Abstract
We describe a recently developed method to measure mechanical properties of the surfaces of plant tissues using atomic force microscopy (AFM) micro/nano-indentations, for a JPK AFM. Specifically, in this protocol we measure the apparent Young’s modulus of cell walls at subcellular resolutions across regions of up to 100 µm x 100 µm in floral meristems, hypocotyls, and roots. This requires careful preparation of the sample, the correct selection of micro-indenters and indentation depths. To account for cell wall properties only, measurements are performed in highly concentrated solutions of mannitol in order to plasmolyze the cells and thus remove the contribution of cell turgor pressure.
In contrast to other extant techniques, by using different indenters and indentation depths, this method allows simultaneous multiscale measurements, i.e. at subcellular resolutions and across hundreds of cells comprising a tissue. This means that it is now possible to spatially-temporally characterize the changes that take place in the mechanical properties of cell walls during development, enabling these changes to be correlated with growth and differentiation. This represents a key step to understand how coordinated microscopic cellular changes bring about macroscopic morphogenetic events.
However, several limitations remain: the method can only be used on fairly small samples (around 100 µm in diameter) and only on external tissues; the method is sensitive to tissue topography; it measures only certain aspects of the tissue’s complex mechanical properties. The technique is being developed rapidly and it is likely that most of these limitations will be resolved in the near future.
Keywords: Plant Biology, Issue 89, Tissue growth, Cell wall, Plant mechanics, Elasticity, Young’s modulus, Root, Apical meristem, Hypocotyl, Organ formation, Biomechanics, Morphogenesis
Introduction
Growth in plants is achieved by the coordinated expansion of the rigid cell walls that surround each and every cell of the organism. Accumulating evidence indicates that it is through the modification of cell wall chemistry that plants locally control this expansion. The expansion is thought to be driven primarily by strain on the cell walls, caused by the cell’s high turgor pressure; this strain response to turgor pressure is governed by the mechanical properties of the cell walls1. Little is known of these mechanical properties and how they change during development. Furthermore little is known of how these mechanical properties are controlled and whether feedbacks contribute to alter cell wall chemistry in a manner that is apparently coordinated across a tissue. If we are to understand the connection between chemical and mechanical changes in plant cell walls during development, and ultimately how these microscopic interactions govern a plant’s macroscopic growth, a method that can monitor mechanical properties of cell walls in developing organs at the cellular or tissue scale is required.
The atomic force microscopy (AFM) method described here, which is based on micrometer or nanometer tissue compressions or indentations, was developed precisely to measure the mechanical properties of cell walls in developing organs simultaneously at subcellular resolutions and across entire regions of tissue. Other methods have either a resolution that is too low or too high: the extensometer is only able to measure the average mechanical properties of a whole tissue at the millimeter scale2-4, a scale that is for instance too large to measure early events in organogenesis; the microindenter can take measurements at subcellular resolution at the nanometer scale, but it is restricted to measuring isolated cells and not groups of cells or organs5-7. With the AFM, the required tissue, cellular, and subcellular resolutions can be achieved8-10. Recently several protocols have been developed specifically to measure mechanics of plants tissue that could also be used11,12.
We will present here how to evaluate the elasticity of the tissue through measurement of the apparent Young’s modulus13.
The Young modulus is commonly used to describe the stiffness of a material. During small deformation the force required to deform a material is proportional to the area of indentation. The Young modulus is this coefficient. In the case of a continuous homogenous material the same coefficient will be measured regardless of the indentation type (size and shape) but will change with the speed of the measurement. In the case of the complex structure of plants tissue, we have observed so far that the force is proportional to the deformation allowing the determination of a coefficient of proportionality that we name “apparent young modulus”. In contrast from continuous medias in the plants, this apparent young modulus is sensitive to the size of the indentation. It does not correspond to the young moduli of a pure cell wall. It best describes the elasticity of the scaffolding of the cell-wall of the tissue.
Protocol
1. Prepare Glass Slides for Mounting Sample
Prepare imbedding agarose media: 0.7% low melting agarose in 10% mannitol (in water).
Using a strong metal instrument (e.g. drill tip, lime), etch out a 0.5 x 0.5 cm area in the center of a microscopy glass slide. Or instead, glue a small piece of glass lamella (about 20 x 200 µm) to the glass slide using Araldite glue. NOTE: This roughens the surface in order to facilitate the adhesion of the agarose to ensure that the agarose media sticks or fixes to the slide. This slide can be reused.
