Supplemental Digital Content is available in the text.
Keywords: respiratory infections, influenza, RSV, infants, epidemiology, cohort study
Abstract
Background:
Understanding viral etiology and age-specific incidence of acute respiratory infections in infants can help identify risk groups and inform vaccine delivery, but community-based data is lacking from tropical settings.
Methods:
One thousand four hundred and seventy-eight infants in urban Ho Chi Minh City and 981 infants in a semi-rural district in southern Vietnam were enrolled at birth and followed to 1 year of age. Acute respiratory infection (ARI) episodes were identified through clinic-based illness surveillance, hospital admissions and self-reports. Nasopharyngeal swabs were collected from infants with respiratory symptoms and tested for 14 respiratory pathogens using multiplex reverse transcription-polymerase chain reaction.
Results:
Estimated incidence of ARI was 542 and 2691 per 1000 infant-years, and hospitalization rates for ARI were 81 and 138 per 1000 infant-years, in urban and semi-rural cohorts, respectively, from clinic- and hospital-based surveillance. However self-reported ARI episodes were just 1.5-fold higher in the semi-rural versus urban cohort, indicating that part of the urban–rural difference was explained by under-ascertainment in the urban cohort. Incidence was higher in infants ≥6 months of age than <6 months, but this was pathogen-specific. One or more viruses were detected in 53% (urban) and 64% (semi-rural) of samples from outpatients with ARI and in 78% and 66% of samples from hospitalized ARI patients, respectively. The most frequently detected viruses were rhinovirus, respiratory syncytial virus, influenza virus A and bocavirus. ARI-associated hospitalizations were associated with longer stays and more frequent ICU admission than other infections.
Conclusions:
ARI is a significant cause of morbidity in Vietnamese infants and influenza virus A is an under-appreciated cause of vaccine-preventable disease and hospitalizations in this tropical setting. Public health strategies to reduce infant ARI incidence and hospitalization rates are needed.
Acute respiratory infections (ARI) remain a leading cause of morbidity and mortality in young children globally, and in particular in low-income countries. An estimated 1.9 million children die from ARI worldwide annually, 70% of them in Africa and Southeast Asia.1
Studies to define the incidence, age-distribution and etiology of respiratory infections in children can help to identify risk groups and to understand the potential impact of current and future vaccines. Although there exists a large hospital-based literature,2–5 including studies from Vietnam,6,7 there are limited data on the epidemiology and etiology of ARI from community-based studies, particularly in low income settings.8–11 Population-based studies of respiratory syncytial virus infection9,12 and influenza13 have demonstrated a substantial burden of disease, including severe disease, in an outpatient setting that would be missed by hospital-based studies. Longitudinal community-based cohort studies have the benefit of capturing this full clinical spectrum, as well as permitting estimates of incidence, detection of repeated infections and identification of risk factors for infection and disease. However, many previous studies have been limited because they were hospital-based, cross-sectional, small in size, did not include year-round case detection, or focused only on the detection of a specific pathogen.
Our study aimed to describe the epidemiology and viral etiology of ARI in the first year of life within an ongoing prospective infant cohort in southern Vietnam.
METHODS
Study Location
The catchment area for this southern Vietnam birth cohort study includes a highly urban district in central Ho Chi Minh City (HCMC; District 8, population 408,772 in the 2009 census; population density approx. 20,000/km2), and a mixed urban/rural district in Dong Thap province, 150 km south-west of HCMC (Cao Lanh district, population 161,292; population density 1500/km2). Four hospitals in HCMC (Hospital for Tropical Diseases, Hung Vuong obstetric hospital, District 8 hospital, Children’s Hospital 1) and Dong Thap provincial hospital participated in the study. The protocol was approved by the institutional review boards of these hospitals and the Oxford Tropical Research Ethics Committee.
Study Design
The design and methods of the southern Vietnam birth cohort study have been described previously.14 In brief, women delivering at Hung Vuong obstetric hospital in HCMC or the provincial hospital in Dong Thap province, and resident in the relevant catchment districts, were invited to enroll during an antenatal visit in the 9th month of pregnancy or on admission for delivery. Written informed consent was obtained from all participants. Infants were enrolled within 72 hours of birth, before discharge from hospital. Infants attended scheduled follow-up visits at 2, 4, 6, 9 and 12 months of age. In HCMC, infants had 2 additional follow-up visits at 1 and 3 months of age. At each visit, data was collected on any illness episodes since the last routine visit. Infants were enrolled from July 1, 2009 to Dec 31, 2013 in HCMC and August 1, 2009 to Dec 31, 2012 in Dong Thap; this analysis is based on the subset of the infant cohort who had reached 12 months of age on or before August 31, 2011.
Diagnosis of ARIs
Families were asked to attend a nominated study clinic whenever an infant was unwell. Brief clinical data and a presumptive diagnosis were recorded and a nasopharyngeal swab (NPS) was collected from infants presenting with respiratory symptoms. We subsequently coded illness episodes as ARI based on either an ARI diagnosis recorded by the treating physician, or a recorded diagnosis of “viral infection” or “infection of unknown origin” in the presence of one or more respiratory symptoms (cough, coryza, difficulty breathing), and with a NPS sample collected. A new ARI episode was defined by ≥7 days between symptom onset dates. If infants were admitted to hospital with a suspected infectious disease, detailed clinical data were collected daily during hospitalization, and ARI episodes were classified as above. NPS were collected on admission only if not already collected at an outpatient visit.
To quantify and account for the under-ascertainment of ARI episodes inherent in the clinic-based surveillance, we utilized 2 additional data sources. The first was self-reported illness episodes documented at each routine follow-up visit for the intervening period since the last visit, with ARI classified on the basis of a self-reported diagnosis. We did not attempt to merge these data with the ARI episodes detected through clinic-based surveillance, rather analyzed them as a separate alternative endpoint. The second was data on admissions to 3 hospitals in HCMC (Hospital for Tropical Diseases, District 8 Hospital and Children’s Hospital 1) and the provincial hospital in Dong Thap, on all infants under 1 year of age resident in our catchment districts, within the study period. These are the hospitals to which cohort infants are most likely to be admitted. Using name, date of birth and address, we matched these hospital datasets to our enrolment data to identify admissions of cohort infants that had been missed through our clinic-based surveillance, and classified ARI episodes on the basis of the ICD10 code (codes J00–J22 were included). These additional ARI admissions were merged with our existing dataset, and all analyses of hospitalized ARI relate to this merged dataset, unless specified otherwise.
