska V et al. Sulthiame but not levetiracetam exerts neurotoxic effect in the developing rat brain. Exp Neurol. 2005;193(2):497–503. doi: 10.1016/j.expneurol.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Kilicdag H, Daglioglu K, Erdogan S et al. The effect of levetiracetam on neuronal apoptosis in neonatal rat model of hypoxic ischemic brain injury. Early Hum Dev. 2013;89(5):355–360. doi: 10.1016/j.earlhumdev.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Hmaimess G, Kadhim H, Nassogne MC et al. Levetiracetam in a neonate with malignant migrating partial seizures. Pediatr Neurol. 2006;34(1):55–59. doi: 10.1016/j.pediatrneurol.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Shoemaker MT, Rotenberg JS. Levetiracetam for the treatment of neonatal seizures. J Child Neurol. 2007;22(1):95–98. doi: 10.1177/0883073807299973. [DOI] [PubMed] [Google Scholar]
- 43.Khan O, Chang E, Cipriani C et al. Use of intravenous levetiracetam for management of acute seizures in neonates. Pediatr Neurol. 2011;44(4):265–269. doi: 10.1016/j.pediatrneurol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Abend NS, Gutierrez-Colina AM, Monk HM et al. Levetiracetam for treatment of neonatal seizures. J Child Neurol. 2011;26(4):465–470. doi: 10.1177/0883073810384263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramantani G, Ikonomidou C, Walter B et al. Levetiracetam: safety and efficacy in neonatal seizures. Eur J Paediatr Neurol. 2011;15(1):1–7. doi: 10.1016/j.ejpn.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Maitre NL, Smolinsky C, Slaughter JC et al. Adverse neurodevelopmental outcomes after exposure to phenobarbital and levetiracetam for the treatment of neonatal seizures. J Perinatol. 2013;33(11):841–846. doi: 10.1038/jp.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiemann-Delgado J, Yang H, Loge Cde L et al. A long-term open-label extension study assessing cognition and behavior, tolerability, and safety, and efficacy of adjunctive levetiracetam in children aged 4 to 16 years with partial-onset seizures. J Child Neurol. 2012;27(1):80–89. doi: 10.1177/0883073811417183. [DOI] [PubMed] [Google Scholar]
- 48.Patsalos PN, Berry DJ, Bourgeois BF et al. Antiepileptic drugs—best practice guidelines for therapeutic drug monitoring: a position paper by the subcommission on therapeutic drug monitoring, ILAE Commission on Therapeutic Strategies. Epilepsia. 2008;49(7):1239–1276. doi: 10.1111/j.1528-1167.2008.01561.x. [DOI] [PubMed] [Google Scholar]
- 49.Kliegman RM, Stanton BF, St. Geme JW Nelson Textbook of Pediatrics. 19th ed. Philadelphia: WB Saunders; 2011. Seizures in childhood; pp. 2013–2039. [Google Scholar]
- 50.Giroux PC, Salas-Prato M, Theroret Y et al. Levetiracetam in children with refractory epilepsy: lack of correlation between plasma concentration and efficacy. Seizure. 2009;18(8):559–563. doi: 10.1016/j.seizure.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Grim SA, Ryan M, Miles MV et al. Correlation of levetiracetam concentrations between serum and saliva. Ther Drug Monit. 2003;25(1):61–66. doi: 10.1097/00007691-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. Guidelines on neonatal seizures. 2011. pp. 1–100. http://www.who.int/mental_health/publications/guidelines_neonatal_seizures/en/index.html. Accessed March 10, 2013. [PubMed]
- 53.Queensland Department of Health. Queensland Clinical guidelines. Translating evidence into best clinical practice: maternity clinical guidelines. http://www.health.qld.gov.au/qcg/html/publications.asp#Neonatal. Accessed March 10, 2013.
- 54.Grosso S, Cordelli DM, Franzoni E et al. Efficacy and safety of levetiracetam in infants and young children with refractory epilepsy. Seizure. 2007;16(4):345–350. doi: 10.1016/j.seizure.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Sharpe CM, Capparelli EV, Mower A et al. A seven-day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked changes in pharmacokinetics occur during the first week of life. Pediatr Res. 2012;72(1):43–49. doi: 10.1038/pr.2012.51. [DOI] [PubMed] [Google Scholar]
- 56.Tulloch JK, Carr RR, Ensom MHH. A systematic review of the pharmacokinetics of antiepileptic drugs in neonates with refractory seizures. J Pediatr Pharmacol Ther. 2012;17(1):31–44. doi: 10.5863/1551-6776-17.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]