Position a droplet of about 20 µl of imbedding agarose media onto the etched region of the glass slide. This gives a thin film of agarose.
Store the glass slide in a humid box and wait for the agarose media to solidify.
2. Dissecting and Mounting Meristem Samples
Microdissect meristems for confocal imaging: remove the floral buds one by one from the stem by pulling them down with ultra fine tweezers. It is important to remove all the floral buds up to the buds that do not exhibit sepals (i.e. the P1 primordia14).
Using a razor blade or the tip of the tweezers, cut the meristem away from the stem. The cut should be parallel to the region of the meristem surface that is to be measured with the AFM. The obtained apex should be between 300 µm and 600 µm high to fit under the AFM tip.
Push the apex into the film of agarose media prepared at step 1.1.4. Choose a position on the glass slide/agarose and push gently such that the meristem is both directly in contact with the glass slide and protrudes out from the agarose. It is crucial to position the meristem in the agarose within the few seconds that follow its cut from the stem. NOTE: This is in order to prevent the meristem from drying. At this stage, the meristem will be standing in a crack because the agarose fractured upon the application of pressure.
Prepare several meristems this way for batch imaging, providing that they are of the same height and are positioned less than 500 µm from one another on the slide. Keep the prepared meristems in the humid box while dissecting further samples. NOTE: It limits the variation coming from the calibration. Ideally 2 samples of each condition could be prepared and measured randomly. So far there is no observed effect of batch imaging on the measurement (stress induced response). This could be thanks to the dilution of stress signalling molecules in the agar and/or the mounting solution. Alternatively the stress response is the same regardless to the amount of sample prepared.
Before moving on to the next step, make sure that the boundary between the meristem and the floral buds is dry. If not, gently remove the excess water using filter paper. This should prevent in the next stage that the melted agarose floods the sample due to capillarity forces.
With a 20 µl pipette, add enough melted mounting agarose to fill in the crack created at step 1.2.3. and to surround each meristem. The agarose should reach the level of the scar of the removed flower bud (primordium). To achieve this, it may be necessary to add agarose at each end of the crack.
When the agarose is added, it will quickly flood into the crack; using the pipette, suck off part of the melted mounting agarose to ensure that the meristem is the highest point on the slide. This fixes the meristem so that it cannot move during imaging.
3. Mounting Root or Hypocotyl Samples
For roots and hypocotyls, no dissection is necessary. In the thin film of agarose on the prepared glass slide, make a groove either directly above an etch in the glass or along a glass lamella. Position the root or hypocotyl in this groove ensuring that it is in contact with the glass along its whole length.
Before moving on to the next step, make sure that the sample is dry at its surface. If not, gently remove the excess of water using filter paper.
Add melted mounting agarose around the sample as in step 1.2.6.
4. AFM Preparation and Sensitivity Calibration (for a JPK Nanowizard AFM)
- Mount a cantilever on the AFM. The cantilever should be mounted with round shaped indenter. The radius will determine the x, y, z resolution and the indentation depth at which accurate measurement could be archived.
- To take meso—nanoscale measurements at the epidermis’s surface, use a 50 nm radius round indenter; to take mesoscale measurements, use a 0.5 µm radius round indenter; to take microscale measurements (across 2-3 cells), use a 2.5 µm radius round indenter.
Position the laser at the tip of the cantilever. Align the laser to the middle of the captor using the mirror.
Position a clean glass under the AFM. NOTE: the following is set to transform the signal of the laser position on the AFM receptor into a deformation of the cantilever and, by evaluating the spring constant of the cantilever, to translate it into a force.
Approach with a target force “set point” of 1 V.
Select “force spectroscopy”. Do an indentation by setting the maximal “relative set point” to 2 V, the “extend speed” to 40 µm/sec, and the “z length” to 5 µm.
From the “calibration manager” menu, select the linear part of the forward curve to fit the sensitivity using the “select fit range” button. The “relative set point” should be transformed from “volt” to “meter”.
From the “calibration manager” menu, select “spring constant” to evaluate the elasticity of the cantilever using thermal fluctuations. Press “run” to obtain the spectral density plot of the cantilever. Find the resonance peak with the lowest frequency. Fit it using the “select fit range” button. NOTE 1: For more details on the thermal fluctuations tuning, read Cook et al.15 NOTE 2: It is preferable to use a direct method to evaluate the elasticity of the cantilever using a reference cantilever (or material with known stiffness). The one used here was kindly donated by Atef Asnacios16.