Laboratory Diagnostic Tests
All samples were collected in viral transport medium and stored at 4°C immediately after collection, then transported within 24 hours for storage at -20°C pending further testing. Nucleic acids were extracted on a MagnaPURE 96 platform (Roche, Mannheim, Germany). Reverse transcription was done using Superscript III (Invitrogen, Carlsbad, CA) and random hexamers (Roche). Multiplex polymerase chain reaction (PCR) was used to identify 14 viral respiratory pathogens: influenza A (Flu A), influenza B (Flu B), adenovirus (AdV), enterovirus (ENT), respiratory syncytial virus A and B (RSV), human metapneumovirus (MPV), rhinovirus (Rhi), parainfluenzavirus 1, 2, 3 and 4 (PIV1–4), coronavirus (Cor, including HKU1, NL63, 229E and OC43), human bocavirus (Boca) and parechovirus (PEV). The PCR was performed as a 4-tube assay on a Roche Lightcycler 480 II using primers and probes as described previously,15 except for the rhinovirus primers and probes which were: AgSCTgCgTggCKgCC (forward); ACACggACACCCAAAgTAgT (reverse); CYAN500-TCCTCCggCCCCTgAATgYggCTAAYC-DB (probe). Equine arteritis virus was used as an internal control. Bacterial diagnostics were not performed.
All NPS samples collected from infants hospitalized with ARI were tested. Due to the large number of samples collected from infants with ARI who were managed as outpatients, we tested only a random sample of 566/2592 of the total (22%), stratified by study sites and study years, selected by random number generation. NPS samples were not available from ARI episodes identified retrospectively from self-reports or from hospital admission records.
Data Analysis
Infant-years of observation (IYO) were calculated as the sum of each infant’s duration of follow-up, from birth until the first one of the following: (1) the first birthday; (2) the date of early exit from the cohort for any reason or (3) the date of the last routine visit attended, or last presentation to a study clinic, whichever occurred later, if the infant did not attend the final 12-month visit. The incidence of ARI was calculated separately based on clinical presentations to a study clinic or self-reported episodes, with IYO as the denominator. The incidence of hospitalized ARI was calculated using the merged dataset of admissions detected through surveillance and/or retrospectively identified from hospital records.
Pathogen-specific incidence estimates were calculated by multiplying the proportion of tested samples that were positive for each pathogen by the overall incidence of ARI, using the survey procedure in STATA to account for the fact that only a random sample of specimens collected from outpatient ARI visits were tested.
We examined the distribution of infant observation time, and the age-structure, by calendar month to investigate whether our incidence estimates and age- and season-related observations might be biased by an interaction between the cohort denominator, infant age distribution and virus seasonality. Although the observation-time was evenly distributed across calendar months (see Fig. A, Supplemental Digital Content 1, http://links.lww.com/INF/C70), the age-structure of the infants under observation did vary considerably across calendar months (see Fig. B, Supplemental Digital Content 1, http://links.lww.com/INF/C70) likely due to monthly fluctuations in enrolment rate and the restriction of this dataset to only those infants enrolled during the first 14 months of the study. We therefore calculated ARI incidence stratified by age group (≤6 or >6 months) and calendar quarter, overall and for the 6 most common pathogens. A weighted ARI incidence estimate for all-cause ARI was calculated by combining the stratum-specific estimates, weighted by stratum-specific observation times. Weighted pathogen-specific incidence estimates could not be calculated because the total number of infections for most viruses was too small to stratify by study site as well as age-group and season, and it was not informative to combine 2 sites with such different ARI incidences.
Multivariable negative binominal regression models were used to examine factors associated with ARI, separately for the 2 study sites. Potential factors were selected based on clinical experience and results from prior studies16–19 including sociodemographic characteristics, medical history and infants’ characteristics at birth.
Selected clinical characteristics of infants hospitalized with ARI and with other diagnoses were compared using χ2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. One-way analysis of variance was used to test for differences in the mean age of infection between viral pathogens. All analyses were performed using STATA 11.0.
RESULTS
Study Population
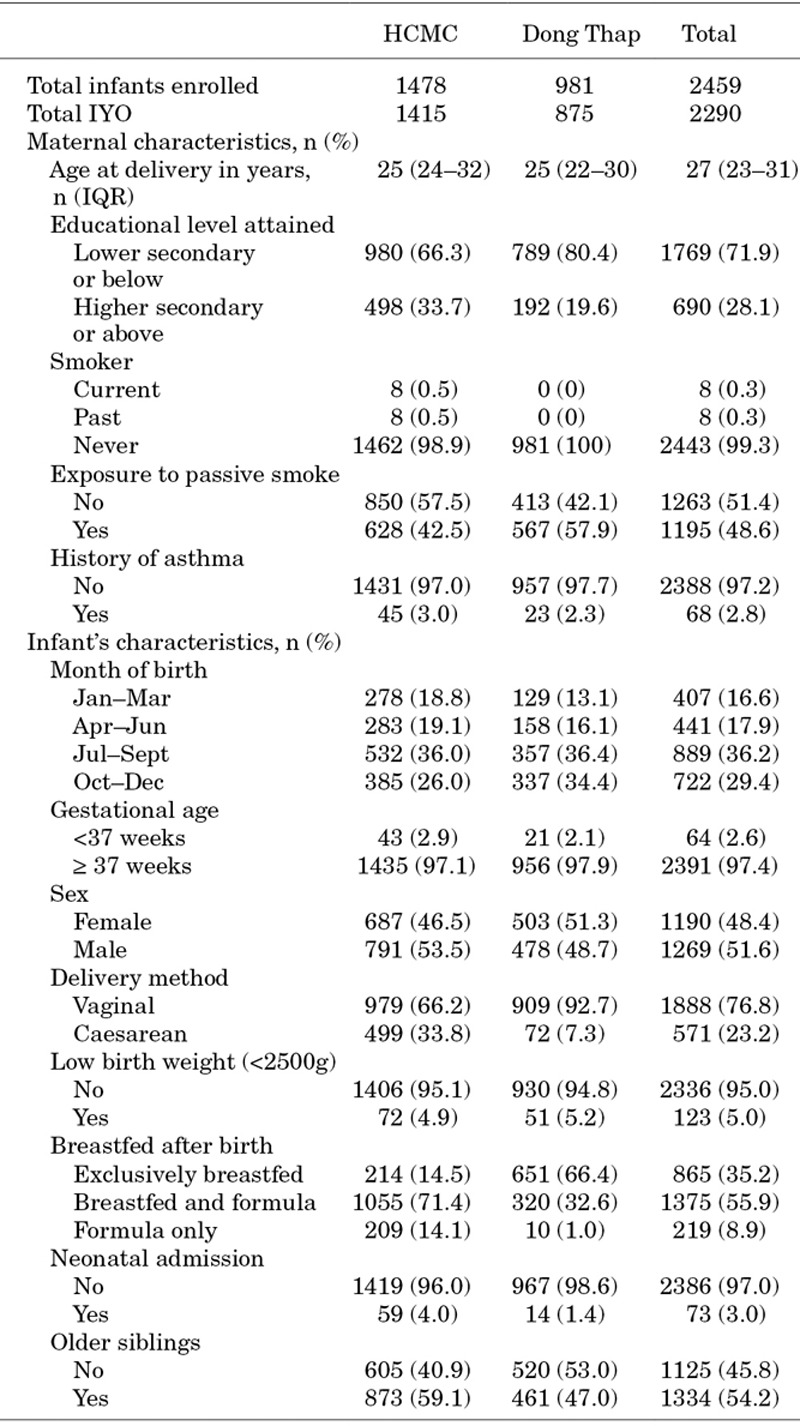
Between July 1, 2009 and August 31, 2010, 2459 infants born to 2445 women (including 14 sets of twins) were enrolled in the cohort and included in this analysis. One thousand four hundred and seventy-eight of infants (60%) were resident in the urban catchment in HCMC, and 981 (40%) in the urban/rural catchment in Dong Thap. Eighty-four percent of infants (2072/2459) completed the full 12-month follow-up period. Of those who exited the cohort early, 63 (16%) moved, 197 (51%) were lost to follow-up or failed to attend the final study visit, 113 (29%) withdrew from the study, and 14 (3.6%) died, giving a total of 2290 IYO (1415 IYO in HCMC and 875 in Dong Thap). Baseline cohort characteristics are presented in Table 1.
TABLE 1.
Characteristics of Study Participants
Incidence of ARI
There were 3121 ARI episodes detected through clinical surveillance and hospital admission records, corresponding to an overall incidence of 1363 ARI presentations per 1000 IYO during the first year of life. This differed markedly between the 2 study sites: in HCMC, 767 ARI episodes were diagnosed (incidence 542/1000 IYO), compared with 2354 ARI episodes (2691/1000 IYO) in DT. When the incidence estimates were weighted for differences in cohort age-structure throughout the year, there was little change (538/1000 IYO in HCMC; 2755/1000 IYO in Dong Thap). The incidence of ARI based on episodes self-reported at follow-up visits was more similar between the study sites: 1036 episodes in HCMC (732/1000 IYO) and 1024 in Dong Thap (1171/1000 IYO).
There were 236 hospital admissions for ARI; 115 in HCMC (in 102 infants) and 121 in DT (in 90 infants), corresponding to admission rates of 81 and 138 per 1000 IYO, respectively [crude incidence rate ratio (IRR) in DT versus HCMC (95% confidence interval {CI}): 1.7 (1.3–2.2)].
Risk Factors for ARI in Infancy
The results of the multivariable regression models of ARI risk are shown in Table 2. In the urban cohort, ARI incidence was significantly lower in infants whose mothers were educated to higher secondary level or above (adjusted IRR: 0.72; 95% CI: 0.52–0.99), and significantly higher in males (adjusted IRR: 1.5; 95% CI: 1.1–2.0) and infants with older siblings (adjusted IRR: 1.6; 95% CI: 1.2–2.2; Table 3).
TABLE 2.