Add 200 µl of 10% mannitol solution under the cantilever tip. Realign the laser to the middle of the captor using the mirror.
Recalibrate the sensitivity: from the “calibration manager” menu, click on the “unknown” button; repeat steps 2.3 through to 2.5.
5. Data Acquisition: Apparent Young’s Modulus Cartography
Position the mounted samples under the AFM and add a 500 µl droplet of 10% mannitol solution onto the sample. The mannitol solution plasmolyzes the cells within minutes.
Approach with a target force (“set point”) of 20 nN (“set point” should not be more than 3 V)
Indent with a “relative set point” of 200 nN, an “extend speed” of 40 µm/sec, and “z length” of 5 µm.
Adjust the “relative set point” in order to obtain an indentation of 100 nm for the 200 nm mounted cantilever or 0.5 µm for the 1 µm/5 µm mounted cantilever.
In “force mapping” mode, select a region to scan: for meristems, 70 µm x 70 µm with a resolution (“pixels”) of 64 x 64; for hypocotyls and roots, 100 µm x 100 µm with a resolution of 64 x 64.
Press scan to launch the experiments. Save the output.
6. Data Analysis: Apparent Young’s Modulus Calculations
Open the data file in the data analysis software.
Select “batch processing” to apply the same analysis to all the curves of a single map.
Select “correct the height for the bending of the cantilever” so to extract the indentation depth.
Select “Young’s modulus fit function” that will assume the force is proportional to the area of indentation. Set the “tip shape” to parabolic to assume that the indentation shape is a parabolic.
Indicate the radius of the tip.
Select preferentially the “retract” curve for the analysis because it is less sensitive to topography than the “extend” curve. However, if there is significant sticking of the probe during the “retract,” use the “extend” curve, which is insensitive to sticking.
Set the Poisson ratio to 0.5. Press on analyze and save the output.
Representative Results
In Figure 1 we present typical Young moduli maps of floral meristems (Figures 1A and 1B), young and old hypocotyls (Figures 1C-F), and root meristem (Figure 1G and 1H). In all experiments the indenter is hemispherical, but its radius differs so that different spatial resolutions can be achieved. Figures 1C and 1D show typical results for meso-nanoscale indenters (50 nm radius) with meso-nanoscale indentations (50 nm depth); the cartographs reveal microscale heterogeneities in surface cell walls of hypocotyl epidermal cells. Figures 1A, 1E, and 1G show typical results for mesoscale indenters (500 nm radius) with mesoscale indentations (500 nm depth) for floral meristem, hypocotyl, and root; note that because the indenter radius exceeds the diameter of the cell wall this meso-indentation evaluates ‘apparent’ Young moduli of anticlinal cell walls (see Peaucelle et al.9,17 and Braybrook et al.10 for details). Lastly, Figure 1B shows a cartograph for microscale indenters (2.5 µm radius) with mesoscale indentations (500 nm depth); interestingly, imaging at this resolution revealed tissue softening during organ initiation that preceded any visible growth9.
We now highlight and propose partial solutions to four technical difficulties that commonly arise when using the AFM to measure properties of developing plant organs. First, Samples may be improperly imbedded in the agarose: regions that are covered with agarose cannot be properly measured (see edges of Figure 1E arrow). To resolve this, try repeating step 1.2.6. Second, samples that are improperly fixed to the glass slide may move during the experiment; then aberrant stripes appear in the images owing to decorrelation between the left and right advancing scans (Figure 1F). To resolve this, try carefully repeating the Sample Preparation. Third, The z-range of the sample’s height must be below a certain threshold. There is no restriction on the sample’s average height in the vertical z-direction, but the range in height in the z-direction (distance between the highest and lowest points) must be less than 15 µm, otherwise the scan will be aborted (see Figure 1B; settings are in the AFM nanowizard). This is because, during the scan, the AFM automatically adjusts the cantilever’s height to match the height of the sample, but in a single scan the cantilever’s height can vary by only 15 µm. To resolve this, use the JPK cellHesion module, which increases the z-range to 100 µm, as demonstrated in Figure 1E-G. Fourth, the method is sensitive to surface topographies: the more the sample surface is oriented parallel to the direction of indentation, the less reliable the measurements. In the root sample presented in Figure 1G-H, the cell walls parallel to the long axis of the root are not visible at the top and bottom edges of the scan: here the root surface is far from being perpendicular to the indentation. In the meristem, similarly, cell walls at the edges of flowering buds were not properly measured (Figure 1A). For meso-nanoscale indentations (Figure 1C), again this topographical effect is observed: cells’ edges are incorrectly measured as being soft precisely where the cells curve away from the plane of the cantilever. A data analysis tool that is able to account for these topographical effects could resolve this artifact in the future, but such a tool is not available yet.