Risk Factors for ARI in Infancy in HCMC
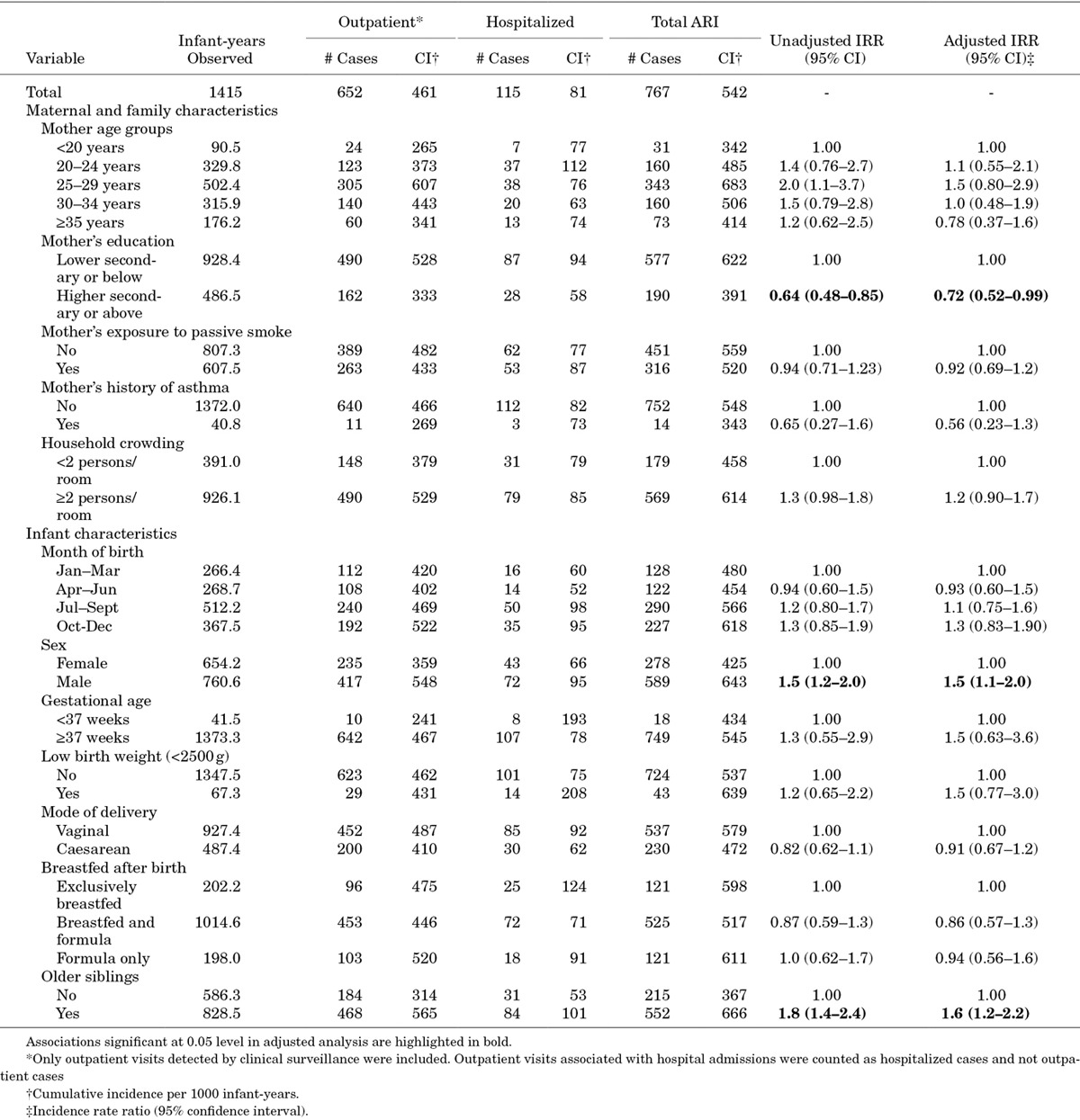
TABLE 3.
Risk Factors for ARI in Infancy in Dong Thap

In the semi-rural cohort, ARI incidence was also significantly lower in infants whose mothers were more highly educated (adjusted IRR: 0.57; 95% CI: 0.47–0.70), as well as in infants whose mothers were aged ≥35 years at delivery (adjusted IRR: 0.49; 95% CI: 0.33–0.72) and in infants born between Oct and Dec, which is the end of the rainy season/beginning of the cooler dry season (adjusted IRR: 0.75; 95% CI :0.60–0.94; Table 3). ARI incidence was again significantly higher in infants with older siblings than in those without (adjusted IRR: 1.2; 95% CI: 1.0–1.4).
The risk factor analysis considered ARI episodes detected through clinical surveillance and hospital admission records, not self-reported episodes. Because the number of hospital admissions with ARI was small, this endpoint was not examined separately in the regression analysis.
Clinical Characteristics of Infants Hospitalized with ARI
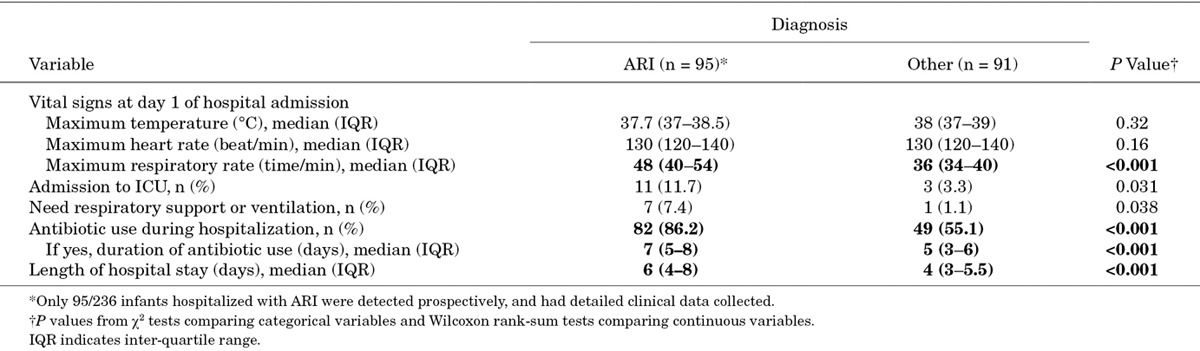
Among the hospitalized infants detected prospectively, on whom detailed clinical data could be collected, infants hospitalized with ARI were more likely to be admitted to the intensive care unit and to be treated with antibiotics compared with the group of infants admitted with other infectious diagnoses, which included dengue (6.6% of other diagnoses), diarrheal disease (52.7%), hand foot and mouth disease (9.9%), sepsis (3.3%), other systemic febrile illness (14.3%) and others (13.2%; Table 4). Infants admitted with ARI tended to have a longer duration of antibiotic use and a longer length of hospital stay than infants with other diagnoses.
TABLE 4.
Clinical Characteristics of Infants Hospitalized with ARI Compared with Other Infections
Viral Etiology of ARI
Outpatient ARI
Among a random sample of 566 NPS collected from infants with ARI who were managed as outpatients (22% of the total samples collected), at least one respiratory virus was detected in 347 samples (61%). In HCMC, 63/119 samples (53%) were positive; and in Dong Thap, 284/447 samples (64%) were positive. The most common viruses identified were rhinovirus (54% and 62% of total positive samples in HCMC and Dong Thap, respectively), coronavirus (11% and 9%), Flu A (11% and 8%), RSV (10% and 9%), Boca (8% and 9%; Fig. 1A). Co-detections (2 or 3 viruses) were found in 11% and 17% of positive samples in HCMC and Dong Thap, respectively; the most common viral co-infections were Rhi and ENT, followed by Rhi and Boca, and Rhi and AdV.
FIGURE 1.
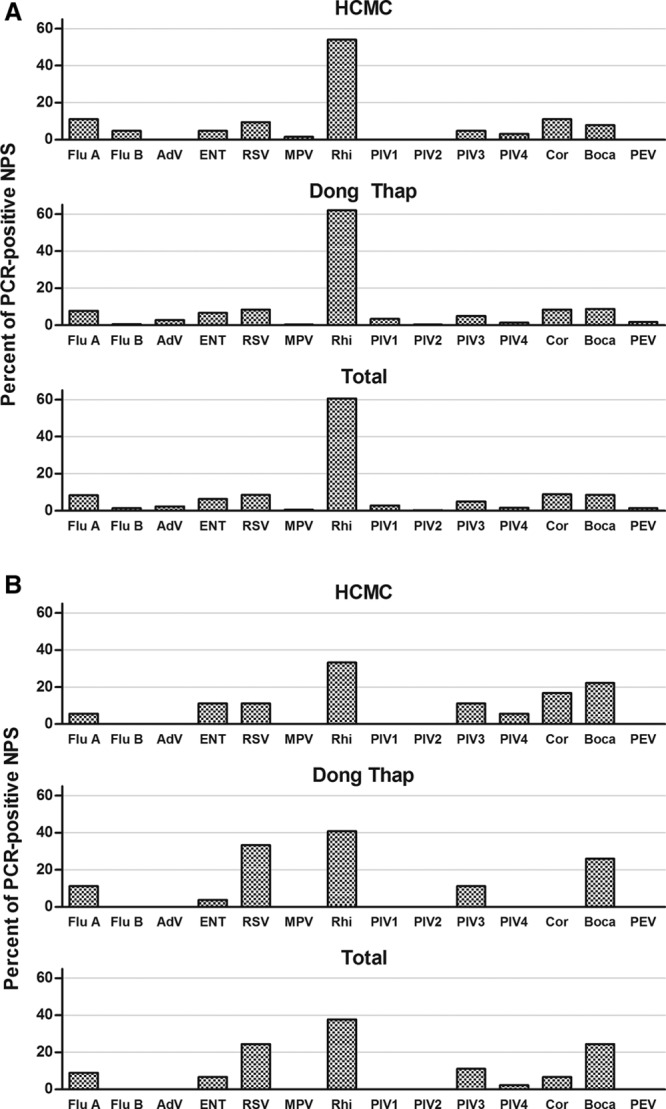
Prevalence of respiratory viruses detected in positive NPS from infants with ARI. Bars The proportion of all PCR-positive swabs collected from (A) infants with ARI treated as outpatients or (B) infants with ARI admitted to hospital that were positive for each of the viral pathogens (n = 14, on x axis) by multiplex PCR, in each of the study sites and in the total cohort. Flu A indicates Influenza A; Flu B, Influenza B; AdV, Adenovirus; ENT, Enterovirus; RSV, Respiratory syncytial virus (A & B); MPV, Metapneumovirus; Rhi, Rhinovirus (A, B & C); PIV1, Parainfluenzavirus 1; PIV2, Parainfluenzavirus 2; PIV3, Parainfluenzavirus 3; PIV4, Parainfluenzavirus 4; Cor, Coronavirus (1 & 2); Boca, Human Bocavirus; PEV, Parechovirus.
Hospitalized ARI
NPS samples were collected from only 64/236 infants hospitalized with ARI and all were tested. The main reason for noncollection was that the admission was identified only retrospectively, so study staff was not alerted to collect acute specimens. One or more viruses were detected in 45 samples (70%); 18/23 (78%) in HCMC and 27/41 (66%) in Dong Thap. The most common viruses were rhinovirus (33% and 41% of total positive samples in HCMC and Dong Thap, respectively), RSV (11% and 33%), Boca (22% and 26%), PIV3 (11% and 11%) and Flu A (6% and 11%; Fig. 1B). Codetections (2 or 3 viruses) were found in 17% and 22% of positive samples in HCMC and Dong Thap, respectively; the most common viral clusters were Rhi and ENT, Rhi and Boca, and RSV and Boca.
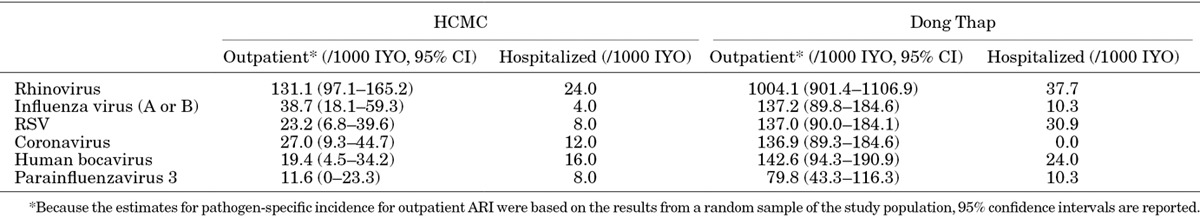
Estimates of Virus-specific Incidence
The above PCR results were extrapolated to estimate the virus-specific incidence of illness and hospitalization during the first year of life in our study population (Table 5).
TABLE 5.
Pathogen-specific Minimum Estimates of Incidence of Illness and Hospital Admission in the First Year of Life
Age Distribution and Seasonality of ARI
Approximately two-thirds of all ARI episodes occurred in infants ≥6 months; there were 1148 ARI episodes per 1000 IYO in infants ≤6 months and 1592/1000 IYO in infants >6 months. Infants with coronavirus- or parainfluenza 3-associated ARI were significantly younger than those with influenza (A or B)-, bocavirus-, RSV- or rhinovirus-associated ARI (mean age: Cor 6.2 and PIV-3 6.5 months versus Flu 8.7, Boca 7.7, RSV 7.2 and Rhi 7.2 months; one-way analysis of variance; P = 0.016).