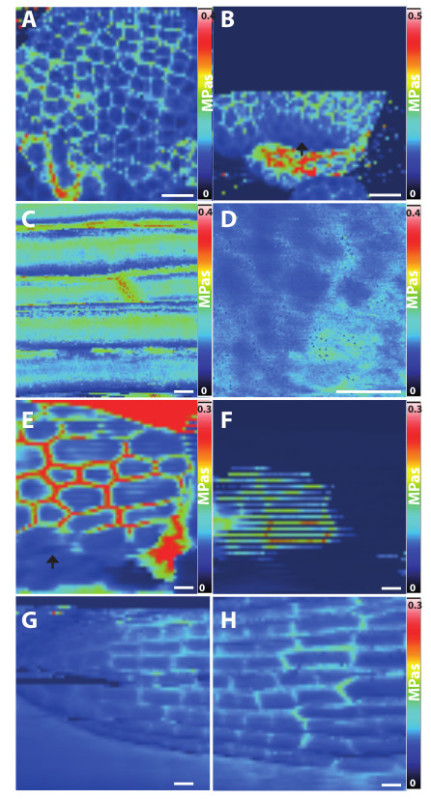 Figure 1. Typical AFM Young moduli measurements on plasmolyzed developing organs of Arabidopsis thaliana. (A, B) Floral meristem, (C-F) dark grown hypocotyl, (G, H) root meristem. (A, E, F, G, H) Imaging using a cantilever with a 0.5 µm radius spherical tip which measures the Young moduli of anticlinal cell walls at the mesoscale. (B) Floral meristem measured using a cantilever with a 2.5 µm radius spherical tip, reveals a reduction of tissular ‘apparent’ Young moduli at the positions of future organs, see arrow. (C, D) Subcellular resolution of surface cell wall Young moduli in dark-grown hypocotyls measured using a cantilever with a 50 nm radius spherical tip. (B) A scan abortion due to the range in sample height exceeding the maximum z-range of the AFM. (F) A blurry scan, resulting from sample movement during the experiment due to improper fixation. (E) A scan when a thin film of agarose is on top of the sample (arrow). Scale bar (A-C, E-H) 10 µm, (D) 1 µm. Please click here to view a larger version of this figure.
Figure 1. Typical AFM Young moduli measurements on plasmolyzed developing organs of Arabidopsis thaliana. (A, B) Floral meristem, (C-F) dark grown hypocotyl, (G, H) root meristem. (A, E, F, G, H) Imaging using a cantilever with a 0.5 µm radius spherical tip which measures the Young moduli of anticlinal cell walls at the mesoscale. (B) Floral meristem measured using a cantilever with a 2.5 µm radius spherical tip, reveals a reduction of tissular ‘apparent’ Young moduli at the positions of future organs, see arrow. (C, D) Subcellular resolution of surface cell wall Young moduli in dark-grown hypocotyls measured using a cantilever with a 50 nm radius spherical tip. (B) A scan abortion due to the range in sample height exceeding the maximum z-range of the AFM. (F) A blurry scan, resulting from sample movement during the experiment due to improper fixation. (E) A scan when a thin film of agarose is on top of the sample (arrow). Scale bar (A-C, E-H) 10 µm, (D) 1 µm. Please click here to view a larger version of this figure.
Discussion
In plants, changing mechanical properties play a major role in directing growth and morphogenesis. To date there has been great progress in unraveling the genetic and chemical networks that control plant growth, but our knowledge of how these networks contribute to and are affected by changes in mechanical properties is rudimentary. This method should enable us to fill this gap, and so it should be of strong interest to scientists studying any aspect of plant growth or morphogenesis. We now summarize the challenges and limitations of the method, and the future outlook.