No clear seasonal pattern was observed in the overall incidence of ARI presentations; however, there were pathogen-specific seasonal trends (Fig. 2). Influenza virus A and RSV infections were highly clustered in the rainy months between July and October 2010, with a second cluster of influenza virus A infections in the dry season between February and May of 2011, whereas other viruses were detected throughout the study period.
FIGURE 2.

Seasonality of virus detection in NPS from infants with ARI. Bars The number of NPS samples from infants with ARI that were positive (black) or negative (white) for selected viral pathogens in multiplex PCR, by month and study site.
ARI incidence stratified by calendar quarter and age-group (≤6 or >6 months) confirmed that older infants generally experienced more ARI episodes than younger infants, independent of season (Fig. 3), but this differed between viral etiologies (see Fig., Supplemental Digital Content 2, http://links.lww.com/INF/C71). The highly clustered influenza A infections during July–October occurred almost exclusively in infants >6 months old.
FIGURE 3.
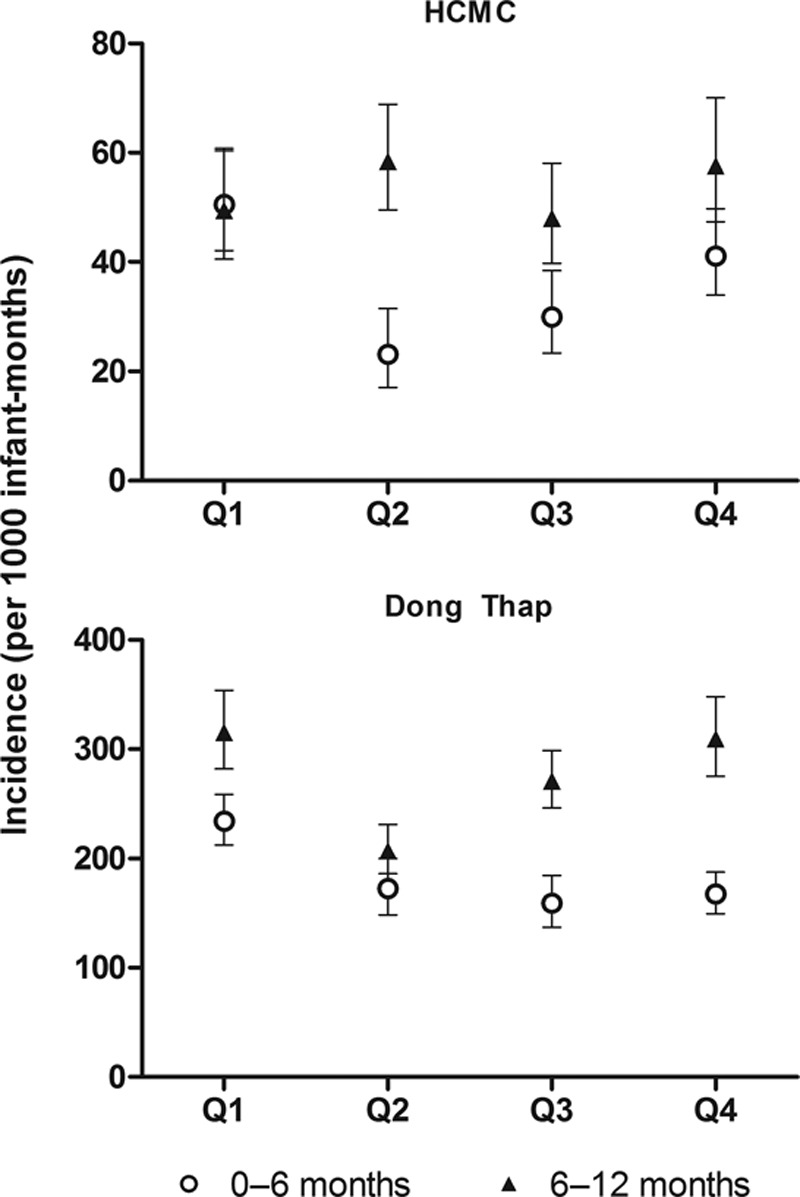
Incidence of ARI stratified by infant age and season. The incidence of ARI in the 2 study sites was calculated within strata of infant age (open circles ≤6 months or filled triangles >6 months) and calendar quarter (Q), to account for any potential bias introduced by time-varying differences in the age-structure of the cohort (see Fig., Supplemental Digital Content 2, http://links.lww.com/INF/C71). Ninety-five percent confidence intervals around the stratum-specific incidence estimates are shown.
DISCUSSION
Our findings from a large prospective infant cohort study, spanning a highly urban and a semi-rural setting in southern Vietnam, demonstrate a high incidence of ARI during the first year of life and a breadth of associated viral pathogens. Other studies from other tropical and sub-tropical countries have demonstrated similar or higher ARI incidence in infants,8,9,20 which highlights the significant burden that respiratory infections place on fragile health care systems in these settings.
The incidence of ARI was higher in infants ≥6 months of age than in those <6 months in our cohort, consistent with other reports.10,13,21,22 This age distribution was pathogen-specific, with coronavirus detected in a greater proportion of ARI in younger infants than older infants, influenza viruses detected mainly in infants >6 months, and rhinovirus detected in similar proportions across all age groups. Other authors have reported that the incidence of RSV infection is higher in older infants than in those <6 months,9,23 but that infants <6 months are at increased risk of severe disease and hospitalization.2,4,9,12,23 Although the total number of laboratory-confirmed RSV cases in our cohort was moderately low (n = 41), only 7/30 (23%) of outpatient RSV-associated ARI occurred in the first 6 months of life compared with 6/11 (55%) of RSV-associated hospital admissions in our study (P = 0.06), which is consistent with the pattern of age-dependent disease severity reported by others. These age-related observations are relevant to understanding maternally acquired passive immunity and the targeting of available (eg, influenza) and potential future (eg, RSV) vaccinations against viral respiratory infections in young children.