The most challenging step in the protocol is sample preparation. The sample must be firmly fixed to the glass slide with agarose but this agarose must not cover any areas of the sample that are to be measured. Furthermore, the preparation has to be completed quickly enough (within minutes) so as to prevent drying and delicately so as to prevent tissue damage: wounding and drought are predicted to lead to irreversible changes in the mechanical properties of the cell wall18. Another challenging step is to select the correct AFM micro-indentation settings: depending on the scientific question, tissue, cellular, or subcellular resolution can be achieved with different indenters and indentation depths. For further information, see9,10.
The method has several limitations. First, the method is limited to measuring mechanical properties at the surfaces of organs; an attempt to develop a protocol for measuring interior regions of resected organs is underway in our laboratory. Second, differentiated tissues with a highly variable topography are very difficult to measure; for example, trichomes block the AFM tip’s access to the epidermis of leaves and stems, and similarly, root hairs block the AFM tip’s access to root epidermis. Third, the method is best applied to samples of length 100 µm or less; it may be used on larger samples up to 1 mm, but this requires time-consuming sequential cartographic measurements (as shown in Figure 1D and 1E). Finally, the AFM measures only part of the mechanical complexity of the cell wall: it is limited to compressive indentation experiments, while cell walls are complex gels that may show different mechanical properties depending on the type of deformation (e.g. tension vs compression). At first, this method limited to compression seems to be non- or remotely capable to inform on growth, which is a turgor derived cell wall stretching process. Yet to our own supersize measurement on the meristem9,19 or on the hypocotyl (unpublished data) indicates an amazing correlation between the elasticity measured through the apparent young modulus and growth. We hope that in the future, development of this technique will help understanding this enigma.
The method presented here only measured certain aspects of the elastic mechanical properties of plants tissues, as explained above. But living tissues also behave viscously, and the combined visco- and elastic behaviour is predicted to be important for growth and development16,20. Hitherto unmentioned, we observed responses of cell walls to AFM micro-indentations that were time-dependent over tens of seconds - this response was characterized as viscoelastic. In our previous work we presented one AFM method to evaluate this viscoelastic response9. Another important growth-mediating property of a tissue is the local turgor pressure of cells. For measuring turgor pressure, we face similar challenges: a technique that measures at mesoscale resolution across tissues does not yet exist. The pressure microprobes used in plants were, up until recently, limited by the glass tip radius, which is the size of meristematic cells (5 µm)12,21. Another very promising avenue of research is to develop this AFM method for use on non-plasmolyzed tissue; we hope, in this way, to reveal the relation between single cell turgor pressure and tissue growth. This method will basically apply to the already developed AFM turgor measurement through indentation using other devices and used on individual cells 22-24.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We give special thanks to Yves Couder for many helpful discussions. We thank Atef Asnacios for the calibration of the cantilevers and discussion. We thank Lisa Willis, Elliot Meyerowitz, and Oliver Hamant for critical reading. This work was funded in part by Human Frontier Science Program grant RGP0062/2005-C; the Agence Nationale de la Recherche projects ‘‘Growpec,’’ and ‘‘Mechastem’’.