A viral pathogen was detected in the majority of respiratory specimens collected from infants presenting with ARI, and the overall proportion positive (61% in outpatients and 70% in inpatients) was within the same range as other studies that used comparable sampling and molecular diagnostic tests (61%2 and 72%–82%7,24). Rhinovirus was the predominant virus detected both in infants admitted to hospital and those seen as outpatients, where it accounted for more than half of the PCR-positive samples. RSV, bocavirus, influenza virus A, coronavirus and parainfluenzavirus 3 (predominantly in inpatients) were the next most commonly detected viruses, which broadly reflects the etiological patterns reported from other settings.7,8,24–27
We extrapolated from the subset of respiratory specimens tested by PCR, to infer virus-specific minimum incidence rates in the first year of life for the predominant pathogens. These estimates are subject to the limitation of case ascertainment, discussed further below, which partly explains the differences between study sites and means that these will be underestimates of the true virus-specific incidence. This extrapolation furthermore assumes a similar pathogen distribution among the ARI episodes from which no specimen was available [293/2885 (10%) of outpatient ARI and 172/236 (73%) of inpatient ARI episodes] as among those specimens tested. An interaction between the time-varying age-distribution of infants under observation and the seasonality of the viruses could conceivably have biased these estimates in either direction, but we were not able to weight the incidence estimates to adjust for this due to very small counts of pathogen-specific infections in many strata when stratifying by study site, inpatient/outpatient, age group and calendar quarter. Nonetheless, these data illustrate the high rates of respiratory virus infection experienced by infants during their first year of life, especially in the semi-rural cohort. The estimated incidence of influenza virus-associated ARI in our Dong Thap cohort is very similar to an infant cohort in rural India8 where cases were actively detected through household visits, and 1.5- to threefold higher than reported from a clinic-based study in the US.12 Our estimates of RSV-associated ARI hospitalizations in the semi-rural cohort are also very similar to those from a community-based cohort in Kenya9 and a clinic-based study in the US,12 although the overall incidence of RSV in our study was substantially lower than reported from the Kenyan cohort or the rural Indian cohort.
To the best of our knowledge, this study is the first birth cohort study in tropical Southeast Asia including surveillance for infectious diseases, and its strengths lie in its longitudinal population-based design, large sample size from both urban and semi-rural settings, and linkage between sociodemographic, clinical and laboratory data. There are however some limitations. First, our ascertainment of acute illness episodes was passive and consequently incomplete, particularly in HCMC, as compared with self-reported episodes or with hospital admission records. This is likely due to the abundance of health care providers in the city that families might use in preference to our study clinic. As a result, it is highly likely that the incidence of ARI in the HCMC cohort was considerably underestimated. The ARI incidence in our semi-rural cohort was also somewhat lower that has been reported in other studies where active case detection was employed,10 indicating that our passive clinic-based case detection might have resulted in some under-ascertainment also in the semi-rural cohort. Second, the study population was drawn from women delivering at one large hospital in each study site, who may not constitute a representative cross-section of the catchment population. Third, the great majority of infants were ≥37 weeks gestation and therefore we cannot generalize our observations to preterm babies, who may be at higher risk for ARI during infancy.28–30 Fourth, the detection of respiratory viruses by PCR in ARI patients does not necessarily imply a causative role. Rhinovirus and coronavirus have been detected frequently also in the nasopharynx of asymptomatic children;27,31–35 however, RSV and influenza viruses are rarely detected in healthy controls,31,34,35 suggesting these pathogens are causally linked to the ARI episode. Finally, bacterial pathogens are known to be contributors to ARI in young children36,37 but were not investigated in this study due to logistical and financial constraints.
CONCLUSIONS
We have demonstrated a high burden of ARI during the first year of life in southern Vietnam. A range of known viral respiratory pathogens contribute to this burden, including viruses for which vaccines are available (influenza) or in development (RSV), and the relative contribution of these viruses was broadly similar in the urban and semi-rural populations. Such age-specific data on the incidence and viral etiology of ARI in infants can inform efforts for the development and implementation of infant and maternal vaccines and other interventions to reduce ARI incidence and hospitalization rates for infants, particularly in semi-rural and rural settings.
ACKNOWLEDGMENTS
The authors are grateful to all the cohort participants and their families, the physicians, nurses and study staff at Hung Vuong Hospital, District 8 Hospital, Hospital for Tropical Diseases, Children’s Hospital No. 1, and Dong Thap Hospital, and the laboratory and support staff at OUCRU for their contributions to this study.
Supplementary Material
Footnotes
This study was supported by the Wellcome Trust (Grant 084368/Z/07/Z to C.P.S).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Williams BG, Gouws E, Boschi-Pinto C, et al. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 2.Iwane MK, Edwards KM, Szilagyi PG, et al. New Vaccine Surveillance Network. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 3.Izurieta HS, Thompson WW, Kramarz P, et al. Influenza and the rates of hospitalization for respiratory disease among infants and young children. N Engl J Med. 2000;342:232–239. doi: 10.1056/NEJM200001273420402. [DOI] [PubMed] [Google Scholar]
- 4.Jennings LC, Anderson TP, Werno AM, et al. Viral etiology of acute respiratory tract infections in children presenting to hospital. Pediatr Infect Dis J. 2004;23(11):1003–1007. doi: 10.1097/01.inf.0000143648.04673.6c. [DOI] [PubMed] [Google Scholar]
- 5.Bender JM, Ampofo K, Gesteland P, et al. Influenza virus infection in infants less than three months of age. Pediatr Infect Dis J. 2010;29:6–9. doi: 10.1097/INF.0b013e3181b4b950. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida LM, Suzuki M, Yamamoto T, et al. Viral pathogens associated with acute respiratory infections in central Vietnamese children. Pediatr Infect Dis J. 2010;29:75–77. doi: 10.1097/INF.0b013e3181af61e9. [DOI] [PubMed] [Google Scholar]
- 7.Do AH, van Doorn HR, Nghiem MN, et al. Viral etiologies of acute respiratory infections among hospitalized Vietnamese children in Ho Chi Minh City, 2004-2008. PLoS One. 2011;6:e18176. doi: 10.1371/journal.pone.0018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broor S, Parveen S, Bharaj P, et al. A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS One. 2007;2:e491. doi: 10.1371/journal.pone.0000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nokes DJ, Okiro EA, Ngama M, et al. Respiratory syncytial virus infection and disease in infants and young children observed from birth in Kilifi District, Kenya. Clin Infect Dis. 2008;46:50–57. doi: 10.1086/524019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selwyn B. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries. Rev Infect Dis. 1990;12:870–888. doi: 10.1093/clinids/12.supplement_s870. [DOI] [PubMed] [Google Scholar]
- 11.Roca A, Quintó L, Saúte F, et al. Community incidences of respiratory infections in an actively followed cohort of children <1 year of age in Manhiça, a rural area of southern Mozambique. Trop Med Int Health. 2006;11:373–380. doi: 10.1111/j.1365-3156.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- 12.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poehling KA, Edwards KM, Weinberg GA, et al. New Vaccine Surveillance Network. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 14.Anders KL, Nguyen NM, Van Thuy NT, et al. A birth cohort study of viral infections in Vietnamese infants and children: study design, methods and characteristics of the cohort. BMC Public Health. 2013;13:937. doi: 10.1186/1471-2458-13-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen RR, Schinkel J, Koekkoek S, et al. Development and evaluation of a four-tube real time multiplex PCR assay covering fourteen respiratory viruses, and comparison to its corresponding single target counterparts. J Clin Virol. 2011;51:179–185. doi: 10.1016/j.jcv.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houben ML, Bont L, Wilbrink B, et al. Clinical prediction rule for RSV bronchiolitis in healthy newborns: prognostic birth cohort study. Pediatrics. 2011;127:35–41. doi: 10.1542/peds.2010-0581. [DOI] [PubMed] [Google Scholar]
- 17.Moore HC, de Klerk N, Richmond P, et al. A retrospective population-based cohort study identifying target areas for prevention of acute lower respiratory infections in children. BMC Public Health. 2010;10:757. doi: 10.1186/1471-2458-10-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143(5 Suppl):S118–S126. doi: 10.1067/s0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 19.Saunders NR, Tennis O, Jacobson S, et al. Parents’ responses to symptoms of respiratory tract infection in their children. CMAJ. 2003;168:25–30. [PMC free article] [PubMed] [Google Scholar]
- 20.Simões EA, Mutyara K, Soh S, et al. The epidemiology of respiratory syncytial virus lower respiratory tract infections in children less than 5 years of age in Indonesia. Pediatr Infect Dis J. 2011;30:778–784. doi: 10.1097/INF.0b013e318218ab9e. [DOI] [PubMed] [Google Scholar]
- 21.von Linstow ML, Holst KK, Larsen K, et al. Acute respiratory symptoms and general illness during the first year of life: a population-based birth cohort study. Pediatr Pulmonol. 2008;43:584–593. doi: 10.1002/ppul.20828. [DOI] [PubMed] [Google Scholar]
- 22.Kusel MM, de Klerk NH, Holt PG, et al. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 23.Ohuma EO, Okiro EA, Ochola R, et al. The natural history of respiratory syncytial virus in a birth cohort: the influence of age and previous infection on reinfection and disease. Am J Epidemiol. 2012;176:794–802. doi: 10.1093/aje/kws257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurent C, Dugué AE, Brouard J, et al. Viral epidemiology and severity of respiratory infections in infants in 2009: a prospective study. Pediatr Infect Dis J. 2012;31:827–831. doi: 10.1097/INF.0b013e3182566005. [DOI] [PubMed] [Google Scholar]
- 25.Annamalay AA, Khoo SK, Jacoby P, et al. Kalgoorlie Otitis Media Research Project Team. Prevalence of and risk factors for human rhinovirus infection in healthy aboriginal and non-aboriginal Western Australian children. Pediatr Infect Dis J. 2012;31:673–679. doi: 10.1097/INF.0b013e318256ffc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fattouh AM, Mansi YA, El-Anany MG, et al. Acute lower respiratory tract infection due to respiratory syncytial virus in a group of Egyptian children under 5 years of age. Ital J Pediatr. 2011;37:14. doi: 10.1186/1824-7288-37-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berkley JA, Munywoki P, Ngama M, et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303:2051–2057. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paramore LC, Mahadevia PJ, Piedra PA. Outpatient RSV lower respiratory infections among high-risk infants and other pediatric populations. Pediatr Pulmonol. 2010;45:578–584. doi: 10.1002/ppul.21224. [DOI] [PubMed] [Google Scholar]
- 29.Lacaze-Masmonteil T, Truffert P, Pinquier D, et al. Lower respiratory tract illness and RSV prophylaxis in very premature infants. Arch Dis Child. 2004;89:562–567. doi: 10.1136/adc.2003.028282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joffe S, Escobar GJ, Black SB, et al. Rehospitalization for respiratory syncytial virus among premature infants. Pediatrics. 1999;104(4 Pt 1):894–899. doi: 10.1542/peds.104.4.894. [DOI] [PubMed] [Google Scholar]
- 31.Jansen RR, Wieringa J, Koekkoek SM, et al. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Zalm MM, Wilbrink B, van Ewijk BE, et al. Highly frequent infections with human rhinovirus in healthy young children: a longitudinal cohort study. J Clin Virol. 2011;52:317–320. doi: 10.1016/j.jcv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Fry AM, Lu X, Olsen SJ, et al. Human rhinovirus infections in rural Thailand: epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS One. 2011;6:e17780. doi: 10.1371/journal.pone.0017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwane MK, Prill MM, Lu X, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011;204:1702–1710. doi: 10.1093/infdis/jir634. [DOI] [PubMed] [Google Scholar]
- 35.Hammitt LL, Kazungu S, Morpeth SC, et al. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis. 2012;54(Suppl 2):S190–S199. doi: 10.1093/cid/cir1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vu HT, Yoshida LM, Suzuki M, et al. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr Infect Dis J. 2011;30:11–18. doi: 10.1097/INF.0b013e3181f111a2. [DOI] [PubMed] [Google Scholar]