References
- Cosgrove DJ. Measuring in vitro extensibility of growing plant cell walls. Methods in molecular biology. 2011;715:291–303. doi: 10.1007/978-1-61779-008-9_20. [DOI] [PubMed] [Google Scholar]
- Durachko DM, Cosgrove DJ. Measuring plant cell wall extension (creep) induced by acidic pH and by alpha-expansin. Journal of visualized experiments : JoVE. 2009. p. 1263. [DOI] [PMC free article] [PubMed]
- Durachko DanielM, C DJ. Measuring Plant Cell Wall Extension (Creep) Induced by Acidic pH and by Alpha-Expansin. J. Vis. Exp. 2009. p. 25. [DOI] [PMC free article] [PubMed]
- Suslov D, Verbelen JP, Vissenberg K. Onion epidermis as a new model to study the control of growth anisotropy in higher plants. Journal of experimental botany. 2009;60:4175–4187. doi: 10.1093/jxb/erp251. [DOI] [PubMed] [Google Scholar]
- Parre E, Geitmann A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta. 2005;220:582–592. doi: 10.1007/s00425-004-1368-5. [DOI] [PubMed] [Google Scholar]
- Zerzour R, Kroeger J, Geitmann A. Polar growth in pollen tubes is associated with spatially confined dynamic changes in cell mechanical properties. Developmental biology. 2009;334:437–446. doi: 10.1016/j.ydbio.2009.07.044. [DOI] [PubMed] [Google Scholar]
- Radotic K, et al. Atomic force microscopy stiffness tomography on living Arabidopsis thaliana cells reveals the mechanical properties of surface and deep cell-wall layers during growth. Biophysical journal. 2012;103:386–394. doi: 10.1016/j.bpj.2012.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani P, et al. In vivo analysis of local wall stiffness at the shoot apical meristem in Arabidopsis using atomic force microscopy. The Plant journal : for cell and molecular biology. 2011;67:1116–1123. doi: 10.1111/j.1365-313X.2011.04649.x. [DOI] [PubMed] [Google Scholar]
- Peaucelle A, et al. Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Current biology : CB. 2011;21:1720–1726. doi: 10.1016/j.cub.2011.08.057. [DOI] [PubMed] [Google Scholar]
- Braybrook SA, Hofte H, Peaucelle A. Probing the mechanical contributions of the pectin matrix: Insights for cell growth. Plant signaling & behavior. 2012;7:1037–1041. doi: 10.4161/psb.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routier-Kierzkowska AL, et al. Cellular force microscopy for in vivo measurements of plant tissue mechanics. Plant physiology. 2012;158:1514–1522. doi: 10.1104/pp.111.191460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agudelo CG, et al. TipChip: a modular, MEMS-based platform for experimentation and phenotyping of tip-growing cells. The Plant journal : for cell and molecular biology. 2013;73:1057–1068. doi: 10.1111/tpj.12093. [DOI] [PubMed] [Google Scholar]
- Miedes E, et al. Xyloglucan endotransglucosylase/hydrolase (XTH) overexpression affects growth and cell wall mechanics in etiolated Arabidopsis hypocotyls. Journal of experimental botany. 2013;64:2481–2497. doi: 10.1093/jxb/ert107. [DOI] [PubMed] [Google Scholar]
- Byrne ME, et al. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature. 2000;408:967–971. doi: 10.1038/35050091. [DOI] [PubMed] [Google Scholar]
- Cook SM, et al. Practical implementation of dynamic methods for measuring atomic force microscope cantilever spring constants. Nanotechnology. 2006;17:20135–22145. [Google Scholar]
- Desprat N, Richert A, Simeon J, Asnacios A. Creep function of a single living cell. Biophysical journal. 2005;88:2224–2233. doi: 10.1529/biophysj.104.050278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A, Braybrook S, Hofte H. Cell wall mechanics and growth control in plants: the role of pectins revisited. Frontiers in plant science. 2012;3:121. doi: 10.3389/fpls.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, et al. ROS signaling: the new wave. Trends in plant science. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Braybrook SA, Peaucelle A. Mechano-chemical aspects of organ formation in Arabidopsis thaliana: the relationship between auxin and pectin. PLoS. 2013;8:e57813. doi: 10.1371/journal.pone.0057813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnacios A, Hamant O. The mechanics behind cell polarity. Trends in cell biology. 2012;22:584–591. doi: 10.1016/j.tcb.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Meister A, et al. FluidFM: combining atomic force microscopy and nanofluidics in a universal liquid delivery system for single cell applications and beyond. Nano letters. 2009;9:2501–2507. doi: 10.1021/nl901384x. [DOI] [PubMed] [Google Scholar]
- Lintilhac PM, Wei C, Tanguay JJ, Outwater JO. Ball tonometry: a rapid, nondestructive method for measuring cell turgor pressure in thin-walled plant cells. Journal of plant growth regulation. 2000;19:90–97. doi: 10.1007/s003440000009. [DOI] [PubMed] [Google Scholar]
- Kroeger JH, Zerzour R, Geitmann A. Regulator or driving force? The role of turgor pressure in oscillatory plant cell growth. PloS one. 2011;6:e18549. doi: 10.1371/journal.pone.0018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouzesh E, Goel A, Mackenzie SA, Turner JA. In vivo extraction of Arabidopsis cell turgor pressure using nanoindentation in conjunction with finite element modeling. The Plant journal : for cell and molecular biology. 2013;73:509–520. doi: 10.1111/tpj.12042. [DOI] [PubMed] [Google Scholar]


